Post-Glycosylation Modification of Sialic Acid and Its Role in Virus Pathogenesis
Abstract
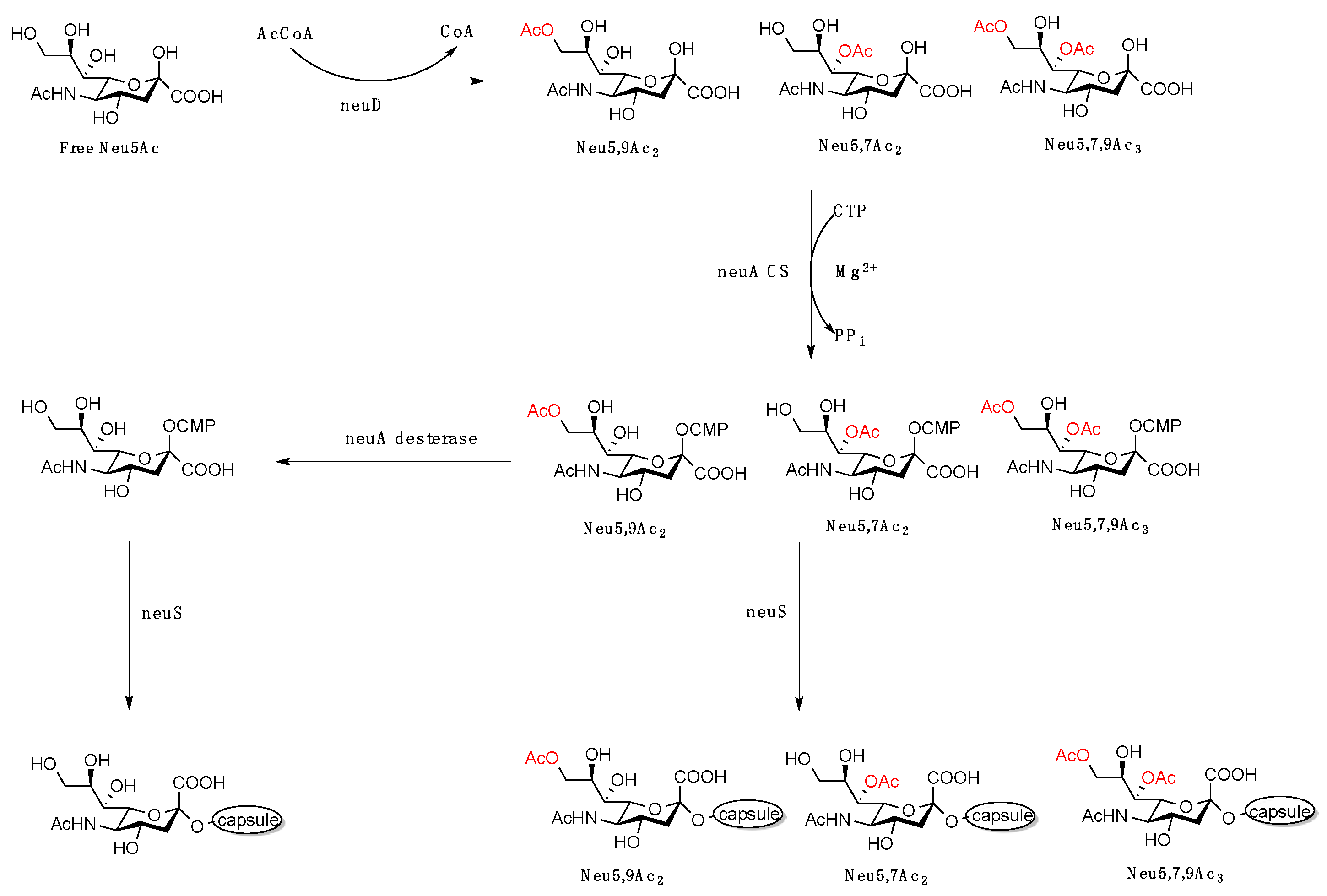
1. Introduction
2. Extraction and Detection Methods of O-Acetylated Neu5Ac
3. Biological Implications of C7–9 and C4 O-Acetylated Neu5Ac
4. Chemical Modifications of C4 Sia
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Blix, G. Concerning the carbohydrate groups of submaxillary mucin. Hoppe-Seylers Zeitschrift fur Physiologische Chemie 1936, 240, 43–54. [Google Scholar] [CrossRef]
- Schauer, R. Chemistry, metabolism, and biological functions of sialic acids. Adv. Carbohydr. Chem. Biochem. 1982, 40, 131–234. [Google Scholar] [PubMed]
- Varki, A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature 2007, 446, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Byrd-Leotis, L.; Liu, R.; Bradley, K.C.; Lasanajak, Y.; Cummings, S.F.; Song, X.; Heimburg-Molinaro, J.; Galloway, S.E.; Culhane, M.R.; Smith, D.F.; et al. Shotgun glycomics of pig lung identifies natural endogenous receptors for influenza viruses. Proc. Natl. Acad. Sci. USA 2014, 111, E2241–E2250. [Google Scholar] [CrossRef]
- Rogers, G.N.; Paulson, J.C. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology 1983, 127, 361–373. [Google Scholar] [CrossRef]
- Rogers, G.N.; D’Souza, B.L. Receptor binding properties of human and animal H1 influenza virus isolates. Virology 1989, 173, 317–322. [Google Scholar] [CrossRef]
- Connor, R.J.; Kawaoka, Y.; Webster, R.G.; Paulson, J.C. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology 1994, 205, 17–23. [Google Scholar] [CrossRef]
- Russell, R.J.; Stevens, D.J.; Haire, L.F.; Gamblin, S.J.; Skehel, J.J. Avian and human receptor binding by hemagglutinins of influenza A viruses. Glycoconj. J. 2006, 23, 85–92. [Google Scholar] [CrossRef]
- Kumari, K.; Gulati, S.; Smith, D.F.; Gulati, U.; Cummings, R.D.; Air, G.M. Receptor binding specificity of recent human H3N2 influenza viruses. Virol. J. 2007, 4, 42. [Google Scholar] [CrossRef]
- Bradley, K.C.; Jones, C.A.; Tompkins, S.M.; Tripp, R.A.; Russell, R.J.; Gramer, M.R.; Heimburg-Molinaro, J.; Smith, D.F.; Cummings, R.D.; Steinhauer, D.A. Comparison of the receptor binding properties of contemporary swine isolates and early human pandemic H1N1 isolates (Novel 2009 H1N1). Virology 2011, 413, 169–182. [Google Scholar] [CrossRef]
- Varki, A. Diversity in the sialic acids. Glycobiology 1992, 2, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Angata, T.; Varki, A. Chemical diversity in the sialic acids and related α-keto acids: an evolutionary perspective. Chem. Rev. 2002, 102, 439–469. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Gervay-Hague, J. Synthesis of Partially O-Acetylated N-Acetylneuraminic Acid Using Regioselective Silyl Exchange Technology. Org. Lett. 2014, 16, 5044–5047. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Hsieh, H.W.; Gervay-Hague, J. Anomeric O-Functionalization of Carbohydrates for Chemical Conjugation to Vaccine Constructs. Molecules 2018, 23, 1742. [Google Scholar] [CrossRef]
- Mandal, C.; Schwartz-Albiez, R.; Vlasak, R. Functions and biosynthesis of o-acetylated sialic acids. Top. Curr. Chem. 2012, 366, 1–30. [Google Scholar]
- Schauer, R.; Srinivasan, G.V.; Wipfler, D.; Kniep, B.; Schwartz-Albiez, R. O-Acetylated sialic acids and their role in immune defense. Adv. Exp. Med. Biol. 2011, 705, 525–548. [Google Scholar]
- Schauer, R.; Schmid, H.; Pommerencke, J.; Iwersen, M.; Kohla, G. Metabolism and role of O-acetylated sialic acids. Adv. Exp. Med. Biol. 2001, 491, 325–342. [Google Scholar]
- Houliston, R.S.; Endtz, H.P.; Yuki, N.; Li, J.J.; Jarrell, H.C.; Koga, M.; van Belkum, A.; Karwaski, M.F.; Wakarchuk, W.W.; Gilbert, M. Identification of a sialate O-acetyltransferase from Campylobacter jejuni—Demonstration of direct transfer to the C-9 position of terminal α-2,8-linked sialic acid. J. Biol. Chem. 2006, 281, 11480–11486. [Google Scholar] [CrossRef]
- Schauer, R.; Stoll, S.; Reuter, G. Differences in the amount of N-acetyl- and N-glycoloyl-neuraminic acid, as well as O-acylated sialic acids, of fetal and adult bovine tissues. Carbohydr. Res. 1991, 213, 353–359. [Google Scholar] [CrossRef]
- Schauer, R.; Casalsstenzel, J.; Corfield, A.P.; Veh, R.W. Subcellular Site of the Biosynthesis of O-Acetylated Sialic Acids in Bovine Submandibular-Gland. Glycoconj. J. 1988, 5, 257–270. [Google Scholar] [CrossRef]
- Schauer, R.; deFreese, A.; Gollub, M.; Iwersen, M.; Kelm, S.; Reuter, G.; Schlenzka, W.; VandammeFeldhaus, V.; Shaw, L. Functional and biosynthetic aspects of sialic acid diversity. Indian J. Biochem. Biophys. 1997, 34, 131–141. [Google Scholar] [PubMed]
- Iwersen, M.; Vandamme-Feldhaus, V.; Schauer, R. Enzymatic 4-O-acetylation of N-acetylneuraminic acid in guinea-pig liver. Glycoconj. J. 1998, 15, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Iwersen, M.; Dora, H.; Kohla, G.; Gasa, S.; Schauer, R. Solubilisation and properties of the sialate-4-O-acetyltransferase from guinea pig liver. Biol. Chem. 2003, 384, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Vandamme-Feldhaus, V.; Schauer, R. Characterization of the enzymatic 7-O-acetylation of sialic acids and evidence for enzymatic O-acetyl migration from C-7 to C-9 in bovine submandibular gland. J. Biochem. Tokyo 1998, 124, 111–121. [Google Scholar] [CrossRef]
