Succinate Coenzyme A Ligase Beta-Like Protein from Trichinella spiralis Suppresses the Immune Functions of Rat PBMCs In Vitro and Inhibits the Secretions of Interleukin-17 In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Parasites
2.2. Serum Samples
2.3. Cloning and Sequence Analysis of SUCLA-β Gene
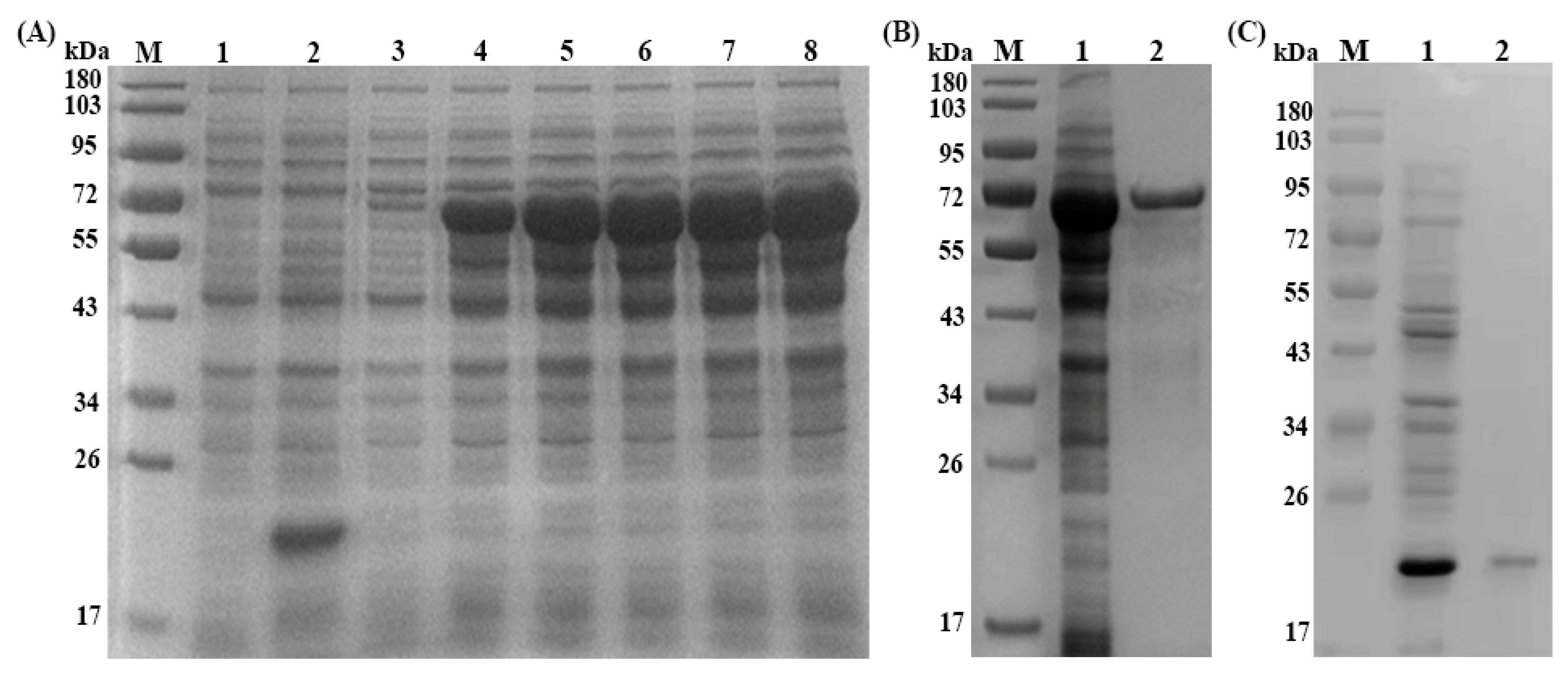
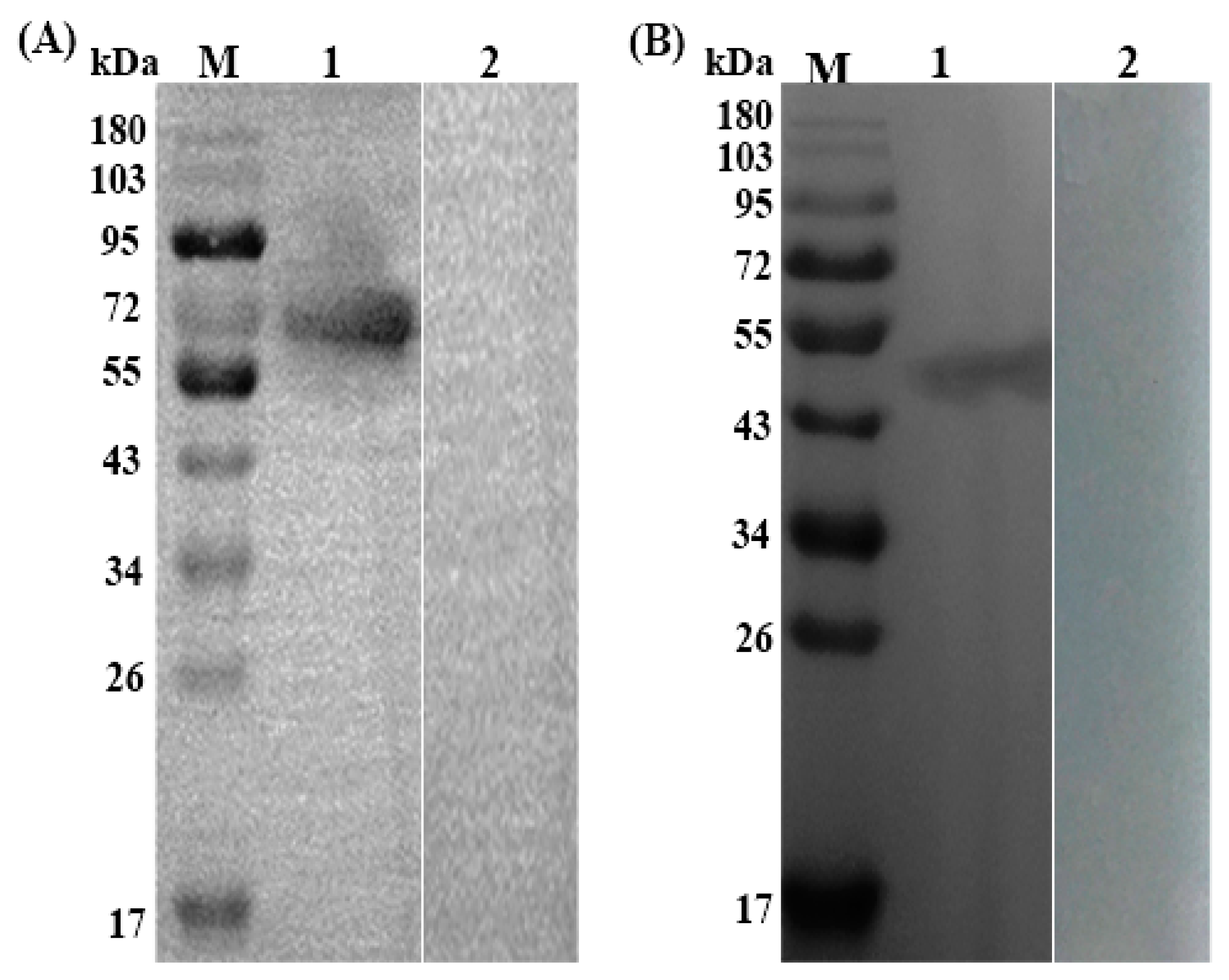
2.4. Expression and Purification of Recombinant SUCLA-β
2.5. Generation of Polyclonal Antibodies
2.6. Immunoblotting Analysis
2.7. Separation of Rat PBMCs
2.8. PBMCs Binding Assay
2.9. Cell Proliferation Assay
2.10. Cell Migration Assay
2.11. Nitric Oxide Production Assay
2.12. Determination of Phagocytic Activity
2.13. Cell Apoptosis Assay
2.14. Effect of rTs-SUCLA-β on Cytokines Expression In Vivo
2.15. Immune Protective Effects of rTs-SUCLA-β In Vivo
2.16. Statistical Analysis
3. Results
3.1. Molecular Cloning and Sequence Analysis of Ts-SUCLA-β Gene
3.2. Expression, Purification and Immunoblotting of rTs-SUCLA-β
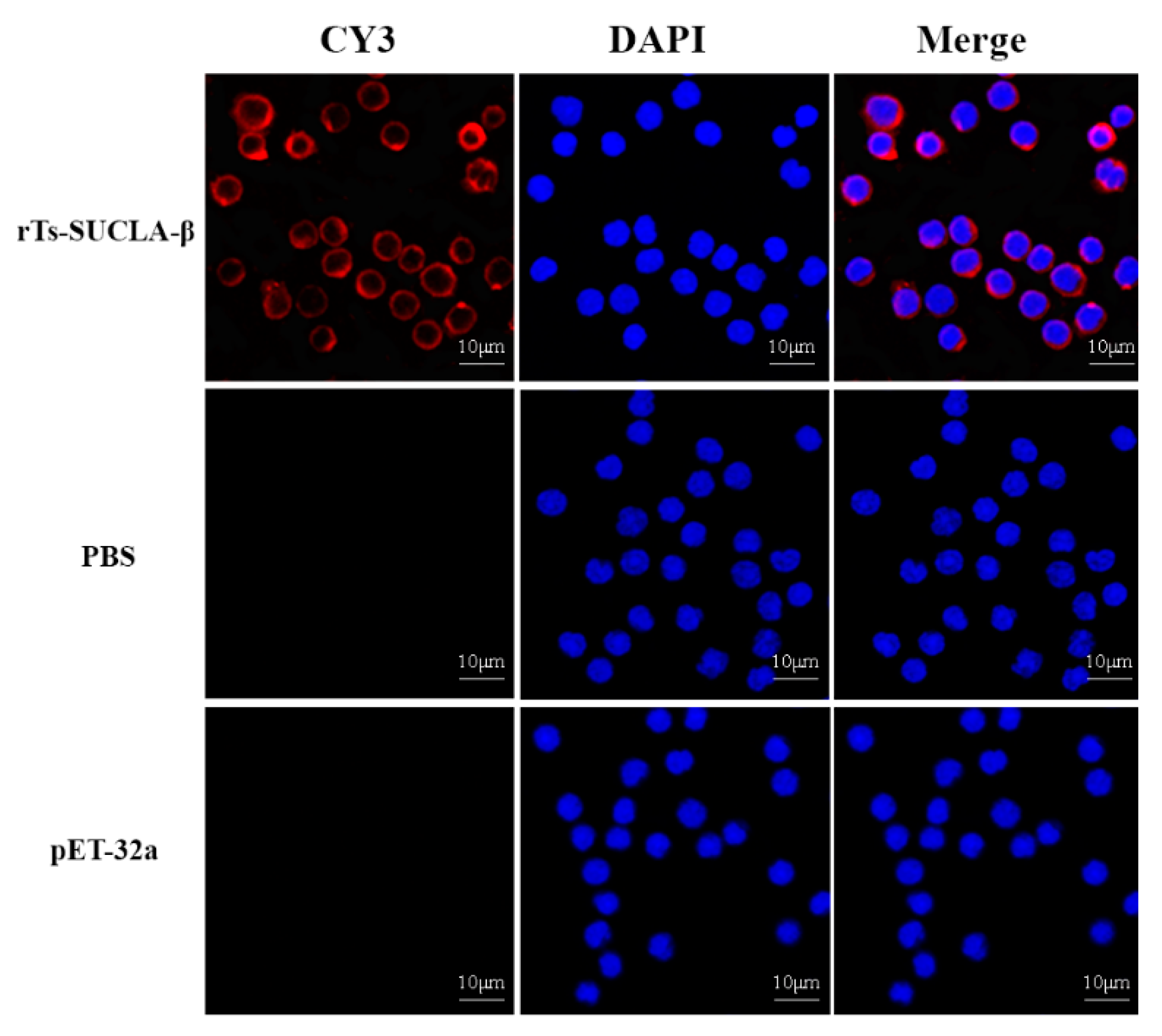
3.3. Binding of rTs-SUCLA-β to Rat PBMCs
3.4. Effect of rTs-SUCLA-β on Proliferation of PBMCs
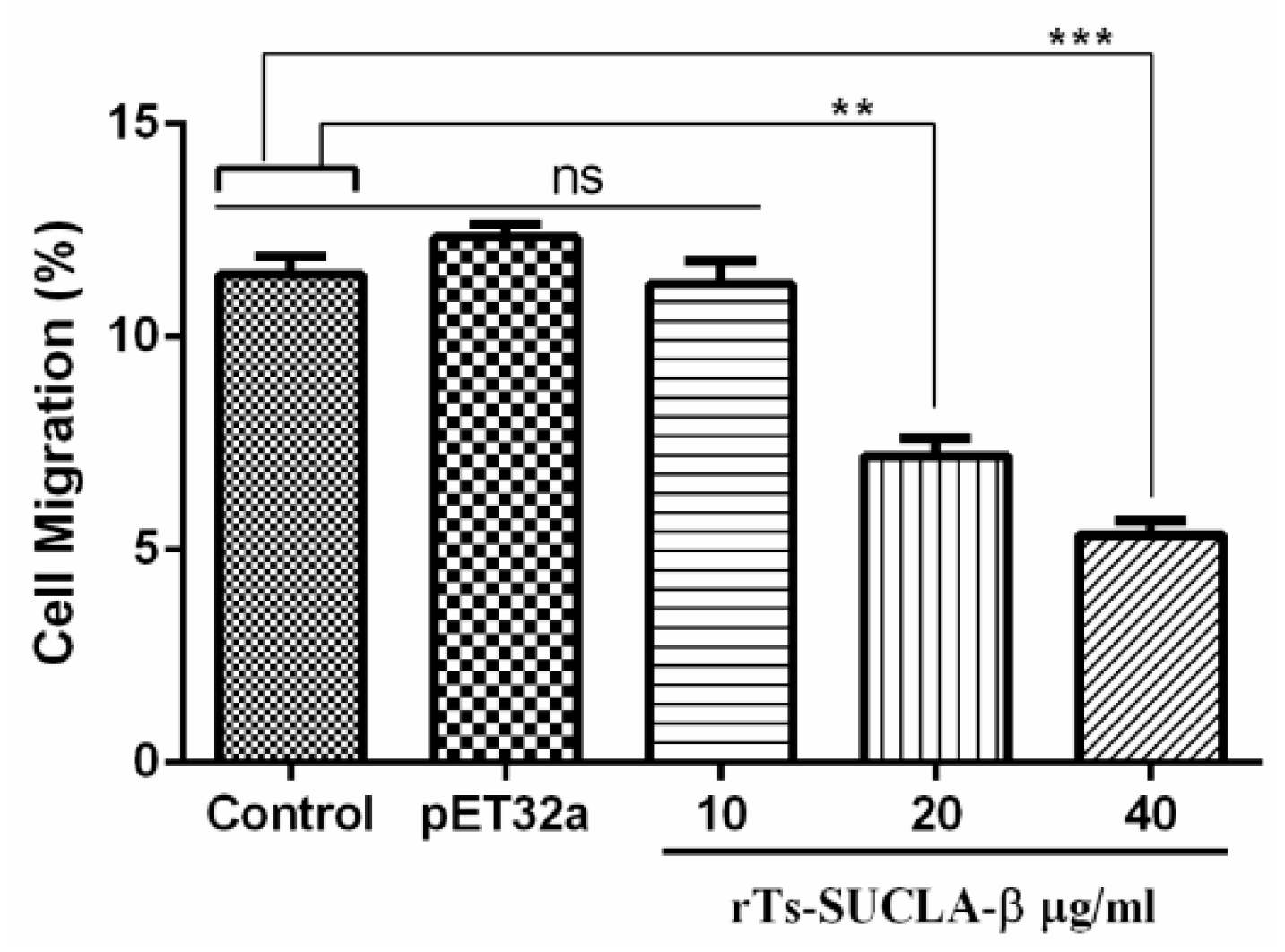
3.5. Effect of rTs-SUCLA-β on PBMCs Migration
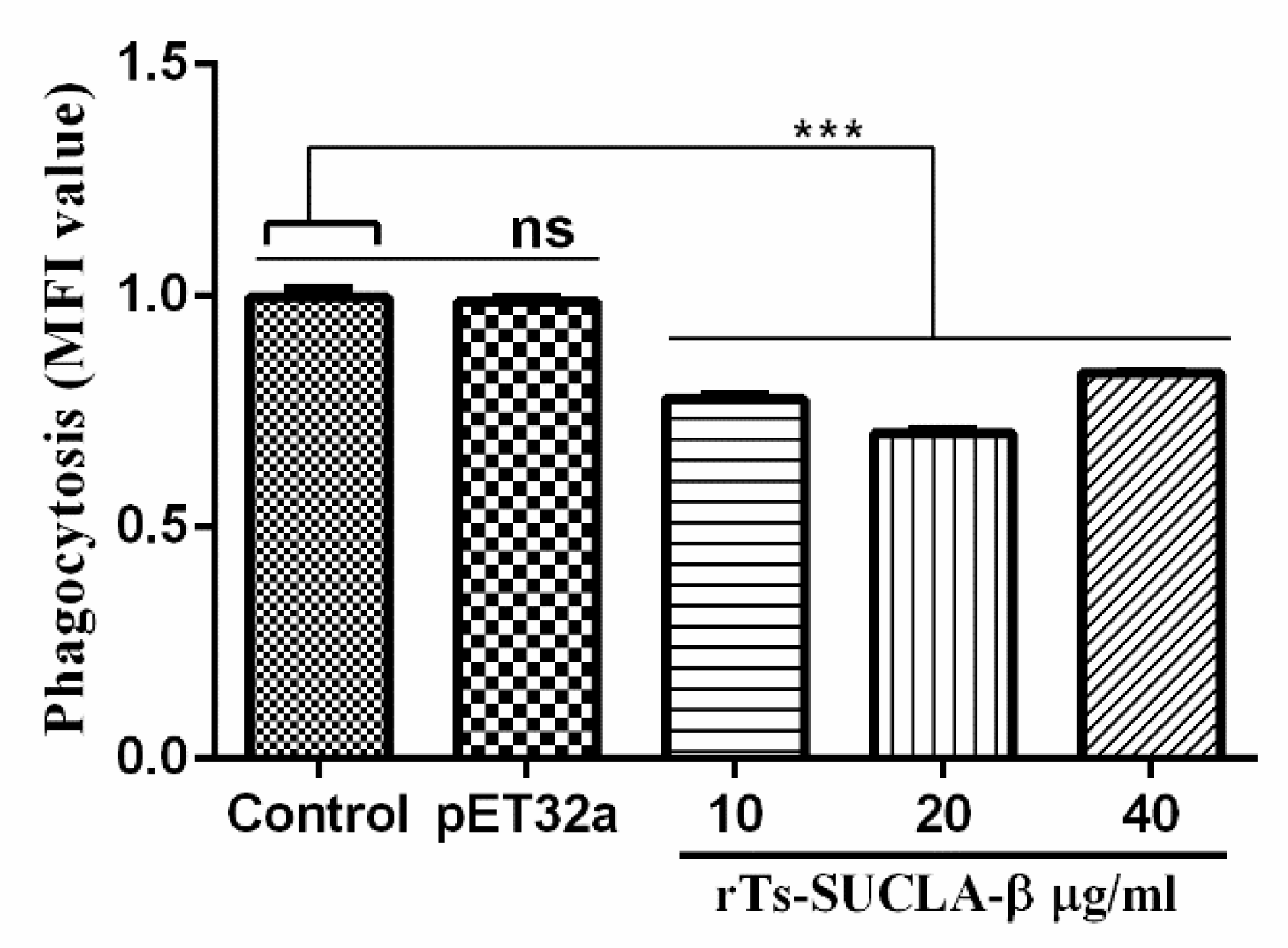
3.6. Phagocytic Activity of rTs-SUCLA-β
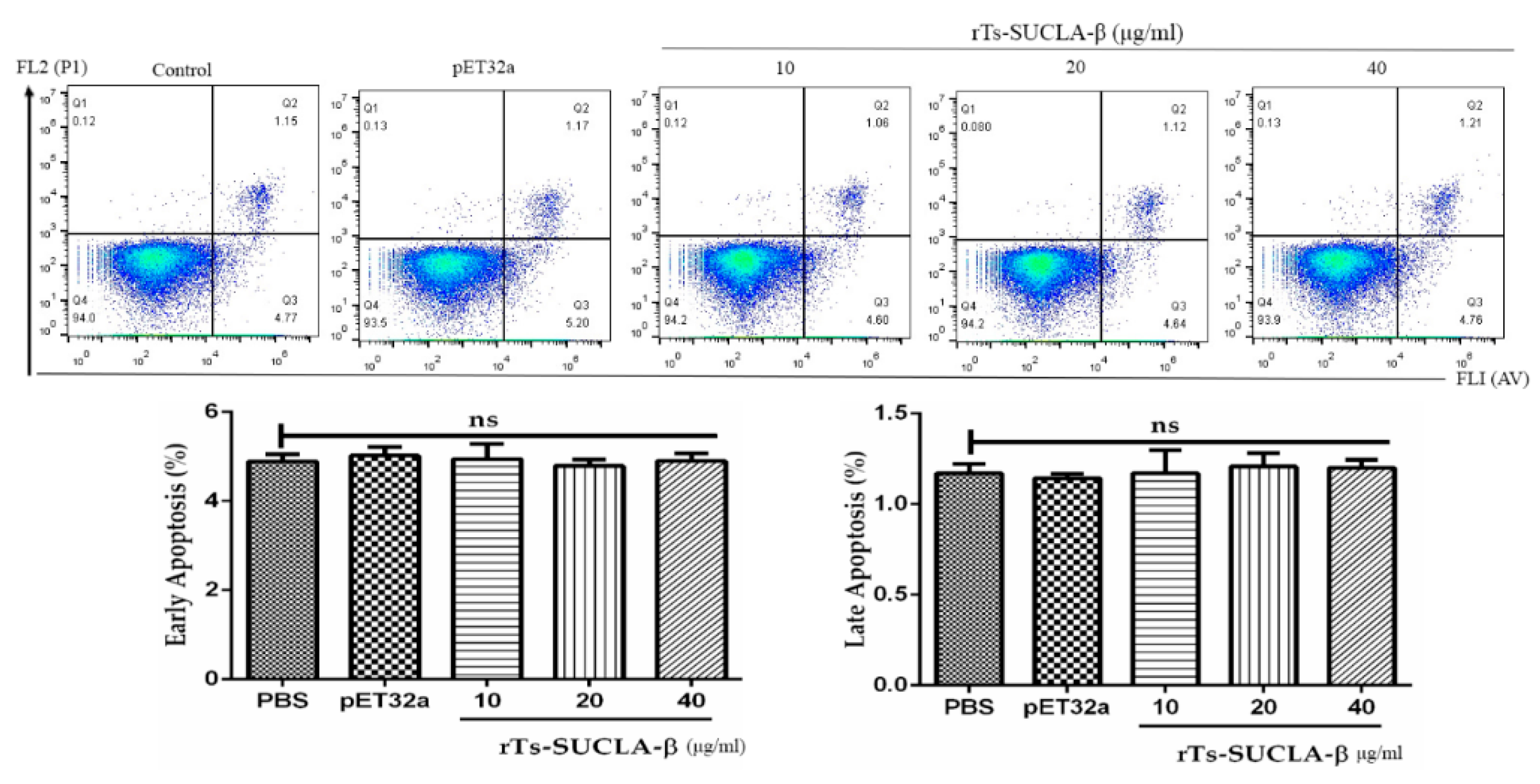
3.7. Effect of rTs-SUCLA-β on PBMCs Viability and Apoptosis
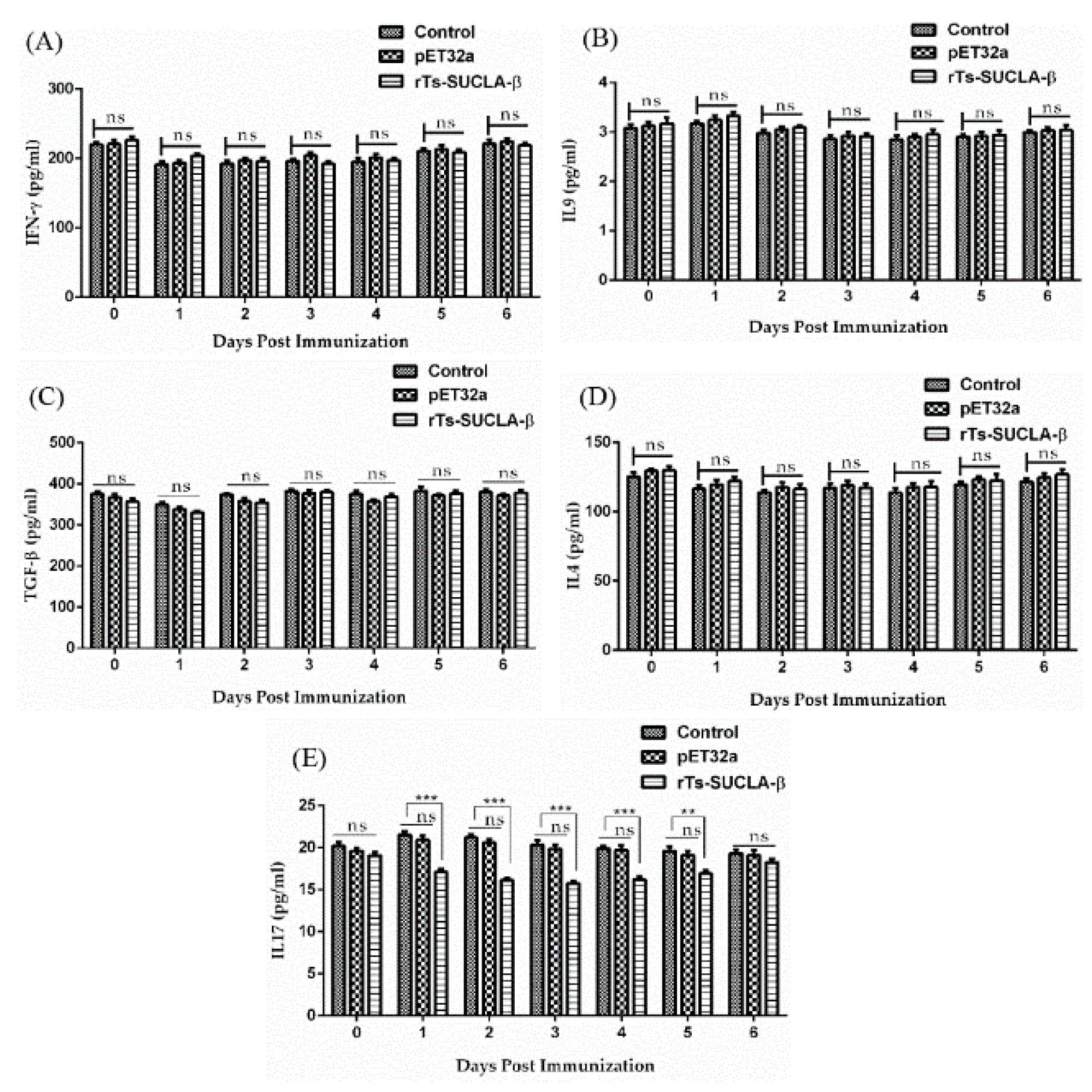
3.8. Effect of rTs-SUCLA-β on Cytokines Expression in Vivo
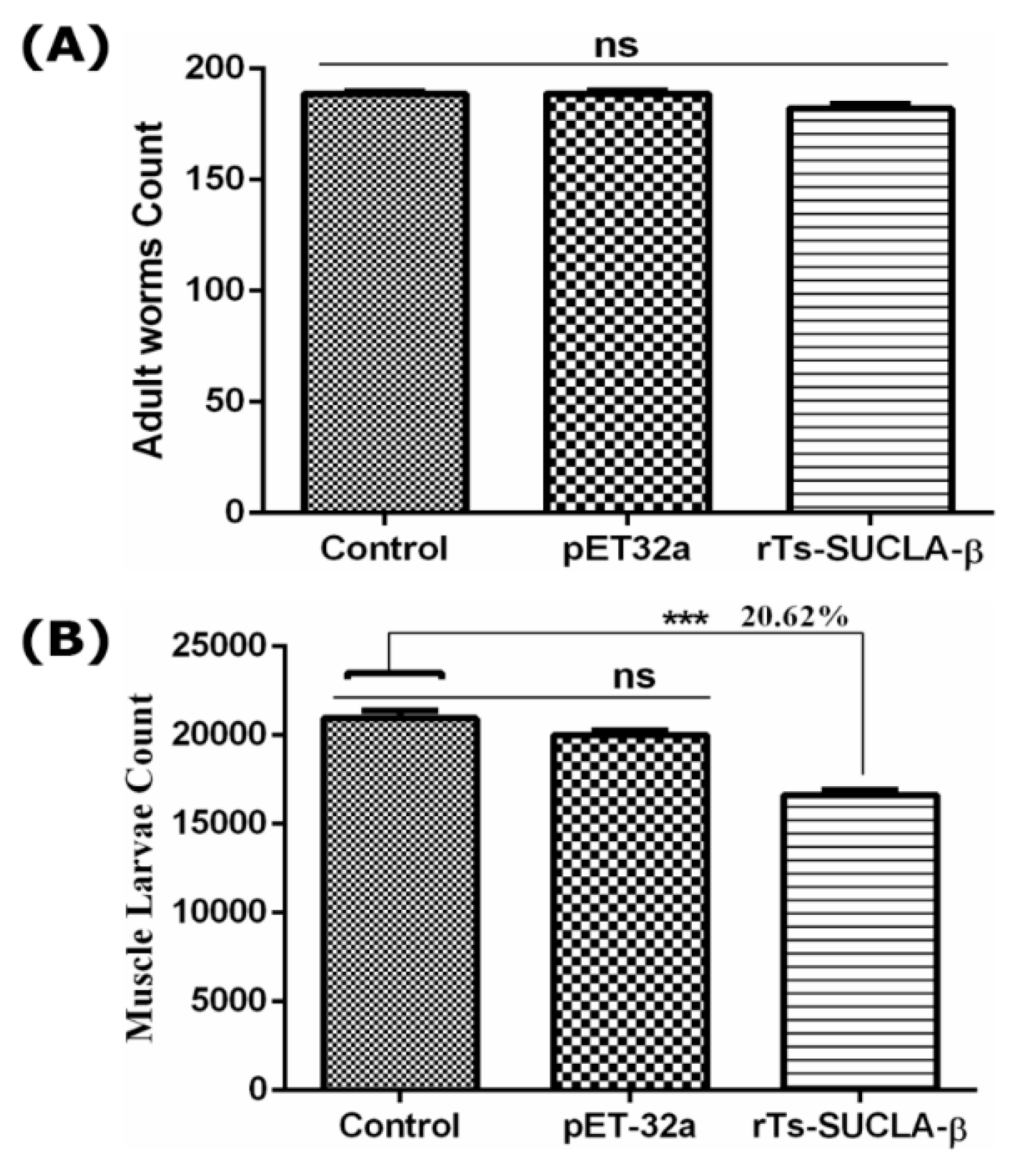
3.9. Parasite Burden Assessment
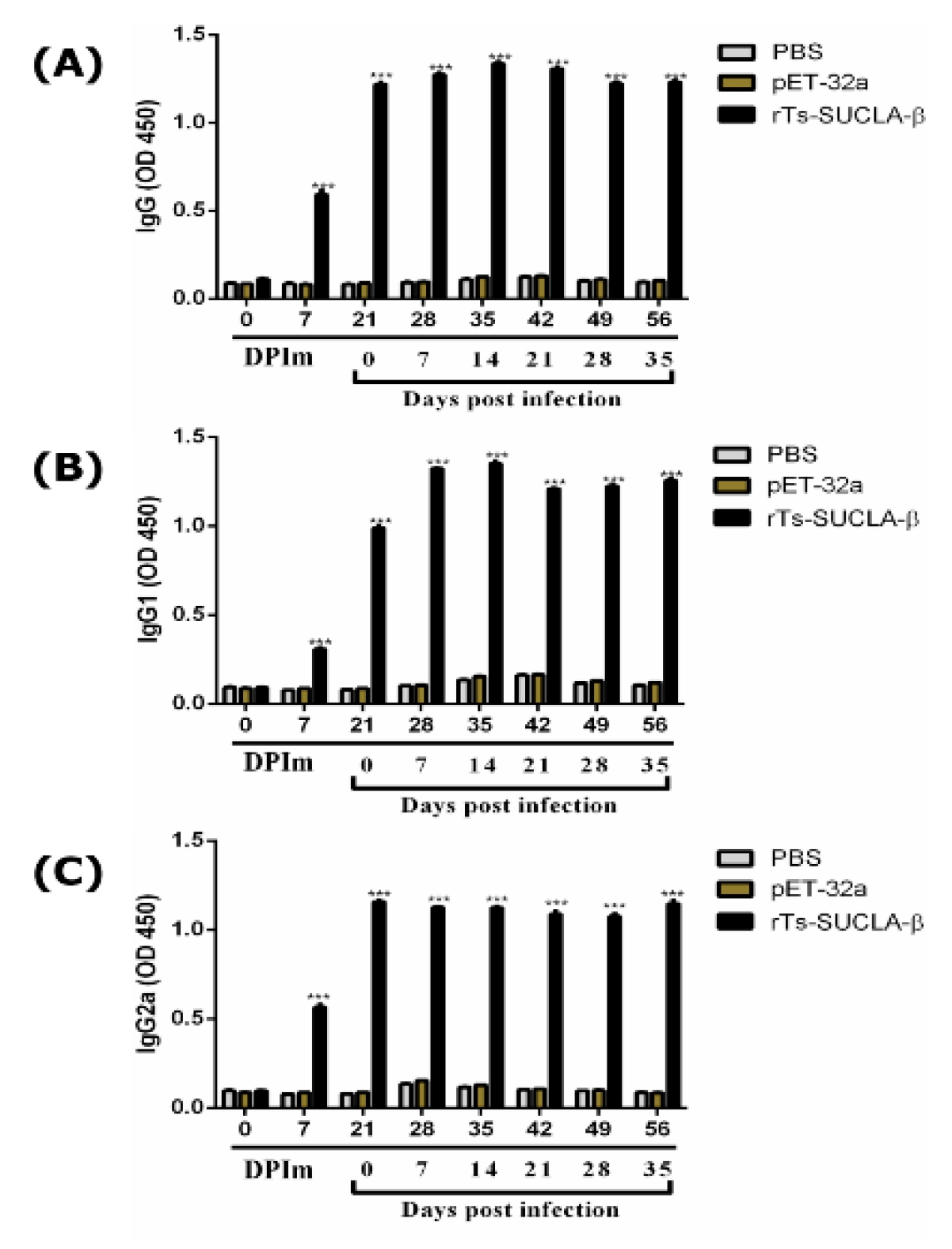
3.10. Antibody Detection
4. Discussion
5. Conclusions
6. Ethics Approval and Consent to Participate
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SUCLA-β | Succinate Coenzyme A ligase beta-like protein |
| PBMCs | Peripheral blood mononuclear cells |
| Th | T helper |
| IL | Interleukin |
| TGF-β | Transforming growth factor-beta |
| ML | Muscle larvae |
| ESPs | Excretory and secretory products |
| NBL | New born larvae |
| ORF | Open reading frame |
| CDS | Conserved domain sequences |
| LB | Luria Bertini |
| PVDF | Polyvinyl Difluoride |
| IPTG | Isopropyl-b-D-thiogalactopyranoside |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophore |
