Integrated Bioinformatic Analyses and Immune Characterization of New Neisseria gonorrhoeae Vaccine Antigens Expressed during Natural Mucosal Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Candidate Antigen Selection Strategy (CASS) and Computational Tools
2.2. Bacterial Strains and Growth Conditions
2.3. Cloning and Expression of Recombinant Gonococcal Hypothetical Proteins
2.4. Chromatography Purification of Recombinant Hypothetical Proteins
2.5. Immunization of Mice
2.6. Immunoblotting
2.7. Antibody ELISA
2.8. Cytokine ELISA
2.9. Flow Cytometry
2.10. Bactericidal Assay (SBA)
2.11. Statistical Analysis
3. Results
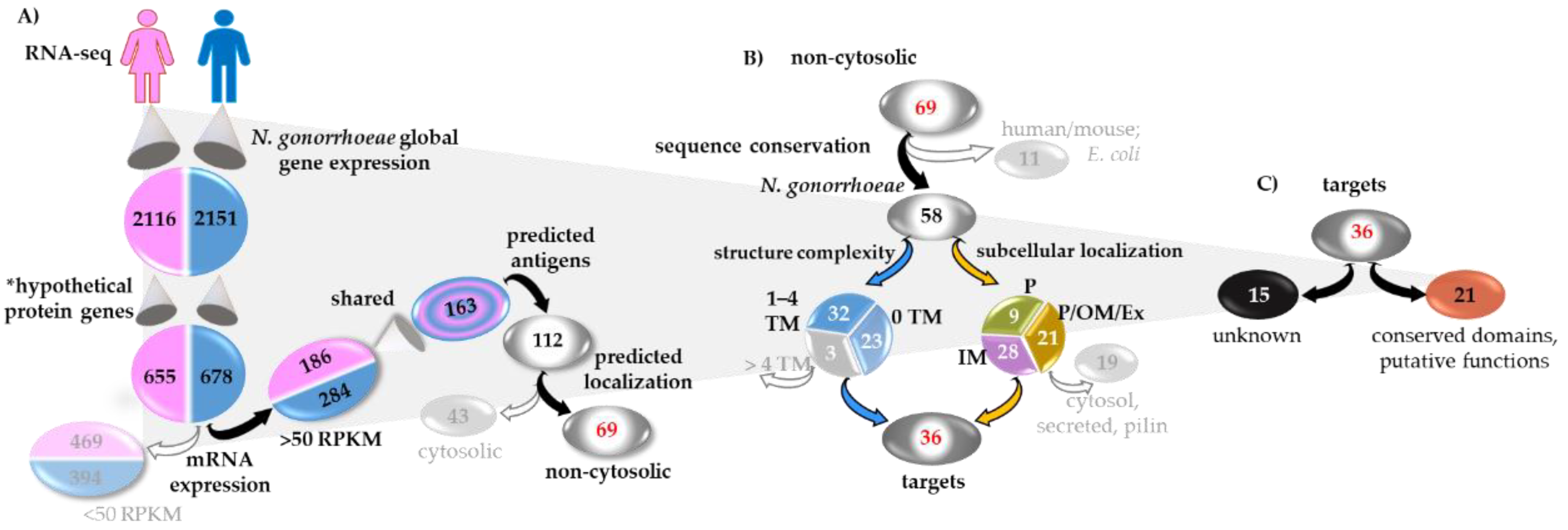
3.1. Gonococcal Candidate Antigen Selection Strategy (CASS)
Discovery and Analysis Phase (DAP)
3.2. Sequence Analysis and Conservation
3.3. Network Analysis
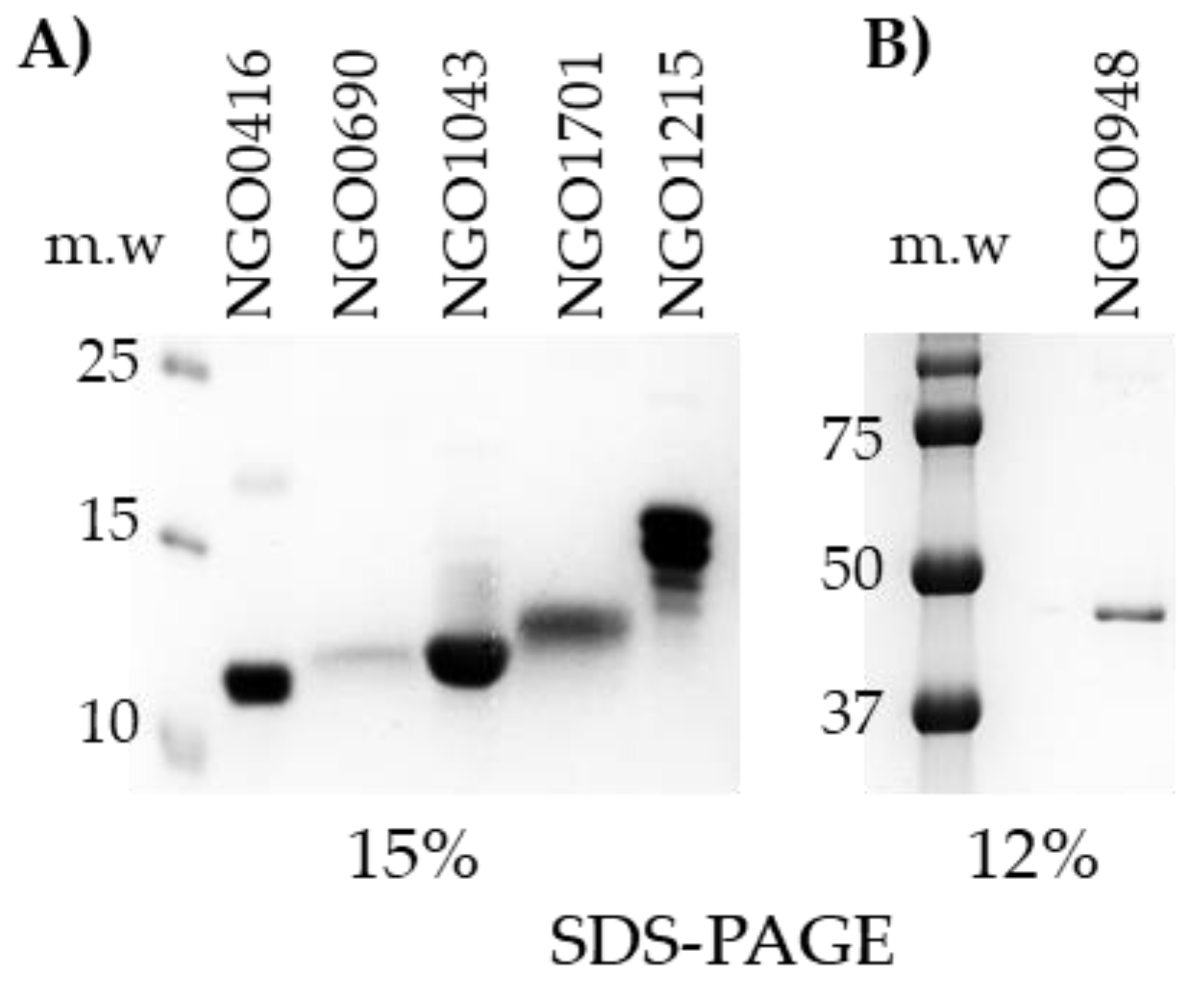
3.4. Candidate Antigens Cloning, Expression and Purification
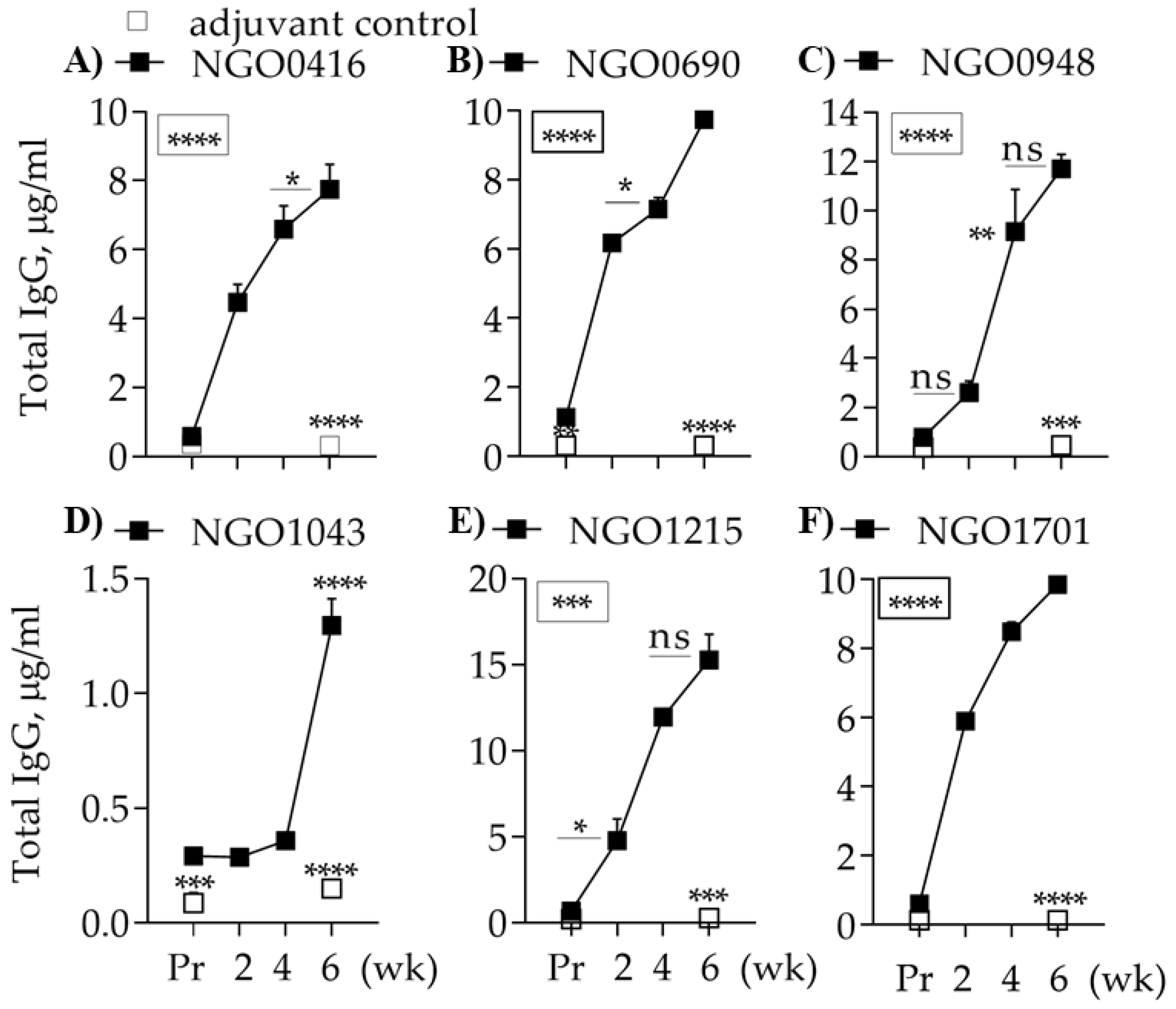
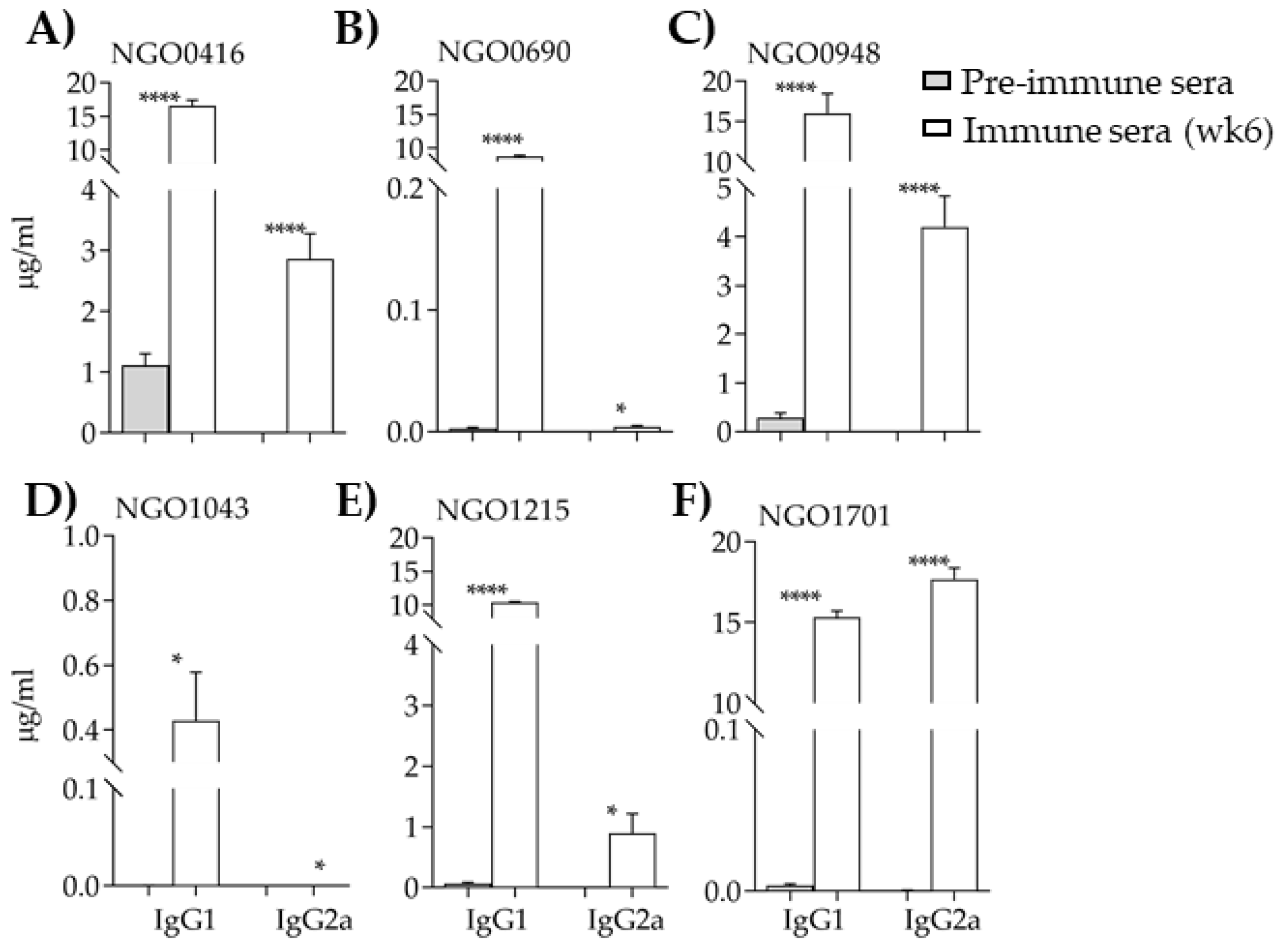
3.5. Characterization of the Mouse Immune Responses to the Candidate Antigens
3.5.1. Total Antibody Responses