- Kamerling, J.P.; Schauer, R.; Shukla, A.K.; Stoll, S.; Vanhalbeek, H.; Vliegenthart, J.F.G. Migration of O-Acetyl Groups in N,O-Acetylneuraminic Acids. Eur. J. Biochem. 1987, 162, 601–607. [Google Scholar] [CrossRef]
- Jennings, H.J.; Bhattacharjee, A.K.; Bundle, D.R.; Kenny, C.P.; Martin, A.; Smith, I.C. Strucutres of the capsular polysaccharides of Neisseria meningitidis as determined by 13C-nuclear magnetic resonance spectroscopy. J. Infect. Dis. 1977, 136 (Suppl. 1), S78–S83. [Google Scholar] [CrossRef]
- Ravenscroft, N.; Averani, G.; Bartoloni, A.; Berti, S.; Bigio, M.; Carinci, V.; Costantino, P.; D’Ascenzi, S.; Giannozzi, A.; Norelli, F.; et al. Size determination of bacterial capsular oligosaccharides used to prepare conjugate vaccines. Vaccine 1999, 17, 2802–2816. [Google Scholar] [CrossRef]
- Lemercinier, X.; Jones, C. Full 1H NMR assignment and detailed O-acetylation patterns of capsular polysaccharides from Neisseria meningitidis used in vaccine production. Carbohydr. Res. 1996, 296, 83–96. [Google Scholar] [CrossRef]
- Diaz, S.; Higa, H.H.; Hayes, B.K.; Varki, A. O-acetylation and de-O-acetylation of sialic acids. 7- and 9-O-acetylation of α-2,6-linked sialic acids on endogenous N-linked glycans in rat liver Golgi vesicles. J. Biol. Chem. 1989, 264, 19416–19426. [Google Scholar]
- Wasik, B.R.; Barnard, K.N.; Parrish, C.R. Effects of Sialic Acid Modifications on Virus Binding and Infection. Trends Microbiol. 2016, 24, 991–1001. [Google Scholar] [CrossRef]
- Byrd-Leotis, L.; Jia, N.; Dutta, S.; Trost, J.F.; Gao, C.; Cummings, S.F.; Braulke, T.; Muller-Loennies, S.; Heimburg-Molinaro, J.; Steinhauer, D.A.; et al. Influenza binds phosphorylated glycans from human lung. Sci. Adv. 2019, 5, eaav2554. [Google Scholar] [CrossRef] [PubMed]
- Bosch, A.A.; Biesbroek, G.; Trzcinski, K.; Sanders, E.A.; Bogaert, D. Viral and bacterial interactions in the upper respiratory tract. PLoS Pathog 2013, 9, e1003057. [Google Scholar] [CrossRef] [PubMed]
- McCullers, J.A. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat. Rev. Microbiol. 2014, 12, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Langereis, M.A.; Bakkers, M.J.; Deng, L.; Padler-Karavani, V.; Vervoort, S.J.; Hulswit, R.J.; van Vliet, A.L.; Gerwig, G.J.; de Poot, S.A.; Boot, W.; et al. Complexity and Diversity of the Mammalian Sialome Revealed by Nidovirus Virolectins. Cell Rep. 2015, 11, 1966–1978. [Google Scholar] [CrossRef] [PubMed]
- Vlasak, R.; Luytjes, W.; Spaan, W.; Palese, P. Human and bovine coronaviruses recognize sialic acid-containing receptors similar to those of influenza C viruses. Proc. Natl. Acad. Sci. USA 1988, 85, 4526–4529. [Google Scholar] [CrossRef] [PubMed]
- Kazi, L.; Lissenberg, A.; Watson, R.; de Groot, R.J.; Weiss, S.R. Expression of hemagglutinin esterase protein from recombinant mouse hepatitis virus enhances neurovirulence. J. Virol. 2005, 79, 15064–15073. [Google Scholar] [CrossRef] [PubMed]
- Vimr, E.R. Unified theory of bacterial sialometabolism: how and why bacteria metabolize host sialic acids. ISRN Microbiol. 2013, 2013, 816713. [Google Scholar] [CrossRef]
- Kamerling, J.P.; Dorland, L.; van Halbeek, H.; Vliegenthart, J.F.; Messer, M.; Schauer, R. Structural studies of 4-O-acetyl-α-N-acetylneuraminyl-(2→3)-lactose, the main oligosaccharide in echidna milk. Carbohydr. Res. 1982, 100, 331–340. [Google Scholar] [CrossRef]
- Hanaoka, K.; Pritchett, T.J.; Takasaki, S.; Kochibe, N.; Sabesan, S.; Paulson, J.C.; Kobata, A. 4-O-acetyl-N-acetylneuraminic acid in the N-linked carbohydrate structures of equine and guinea pig α2-macroglobulins, potent inhibitors of influenza virus infection. J. Biol. Chem. 1989, 264, 9842–9849. [Google Scholar]
- Inoue, S.; Iwasaki, M.; Ishii, K.; Kitajima, K.; Inoue, Y. Isolation and structures of glycoprotein-derived free sialooligosaccharides from the unfertilized eggs of Tribolodon hakonensis, a dace. Intracellular accumulation of a novel class of biantennary disialooligosaccharides. J. Biol. Chem. 1989, 264, 18520–18526. [Google Scholar]
- Lochnit, G.; Geyer, R. Carbohydrate structure analysis of batroxobin, a thrombin-like serine protease from Bothrops moojeni venom. Eur. J. Biochem. 1995, 228, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Roussel, P. O-acetylation of sialic acids. Biochimie 1998, 80, 49–57. [Google Scholar] [CrossRef]
- Zanetta, J.P.; Srinivasan, V.; Schauer, R. Analysis of monosaccharides, fatty constituents and rare O-acetylated sialic acids from gonads of the starfish Asterias rubens. Biochimie 2006, 88, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Aamelfot, M.; Dale, O.B.; Weli, S.C.; Koppang, E.O.; Falk, K. Expression of the Infectious Salmon Anemia Virus Receptor on Atlantic Salmon Endothelial Cells Correlates with the Cell Tropism of the Virus. J. Virol. 2012, 86, 10571–10578. [Google Scholar] [CrossRef]
- Aamelfot, M.; Dale, O.B.; Weli, S.C.; Koppang, E.O.; Falk, K. The in situ distribution of glycoprotein-bound 4-O-acetylated sialic acids in vertebrates. Glycoconj. J. 2014, 31, 327–335. [Google Scholar] [CrossRef]
- Reuter, G.; Schauer, R. Determination of sialic acids. Methods Enzymol. 1994, 230, 168–199. [Google Scholar]