| RPMI | Roswell Park Memorial Institute |
| DPI | Days post infection |
| DPIm | Days post immunization |
| ELISA | Enzyme-linked immunosorbent assay |
References
- Kuwabara, T.; Ishikawa, F.; Kondo, M.; Kakiuchi, T. The Role of IL-17 and Related Cytokines in Inflammatory Autoimmune Diseases. Mediat. Inflamm. 2017, 2017, 3908061. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Lu, M.; Wang, W.; Jia, C.; Muhammad, E.; Yan, R.; Xu, L.; Song, X.; Li, X. HcTTR: A novel antagonist against goat interleukin 4 derived from the excretory and secretory products of Haemonchus contortus. Vet. Res. 2019, 50, 42. [Google Scholar] [CrossRef] [PubMed]
- Gruden-Movsesijan, A.; Ilic, N.; Mostarica-Stojkovic, M.; Stosic-Grujicic, S.; Milic, M.; Sofronic-Milosavljevic, L. Mechanisms of modulation of experimental autoimmune encephalomyelitis by chronic Trichinella spiralis infection in Dark Agouti rats. Parasite Immunol. 2010, 32, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Wang, Z.Q.; Li, L.G.; Cui, J. Molecular characterization of Trichinella spiralis aminopeptidase and its potential as a novel vaccine candidate antigen against trichinellosis in BALB/c mice. Parasites Vectors 2013, 6, 246. [Google Scholar] [CrossRef] [PubMed]
- Sofronic-Milosavljevic, L.; Radovic, I.; Ilic, N.; Majstorovic, I.; Cvetkovic, J.; Gruden-Movsesijan, A. Application of dendritic cells stimulated with Trichinella spiralis excretory-secretory antigens alleviates experimental autoimmune encephalomyelitis. Med. Microbiol. Immunol. 2013, 202, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Pozio, E.; Zarlenga, D.S. New pieces of the Trichinella puzzle. Int. J. Parasitol. 2013, 43, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Despommier, D.D. How does Trichinella spiralis make itself at home? Parasitol. Today 1998, 14, 318–323. [Google Scholar] [CrossRef]
- Wu, Z.; Sofronic-Milosavljevic, L.; Nagano, I.; Takahashi, Y. Trichinella spiralis: Nurse cell formation with emphasis on analogy to muscle cell repair. Parasites Vectors 2008, 1, 27. [Google Scholar] [CrossRef]
- Sofronic-Milosavljevic, L.; Ilic, N.; Pinelli, E.; Gruden-Movsesijan, A. Secretory products of Trichinella spiralis muscle larvae and immunomodulation: Implication for autoimmune diseases, allergies, and malignancies. J. Immunol. Res. 2015, 2015, 523875. [Google Scholar] [CrossRef]
