Recombinant Lactobacillus casei Expressing Capsid Protein VP60 can Serve as Vaccine Against Rabbit Hemorrhagic Disease Virus in Rabbits
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain, Virus, Primers, and Plasmid
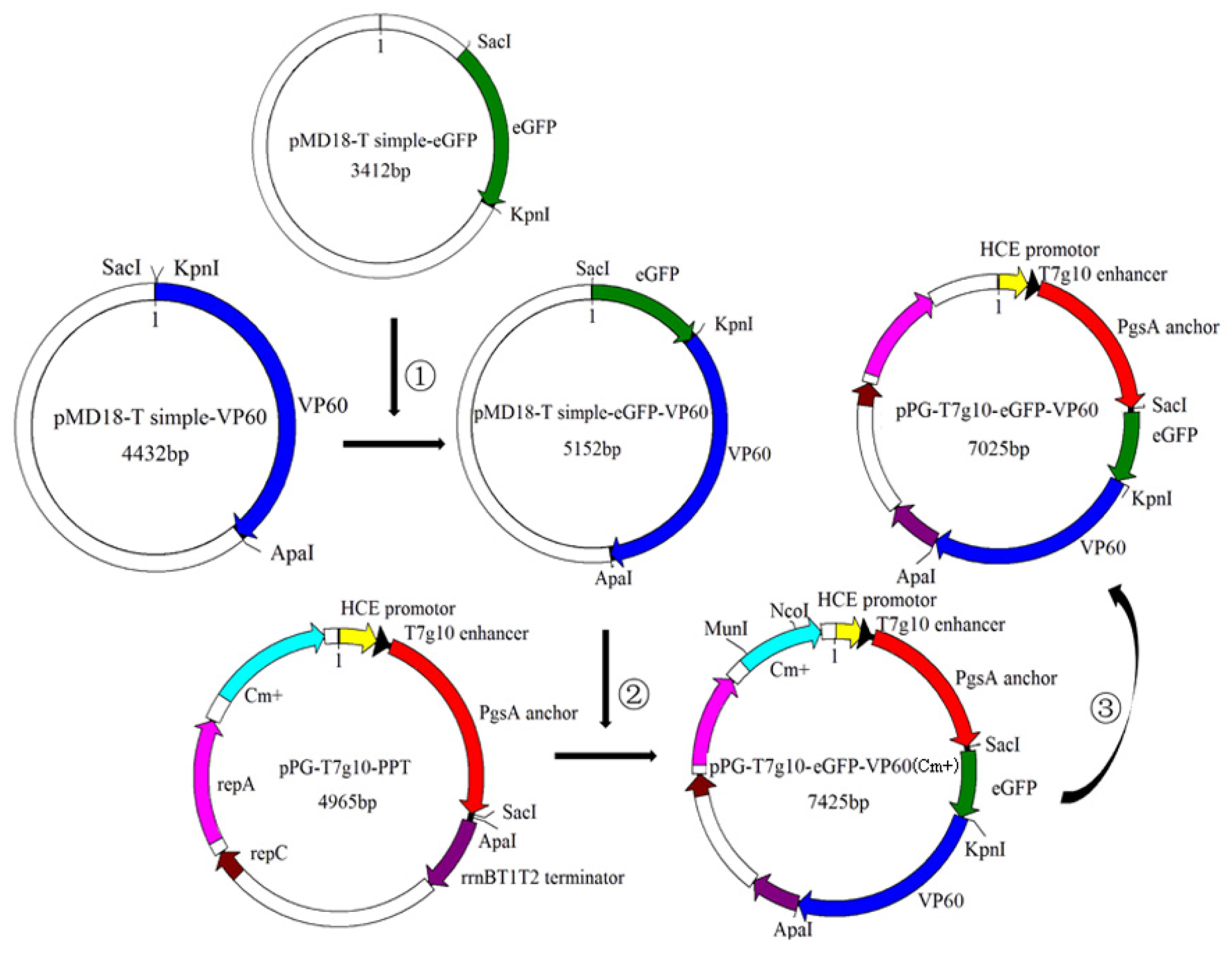
2.2. Construction of Recombinant L. casei (pPG-T7g10-eGFP-VP60/LC393)
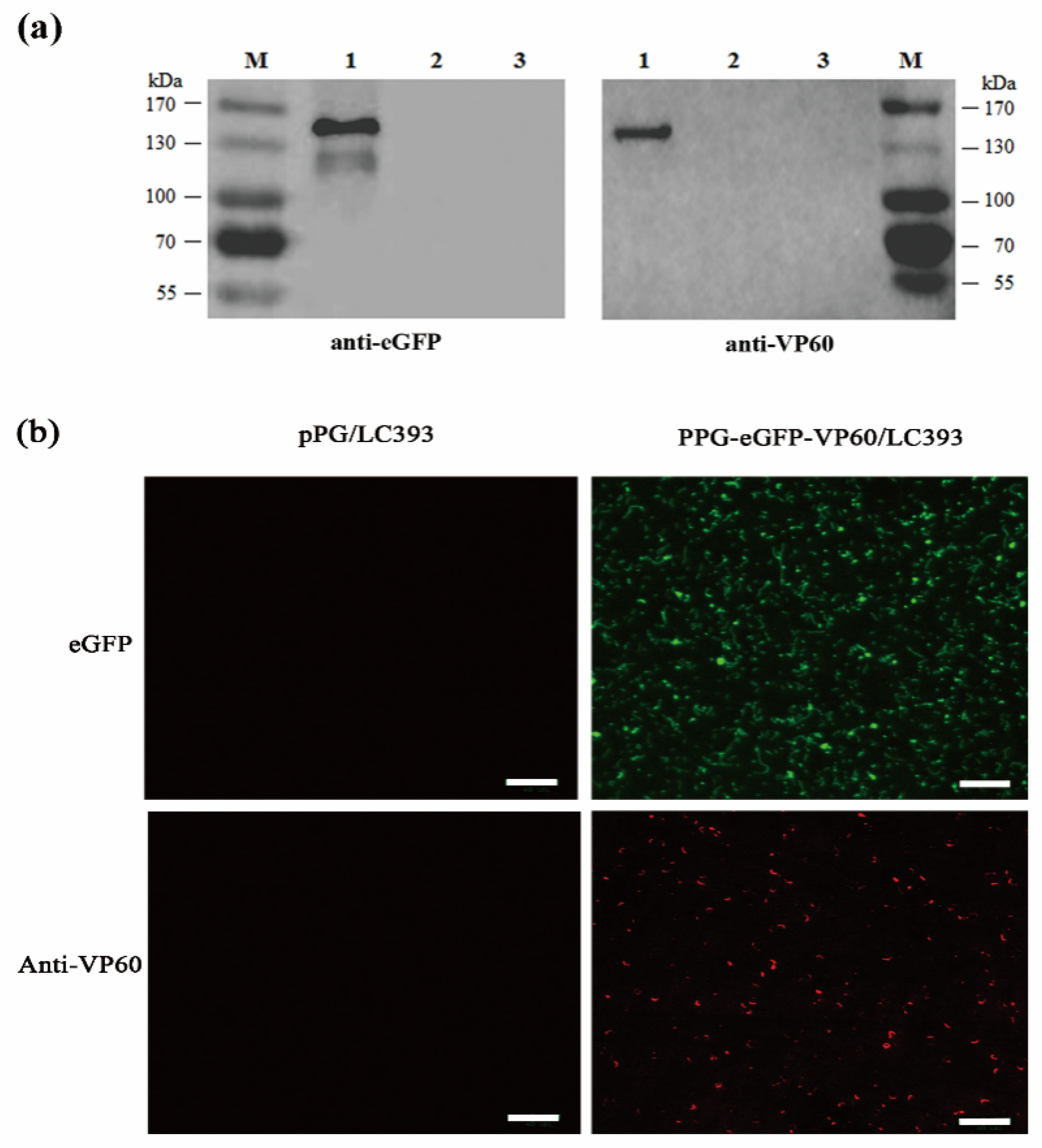
2.3. Analysis of Protein Expression by Western Blotting
2.4. Analysis of Protein Expression by Indirect Immunofluorescence
2.5. Immunization, Specimen Collection, and RHDV Challenge
2.6. ELISA Immunoassay
2.7. Histopathological Analysis
2.8. Statistical Analysis
3. Results
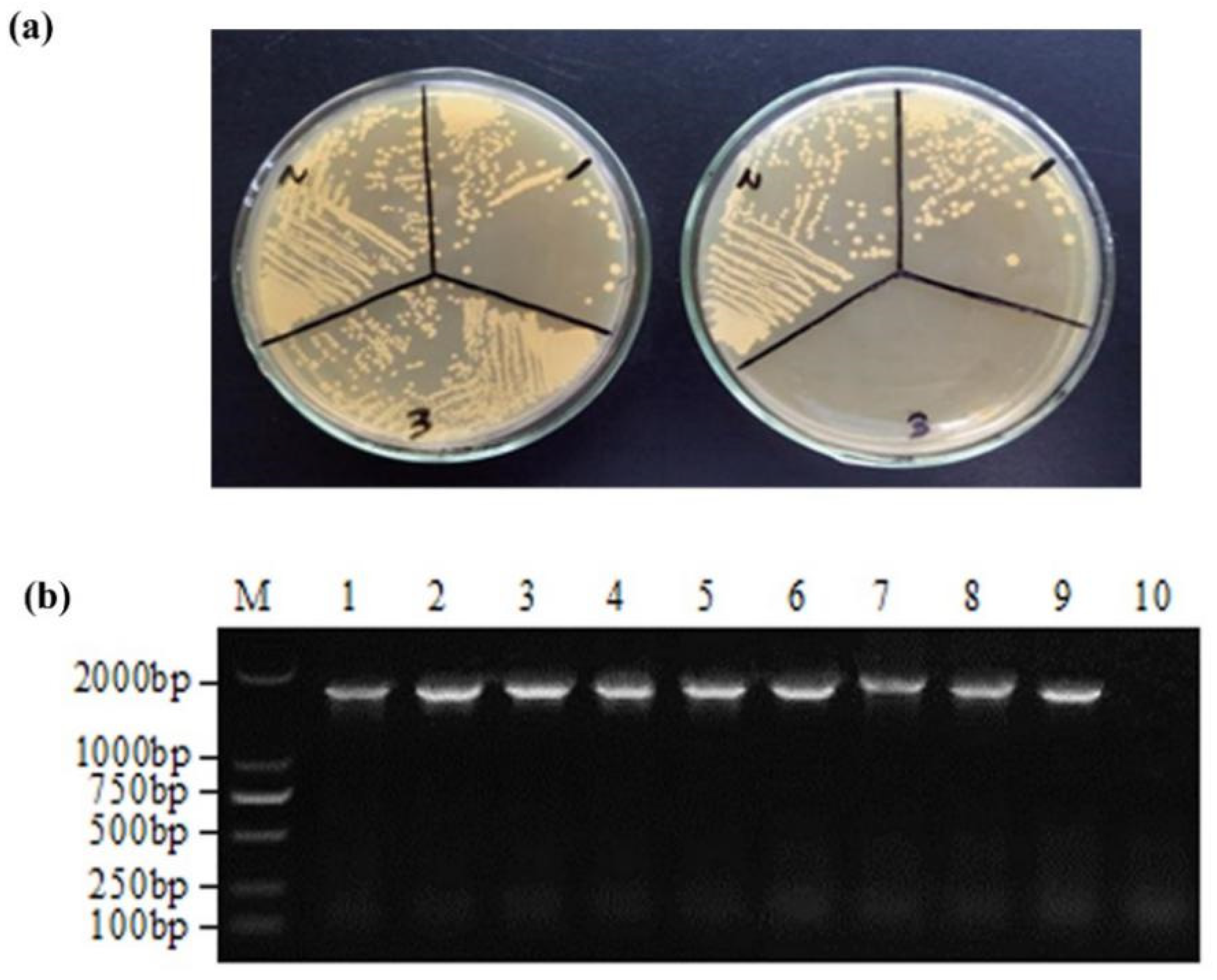
3.1. Determination of Chloramphenicol Resistance and Inherited Stability of Recombinant L. casei
3.2. Expression of the RHDV VP60(VP1) by Recombinant L. casei
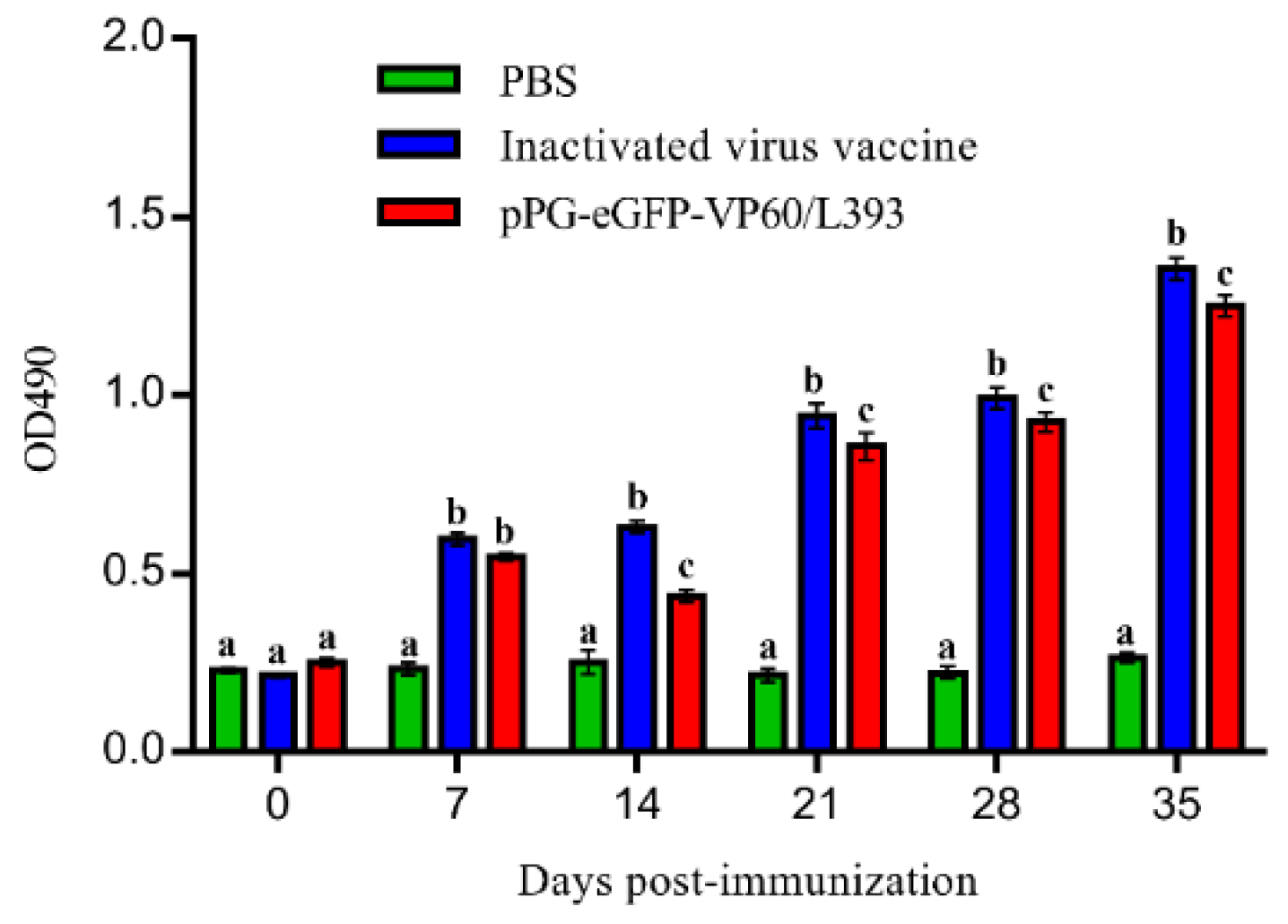
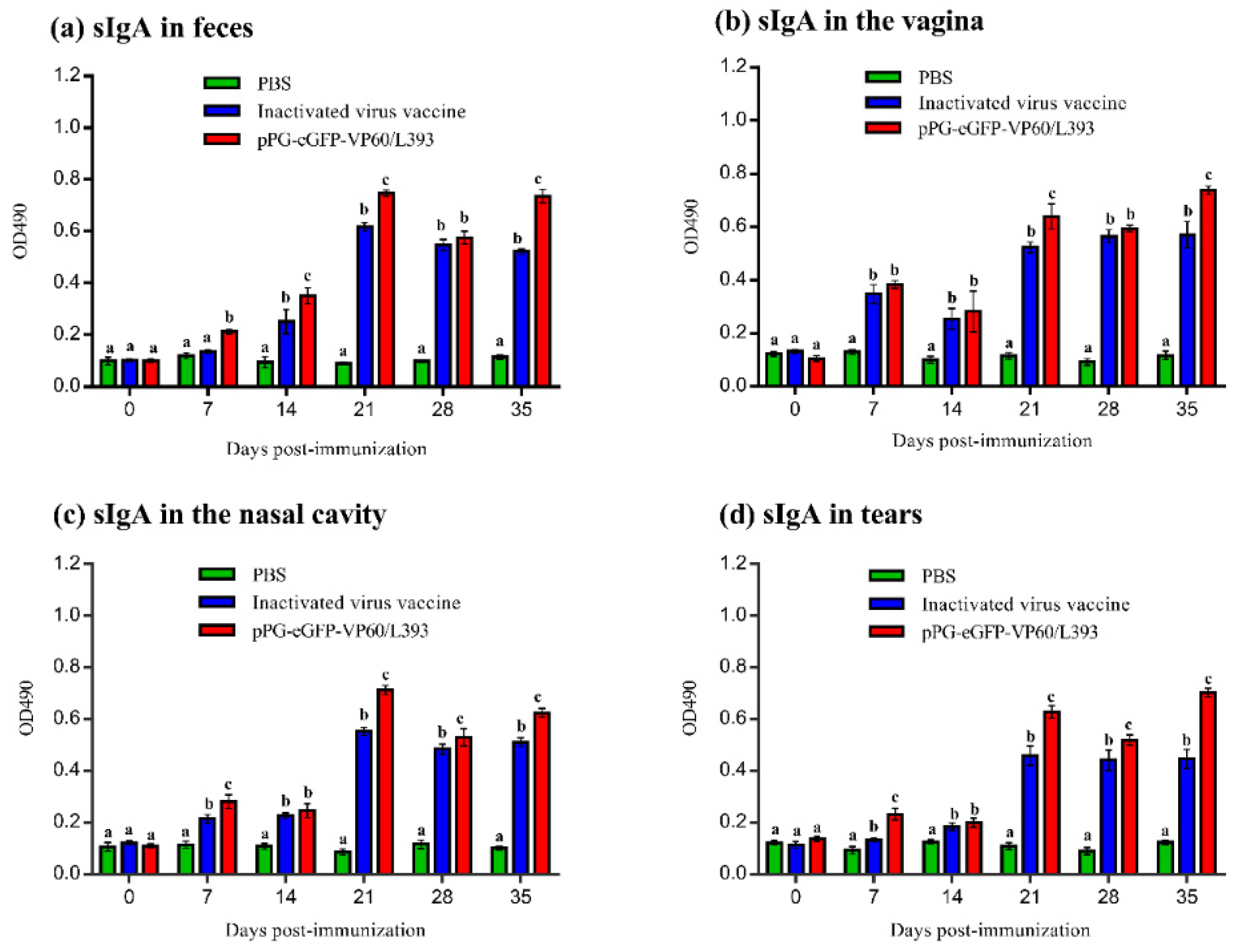
3.3. Induction of Systemic and Mucosal Antibody Responses
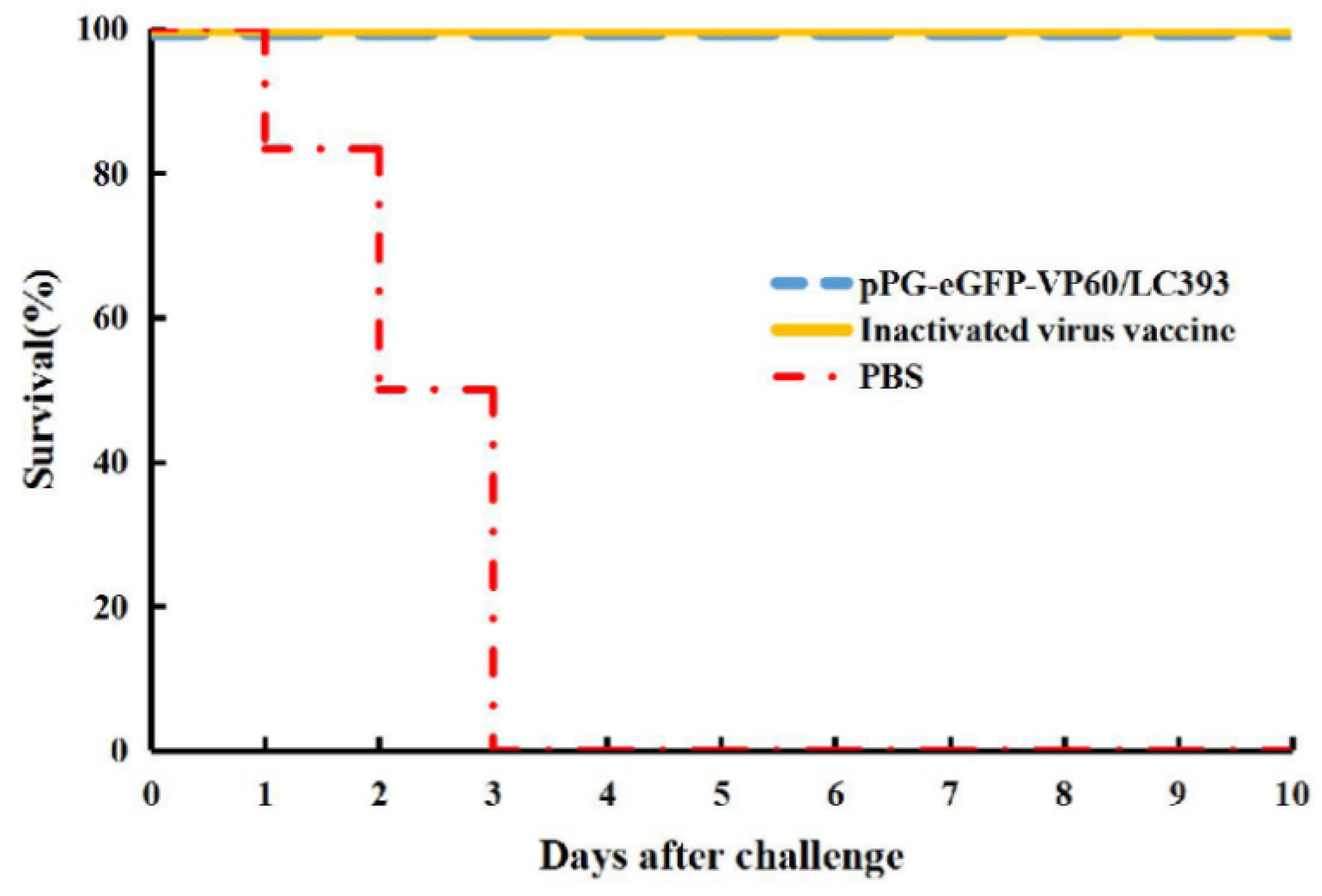
3.4. Immune Protection Against RHDV Challenge
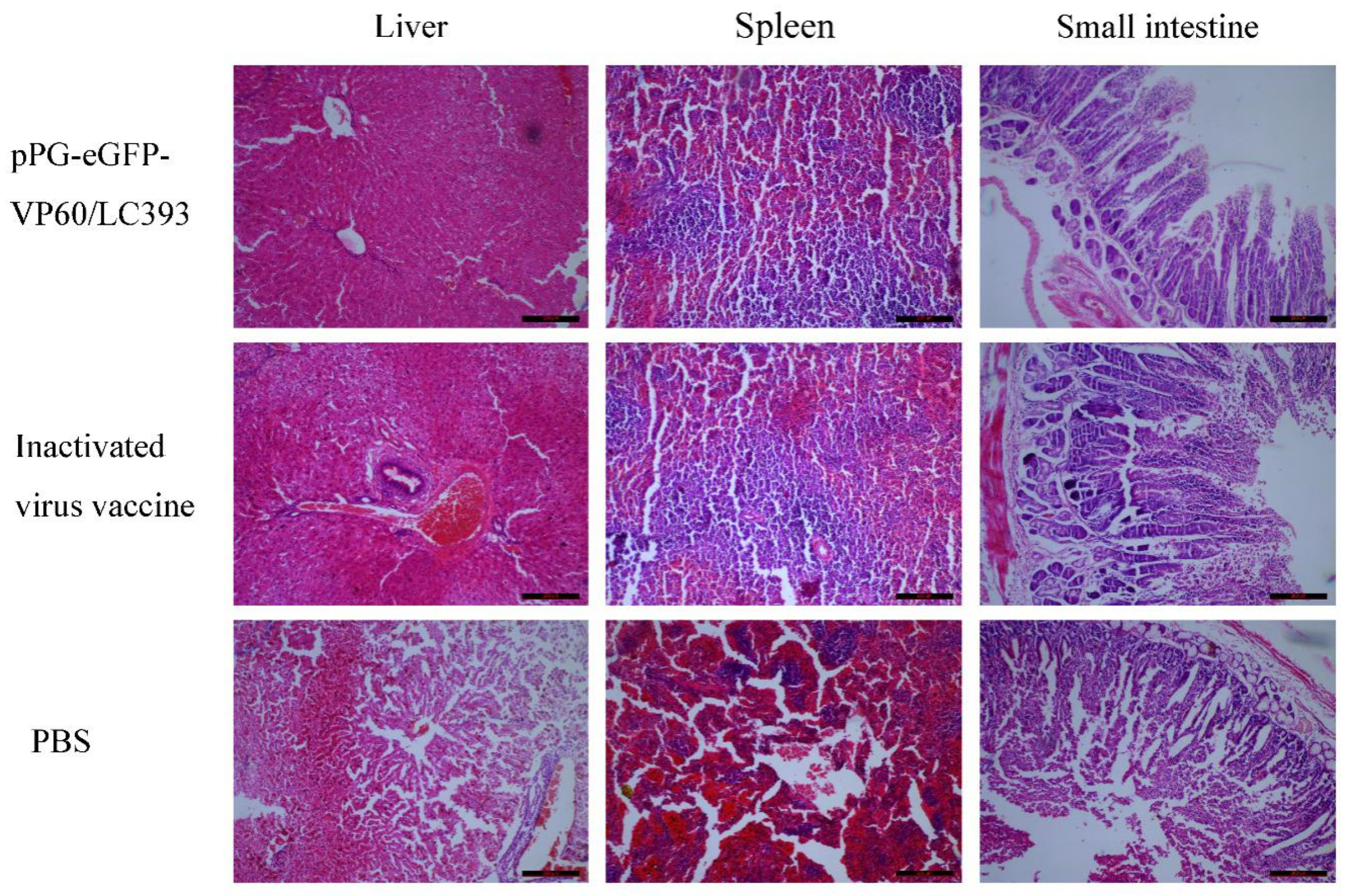
3.5. Histopathological Analysis on Different Tissues of the Infected Rabbits
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abrantes, J.; van der Loo, W.; Le Pendu, J.; Esteves, P.J. Rabbit haemorrhagic disease (RHD) and rabbit haemorrhagic disease virus (RHDV): A review. Vet. Res. 2012, 43, 12. [Google Scholar] [CrossRef]
- Gregg, D.A.; House, C.; Meyer, R.; Berninger, M. Viral haemorrhagic disease of rabbits in Mexico: Epidemiology and viral characterization. Rev. Sci. Tech. 1991, 10, 435–451. [Google Scholar] [CrossRef]
- Le Gall-Recule, G.; Zwingelstein, F.; Boucher, S.; Le Normand, B.; Plassiart, G.; Portejoie, Y.; Decors, A.; Bertagnoli, S.; Guerin, J.L.; Marchandeau, S. Detection of a new variant of rabbit haemorrhagic disease virus in France. Vet. Rec. 2011, 168, 137–138. [Google Scholar] [CrossRef] [PubMed]
- Le Pendu, J.; Abrantes, J.; Bertagnoli, S.; Guitton, J.S.; Le Gall-Recule, G.; Lopes, A.M.; Marchandeau, S.; Alda, F.; Almeida, T.; Celio, A.P.; et al. Proposal for a unified classification system and nomenclature of lagoviruses. J. Gen. Virol. 2017, 98, 1658–1666. [Google Scholar] [CrossRef] [PubMed]
