Parenterally Administered P24-VP8* Nanoparticle Vaccine Conferred Strong Protection against Rotavirus Diarrhea and Virus Shedding in Gnotobiotic Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Rotavirus
2.2. Vaccine
2.3. Gn Pigs and Treatments
2.4. Assessment of Diarrhea and Detection of RV Shedding in Feces by Rotavirus Antigen ELISA and CCIF
2.5. RV-Specific Serum VN, and VP8*-Specific Serum and Intestinal IgA and IgG Antibody Titration
2.6. Flow Cytometry
2.7. Statistical Analysis
3. Results
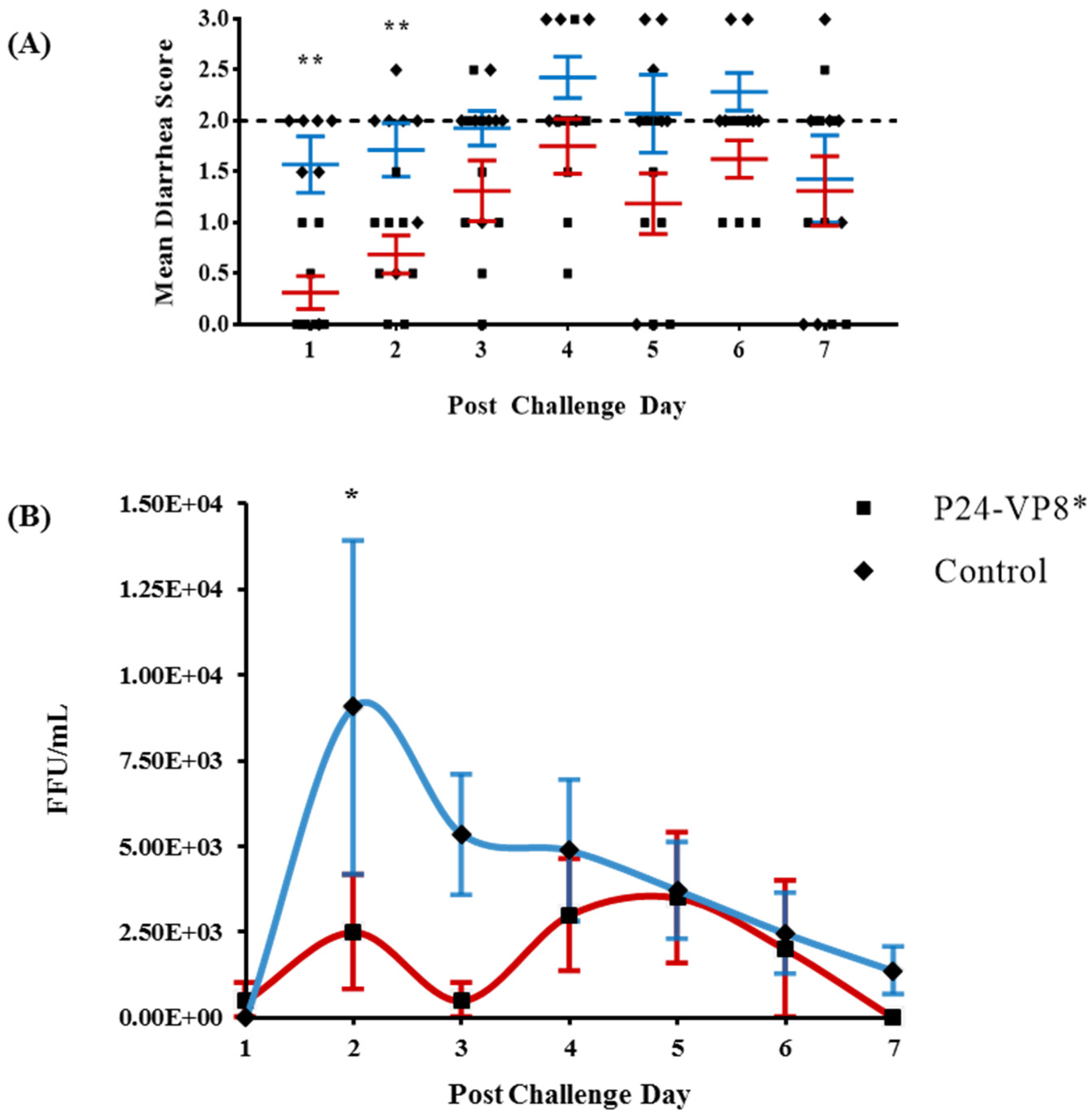
3.1. Protection against Diarrhea and Virus Shedding upon Challenge with VirHRV
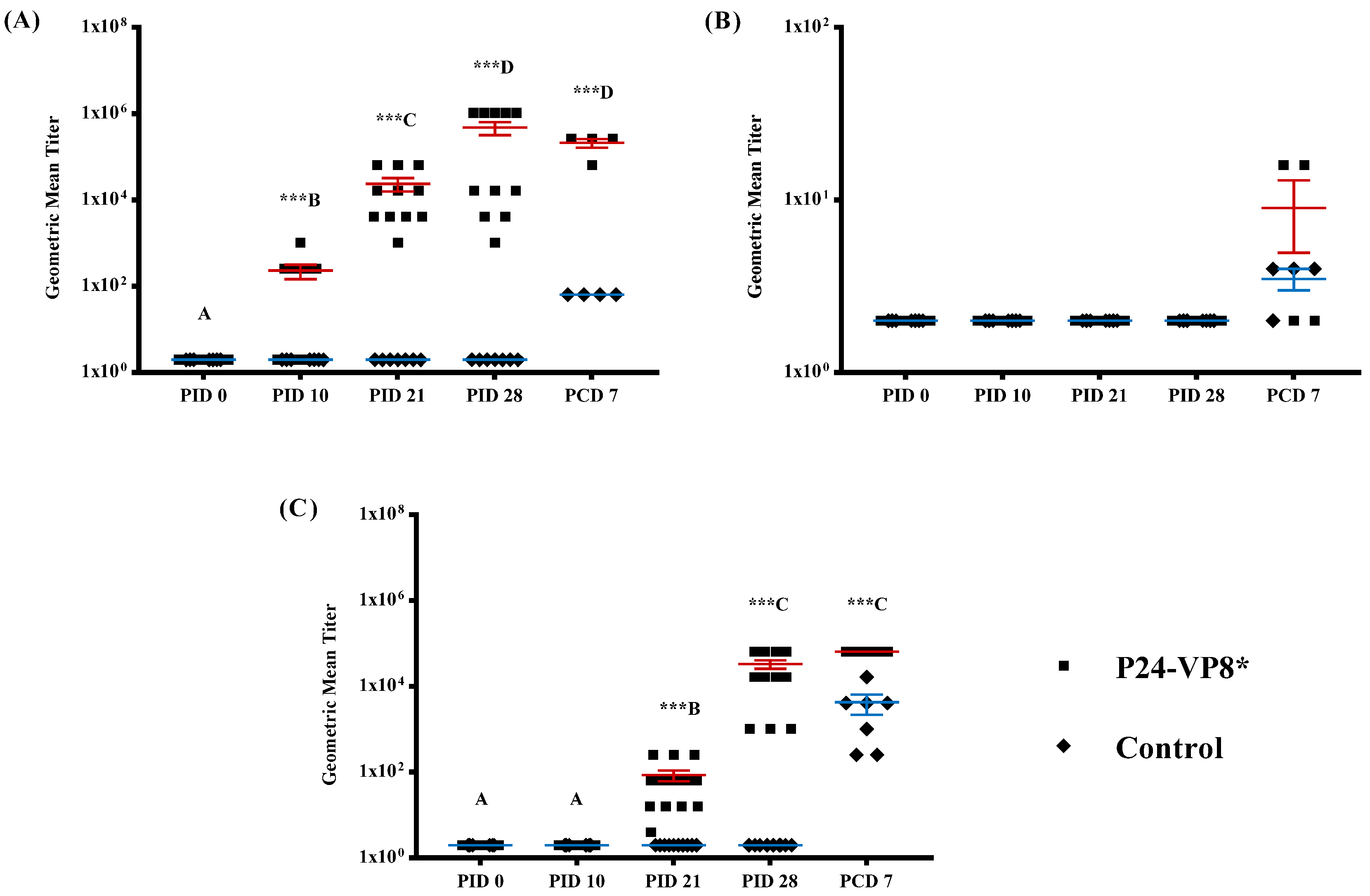
3.2. Strong VP8*-Specific IgG and Virus Neutralizing, but Lack of IgA, Antibody Responses in Serum