3.5.2. Antibody Subclasses
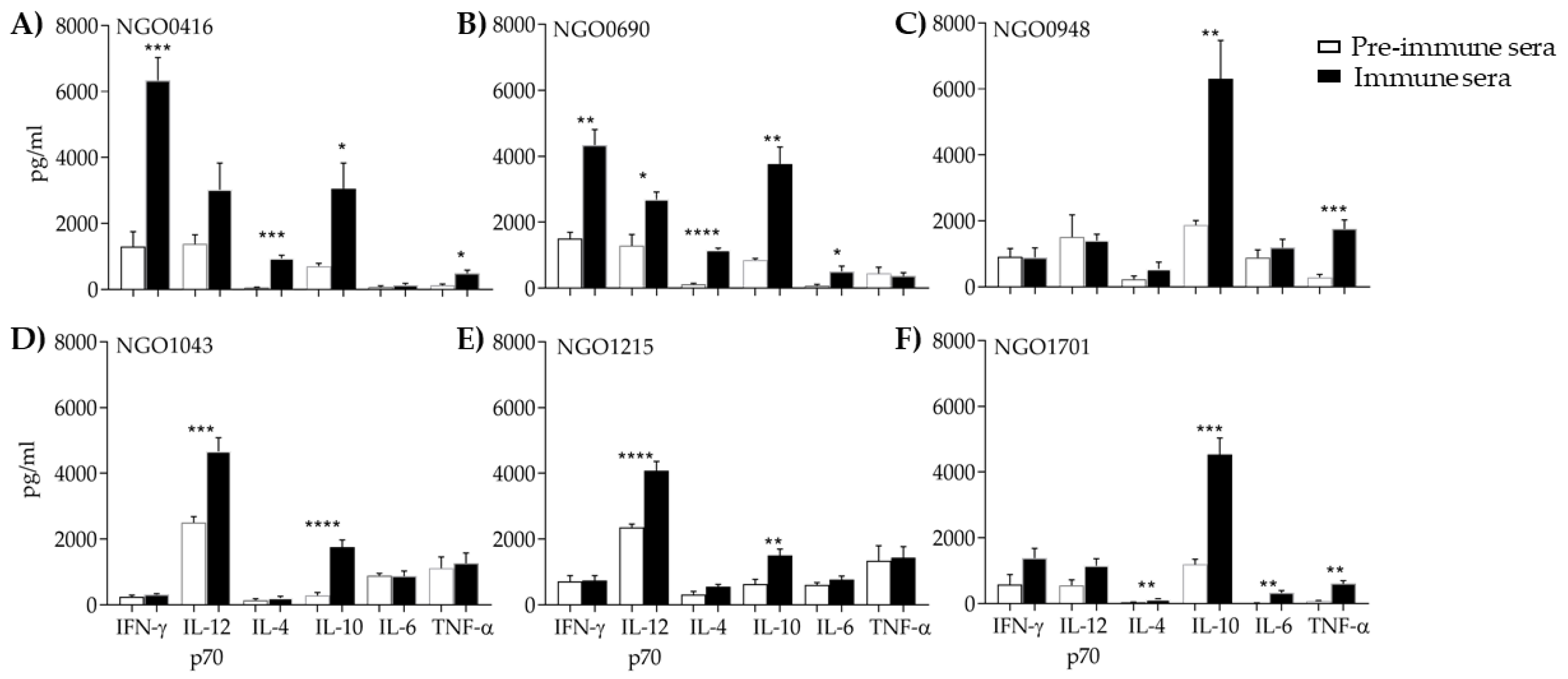
3.5.3. Serum Cytokines
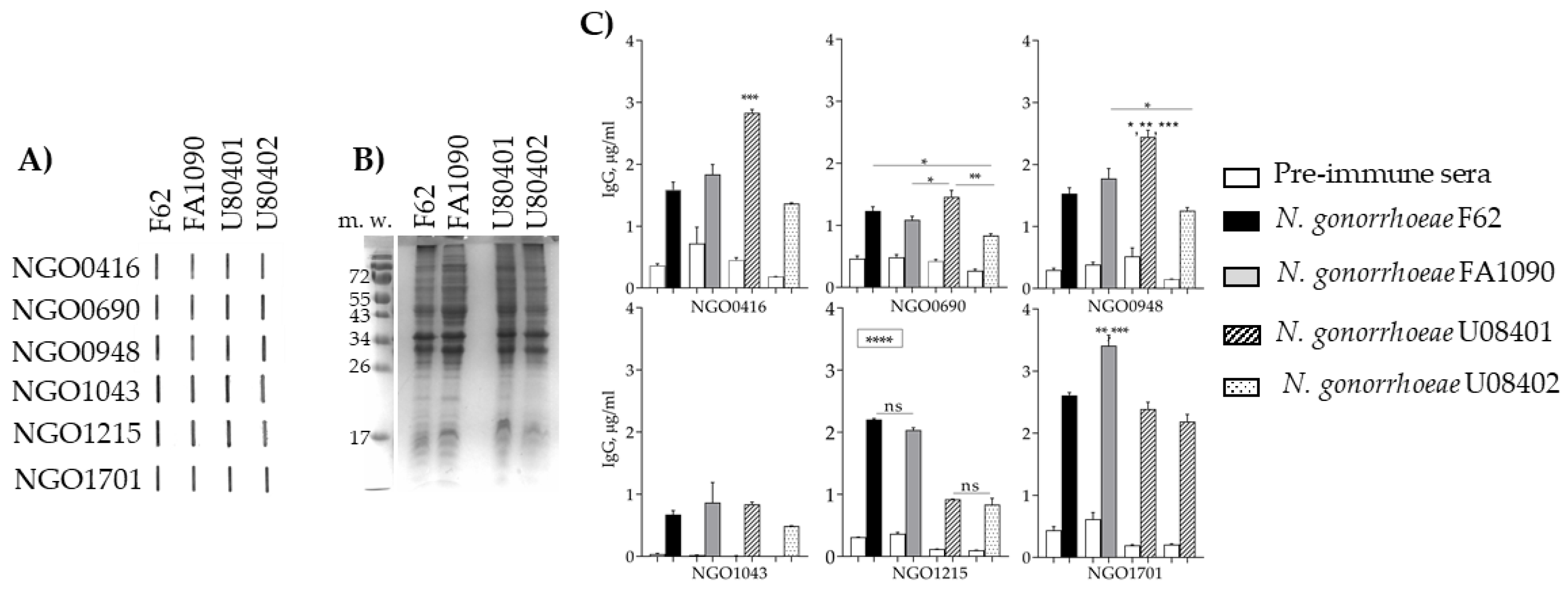
3.5.4. Antibody Cross-Reactivity with N. gonorrhoeae Strains
3.6. Sub-Cellular Localization of the Candidate Antigens
3.7. Antibody Bactericidal Activity
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- CDC. Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2018; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2019.
- Rice, P.A.; Shafer, W.M.; Ram, S.; Jerse, A.E. Neisseria gonorrhoeae: Drug Resistance, Mouse Models, and Vaccine Development. Annu. Rev. Microbiol. 