- Embury-Hyatt, C.; Postey, R.; Hisanaga, T.; Burton, L.; Hooper-McGrevy, K.; McIntyre, L.; Millar, K.; Pasick, J. The first reported case of rabbit hemorrhagic disease in Canada. Can. Vet. J. 2012, 53, 998–1002. [Google Scholar] [PubMed]
- Mitro, S.; Krauss, H. Rabbit hemorrhagic disease: A review with special reference to its epizootiology. Eur. J. Epidemiol. 1993, 9, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Morisse, J.P.; Le Gall, G.; Boilletot, E. Hepatitis of viral origin in Leporidae: Introduction and aetiological hypotheses. Rev. Sci. Tech. 1991, 10, 269–310. [Google Scholar] [CrossRef] [PubMed]
- Dalton, K.P.; Nicieza, I.; Balseiro, A.; Muguerza, M.A.; Rosell, J.M.; Casais, R.; Alvarez, A.L.; Parra, F. Variant rabbit hemorrhagic disease virus in young rabbits, Spain. Emerg. Infect. Dis. 2012, 18, 2009–2012. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.M.; Correia, J.; Abrantes, J.; Melo, P.; Ramada, M.; Magalhaes, M.J.; Alves, P.C.; Esteves, P.J. Is the new variant RHDV replacing genogroup 1 in Portuguese wild rabbit populations? Viruses 2014, 7, 27–36. [Google Scholar] [CrossRef]
- Martin-Alonso, A.; Martin-Carrillo, N.; Garcia-Livia, K.; Valladares, B.; Foronda, P. Emerging rabbit haemorrhagic disease virus 2 (RHDV2) at the gates of the African continent. Infect. Genet. Evol. 2016, 44, 46–50. [Google Scholar] [CrossRef]
- Meyers, G.; Wirblich, C.; Thiel, H.J. Genomic and subgenomic RNAs of rabbit hemorrhagic disease virus are both protein-linked and packaged into particles. Virology 1991, 184, 677–686. [Google Scholar] [CrossRef]
- Meyers, G.; Wirblich, C.; Thiel, H.J. Rabbit hemorrhagic disease virus—molecular cloning and nucleotide sequencing of a calicivirus genome. Virology 1991, 184, 664–676. [Google Scholar] [CrossRef]
- Rohde, J.; Schirrmeier, H.; Granzow, H.; Rziha, H.J. A new recombinant Orf virus (ORFV, Parapoxvirus) protects rabbits against lethal infection with rabbit hemorrhagic disease virus (RHDV). Vaccine 2011, 29, 9256–9264. [Google Scholar] [CrossRef]