- Sorobetea, D.; Svensson-Frej, M.; Grencis, R. Immunity to gastrointestinal nematode infections. Mucosal Immunol. 2018, 11, 304–315. [Google Scholar] [CrossRef] [Green Version]
- Rudner, X.L.; Happel, K.I.; Young, E.A.; Shellito, J.E. Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection. Infect. Immun. 2007, 75, 3055–3061. [Google Scholar] [CrossRef] [PubMed]
- Stassen, M.; Schmitt, E.; Bopp, T. From interleukin-9 to T helper 9 cells. Ann. N. Y. Acad. Sci. 2012, 1247, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Sun, B. T Helper Cell Differentiation and Their Function; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Lenarczyk, A.; Helsloot, J.; Farmer, K.; Peters, L.; Sturgess, A.; Kirkham, B. Antigen-induced IL-17 response in the peripheral blood mononuclear cells (PBMC) of healthy controls. Clin. Exp. Immunol. 2000, 122, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Ghoreschi, K.; Laurence, A.; Yang, X.P.; Tato, C.M.; McGeachy, M.J.; Konkel, J.E.; Ramos, H.L.; Wei, L.; Davidson, T.S.; Bouladoux, N.; et al. Generation of pathogenic TH17 cells in the absence of TGF-β 2 signalling. Nature 2010, 467, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Gottstein, B.; Pozio, E.; Nöckler, K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin. Microbiol. Rev. 2009, 22, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Cortés, A.; Muñoz-Antoli, C.; Esteban, J.G.; Toledo, R. Th2 and Th1 Responses: Clear and Hidden Sides of Immunity Against Intestinal Helminths. Trends Parasitol. 2017, 33, 678–693. [Google Scholar] [CrossRef] [PubMed]
- Harris, N.L.; Loke, P. Recent Advances in Type-2-Cell-Mediated Immunity: Insights from Helminth Infection. Immunity 2017, 47, 1024–1036. [Google Scholar] [CrossRef] [Green Version]
- Fraser, M.E.; Hayakawa, K.; Hume, M.S.; Ryan, D.G.; Brownie, E.R. Interactions of GTP with the ATP-grasp domain of GTP-specific succinyl-CoA synthetase. J. Biol. Chem. 2006, 281, 11058–11065. [Google Scholar] [CrossRef]
- Birney, M.; Um, H.D.; Klein, C. Novel mechanisms of Escherichia coli succinyl-coenzyme A synthetase regulation. J. Bacteriol. 1996, 178, 2883–2889. [Google Scholar] [CrossRef]