- Varki, A.; Diaz, S. The release and purification of sialic acids from glycoconjugates: methods to minimize the loss and migration of O-acetyl groups. Anal. Biochem. 1984, 137, 236–247. [Google Scholar] [CrossRef]
- Mandal, C.; Mandal, C. Identification and analysis of O-acetylated sialoglycoproteins. Methods Mol. Biol. 2013, 981, 57–93. [Google Scholar]
- Ravindranaths, M.H.; Paulson, J.C.; Irie, R.F. Human melanoma antigen O-acetylated ganglioside GD3 is recognized by Cancer antennarius lectin. J. Biol. Chem. 1988, 263, 2079–2086. [Google Scholar]
- Colsch, B.; Jackson, S.N.; Dutta, S.; Woods, A.S. Molecular Microscopy of Brain Gangliosides: Illustrating their Distribution in Hippocampal Cell Layers. ACS Chem. Neurosci. 2011, 2, 213–222. [Google Scholar] [CrossRef]
- Urashima, T.; Inamori, H.; Fukuda, K.; Saito, T.; Messer, M.; Oftedal, O.T. 4-O-Acetyl-sialic acid (Neu4,5Ac2) in acidic milk oligosaccharides of the platypus (Ornithorhynchus anatinus) and its evolutionary significance. Glycobiology 2015, 25, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, N.; Nakano, M.; Kinoshita, M.; Kawabata, A.; Morita, M.; Oda, Y.; Kuroda, R.; Kakehi, K. Specific distribution of sialic acids in animal tissues as examined by LC-ESI-MS after derivatization with 1,2-diamino-4,5-methylenedioxybenzene. Anal. Chem. 2001, 73, 5422–5428. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.K.; Scholz, N.; Reimerdes, E.H.; Schauer, R. High-performance liquid chromatography of N,O-acylated sialic acids. Anal. Biochem. 1982, 123, 78–82. [Google Scholar] [CrossRef]
- Hara, S.; Yamaguchi, M.; Takemori, Y.; Furuhata, K.; Ogura, H.; Nakamura, M. Determination of mono-O-acetylated N-acetylneuraminic acids in human and rat sera by fluorometric high-performance liquid chromatography. Anal. Biochem. 1989, 179, 162–166. [Google Scholar] [CrossRef]
- Manzi, A.E.; Diaz, S.; Varki, A. High-pressure liquid chromatography of sialic acids on a pellicular resin anion-exchange column with pulsed amperometric detection: a comparison with six other systems. Anal. Biochem. 1990, 188, 20–32. [Google Scholar] [CrossRef]
- Sinha, D.; Bhattacharya, D.K.; Mandal, C. A colorimetric assay to evaluate the chemotherapeutic response of children with acute lymphoblastic leukemia (ALL) employing achatininH: a 9-O-acetyl sialic acid binding lectin. Leuk. Res. 1999, 23, 803–809. [Google Scholar] [CrossRef]
- Mlinac, K.; Fabris, D.; Vukelic, Z.; Rozman, M.; Heffer, M.; Bognar, S.K. Structural analysis of brain ganglioside acetylation patterns in mice with altered ganglioside biosynthesis. Carbohydr. Res. 2013, 382, 1–8. [Google Scholar] [CrossRef]
- Schauer, R.; Corfield, A. Colorimetry and Thin-Layer Chromatography of Sialic Acids. In Sialic Acids; Schauer, R., Ed.; Springer: Vienna, Austria, 1982; Volume 10, pp. 77–94. [Google Scholar]
- Klein, A.; Diaz, S.; Ferreira, I.; Lamblin, G.; Roussel, P.; Manzi, A.E. New sialic acids from biological sources identified by a comprehensive and sensitive approach: liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS) of SIA quinoxalinones. Glycobiology 1997, 7, 421–432. [Google Scholar] [CrossRef]
- Kamerling, J.P.; Vliegenthart, J.F. Identification of O-acetylated N-acylneuraminic acids by mass spectrometry. Carbohydr. Res. 1975, 41, 7–17. [Google Scholar] [CrossRef]
- Haverkamp, J.; van Halbeek, H.; Dorland, L.; Vliegenthart, J.F.; Pfeil, R.; Schauer, R. High-resolution 1H-NMR spectroscopy of free and glycosidically linked O-acetylated sialic acids. Eur. J. Biochem. 1982, 122, 305–311. [Google Scholar] [CrossRef]
- Schauer, R. Characterization of sialic acids. Methods Enzymol. 1978, 50, 64–89. [Google Scholar] [PubMed]
- Mawhinney, T.P.; Chance, D.L. Hydrolysis of sialic acids and O-acetylated sialic acids with propionic acid. Anal. Biochem. 1994, 223, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Fahr, C.; Schauer, R. Detection of sialic acids and gangliosides with special reference to 9-O-acetylated species in basaliomas and normal human skin. J. Investig. Dermatol. 2001, 116, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Culling, C.F.; Reid, P.E.; Clay, M.G.; Dunn, W.L. The histochemical demonstration of O-acylated sialic acid in gastrointestinal mucins. Their association with the potassium hydroxide-periodic acid-schiff effect. J. Histochem. Cytochem. 1974, 22, 826–831. [Google Scholar] [CrossRef]
- Shukla, A.K.; Schauer, R. Fluorimetric determination of unsubstituted and 9(8)-O-acetylated sialic acids in erythrocyte membranes. Hoppe-Seyler’s Zeitschrift fur Physiologische Chemie 1982, 363, 255–262. [Google Scholar] [CrossRef]
- Mandal, C.; Basu, S. An unique specificity of a sialic acid binding lectin AchatininH, from the hemolymph of Achatina fulica snail. Biochem. Biophys. Res. Commun. 1987, 148, 795–801. [Google Scholar] [CrossRef]
- Sen, G.; Mandal, C. The specificity of the binding site of AchatininH, a sialic acid-binding lectin from Achatina fulica. Carbohydr. Res. 1995, 268, 115–125. [Google Scholar] [CrossRef]
- Sinha, D.; Mandal, C.; Bhattacharya, D.K. Identification of 9-O acetyl sialoglycoconjugates (9-OAcSGs) as biomarkers in childhood acute lymphoblastic leukemia using a lectin, AchatininH, as a probe. Leukemia 1999, 13, 119–125. [Google Scholar] [CrossRef]
- Mandal, C.; Basu, S.; Mandal, C. Physiochemical studies on achatininH, a novel sialic acid-binding lectin. Biochem. J. 1989, 257, 65–71. [Google Scholar] [CrossRef]