3.3. Lack of P24-VP8* Specific Antibody Responses in the Intestines
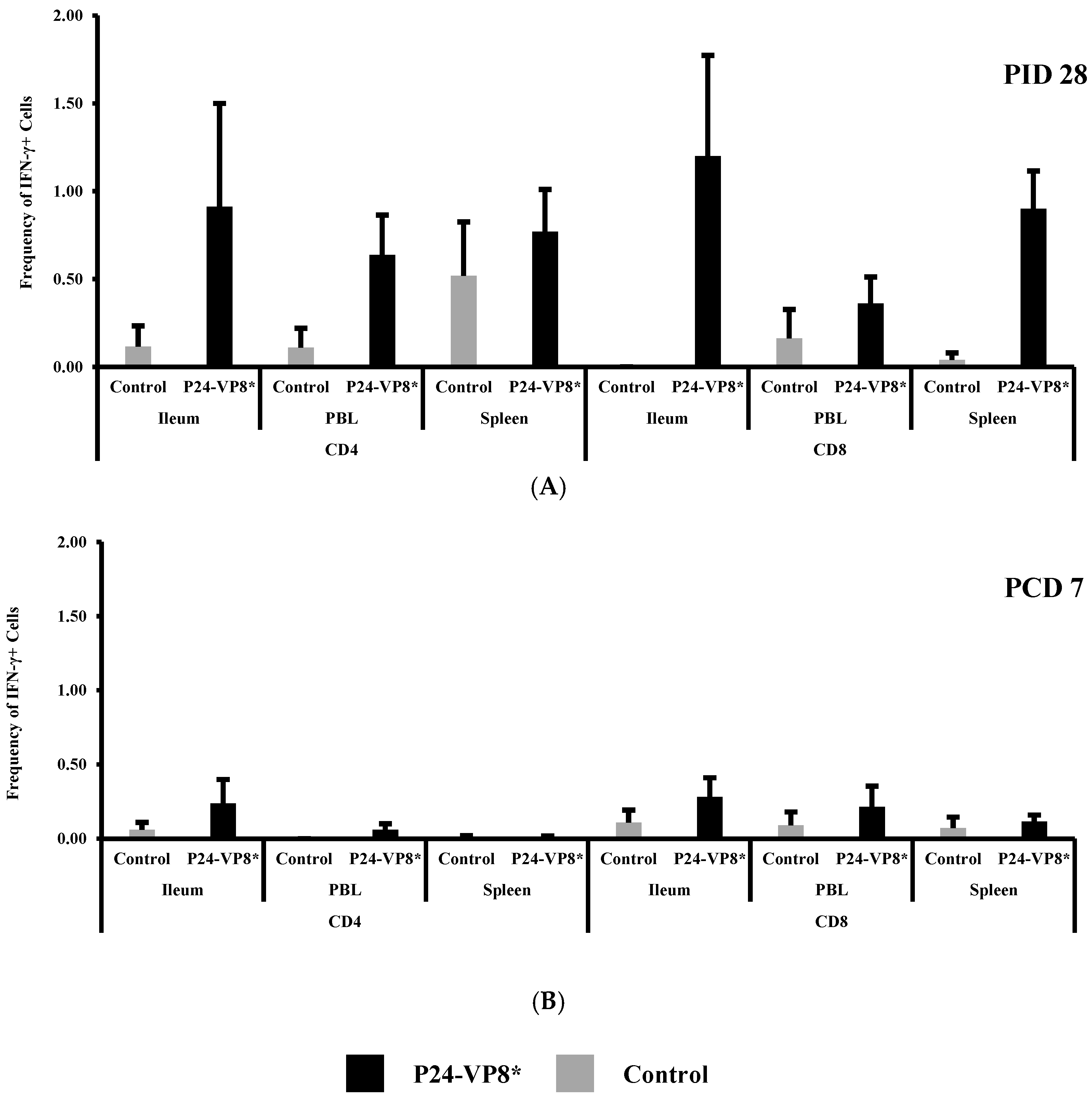
3.4. P24-VP8* Vaccine did not Induce Strong VP8*-Specific Effector T Cell Responses in Intestinal and Systemic Lymphoid Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Approval
References
- IVAC. Current Vaccine Intro Status. Available online: www.view-hub.org (accessed on 11 June 2019).