- Huang, X.; Bedoyan, J.K.; Demirbas, D.; Harris, D.J.; Miron, A.; Edelheit, S.; Grahame, G.; DeBrosse, S.D.; Wong, L.J.; Hoppel, C.L.; et al. Succinyl-CoA synthetase (SUCLA2) deficiency in two siblings with impaired activity of other mitochondrial oxidative enzymes in skeletal muscle without mitochondrial DNA depletion. Mol. Genet. Metab. 2017, 120, 213–222. [Google Scholar] [CrossRef]
- Wang, L.; Cui, J.; Hu, D.D.; Liu, R.D.; Wang, Z.Q. Identification of early diagnostic antigens from major excretory-secretory proteins of Trichinella spiralis muscle larvae using immunoproteomics. Trop. Biomed. 2014, 31, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Ribicich, M.; Gamble, H.R.; Bolpe, J.; Scialfa, E.; Krivokapich, S.; Cardillo, N.; Betti, A.; Cambiaggi Holzmann, M.L.; Pasqualetti, M.; Fariña, F.; et al. Trichinella infection in wild animals from endemic regions of Argentina. Parasitol. Res. 2010, 107, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Gadahi, J.A.; Li, B.; Ehsan, M.; Wang, S.; Zhang, Z.; Wang, Y.; Hasan, M.W.; Yan, R.; Song, X.; Xu, L.; et al. Recombinant Haemonchus contortus 24 kDa excretory/secretory protein (rHcES-24) modulate the immune functions of goat PBMCs in vitro. Oncotarget 2016, 7, 83926–83937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.C.; Huang, J.W.; Li, M.H.; Sui, Y.X.; Wang, S.; Liu, L.R.; Xu, L.X.; Yan, R.F.; Song, X.K.; Li, X.R. Identification and molecular characterization of microneme 5 of Eimeria acervulina. PLoS ONE 2014, 9, e115411. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of proteian-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Naqvi, M.A.; Naqvi, S.Z.; Memon, M.A.; Aimulajiang, K.; Haseeb, M.; Xu, L.; Song, X.; Li, X.; Ruofeng, Y. Combined use of indirect ELISA and western blotting with recombinant Hepatocellular carcinoma-associated antigen 59 is a potential immunodiagnostic tool for the detection of prepatent Haemonchus contortus infection in goat. Animals 2019, 9, 548. [Google Scholar] [CrossRef]
- Adefegha, S.A.; Leal, D.B.R.; Doleski, P.H.; Ledur, P.C.; Ecker, A. Peripheral blood mononuclear cells from rat model of pleurisy: The effects of hesperidin on ectoenzymes activity, apoptosis, cell cycle and reactive oxygen species production. Biomed. Pharmacother. 2017, 91, 278–286. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2001. [Google Scholar] [CrossRef]