- Strasser, P.; Unger, U.; Strobl, B.; Vilas, U.; Vlasak, R. Recombinant viral sialate-O-acetylesterases. Glycoconj. J. 2004, 20, 551–561. [Google Scholar] [CrossRef]
- Alviano, D.S.; Rodrigues, M.L.; Almeida, C.A.; Santos, A.L.; Couceiro, J.N.; Soares, R.M.; Travassos, L.R.; Alviano, C.S. Differential expression of sialylglycoconjugates and sialidase activity in distinct morphological stages of Fonsecaea pedrosoi. Arch. Microbiol. 2004, 181, 278–286. [Google Scholar] [PubMed]
- Hubl, U.; Ishida, H.; Kiso, M.; Hasegawa, A.; Schauer, R. Studies on the specificity and sensitivity of the influenza C virus binding assay for 9-O-acetylated sialic acids and its application to human melanomas. J. Biochem. 2000, 127, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- do Valle Matta, M.A.; Sales Alviano, D.; dos Santos Silva Couceiro, J.N.; Nazareth, M.; Meirelles, L.; Sales Alviano, C.; Angluster, J. Cell-surface sialoglycoconjugate structures in wild-type and mutant Crithidia fasciculata. Parasitol. Res. 1999, 85, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Harms, G.; Reuter, G.; Corfield, A.P.; Schauer, R. Binding specificity of influenza C-virus to variably O-acetylated glycoconjugates and its use for histochemical detection of N-acetyl-9-O-acetylneuraminic acid in mammalian tissues. Glycoconj. J. 1996, 13, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, G.; Suguri, T.; Reuter, G.; Yu, R.K.; Schauer, R.; Herrler, G. Modification of sialic acids by 9-O-acetylation is detected in human leucocytes using the lectin property of influenza C virus. Glycobiology 1994, 4, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Krishna, M.; Varki, N.M.; Varki, A. 9-O-acetylated sialic acids have widespread but selective expression: analysis using a chimeric dual-function probe derived from influenza C hemagglutinin-esterase. Proc. Natl. Acad. Sci. USA 1994, 91, 7782–7786. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, G.; Reuter, G.; Schauer, R. Use of influenza C virus for detection of 9-O-acetylated sialic acids on immobilized glycoconjugates by esterase activity. Eur. J. Biochem. 1992, 204, 209–215. [Google Scholar] [CrossRef]
- Muchmore, E.A.; Varki, A. Selective inactivation of influenza C esterase: a probe for detecting 9-O-acetylated sialic acids. Science 1987, 236, 1293–1295. [Google Scholar] [CrossRef]
- Manuguerra, J.C.; DuBois, C.; Hannoun, C. Analytical detection of 9(4)-O-acetylated sialoglycoproteins and gangliosides using influenza C virus. Anal. Biochem. 1991, 194, 425–432. [Google Scholar] [CrossRef]
- Hellebo, A.; Vilas, U.; Falk, K.; Vlasak, R. Infectious salmon anemia virus specifically binds to and hydrolyzes 4-O-acetylated sialic acids. J. Virol. 2004, 78, 3055–3062. [Google Scholar] [CrossRef]
- Falk, K.; Aspehaug, V.; Vlasak, R.; Endresen, C. Identification and characterization of viral structural proteins of infectious salmon anemia virus. J. Virol. 2004, 78, 3063–3071. [Google Scholar] [CrossRef] [PubMed]
- Regl, G.; Kaser, A.; Iwersen, M.; Schmid, H.; Kohla, G.; Strobl, B.; Vilas, U.; Schauer, R.; Vlasak, R. The hemagglutinin-esterase of mouse hepatitis virus strain S is a sialate-4-O-acetylesterase. J. Virol. 1999, 73, 4721–4727. [Google Scholar] [PubMed]
- Wurzer, W.J.; Obojes, K.; Vlasak, R. The sialate-4-O-acetylesterases of coronaviruses related to mouse hepatitis virus: A proposal to reorganize group 2 Coronaviridae. J. Gen. Virol. 2002, 83, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Smits, S.L.; Gerwig, G.J.; van Vliet, A.L.; Lissenberg, A.; Briza, P.; Kamerling, J.P.; Vlasak, R.; de Groot, R.J. Nidovirus sialate-O-acetylesterases: evolution and substrate specificity of coronaviral and toroviral receptor-destroying enzymes. J. Biol. Chem. 2005, 280, 6933–6941. [Google Scholar] [CrossRef] [PubMed]
- Langereis, M.A.; Zeng, Q.; Gerwig, G.J.; Frey, B.; von Itzstein, M.; Kamerling, J.P.; de Groot, R.J.; Huizinga, E.G. Structural basis for ligand and substrate recognition by torovirus hemagglutinin esterases. Proc. Natl. Acad. Sci. USA 2009, 106, 15897–15902. [Google Scholar] [CrossRef] [PubMed]
- Schultze, B.; Gross, H.J.; Brossmer, R.; Klenk, H.D.; Herrler, G. Hemagglutinating encephalomyelitis virus attaches to N-acetyl-9-O-acetylneuraminic acid-containing receptors on erythrocytes: comparison with bovine coronavirus and influenza C virus. Virus Res. 1990, 16, 185–194. [Google Scholar] [CrossRef]
- Schultze, B.; Wahn, K.; Klenk, H.D.; Herrler, G. Isolated HE-protein from hemagglutinating encephalomyelitis virus and bovine coronavirus has receptor-destroying and receptor-binding activity. Virology 1991, 180, 221–228. [Google Scholar] [CrossRef]
- Klausegger, A.; Strobl, B.; Regl, G.; Kaser, A.; Luytjes, W.; Vlasak, R. Identification of a coronavirus hemagglutinin-esterase with a substrate specificity different from those of influenza C virus and bovine coronavirus. J. Virol. 1999, 73, 3737–3743. [Google Scholar]
- Kunkel, F.; Herrler, G. Structural and Functional-Analysis of the Surface Protein of Human Coronavirus OC43. Virology 1993, 195, 195–202. [Google Scholar] [CrossRef]
- Krempl, C.; Schultze, B.; Laude, H.; Herrler, G. Point mutations in the S protein connect the sialic acid binding activity with the enteropathogenicity of transmissible gastroenteritis coronavirus. J. Virol. 1997, 71, 3285–3287. [Google Scholar]