- Gilmartin, A.A.; Petri, W.A. Exploring the role of environmental enteropathy in malnutrition, infant development and oral vaccine response. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140143. [Google Scholar] [CrossRef]
- Boom, J.A.; Tate, J.E.; Sahni, L.C.; Rench, M.A.; Hull, J.J.; Gentsch, J.R.; Patel, M.M.; Baker, C.J.; Parashar, U.D. Effectiveness of Pentavalent Rotavirus Vaccine in a Large Urban Population in the United States. Pediatrics 2010, 125, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Vesikari, T.; Karvonen, A.; Prymula, R.; Schuster, V.; Tejedor, J.C.; Cohen, R.; Meurice, F.; Han, H.; Damaso, S.; Bouckenooghe, A. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: Randomised, double-blind controlled study. Lancet 2007, 370, 1757–1763. [Google Scholar] [CrossRef]
- Armah, G.E.; O Sow, S.; Breiman, R.F.; Dallas, M.J.; Tapia, M.D.; Feikin, D.R.; Binka, F.N.; Steele, A.D.; Laserson, K.F.; Ansah, N.A.; et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: A randomised, double-blind, placebo-controlled trial. Lancet 2010, 376, 606–614. [Google Scholar] [CrossRef]
- Patel, M.M.; Glass, R.; Desai, R.; E Tate, J.; Parashar, U.D. Fulfilling the promise of rotavirus vaccines: How far have we come since licensure? Lancet Infect. Dis. 2012, 12, 561–570. [Google Scholar] [CrossRef]
- Lamberti, L.M.; Ashraf, S.; Walker, C.L.F.; Black, R.E. A Systematic Review of the Effect of Rotavirus Vaccination on Diarrhea Outcomes among Children Younger Than 5 Years. Pediatr. Infect. Dis. J. 2016, 35, 992–998. [Google Scholar] [CrossRef]
- Arnold, M.M. Rotavirus Vaccines: Why Continued Investment in Research Is Necessary. Curr. Clin. Microbiol. Rep. 2018, 5, 73–81. [Google Scholar] [CrossRef]
- Desselberger, U. Differences of Rotavirus Vaccine Effectiveness by Country: Likely Causes and Contributing Factors. Pathogens 2017, 6, 65. [Google Scholar] [CrossRef]
- Aliabadi, N.; Tate, J.; Parashar, U. Potential safety issues and other factors that may affect the introduction and uptake of rotavirus vaccines. Clin. Microbiol. Infect. 2016, 22, S128–S135. [Google Scholar] [CrossRef]
- World Health Organization. Safety of rotavirus vaccines: Postmarketing surveillance in the WHO Region of the Americas. Relev. Epidemiol. Hebd. 2011, 86, 66–72. [Google Scholar]
- Pecenka, C.; Debellut, F.; Bar-Zeev, N.; Anwari, P.; Nonvignon, J.; Shamsuzzaman, M.; Clark, A. Re-evaluating the cost and cost-effectiveness of rotavirus vaccination in Bangladesh, Ghana, and Malawi: A comparison of three rotavirus vaccines. Vaccine 2018, 36, 7472–7478. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, D.E.; Parashar, U.; Jiang, B. Decreased performance of live attenuated, oral rotavirus vaccines in low-income settings: Causes and contributing factors. Expert Rev. Vaccines 2018, 17, 145–161. [Google Scholar] [CrossRef] [PubMed]
- O’Ryan, M.; A Lopman, B. Parenteral protein-based rotavirus vaccine. Lancet Infect. Dis. 2017, 17, 786–787. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, P.; Tan, M.; Liu, Y.; Biesiada, J.; Meller, J.; Castello, A.A.; Jiang, B.; Jiang, X. Rotavirus VP8: Phylogeny, Host Range, and Interaction with Histo-Blood Group Antigens. J. Virol. 2012, 86, 9899–9910. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Jiang, X. Histo-blood group antigens: A common niche for norovirus and rotavirus. Expert Rev. Mol. Med. 2014, 16, e5. [Google Scholar] [CrossRef] [PubMed]