- Jaques, J.A.; Rezer, J.F.P.; Ruchel, J.B.; Gutierres, J.; Bairros, A.V.; Farias, I.L.G.; Da Luz, S.C.A.; Bertoncheli, C.M.; Schetinger, M.R.C.; Morsch, V.M.; et al. A method for isolation of rat lymphocyte-rich mononuclear cells from lung tissue useful for determination of nucleoside triphosphate diphosphohydrolase activity. Anal. Biochem. 2011, 410, 34–39. [Google Scholar] [CrossRef]
- Yuan, C.; Zhang, H.; Wang, W.; Li, Y.; Yan, R.; Xu, L.; Song, X.; Li, X. Transmembrane protein 63A is a partner protein of Haemonchus contortus galectin in the regulation of goat peripheral blood mononuclear cells. Parasites Vectors 2015, 8, 211. [Google Scholar] [CrossRef]
- Taylor, A.; Verhagen, J.; Blaser, K.; Akdis, M.; Akdis, C.A. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-β: The role of T regulatory cells. Immunology 2006, 117, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Xiu, F.; Stanojcic, M.; Jeschke, M.G. Norepinephrine Inhibits Macrophage Migration by Decreasing CCR2 Expression. PLoS ONE 2013, 8, e69167. [Google Scholar] [CrossRef] [PubMed]
- Ehsan, M.; Gao, W.X.; Gadahi, J.A.; Lu, M.M.; Liu, X.C.; Wang, Y.J.; Yan, R.F.; Xu, L.; Song, X.K.; Li, X.R. Arginine kinase from Haemonchus contortus decreased the proliferation and increased the apoptosis of goat PBMCs in vitro. Parasites Vectors 2017, 10, 311. [Google Scholar] [CrossRef] [PubMed]
- Ehsan, M.; Wang, W.J.; Gadahi, J.A.; Hasan, M.W.; Lu, M.M.; Wang, Y.J.; Liu, X.C.; Haseeb, M.; Yan, R.F.; Xu, L.X.; et al. The serine/Threonine-Protein Phosphatase 1 From Haemonchus contortus is actively involved in suppressive regulatory roles on immune Functions of goat Peripheral Blood Mononuclear cells. Front. Immunol. 2018, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, C.; Wang, L.; Lu, M.; Wang, Y.; Wen, Y.; Yan, R.; Xu, L.; Song, X.; Li, X. Transmembrane protein 147 (TMEM147): Another partner protein of Haemonchus contortus galectin on the goat peripheral blood mononuclear cells (PBMC). Parasites Vectors 2016, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Fallon, P.G.; Alcami, A. Pathogen-derived immunomodulatory molecules: Future immunotherapeutics? Trends Immunol. 2006, 27, 470–476. [Google Scholar] [CrossRef]