- Smid, B.; Valicek, L.; Rodak, L.; Stepanek, J.; Jurak, E. Rabbit haemorrhagic disease: An investigation of some properties of the virus and evaluation of an inactivated vaccine. Vet. Microbiol. 1991, 26, 77–85. [Google Scholar] [CrossRef]
- Asgari, S.; Hardy, J.R.; Sinclair, R.G.; Cooke, B.D. Field evidence for mechanical transmission of rabbit haemorrhagic disease virus (RHDV) by flies (Diptera: Calliphoridae) among wild rabbits in Australia. Virus Res. 1998, 54, 123–132. [Google Scholar] [CrossRef]
- Xu, Z.J.; Chen, W.X. Viral haemorrhagic disease in rabbits: A review. Vet. Res. Commun. 1989, 13, 205–212. [Google Scholar] [CrossRef]
- Bermudez-Humaran, L.G.; Langella, P.; Cortes-Perez, N.G.; Gruss, A.; Tamez-Guerra, R.S.; Oliveira, S.C.; Saucedo-Cardenas, O.; Montes de Oca-Luna, R.; Le Loir, Y. Intranasal immunization with recombinant Lactococcus lactis secreting murine interleukin-12 enhances antigen-specific Th1 cytokine production. Infect. Immun. 2003, 71, 1887–1896. [Google Scholar] [CrossRef]
- Vela Ramirez, J.E.; Sharpe, L.A.; Peppas, N.A. Current state and challenges in developing oral vaccines. Adv. Drug Deliv. Rev. 2017, 114, 116–131. [Google Scholar] [CrossRef]
- Azizi, A.; Kumar, A.; Diaz-Mitoma, F.; Mestecky, J. Enhancing oral vaccine potency by targeting intestinal M cells. PLoS Pathog. 2010, 6, e1001147. [Google Scholar] [CrossRef]
- Mitragotri, S.; Burke, P.A.; Langer, R. Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nat. Rev. Drug Discov. 2014, 13, 655–672. [Google Scholar] [CrossRef]
- Newsted, D.; Fallahi, F.; Golshani, A.; Azizi, A. Advances and challenges in mucosal adjuvant technology. Vaccine 2015, 33, 2399–2405. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, H.; Zhang, X.; Qian, F. Intranasal and oral vaccination with protein-based antigens: Advantages, challenges and formulation strategies. Protein Cell 2015, 6, 480–503. [Google Scholar] [CrossRef] [PubMed]
- Hutton, G.; Tediosi, F. The costs of introducing a malaria vaccine through the expanded program on immunization in Tanzania. Am. J. Trop. Med. Hyg. 2006, 75 (Suppl. 2), 119–130. [Google Scholar] [CrossRef]