- Andres, I.; Rodriguez-Diaz, J.; Buesa, J.; Zueco, J. Yeast expression of the VP8* fragment of the rotavirus spike protein and its use as immunogen in mice. Biotechnol. Bioeng. 2006, 93, 89–98. [Google Scholar] [CrossRef]
- Dunn, S.J.; Fiore, L.; Werner, R.L.; Cross, T.L.; Broome, R.L.; Ruggeri, F.M.; Greenberg, H.B. Immunogenicity, antigenicity, and protection efficacy of baculovirus expressed VP4 trypsin cleavage products, VP5(1) and VP8 from rhesus rotavirus. Arch. Virol. 1995, 140, 1969–1978. [Google Scholar] [CrossRef]
- Fix, A.D.; Harro, C.; McNeal, M.; Dally, L.; Flores, J.; Robertson, G.; Boslego, J.W.; Cryz, S. Safety and immunogenicity of a parenterally administered rotavirus VP8 subunit vaccine in healthy adults. Vaccine 2015, 33, 3766–3772. [Google Scholar] [CrossRef]
- Gil, M.T.; De Souza, C.O.; Asensi, M.; Buesa, J. Homotypic Protection Against Rotavirus-Induced Diarrhea in Infant Mice Breast-Fed by Dams Immunized with the Recombinant VP8 Subunit of the VP4 Capsid Protein. Viral Immunol. 2000, 13, 187–200. [Google Scholar] [CrossRef]
- Marelli, B.; Pérez, A.R.; Banchio, C.; De Mendoza, D.; Magni, C. Oral immunization with live Lactococcus lactis expressing rotavirus VP8* subunit induces specific immune response in mice. J. Virol. Methods 2011, 175, 28–37. [Google Scholar] [CrossRef]
- Rodríguez-Díaz, J.; Montava, R.; Viana, R.; Buesa, J.; Pérez-Martínez, G.; Monedero, V. Oral immunization of mice with Lactococcus lactis expressing the rotavirus VP8* protein. Biotechnol. Lett. 2011, 33, 1169–1175. [Google Scholar] [CrossRef]
- Wen, X.; Cao, D.; Jones, R.W.; Li, J.; Szu, S.; Hoshino, Y. Construction and characterization of human rotavirus recombinant VP8* subunit parenteral vaccine candidates. Vaccine 2012, 30, 6121–6126. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Cao, D.; Jones, R.W.; Hoshino, Y.; Yuan, L. Tandem truncated rotavirus VP8* subunit protein with T cell epitope as non-replicating parenteral vaccine is highly immunogenic. Hum. Vaccines Immunother. 2015, 11, 2483–2489. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.K.; Kim, P.H.; Ko, E.J.; Seo, J.Y.; Seong, S.Y.; Kim, Y.H.; Kwon, I.C.; Jeong, S.Y.; Yang, J.M. Peroral immunization of microencapsulated human VP8 in combination with cholera toxin induces intestinal antibody responses. Mol. Cells 1999, 9, 609–616. [Google Scholar] [PubMed]
- Lentz, E.; Mozgovoj, M.; Bellido, D.; Santos, M.D.; Wigdorovitz, A.; Bravo-Almonacid, F. VP8* antigen produced in tobacco transplastomic plants confers protection against bovine rotavirus infection in a suckling mouse model. J. Biotechnol. 2011, 156, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Wen, K.; Cao, D.; Li, G.; Jones, R.W.; Li, J.; Szu, S.; Hoshino, Y.; Yuan, L. Inclusion of a universal tetanus toxoid CD4(+) T cell epitope P2 significantly enhanced the immunogenicity of recombinant rotavirus DeltaVP8* subunit parenteral vaccines. Vaccine 2014, 32, 4420–4427. [Google Scholar] [CrossRef]
- Xue, M.; Yu, L.; Che, Y.; Lin, H.; Zeng, Y.; Fang, M.; Li, T.; Ge, S.; Xia, N. Characterization and protective efficacy in an animal model of a novel truncated rotavirus VP8 subunit parenteral vaccine candidate. Vaccine 2015, 33, 2606–2613. [Google Scholar] [CrossRef]
- Groome, M.J.; Koen, A.; Fix, A.; Page, N.; Jose, L.; Madhi, S.A.; McNeal, M.; Dally, L.; Cho, I.; Power, M.; et al. Safety and immunogenicity of a parenteral P2-VP8-P[8] subunit rotavirus vaccine in toddlers and infants in South Africa: A randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2017, 17, 843–853. [Google Scholar] [CrossRef]