- Harnett, W.; Harnett, M.M. Therapeutic immunomodulators from nematode parasites. Expert Rev. Mol. Med. 2008, 10, e18. [Google Scholar] [CrossRef]
- Long, S.R.; Wang, Z.Q.; Liu, R.D.; Liu, L.N.; Li, L.G.; Jiang, P.; Zhang, X.; Zhang, Z.F.; Shi, H.N.; Cu, J. Molecular identification of Trichinella spiralis nudix hydrolase and its induced protective immunity against trichinellosis in BALB/c mice. Parasites Vectors 2014, 7, 600. [Google Scholar] [CrossRef]
- Aranzamendi, C.; Bruin, A.D.; Kuiper, R.; Boog, C.J.P.; van Eden, W.; Rutten, V.; Pinelli, E. Protection against allergic airway inflammation during the chronic and acute phases of Trichinella spiralis infection. Clin. Exp. Allergy 2013, 43, 103–115. [Google Scholar] [CrossRef]
- Khan, W.I.; Blennerhasset, P.A.; Varghese, A.K.; Chowdhury, S.K.; Omsted, P.; Deng, Y.; Collins, S.M. Intestinal nematode infection ameliorates experimental colitis in mice. Infect. Immun. 2002, 70, 5931–5937. [Google Scholar] [CrossRef]
- Vincenti, F.; Charpentier, B.; Vanrenterghem, Y.; Rostaing, L.; Bresnahan, B.; Darji, P.; Massari, P.; Mondragon-Ramirez, G.A.; Agarwal, M.; Di Russo, G.; et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT Study). Am. J. Transplant. 2010, 10, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Panzer, M.; Sitte, S.; Wirth, S.; Drexler, I.; Sparwasser, T.; Voehringer, D. Rapid In Vivo Conversion of Effector T Cells into Th2 Cells during Helminth Infection. J. Immunol. 2012, 188, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Smits, H.H.; Everts, B.; Hartgers, F.C.; Yazdanbakhsh, M. Chronic helminth infections protect against allergic diseases by active regulatory processes. Curr. Allergy Asthma Rep. 2010, 10, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; He, L.; Chi, Y.; Zhou, S.; Hoellwarth, J.; Zhang, C.; Zhu, J.; Wu, C.; Dhesi, S.; Wang, X.; et al. Dynamics of Th17 cells and their role in Schistosoma japonicum infection in C57BL/6 mice. PLoS Negl. Trop. Dis. 2011, 5, e1399. [Google Scholar] [CrossRef]
- Gadahi, J.A.; Ehsan, M.; Wang, S.; Zhang, Z.; Yan, R.; Song, X.; Xu, L.; Li, X. Recombinant protein of Haemonchus contortus small GTPase ADP-ribosylation factor 1 (HcARF1) modulate the cell mediated immune response in vitro. Oncotarget 2017, 8, 112211–112221. [Google Scholar] [CrossRef]