- Levine, M.M.; Dougan, G. Optimism over vaccines administered via mucosal surfaces. Lancet 1998, 351, 1375–1376. [Google Scholar] [CrossRef]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Pelaseyed, T.; Bergstrom, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.; Schutte, A.; van der Post, S.; Svensson, F.; Rodriguez-Pineiro, A.M.; Nystrom, E.E.; et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Pessione, E. Lactic acid bacteria contribution to gut microbiota complexity: Lights and shadows. Front. Cell Infect. Microbiol. 2012, 2, 86. [Google Scholar] [CrossRef]
- Mercier-Bonin, M.; Chapot-Chartier, M.P. Surface Proteins of Lactococcus lactis: Bacterial Resources for Muco-adhesion in the Gastrointestinal Tract. Front. Microbiol. 2017, 8, 2247. [Google Scholar] [CrossRef]
- Ogawa, T.; Asai, Y.; Sakamoto, H.; Yasuda, K. Oral immunoadjuvant activity of Lactobacillus casei subsp. casei in dextran-fed layer chickens. Br. J. Nutr. 2006, 95, 430–434. [Google Scholar] [CrossRef]
- Ho, P.S.; Kwang, J.; Lee, Y.K. Intragastric administration of Lactobacillus casei expressing transmissible gastroentritis coronavirus spike glycoprotein induced specific antibody production. Vaccine 2005, 23, 1335–1342. [Google Scholar] [CrossRef]
- Gao, X.; Ma, Y.; Wang, Z.; Bai, J.; Jia, S.; Feng, B.; Jiang, Y.; Cui, W.; Tang, L. Oral immunization of mice with a probiotic Lactobacillus casei constitutively expressing the alpha-toxoid induces protective immunity against Clostridium perfringens alpha-toxin. Virulence 2019, 10, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Hou, X.; Tang, L.; Jiang, Y.; Ma, G.; Li, Y. A phase trial of the oral Lactobacillus casei vaccine polarizes Th2 cell immunity against transmissible gastroenteritis coronavirus infection. Appl. Microbiol. Biotechnol. 2016, 100, 7457–7469. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, L.; Huang, X.; Wang, X.; Chen, S.; Shi, W.; Qiao, X.; Jiang, Y.; Tang, L.; Xu, Y.; et al. Oral recombinant Lactobacillus vaccine targeting the intestinal microfold cells and dendritic cells for delivering the core neutralizing epitope of porcine epidemic diarrhea virus. Microb. Cell Fact. 2018, 17, 20. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Huang, X.; Ma, S.; Yu, M.; Shi, W.; Qiao, X.; Tang, L.; Xu, Y.; Li, Y. Oral Delivery of Probiotics Expressing Dendritic Cell-Targeting Peptide Fused with Porcine Epidemic Diarrhea Virus COE Antigen: A Promising Vaccine Strategy against PEDV. Viruses 2017, 9, 312. [Google Scholar] [CrossRef]
- Wang, X.N.; Wang, L.; Zheng, D.Z.; Chen, S.; Shi, W.; Qiao, X.Y.; Jiang, Y.P.; Tang, L.J.; Xu, Y.G.; Li, Y.J. Oral immunization with a Lactobacillus casei-based anti-porcine epidemic diarrhoea virus (PEDV) vaccine expressing microfold cell-targeting peptide Co1 fused with the COE antigen of PEDV. J. Appl. Microbiol. 2018, 124, 368–378. [Google Scholar] [CrossRef]
- Yu, M.; Qi, R.; Chen, C.; Yin, J.; Ma, S.; Shi, W.; Wu, Y.; Ge, J.; Jiang, Y.; Tang, L. Immunogenicity of recombinant Lactobacillus casei-expressing F4 (K88) fimbrial adhesin FaeG in conjunction with a heat-labile enterotoxin A (LTAK63) and heat-labile enterotoxin B (LTB) of enterotoxigenic Escherichia coli as an oral adjuvant in mice. J. Appl. Microbiol. 2017, 122, 506–515. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, Z.; Liu, J.; Wang, Z.; Wang, R.; Li, Y.; Wang, L.; Xu, Y.; Tang, L.; Qiao, X. Recombinant Lactobacillus casei expressing Clostridium perfringens toxoids alpha, beta2, epsilon and beta1 gives protection against Clostridium perfringens in rabbits. Vaccine 2017, 35, 4010–4021. [Google Scholar] [CrossRef]
- Parra, F.; Prieto, M. Purification and characterization of a calicivirus as the causative agent of a lethal hemorrhagic disease in rabbits. J. Virol. 1990, 64, 4013–4015. [Google Scholar]
- Farnos, O.; Boue, O.; Parra, F.; Martin-Alonso, J.M.; Valdes, O.; Joglar, M.; Navea, L.; Naranjo, P.; Lleonart, R. High-level expression and immunogenic properties of the recombinant rabbit hemorrhagic disease virus VP60 capsid protein obtained in Pichia pastoris. J. Biotechnol. 2005, 117, 215–224. [Google Scholar] [CrossRef]