- Schultze, B.; Gross, H.J.; Brossmer, R.; Herrler, G. The S protein of bovine coronavirus is a hemagglutinin recognizing 9-O-acetylated sialic acid as a receptor determinant. J. Virol. 1991, 65, 6232–6237. [Google Scholar] [PubMed]
- Schultze, B.; Herrler, G. Bovine coronavirus uses N-acetyl-9-O-acetylneuraminic acid as a receptor determinant to initiate the infection of cultured cells. J. Gen. Virol. 1992, 73 Pt 4, 901–906. [Google Scholar] [CrossRef]
- Zeng, Q.; Langereis, M.A.; van Vliet, A.L.; Huizinga, E.G.; de Groot, R.J. Structure of coronavirus hemagglutinin-esterase offers insight into corona and influenza virus evolution. Proc. Natl. Acad. Sci. USA 2008, 105, 9065–9069. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.S.; Barnstable, C.J. O-acetylation of a cell-surface carbohydrate creates discrete molecular patterns during neural development. Proc. Natl. Acad. Sci. USA 1987, 84, 8716–8720. [Google Scholar] [CrossRef] [PubMed]
- Kniep, B.; Peter-Katalinic, J.; Flegel, W.; Northoff, H.; Rieber, E.P. CDw 60 antibodies bind to acetylated forms of ganglioside GD3. Biochem. Biophys. Res. Commun. 1992, 187, 1343–1349. [Google Scholar] [CrossRef]
- Kniep, B.; Claus, C.; Peter-Katalinic, J.; Monner, D.A.; Dippold, W.; Nimtz, M. 7-O-acetyl-GD3 in human T-lymphocytes is detected by a specific T-cell-activating monoclonal antibody. J. Biol. Chem. 1995, 270, 30173–30180. [Google Scholar]
- Cerato, E.; Birkle, S.; Portoukalian, J.; Mezazigh, A.; Chatal, J.F.; Aubry, J. Variable region gene segments of nine monoclonal antibodies specific to disialogangliosides (GD2, GD3) and their O-acetylated derivatives. Hybridoma 1997, 16, 307–316. [Google Scholar] [CrossRef]
- Song, L.; Zhou, H.; Cai, X.; Li, C.; Liang, J.; Jin, C. NeuA O-acetylesterase activity is specific for CMP-activated O-acetyl sialic acid in Streptococcus suis serotype 2. Biochem. Biophys. Res. Commun. 2011, 410, 212–217. [Google Scholar] [CrossRef]
- Yu, H.; Zeng, J.; Li, Y.; Thon, V.; Shi, B.; Chen, X. Effective one-pot multienzyme (OPME) synthesis of monotreme milk oligosaccharides and other sialosides containing 4-O-acetyl sialic acid. Org. Biomol. Chem. 2016, 14, 8586–8597. [Google Scholar] [CrossRef]
- Corfield, A.P.; Wagner, S.A.; Clamp, J.R.; Kriaris, M.S.; Hoskins, L.C. Mucin degradation in the human colon: production of sialidase, sialate O-acetylesterase, N-acetylneuraminate lyase, arylesterase, and glycosulfatase activities by strains of fecal bacteria. Infect. Immun. 1992, 60, 3971–3978. [Google Scholar]
- Gottschalk, A. Structural Relationship between Sialic Acid, Neuraminic Acid and 2-Carboxy-Pyrrole. Nature 1955, 176, 881–882. [Google Scholar] [CrossRef]
- Pepper, D.S. Sialic acids of horse serum with special reference to their virus inhibitory properties. Biochim. Biophys. Acta 1968, 156, 317–326. [Google Scholar] [CrossRef]
- Haverkamp, J.; Schauer, R.; Wember, M.; Kamerling, J.P.; Vliegenthart, J.F. Synthesis of 9-O-acetyl- and 4,9-di-O-acetyl derivatives of the methyl ester of N-acetyl-β-d-neuraminic acid methylglycoside. Their use as models in periodate oxidation studies. Hoppe-Seyler’s Zeitschrift fur Physiologische Chemie 1975, 356, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Furuhata, K.; Ogura, H. Studies on sialic acids. 19. Syntheses of partially O-acetylated 4-methylcoumarin-7-yl 5-acetamido-3,5-dideoxy-α-d-glycero-d-galacto-2-nonulopyranosidonic acids. Chem. Pharm. Bull. 1989, 37, 2037–2040. [Google Scholar] [CrossRef]
- Roth, A.; Faillard, H. Synthesis of fluorescent 7,8,9-tri-O-acetyl-N-acetylneuraminic acid and 4-O-acetyl-N-acetylneuraminic acid α-thioketosides. Liebigs Ann. Der Chem. 1993, 485–489. [Google Scholar] [CrossRef]
- Reinhard, B.; Faillard, H. Regioselective acetylations of sialic acid α-ketosides. Liebigs Ann. Der Chem. 1994, 193–203. [Google Scholar] [CrossRef]
- Yachida, Y. Synthesis of O-acetylated GM3s containing 9-O-acetyl sialic acid, containing, 4,9-di-O-acetyl sialic acid and containing 6’-O-acetyl galactose. J. Lib. Arts Sci. Sapporo Med. Univ. Sch. Med. 1994, 0, 19–30. [Google Scholar]
- Hasegawa, A.; Murase, T.; Ogawa, M.; Ishida, H.; Kiso, M. Synthetic Studies on Sialoglycoconjugates 17: Synthesis of 4-O-, 9-O-, and 4,9-Di-O-Acetyl-N-Acetylneuraminic Acids and their Derivatives. J. Carbohydr. Chem. 1990, 9, 415–428. [Google Scholar] [CrossRef]
- Clarke, P.A.; Mistry, N.; Thomas, G.H. Synthesis of the complete series of mono acetates of N-acetyl-d-neuraminic acid. Org. Biomol. Chem. 2012, 10, 529–535. [Google Scholar] [CrossRef]
- Manzi, A.E.; Dell, A.; Azadi, P.; Varki, A. Studies of naturally occurring modifications of sialic acids by fast-atom bombardment mass-spectrometry—Analysis of positional isomers by periodate cleavage. J. Biol. Chem. 1990, 265, 8094–8107. [Google Scholar]
- Yachida, Y. Isolation and characterization of novel O-acetylated GM3 containing 4-O-acetyl-N-acetyl sialic acid in equine erythrocytes. J. Lib. Arts Sci. Sapporo Med. Univ. Sch. Med. 1994, 0, 53–60. [Google Scholar]
- Priyadarzini, T.R.K.; Selvin, J.F.A.; Veluraja, K. Molecular Dynamics Simulation Studies on Sialic acid and its Acetylated Derivatives and their Interaction with Vibrio Cholerae Neuraminidase. In Proceedings of the 2009 International Association of Computer Science and Information Technology—Spring Conference, Singapore, 17–20 April 2009; pp. 562–566. [Google Scholar]