- Miljkovic, D.; Cvetkovic, I.; Vuckovic, O.; Stosic-Grujicic, S.; Stojkovic, M.M.; Trajkovic, V. The role of interleukin-17 in inducible nitric oxide synthase-mediated nitric oxide production in endothelial cells. Cell. Mol. Life Sci. 2003, 60, 518–525. [Google Scholar] [CrossRef]
- Grencis, R.K. Cytokine regulation of resistance and susceptibility to intestinal nematode infection—From host to parasite. Vet. Parasitol. 2001, 100, 45–50. [Google Scholar] [CrossRef]
- Deng, G.; Deng, R.; Yao, J.; Liao, B.; Chen, Y.; Wu, Z.; Hu, H.; Zhou, X.; Ma, Y. Trichinella spiralis infection changes immune response in mice performed abdominal heterotopic cardiac transplantation and prolongs cardiac allograft survival time. Parasitol. Res. 2016, 115, 407–414. [Google Scholar] [CrossRef]
- Goswami, R.; Kaplan, M.H. A Brief History of IL-9 Ritobrata. 2012, 186, 3283–3288. J. Immunol. 2012, 186, 3283–3288. [Google Scholar] [CrossRef]
- Vaiyavatjamai, P.; Tongtrongchitr, A. Parasitic infections and allergy—A review. J. Med. Assoc. Thail. 2006, 89, 1551–1559. [Google Scholar]
- Ferretti, S.; Bonneau, O.; Dubois, G.R.; Jones, C.E.; Trifilieff, A. IL-17, Produced by Lymphocytes and Neutrophils, Is Necessary for Lipopolysaccharide-Induced Airway Neutrophilia: IL-15 as a Possible Trigger. J. Immunol. 2003, 170, 2106–2112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beriou, G.; Bradshaw, E.M.; Lozano, E.; Costantino, C.M.; Hastings, W.D.; Orban, T.; Elyaman, W.; Khoury, S.J.; Kuchroo, V.K.; Baecher-Allan, C.; et al. TGF-β Induces IL-9 Production from Human Th17 Cells. J. Immunol. 2010, 185, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Saunders, K.A.; Raine, T.; Cooke, A.; Lawrence, C.E. Inhibition of autoimmune type 1 diabetes by gastrointestinal helminth infection. Infect. Immun. 2007, 75, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Cho, M.K.; Choi, S.H.; Kim, Y.S.; Yu, H.S. Trichinella spiralis: Infection reduces airway allergic inflammation in mice. Exp. Parasitol. 2011, 127, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Maizels, R.M.; Mcsorley, H.J.; Smyth, D.J. Helminths in the hygiene hypothesis: Sooner or later? Clin. Exp. Immunol. 2014, 177, 38–46. [Google Scholar] [CrossRef]
- Mishra, P.K.; Palma, M.; Bleich, D.; Loke, P.; Gause, W.C. Systemic impact of intestinal helminth infections. Mucosal Immunol. 2014, 7, 753–762. [Google Scholar] [CrossRef] [Green Version]