- Arguello Villares, J.L. Viral haemorrhagic disease of rabbits: Vaccination and immune response. Rev. Sci. Tech. 1991, 10, 459–480. [Google Scholar] [CrossRef]
- Perez-Filgueira, D.M.; Resino-Talavan, P.; Cubillos, C.; Angulo, I.; Barderas, M.G.; Barcena, J.; Escribano, J.M. Development of a low-cost, insect larvae-derived recombinant subunit vaccine against RHDV. Virology 2007, 364, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Frantz, F.G.; Ito, T.; Cavassani, K.A.; Hogaboam, C.M.; Lopes Silva, C.; Kunkel, S.L.; Faccioli, L.H. Therapeutic DNA vaccine reduces schistosoma mansoni-induced tissue damage through cytokine balance and decreased migration of myofibroblasts. Am. J. Pathol. 2011, 179, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.; Toledo, J.R.; Chiong, M.; Parra, F.; Rodriguez, E.; Montero, C.; Mendez, L.; Capucci, L.; Farnos, O. Single dose adenovirus vectored vaccine induces a potent and long-lasting immune response against rabbit hemorrhagic disease virus after parenteral or mucosal administration. Vet. Immunol. Immunopathol. 2011, 142, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.; Le Gros, F.X.; Mason, P.W.; Paoletti, E. A recombinant canarypox virus protects rabbits against a lethal rabbit hemorrhagic disease virus (RHDV) challenge. Vaccine 1997, 15, 90–96. [Google Scholar] [CrossRef]
- Marin, M.S.; Martin Alonso, J.M.; Perez Ordoyo Garcia, L.I.; Boga, J.A.; Arguello-Villares, J.L.; Casais, R.; Venugopal, K.; Jiang, W.; Gould, E.A.; Parra, F. Immunogenic properties of rabbit haemorrhagic disease virus structural protein VP60 expressed by a recombinant baculovirus: An efficient vaccine. Virus Res. 1995, 39, 119–128. [Google Scholar] [CrossRef]
- Davitt, C.J.; Lavelle, E.C. Delivery strategies to enhance oral vaccination against enteric infections. Adv. Drug Deliv. Rev. 2015, 91, 52–69. [Google Scholar] [CrossRef]
- Daniel, C.; Roussel, Y.; Kleerebezem, M.; Pot, B. Recombinant lactic acid bacteria as mucosal biotherapeutic agents. Trends Biotechnol. 2011, 29, 499–508. [Google Scholar] [CrossRef]
- Esvaran, M.; Conway, P.L. Factors that Influence the Immunological Adjuvant Effect of Lactobacillus fermentum PC1 on Specific Immune Responses in Mice to Orally Administered Antigens. Vaccines 2016, 4, 24. [Google Scholar] [CrossRef]