- Tan, M.; Huang, P.; Xia, M.; Fang, P.A.; Zhong, W.; McNeal, M.; Wei, C.; Jiang, W.; Jiang, X. Norovirus P Particle, a Novel Platform for Vaccine Development and Antibody Production. J. Virol. 2011, 85, 753–764. [Google Scholar] [CrossRef]
- Yuan, L.; Wen, K. Rotavirus. In Laboratory Models for Foodborne Infections; Liu, D., Ed.; CRC Press, Taylor & Francis: Boca Raton, FL, USA, 2017; pp. 95–107. [Google Scholar]
- Yuan, L.; Saif, L.J. Induction of mucosal immune responses and protection against enteric viruses: Rotavirus infection of gnotobiotic pigs as a model. Veter. Immunol. Immunopathol. 2002, 87, 147–160. [Google Scholar] [CrossRef]
- Saif, L.J.; Ward, L.A.; Yuan, L.; Rosen, B.I.; To, T.L. The gnotobiotic piglet as a model for studies of disease pathogenesis and immunity to human rotaviruses. Viral Gastroenteritis 1996, 12, 153–161. [Google Scholar]
- Birchall, M.A.; Bailey, M.; Barker, E.V.; Rothkötter, H.-J.; Otto, K.; Macchiarini, P. Model for experimental revascularized laryngeal allotransplantation. BJS 2002, 89, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Rothkötter, H.J.; Sowa, E.; Pabst, R. The pig as a model of developmental immunology. Hum. Exp. Toxicol. 2002, 21, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Desselberger, U.; Huppertz, H.I. Immune responses to rotavirus infection and vaccination and associated correlates of protection. J. Infect. Dis. 2011, 203, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Kang, S.Y.; Ward, L.A.; To, T.L.; Saif, L.J. Antibody-Secreting Cell Responses and Protective Immunity Assessed in Gnotobiotic Pigs Inoculated Orally or Intramuscularly with Inactivated Human Rotavirus. J. Virol. 1998, 72, 330–338. [Google Scholar]
- Ward, L.A.; Rosen, B.I.; Yuan, L.; Saif, L.J. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J. Gen. Virol. 1996, 77, 1431–1441. [Google Scholar] [CrossRef]
- Wen, K.; Tin, C.; Wang, H.; Yang, X.; Li, G.; Giri-Rachman, E.; Kocher, J.; Bui, T.; Clark-Deener, S.; Yuan, L. Probiotic Lactobacillus rhamnosus GG Enhanced Th1 Cellular Immunity but Did Not Affect Antibody Responses in a Human Gut Microbiota Transplanted Neonatal Gnotobiotic Pig Model. PLoS ONE 2014, 9, e94504. [Google Scholar] [CrossRef]
- Wentzel, J.F.; Yuan, L.; Rao, S.; Van Dijk, A.A.; O’Neill, H.G. Consensus sequence determination and elucidation of the evolutionary history of a rotavirus Wa variant reveal a close relationship to various Wa variants derived from the original Wa strain. Infect. Genet. Evol. 2013, 20, 276–283. [Google Scholar] [CrossRef]
- Liu, F.; Wen, K.; Li, G.; Yang, X.; Kocher, J.; Bui, T.; Jones, D.; Pelzer, K.; Clark-Deener, S.; Yuan, L. Dual Functions of Lactobacillus acidophilus NCFM at The Intermediate Dose in Protection Against Rotavirus Diarrhea in Gnotobiotic Pigs Vaccinated with a Human Rotavirus Vaccine. J. Pediatric Gastroenterol. Nutr. 2014, 58, 171. [Google Scholar] [CrossRef]
- Yuan, L.; Wen, K.; Azevedo, M.S.; Gonzalez, A.M.; Zhang, W.; Saif, L.J. Virus-specific intestinal IFN-gamma producing T cell responses induced by human rotavirus infection and vaccines are correlated with protection against rotavirus diarrhea in gnotobiotic pigs. Vaccine 2008, 26, 3322–3331. [Google Scholar] [CrossRef]