- Matrosovich, M.N.; Gambaryan, A.S.; Chumakov, M.P. Influenza viruses differ in recognition of 4-O-acetyl substitution of sialic acid receptor determinant. Virology 1992, 188, 854–858. [Google Scholar] [CrossRef]
- Sarris, A.H.; Palade, G.E. Sialoglycoproteins of murine erythrocyte-ghosts—Modified periodic acid-schiff stain procedure staining non-substituted and O-acetylated sialyl residues on glycopeptides. J. Biol. Chem. 1979, 254, 6724–6731. [Google Scholar] [PubMed]
- Accili, D.; Gabrielli, M.G.; Menghi, G.; Materazzi, G. Histoenzymological detection of sialic acids in the rodent salivary glands. Histol. Histopathol. 1996, 11, 647–658. [Google Scholar] [PubMed]
- Yachida, Y.; Tsuchihashi, K.; Gasa, S. Novel di-O-acetylated GM3s from equine erythrocytes, one containing 4,9-di-O-acetyl-N-glycolylneuraminic acid and another containing 4-O-acetyl-N-glycolylneuraminic acid and 6-O-acetyl-d-galactose. Carbohydr. Res. 1997, 298, 201–212. [Google Scholar] [CrossRef]
- Reid, P.E.; Culling, C.F.A.; Tsang, W.C.; Clay, M.G.; Ramey, C.W.; Dunn, W.L. Demonstration of O-acetylated sialic acids in colonic epithelial glycoproteins. Can. J. Biochem. 1975, 53, 388–391. [Google Scholar] [CrossRef]
- Downs, F.; Herp, A. Chemical studies on a hamster sublingual glycoprotein. Int. J. Pept. Protein Res. 1977, 10, 229–234. [Google Scholar] [CrossRef]
- Blix, G.; Lindberg, E. The Sialic Acids of Bovine and Equine Submaxillary mucins. Acta Chem. Scand. 1960, 14, 1809–1814. [Google Scholar] [CrossRef]
- Schauer, R.; Reuter, G.; Stoll, S.; Delrio, F.P.; Herrler, G.; Klenk, H.D. Isolation and characterization of sialate 9(4)-O-acetylesterase from influenza C virus. Biol. Chem. Hoppe Seyler 1988, 369, 1121–1130. [Google Scholar] [CrossRef]
- Sato, C.; Kitajima, K.; Tazawa, I.; Inoue, Y.; Inoue, S.; Troy, F.A. Structural diversity in the α-2,8-linked polysialic acid chains in salmonid fish egg glycoproteins-occurrence of poly(Neu5Ac), poly(Neu5Gc), poly(Neu5Ac, Neu5Gc), poly(KDN), and their partially acetylated forms. J. Biol. Chem. 1993, 268, 23675–23684. [Google Scholar]
- Neuberge, A.; Ratcliff, W. Acid and enzymic hydrolysis of O-acetylated sialic acid residues from rabbit tamm-horsfall glycoprotein. Biochem. J. 1972, 129, 683–693. [Google Scholar]
- Rinninger, A.; Richet, C.; Pons, A.; Kohla, G.; Schauer, R.; Bauer, H.C.; Zanetta, J.P.; Vlasak, R. Localisation and distribution of O-acetylated N-acetylneuraminic acids, the endogenous substrates of the hemagglutinin-esterases of murine coronaviruses, in mouse tissue. Glycoconj. J. 2006, 23, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Schauer, R.; Shukla, A.K. Isolation and properties of two sialate-O-acetylesterases from horse liver with 4- and 9-O-acetyl specificities. Glycoconj. J. 2008, 25, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Oftedal, O.T.; Nicol, S.C.; Davies, N.W.; Sekii, N.; Taufik, E.; Fukuda, K.; Saito, T.; Urashima, T. Can an ancestral condition for milk oligosaccharides be determined? Evidence from the Tasmanian echidna (Tachyglossus aculeatus setosus). Glycobiology 2014, 24, 826–839. [Google Scholar] [CrossRef]
- Varki, A.; Diaz, S. A neuraminidase from streptococcus sanguis that can release O-acetylated sialic acids. J. Biol. Chem. 1983, 258, 2465–2471. [Google Scholar]
- Schauer, R.; Wember, M.; Tschesche, H. Isolation and characterization of an oligosaccharide specific and glycoprotein specific sialidase from human leukocytes. Hoppe-Seylers Zeitschrift fur Physiologische Chemie 1984, 365, 419–426. [Google Scholar] [CrossRef]
- Kleineidam, R.G.; Furuhata, K.; Ogura, H.; Schauer, R. 4-methylumbelliferyl-α-glycosides of partially O-acetylated N-acetylneuraminic acids as substrates of a bacterial and viral sialidases. Biol. Chem. Hoppe Seyler 1990, 371, 715–719. [Google Scholar] [CrossRef]
- Corfield, A.P.; Sanderwewer, M.; Veh, R.W.; Wember, M.; Schauer, R. The action of sialidases on substrates containing O-acetylated sialic acids. Biol. Chem. Hoppe Seyler 1986, 367, 433–439. [Google Scholar] [CrossRef]
- Ahmed, H.; Gabius, H.J. Purification and properties of a Ca2+ independent sialic acid binding lectin from human placenta with preferential affinity to ortho-acetylsialic acids. J. Biol. Chem. 1989, 264, 18673–18678. [Google Scholar]
- Pritchett, T.J.; Paulson, J.C. Basis for the potent inhibition of influenza virus infection by equine and guinea pig α2-macroglobulin. J. Biol. Chem. 1989, 264, 9850–9858. [Google Scholar]
- Schauer, R.; Reuter, G.; Stoll, S. Sialate O-acetylesterases—Key enzymes in sialic acid catabolism. Biochimie 1988, 70, 1511–1519. [Google Scholar] [CrossRef]
- Corfield, A.P.; Ferreiradoamaral, C.; Wember, M.; Schauer, R. Metabolism of Ω-acyl-N-acetylneuraminic acids in bovine and equine submandibular glands. Eur. J. Biochem. 1976, 68, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, N.; Santos, J.M.; Liu, Y.; Palma, A.S.; Leon, E.; Saouros, S.; Kiso, M.; Blackman, M.J.; Matthews, S.; Feizi, T.; et al. Members of a Novel Protein Family Containing Microneme Adhesive Repeat Domains Act as Sialic Acid-binding Lectins during Host Cell Invasion by Apicomplexan Parasites. J. Biol. Chem. 2010, 285, 2064–2076. [Google Scholar] [CrossRef] [PubMed]