- Allen, J.E.; Maizels, R.M. Diversity and dialogue in immunity to helminths. Nat. Rev. Immunol. 2011, 11, 375–388. [Google Scholar] [CrossRef]
- Varyani, F.; Fleming, J.O.; Maizels, R.M. Helminths in the gastrointestinal tract as modulators of immunity and pathology. Am. J. Physiol. Liver Physiol. 2017, 312, G537–G549. [Google Scholar] [CrossRef]
- Lin, Y.; Slight, S.R.; Khader, S.A. Th17 cytokines and vaccine-induced immunity. Semin. Immunopathol. 2010, 32, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Rutitzky, L.I.; Bazzone, L.; Shainheit, M.G.; Joyce-Shaikh, B.; Cua, D.J.; Stadecker, M.J. IL-23 Is Required for the Development of Severe Egg-Induced Immunopathology in Schistosomiasis and for Lesional Expression of IL-17. J. Immunol. 2008, 180, 2486–2495. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Li, Y.; Naqvi, M.A.-u.-H.; Naqvi, S.Z.; Chu, W.; Xu, L.; Song, X.; Li, X.; Yan, R. Succinate Coenzyme A Ligase Beta-Like Protein from Trichinella spiralis Suppresses the Immune Functions of Rat PBMCs In Vitro and Inhibits the Secretions of Interleukin-17 In Vivo. Vaccines 2019, 7, 167. https://doi.org/10.3390/vaccines7040167
Sun X, Li Y, Naqvi MA-u-H, Naqvi SZ, Chu W, Xu L, Song X, Li X, Yan R. Succinate Coenzyme A Ligase Beta-Like Protein from Trichinella spiralis Suppresses the Immune Functions of Rat PBMCs In Vitro and Inhibits the Secretions of Interleukin-17 In Vivo. Vaccines. 2019; 7(4):167. https://doi.org/10.3390/vaccines7040167
Chicago/Turabian StyleSun, Xiaoke, Yin Li, Muhammad Ali-ul-Husnain Naqvi, Sana Zahra Naqvi, Wen Chu, Lixin Xu, Xiaokai Song, Xiangrui Li, and Ruofeng Yan. 2019. "Succinate Coenzyme A Ligase Beta-Like Protein from Trichinella spiralis Suppresses the Immune Functions of Rat PBMCs In Vitro and Inhibits the Secretions of Interleukin-17 In Vivo" Vaccines 7, no. 4: 167. https://doi.org/10.3390/vaccines7040167
APA StyleSun, X., Li, Y., Naqvi, M. A.-u.-H., Naqvi, S. Z., Chu, W., Xu, L., Song, X., Li, X., & Yan, R. (2019). Succinate Coenzyme A Ligase Beta-Like Protein from Trichinella spiralis Suppresses the Immune Functions of Rat PBMCs In Vitro and Inhibits the Secretions of Interleukin-17 In Vivo. Vaccines, 7(4), 167. https://doi.org/10.3390/vaccines7040167