- Wells, J.M.; Mercenier, A. Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat. Rev. Microbiol. 2008, 6, 349–362. [Google Scholar] [CrossRef]
- Tang, L.; Li, Y. Oral immunization of mice with recombinant Lactococcus lactis expressing porcine transmissible gastroenteritis virus spike glycoprotein. Virus Genes 2009, 39, 238–245. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, B.; Niu, C.; Jia, S.; Sun, C.; Wang, Z.; Jiang, Y.; Cui, W.; Wang, L.; Xu, Y. Dendritic Cell Targeting of Bovine Viral Diarrhea Virus E2 Protein Expressed by Lactobacillus casei Effectively Induces Antigen-Specific Immune Responses via Oral Vaccination. Viruses 2019, 11, 575. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.G.; Cui, L.C.; Ma, G.P.; Tang, L.J.; Ge, J.W.; Xia, C.L.; Qiao, X.Y.; Zhao, L.L.; Li, Y.J. The surface display of porcine parvovirus VP2 protein in Lactobacillus casei. Sheng Wu Gong Cheng Xue Bao 2007, 23, 315–318. [Google Scholar]
- Ge, J.W.; Liu, D.Q.; Li, Y.J. Construction of recombinant lactobacilli expressing the core neutralizing epitope (COE) of porcine epidemic diarrhea virus and a fusion protein consisting of COE and Escherichia coli heat-labile enterotoxin B, and comparison of the immune responses by orogastric immunization. Can. J. Microbiol. 2012, 58, 1258–1267. [Google Scholar]
- Meng, F.; Ren, Y.; Suo, S.; Sun, X.; Li, X.; Li, P.; Yang, W.; Li, G.; Li, L.; Schwegmann-Wessels, C.; et al. Evaluation on the efficacy and immunogenicity of recombinant DNA plasmids expressing spike genes from porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus. PLoS ONE 2013, 8, e57468. [Google Scholar] [CrossRef]
- Takala, T.M.; Saris, P.E.; Tynkkynen, S.S. Food-grade host/vector expression system for Lactobacillus casei based on complementation of plasmid-associated phospho-beta-galactosidase gene lacG. Appl. Microbiol. Biotechnol. 2003, 60, 564–570. [Google Scholar] [CrossRef]
- Olins, P.O.; Rangwala, S.H. A novel sequence element derived from bacteriophage T7 mRNA acts as an enhancer of translation of the lacZ gene in Escherichia coli. J. Biol. Chem. 1989, 264, 16973–16976. [Google Scholar]