- Langereis, M.A.; Zeng, Q.; Heesters, B.; Huizinga, E.G.; de Groot, R.J. The Murine Coronavirus Hemagglutinin-esterase Receptor-binding Site: A Major Shift in Ligand Specificity through Modest Changes in Architecture. Plos Pathogens 2012, 8, e1002492. [Google Scholar] [CrossRef]
- Weli, S.C.; Aamelfot, M.; Dale, O.B.; Koppang, E.O.; Falk, K. Infectious salmon anaemia virus infection of Atlantic salmon gill epithelial cells. Virol. J. 2013, 10, 5. [Google Scholar] [CrossRef]
- Aamelfot, M.; Dale, O.B.; Falk, K. Infectious salmon anaemia—Pathogenesis and tropism. J. Fish. Dis. 2014, 37, 291–307. [Google Scholar] [CrossRef]
- Aamelfot, M.; Dale, O.B.; McBeath, A.; Falk, K. Host tropism of infectious salmon anaemia virus in marine and freshwater fish species. J. Fish. Dis. 2015, 38, 687–694. [Google Scholar] [CrossRef]
- Langereis, M.A.; van Vliet, A.L.; Boot, W.; de Groot, R.J. Attachment of mouse hepatitis virus to O-acetylated sialic acid is mediated by hemagglutinin-esterase and not by the spike protein. J. Virol. 2010, 84, 8970–8974. [Google Scholar] [CrossRef]
- de Groot, R.J. Structure, function and evolution of the hemagglutinin-esterase proteins of corona- and toroviruses. Glycoconj. J. 2006, 23, 59–72. [Google Scholar] [CrossRef]
- von Itzstein, M.; Wu, W.Y.; Kok, G.B.; Pegg, M.S.; Dyason, J.C.; Jin, B.; Van Phan, T.; Smythe, M.L.; White, H.F.; Oliver, S.W.; et al. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature 1993, 363, 418–423. [Google Scholar] [CrossRef]
- Tindal, D.J.; Dyason, J.C.; Thomson, R.J.; Suzuki, T.; Ueyama, H.; Kuwahara, Y.; Maki, N.; Suzuki, Y.; von Itzstein, M. Synthesis and evaluation of 4-O-alkylated 2-deoxy-2,3-didehydro-N-acetylneuraminic acid derivatives as inhibitors of human parainfluenza virus type-3 sialidase activity. Bioorg. Med. Chem. Lett. 2007, 17, 1655–1658. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Luo, C.; Qin, G.; Xu, Z.; Li, N.; Liu, H.; Shen, X.; Ma, J.; Wang, Q.; Yang, C.; et al. Why are Oseltamivir and Zanamivir effective against the newly emerged influenza A virus (A/H1N1)? Cell Res 2009, 19, 1221–1224. [Google Scholar] [CrossRef] [PubMed]
- Brandstetter, H.H.; Zbiral, E. Strukturelle Abwandlungen an N-Acetylneuraminsäure, 2. Liebigs Ann. Der Chem. 1983, 1983, 2055–2065. [Google Scholar] [CrossRef]
- Groves, D.R.; Itzstein, M.V. Synthesis of C-4-disubstituted analogues of N-acetylneuraminic acid. J. Chem. Soc. Perkin Trans. 1996, 23, 2817–2821. [Google Scholar] [CrossRef]
- Hagedorn, H.-W.; Brossmer, R. Synthesis and Biological Properties of N-Acetyl-4-deoxy-d-neuraminic Acid. Helv. Chim. Acta 1986, 69, 2127–2132. [Google Scholar] [CrossRef]
- Liu, K.G.; Yan, S.; Wu, Y.L.; Yao, Z.J. Synthesis of 4-azido-4-deoxy-Neu5,7,8,9Ac42en1Me. A key intermediate for the synthesis of GG167 from d-glucono-delta-lactone. Org. Lett. 2004, 6, 2269–2272. [Google Scholar] [CrossRef] [PubMed]
- Khedri, Z.; Xiao, A.; Yu, H.; Landig, C.S.; Li, W.; Diaz, S.; Wasik, B.R.; Parrish, C.R.; Wang, L.P.; Varki, A.; et al. A Chemical Biology Solution to Problems with Studying Biologically Important but Unstable 9-O-Acetyl Sialic Acids. ACS Chem. Biol. 2017, 12, 214–224. [Google Scholar] [CrossRef]



| Sialic Acids | Occurrence a |
|---|---|
| 5-N-Acetyl-4-O-acetylneuraminic acid | V |
| 5-N-Acetyl-7-O-acetylneuraminic acid | V, Pz, B |
| 5-N-Acetyl-8-O-acetylneuraminic acid | V, B |
| 5-N-Acetyl-9-O-acetylneuraminic acid | V, E, Pz, F, B, H [31] |
| 5-N-Acetyl-4,9-di-O-acetylneuraminic acid | V |
| 5-N-Acetyl-7,9-di-O-acetylneuraminic acid | V, B, H [32] |
| 5-N-Acetyl-8,9-di-O-acetylneuraminic acid | V, H [32] |
| 5-N-Acetyl-4,7,9-tri-O-acetylneuraminic acid | V |
| 5-N-Acetyl-7,8,9-tri-O-acetylneuraminic acid | V |
| 5-N-Acetyl-4,7,8,9-tetra-O-acetylneuraminic acid | V |
| 5-N-Acetyl-9-O-lactylneuraminic acid | V |
| 5-N-Acetyl-4-O-acetyl-9-O-lactylneuraminic acid | V |
| 5-N-Acetyl-7-O-acetyl-9-O-lactylneuraminic acid | V |
| 5-N-Acetyl-9-O-acetyl-8-O-methylneuraminic acid | V, E |
| 5-N-Acetyl-4-O-acetyl-8-O-sulfoneuraminic acid | V, E |
| 5-N-Acetyl-9-O-acetyl-2-deoxy-2,3-didehydroneuraminic acid | V |
| 5-N-Acetyl-4,9-di-O-acetylneuraminic acid 1,7-lactone | V |
| Conditions | +/− | Comments | References |
|---|---|---|---|
| Alkaline conditions | – | Acetyl migrations (C7 and C9 OAc’s) | [47,62] |
| Glycan release by NaOH | – | Loss of esters | [48] |
| Acidic hydrolysis with propionic acid vs. acetic acid | / | Loss of esters. Minimal if propionic acid (2M, 4 h, 80 °C) is used | [46,47,63,64] |
| Histochemical staining Mild periodic-Schiff | + | Stain after hydrolytic removal (General method) | [65] |
| Mild oxidation | + | Indirect quantitative determination by formaldehyde production (For C8 and C9 OAcs) | [66] |
| Lectin staining | + | from crab Cancer antennarius (For C9 OAc) | [49] |
| + | from snail Achatina fulica (For C9 OAc) | [67,68,69,70] | |
| Viral lectin staining | + | from influenza C (For C9 and C4 OAc) | [64,71,72,73,74,75,76,77,78,79,80] |
| + | infection salmon anemia virus (For C4 OAc) | [81,82] | |
| Coronavirus | + | Murine coronavirus (For C4 OAc) | [83,84] |
| Porcine torovirus (For C9 OAc) | [85,86] | ||
| Bovine torovirus (For C7(8), C9 OAc) | [85] | ||