- Yuan, L.J.; Jobst, P.M.; Weiss, M. Gnotobiotic Pigs: From Establishing Facility to Modeling Human Infectious Diseases. In Gnotobiotics; Schoeb, T.R., Eaton, K.A., Eds.; Acamedic Press: Cambridge, MA, USA, 2017; pp. 349–368. [Google Scholar]
- Saif, L.; Yuan, L.; Ward, L.; To, T. Comparative Studies of the Pathogenesis, Antibody Immune Responses, and Homologous Protection to Porcine and Human Rotaviruses in Gnotobiotic Piglets. Adv. Exp. Med. Biol. 1997, 412, 397–403. [Google Scholar] [PubMed]
- Azevedo, M.S.; Yuan, L.; Jeong, K.I.; Gonzalez, A.; Nguyen, T.V.; Pouly, S.; Gochnauer, M.; Zhang, W.; Azevedo, A.; Saif, L.J. Viremia and Nasal and Rectal Shedding of Rotavirus in Gnotobiotic Pigs Inoculated with Wa Human Rotavirus. J. Virol. 2005, 79, 5428–5436. [Google Scholar] [CrossRef] [PubMed]
- Parre, V.; Hodgins, D.C.; Saif, L.J.; Yuan, L.; Kang, S.Y.; Yuan, L.; Ward, L.A.; De Arriba, L. Serum and intestinal isotype antibody responses to Wa human rotavirus in gnotobiotic pigs are modulated by maternal antibodies. J. Gen. Virol. 1999, 80, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Twitchell, E.L.; Tin, C.; Wen, K.; Zhang, H.; Becker-Dreps, S.; Azcarate-Peril, M.A.; Vilchez, S.; Li, G.; Ramesh, A.; Weiss, M.; et al. Modeling human enteric dysbiosis and rotavirus immunity in gnotobiotic pigs. Gut Pathog. 2016, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Azevedo, M.; Saif, L.J.; Gentsch, J.R.; Glass, R.I.; Jiang, B. Inactivated rotavirus vaccine induces protective immunity in gnotobiotic piglets. Vaccine 2010, 28, 5432–5436. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Twitchell, E.; Li, G.; Wen, K.; Weiss, M.; Kocher, J.; Lei, S.; Ramesh, A.; Ryan, E.P.; Yuan, L. High protective efficacy of rice bran against human rotavirus diarrhea via enhancing probiotic growth, gut barrier function, and innate immunity. Sci. Rep. 2015, 5, 15004. [Google Scholar] [CrossRef]
- Liu, F.; Li, G.; Wen, K.; Bui, T.; Cao, D.; Zhang, Y.; Yuan, L. Porcine Small Intestinal Epithelial Cell Line (IPEC-J2) of Rotavirus Infection as a New Model for the Study of Innate Immune Responses to Rotaviruses and Probiotics. Viral Immunol. 2010, 23, 135–149. [Google Scholar] [CrossRef]
- Arnold, M.; Patton, J.T.; McDonald, S.M. Culturing, storage, and quantification of rotaviruses. Curr. Protoc. Microbiol. 2009, 15, 15C-3. [Google Scholar]
- Shao, L.; Fischer, D.D.; Kandasamy, S.; Rauf, A.; Langel, S.N.; Wentworth, D.E.; Stucker, K.M.; Halpin, R.A.; Lam, H.C.; Marthaler, D.; et al. Comparative In Vitro and In Vivo Studies of Porcine Rotavirus G9P[13] and Human Rotavirus Wa G1P. J. Virol. 2015, 90, 142–151. [Google Scholar] [CrossRef]
- Saif, L.J.; Yuan, L.; Ward, L.A. Serum and intestinal isotype antibody responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J. Gen. Virol. 1998, 79, 2661–2672. [Google Scholar]
- Yuan, L.; Ward, L.A.; Rosen, B.I.; To, T.L.; Saif, L.J. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J. Virol. 1996, 70, 3075–3083. [Google Scholar] [PubMed]
- Lei, S.; Ryu, J.; Wen, K.; Twitchell, E.; Bui, T.; Ramesh, A.; Weiss, M.; Li, G.; Samuel, H.; Clark-Deener, S.; et al. Increased and prolonged human norovirus infection in RAG2/IL2RG deficient gnotobiotic pigs with severe combined immunodeficiency. Sci. Rep. 2016, 6, 25222. [Google Scholar] [CrossRef] [PubMed]
- HogenEsch, H. Mechanism of Immunopotentiation and Safety of Aluminum Adjuvants. Front. Immunol. 2013, 3, 406. [Google Scholar] [CrossRef] [PubMed]