- Xu, Y.; Li, Y. Induction of immune responses in mice after intragastric administration of Lactobacillus casei producing porcine parvovirus VP2 protein. Appl. Env. Microbiol. 2007, 73, 7041–7047. [Google Scholar] [CrossRef]
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 2005, 11 (Suppl. 4), 45–53. [Google Scholar] [CrossRef]
- Mantis, N.J.; Rol, N.; Corthesy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef]
- Jiang, X.; Yu, M.; Qiao, X.; Liu, M.; Tang, L.; Jiang, Y.; Cui, W.; Li, Y. Up-regulation of MDP and tuftsin gene expression in Th1 and Th17 cells as an adjuvant for an oral Lactobacillus casei vaccine against anti-transmissible gastroenteritis virus. Appl. Microbiol. Biotechnol. 2014, 98, 8301–8312. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Xia, T.; Guo, T.; Ru, Y.; Jiang, Y.; Cui, W.; Zhou, H.; Qiao, X.; Tang, L.; Xu, Y.; et al. Recombinant Lactobacillus casei Expressing Capsid Protein VP60 can Serve as Vaccine Against Rabbit Hemorrhagic Disease Virus in Rabbits. Vaccines 2019, 7, 172. https://doi.org/10.3390/vaccines7040172
Wang L, Xia T, Guo T, Ru Y, Jiang Y, Cui W, Zhou H, Qiao X, Tang L, Xu Y, et al. Recombinant Lactobacillus casei Expressing Capsid Protein VP60 can Serve as Vaccine Against Rabbit Hemorrhagic Disease Virus in Rabbits. Vaccines. 2019; 7(4):172. https://doi.org/10.3390/vaccines7040172
Chicago/Turabian StyleWang, Li, Tian Xia, Tiantian Guo, Yi Ru, Yanping Jiang, Wen Cui, Han Zhou, Xinyuan Qiao, Lijie Tang, Yigang Xu, and et al. 2019. "Recombinant Lactobacillus casei Expressing Capsid Protein VP60 can Serve as Vaccine Against Rabbit Hemorrhagic Disease Virus in Rabbits" Vaccines 7, no. 4: 172. https://doi.org/10.3390/vaccines7040172
APA StyleWang, L., Xia, T., Guo, T., Ru, Y., Jiang, Y., Cui, W., Zhou, H., Qiao, X., Tang, L., Xu, Y., & Li, Y. (2019). Recombinant Lactobacillus casei Expressing Capsid Protein VP60 can Serve as Vaccine Against Rabbit Hemorrhagic Disease Virus in Rabbits. Vaccines, 7(4), 172. https://doi.org/10.3390/vaccines7040172