| Hemagglutinating encephalomyelitis virus (For C9 OAc) | [87,88] | ||
| Puffin coronavirus (For C4 OAc) | [84,89] | ||
| Human coronavirus OC43 (For C9 OAc) | [90,91] | ||
| Bovine coronavirus (For C9 OAc) | [85,92,93,94] | ||
| + | Sialodacryo-adenitis virus (For C4 OAc) | [71] | |
| Monoclonal antibodies (For OAc gangliosides) | |||
| + | MAb Jones | [95] | |
| + | MAb UM4D4 | [96] | |
| + | MAb U5 | [97] | |
| + | MAb 7H2 | [98] | |
| MALDI mass spectrometry imaging | + | (For OAc gangliosides) | [50,51] |
| Topic | References |
|---|---|
| Chemical synthesis | |
| Synthesis of Neu4,5Ac2 and Neu4,5,9Ac3 methylate | [104] |
| Synthesis of Neu4,5Ac2 with methylcoumarin | [105] |
| Synthesis of fluorescent 4-O-acetyl thioketosides Neu5Ac | [106] |
| Synthesis of 4-O-acetyl ketosides of Neu5Ac | [107] |
| Synthesis of 4-O-acetyl containing GM3s | [108] |
| Synthesis of Neu4,5Ac2; Neu4,5,9Ac3; Neu4,5,8,9Ac4; Neu2,4,5,8,9Ac5; Neu4,5,7,8,9Ac5 | [13,109,110] |
| Structural analysis | |
| Identification of Neu4,5Ac2, Neu5,9Ac2, Neu4,5,9Ac3, Neu5,7,9Ac3, Neu4Ac5Gc, Neu9Ac5Gc by mass spectrometry | [60] |
| NMR studies on Neu4,5Ac2(α2→3) lactose | [38] |
| FAB-MS analysis of C4-O-acetylated Neu5Ac | [111] |
| Characterization of O-acetylated GM3s in equine erythrocytes | [112] |
| Computational/conformational studies on Neu4,5Ac2 | [113] |
| Molecular dynamics studies on C4-O-acetylated Neu5Ac on hemagglutinin activity and its receptor binding site | [114] |
| Oxidative studies | |
| Periodate oxidation on Neu4,5Ac2 studies | [104] |
| Periodate oxidation on Neu4,5Ac2 of murine erythrocyte ghosts | [115] |
| Periodate oxidation on O-acetyl sialosides from rat salivary glands | [116] |
| Isolation of C4-O-acetylated containing Neu5Ac from various sources | |
| Isolation from gangliosides | [117] |
| Isolation from colonic epithelial cells | [118] |
| Isolation from hamster sublingual gland | [119] |
| Isolation from murine erythrocyte ghosts (DBA/2, CD-1, B6D2 strains) | [115] |
| Isolation from bovine and equine submaxillary mucins | [120] |
| Isolation from influenza C | [121] |
| Isolation from salmon eggs | [122] |
| Isolation from equine erythrocytes | [112] |
| Isolation from rat submandibular glands | [116] |
| Isolation of 4-O-Ac-GM3 from equine erythrocytes | [117] |
| Isolation from starfish A. rubens | [43] |
| Isolation from vertebrates (fishes) | [45] |
| Acid/Enzyme hydrolysis from rabbit urine glycoprotein | [123] |
| Isolation of O-acetylated Neu5Ac using propionic acid | [63] |
| Isolation from rat coronavirus | [71] |
| Hydrolysis from murine coronaviruses in mouse tissue | [124] |
| O-Acetylesterases from horse liver specific for Neu4,5Ac2 | [125] |
| Hydrolysis from infectious salmon anemia virus (ISAV) | [81] |
| Isolation from echidna and platypus milk | [51,126] |
| Inhibitory activities of C4-O-acetylated containing Neu5Ac | |
| A2 viral strain inhibited by horse serum containing Neu4,5Ac2 | [103] |
| Neuraminidase from S. sanguis cannot cleave C4-O-acetylated Neu5Ac | [127] |
| Sialidase from human leukocytes cannot cleave C4-O-acetylated Neu5Ac | [128] |
| Sialidase from C. perfringens cannot cleave C4-O-acetylated Neu5Ac | [129] |
| Bacterial sialidase activities inhibited by C4-O-acetylated Neu5Ac | [130] |
| Inhibition of rabbit red blood cell agglutination activities by AchatininH | [67] |
| Inhibition of hemagglutinin activities of lectin bindings in human placenta | [131] |
| Influenza viruses (H3N2 strain) binding to Neu4,5Ac2 while unrecognized by B or H1N1 viruses | [114] |
| Viral inhibitory effects by equine and pig sera based on Neu4,5Ac2 located on the α2 macroglobulins | [132] |
| Esterase hydrolysis of acetyl groups | |
| Acetylesterases from horse release Neu4,5Ac2 | [133] |
| ISAV esterases bind and hydrolyze C4-OAc Neu5Ac | [81] |
| C4-OAc Neu5Ac is the preferred binding receptor of ISAV | [44] |
| Metabolism of partially O-acetylated Neu5Ac from bovine and equine submandibular glands | [134] |
| 4-O-Acetylated Neu5Ac in equine and guinea pig α2 macroglobulins | [39] |
| O-Acetyltransferase in C4-OAc Neu5Ac biosynthesis | [21] |
| O-Acetylation on C4-OAc Neu5Ac | [42] |
| Mouse hepatitis virus S esterase cleaves C4-OAc Neu5Ac | [83] |
| Binding studies on C4-O-acetylated Neu5Ac | |
| Micronemes show strong binding preference to C4-OAc Neu5Ac | [135] |
| Mouse hepatitis virus S binds and recognizes C4-OAc Neu5Ac | [136] |
| Infectious Salmon Anaemia Virus (ISAV) binds and recognizes C4-OAc Neu5Ac | [137,138,139] |
| Crab (Cancer antennarius) lectin binds to C4-OAc Neu5Ac | [49] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.S. Post-Glycosylation Modification of Sialic Acid and Its Role in Virus Pathogenesis. Vaccines 2019, 7, 171. https://doi.org/10.3390/vaccines7040171
Park SS. Post-Glycosylation Modification of Sialic Acid and Its Role in Virus Pathogenesis. Vaccines. 2019; 7(4):171. https://doi.org/10.3390/vaccines7040171
Chicago/Turabian StylePark, Simon S. 2019. "Post-Glycosylation Modification of Sialic Acid and Its Role in Virus Pathogenesis" Vaccines 7, no. 4: 171. https://doi.org/10.3390/vaccines7040171
APA StylePark, S. S. (2019). Post-Glycosylation Modification of Sialic Acid and Its Role in Virus Pathogenesis. Vaccines, 7(4), 171. https://doi.org/10.3390/vaccines7040171