- Westerman, L.E.; McClure, H.M.; Jiang, B.; Almond, J.W.; Glass, R.I. Serum IgG mediates mucosal immunity against rotavirus infection. Proc. Natl. Acad. Sci. USA 2005, 102, 7268–7273. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Honma, S.; Kim, I.; Kapikian, A.Z.; Hoshino, Y. Resistance to rotavirus infection in adult volunteers challenged with a virulent G1P1A[8] virus correlated with serum immunoglobulin G antibodies to homotypic viral proteins 7 and 4. J. Infect. Dis. 2009, 200, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Dennehy, P.; Keyserling, H.; Westerman, L.E.; Wang, Y.; Holman, R.C.; Gentsch, J.R.; Glass, R.I.; Jiang, B. Serum Antibody Responses in Children with Rotavirus Diarrhea Can Serve as Proxy for Protection. Clin. Diagn. Lab. Immunol. 2005, 12, 273–279. [Google Scholar] [CrossRef]
- Jiang, B.; Gentsch, J.R.; Glass, R.I. The Role of Serum Antibodies in the Protection against Rotavirus Disease: An Overview. Clin. Infect. Dis. 2002, 34, 1351–1361. [Google Scholar] [CrossRef]
- Steele, A.D.; Neuzil, K.M.; Cunliffe, N.A.; Madhi, S.A.; Bos, P.; Ngwira, B.; Witte, D.; Todd, S.; Louw, C.; Kirsten, M.; et al. Human rotavirus vaccine Rotarix™ provides protection against diverse circulating rotavirus strains in African infants: A randomized controlled trial. BMC Infect. Dis. 2012, 12, 213. [Google Scholar] [CrossRef]
| Group | Number of Pigs | Challenge | Time of Euthanasia |
|---|---|---|---|
| Control | 3 | No | PID 28 |
| Control | 7 | Yes | PCD 7 |
| P24-VP8* Vaccine | 7 | No | PID 28 |
| P24-VP8* Vaccine | 8 | Yes | PCD 7 |
| Diarrhea | Fecal Virus Shedding | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | n | % with Diarrhea a | Mean Days to Onset b | Mean Duration Days c,§ | Mean Cumulative Fecal Score c,* | % Shedding Virus a | Mean Days to Onset b | Mean Duration Days c,* | Mean Peak Titer (FFU/mL) | AUC |
| P24-VP8* | 8 | 87.5 | 4.4 (0.5) d,* | 3.3 (0.75) * | 9.1 (1.23) * | 75 | 4.8 (1.0) | 2.5 (0.89) * | 8500 (2196) * | 11,750 (3172) |
| Control | 7 | 100 | 1.6 (0.3) | 6.0 (0) | 14.3 (0.44) | 85.7 | 1.9 (0.14) | 5.9 (0.14) | 11,492 (4300) | 26,664 (10,489) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramesh, A.; Mao, J.; Lei, S.; Twitchell, E.; Shiraz, A.; Jiang, X.; Tan, M.; Yuan, L. Parenterally Administered P24-VP8* Nanoparticle Vaccine Conferred Strong Protection against Rotavirus Diarrhea and Virus Shedding in Gnotobiotic Pigs. Vaccines 2019, 7, 177. https://doi.org/10.3390/vaccines7040177
Ramesh A, Mao J, Lei S, Twitchell E, Shiraz A, Jiang X, Tan M, Yuan L. Parenterally Administered P24-VP8* Nanoparticle Vaccine Conferred Strong Protection against Rotavirus Diarrhea and Virus Shedding in Gnotobiotic Pigs. Vaccines. 2019; 7(4):177. https://doi.org/10.3390/vaccines7040177
Chicago/Turabian StyleRamesh, Ashwin, Jiangdi Mao, Shaohua Lei, Erica Twitchell, Ashton Shiraz, Xi Jiang, Ming Tan, and Lijuan Yuan. 2019. "Parenterally Administered P24-VP8* Nanoparticle Vaccine Conferred Strong Protection against Rotavirus Diarrhea and Virus Shedding in Gnotobiotic Pigs" Vaccines 7, no. 4: 177. https://doi.org/10.3390/vaccines7040177
APA StyleRamesh, A., Mao, J., Lei, S., Twitchell, E., Shiraz, A., Jiang, X., Tan, M., & Yuan, L. (2019). Parenterally Administered P24-VP8* Nanoparticle Vaccine Conferred Strong Protection against Rotavirus Diarrhea and Virus Shedding in Gnotobiotic Pigs. Vaccines, 7(4), 177. https://doi.org/10.3390/vaccines7040177