2017, 71, 665–686. [Google Scholar] [CrossRef]
- CDC Grand Rounds: The growing threat of multidrug-resistant gonorrhea. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 103–106.
- Unemo, M.; Del Rio, C.; Shafer, W.M. Antimicrobial Resistance Expressed by Neisseria gonorrhoeae: A Major Global Public Health Problem in the 21st Century. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Li, S.; Su, X.H.; Le, W.J.; Jiang, F.X.; Wang, B.X.; Rice, P.A. Antimicrobial susceptibility of Neisseria gonorrhoeae isolates from symptomatic men attending the Nanjing sexually transmitted diseases clinic (2011–2012): Genetic characteristics of isolates with reduced sensitivity to ceftriaxone. BMC Infect. Dis. 2014, 14, 622. [Google Scholar] [CrossRef][Green Version]
- Plummer, F.A.; Simonsen, J.N.; Chubb, H.; Slaney, L.; Kimata, J.; Bosire, M.; Ndinya-Achola, J.O.; Ngugi, E.N. Epidemiologic evidence for the development of serovar-specific immunity after gonococcal infection. J. Clin. Investig. 1989, 83, 1472–1476. [Google Scholar] [CrossRef]
- Schmidt, K.A.; Schneider, H.; Lindstrom, J.A.; Boslego, J.W.; Warren, R.A.; Van de Verg, L.; Deal, C.D.; McClain, J.B.; Griffiss, J.M. Experimental gonococcal urethritis and reinfection with homologous gonococci in male volunteers. Sex. Transm. Dis. 2001, 28, 555–564. [Google Scholar] [CrossRef]
- Mehta, S.D.; Erbelding, E.J.; Zenilman, J.M.; Rompalo, A.M. Gonorrhoea reinfection in heterosexual STD clinic attendees: Longitudinal analysis of risks for first reinfection. Sex. Transm. Infect. 2003, 79, 124–128. [Google Scholar] [CrossRef]
- Hobbs, M.M.; Sparling, P.F.; Cohen, M.S.; Shafer, W.M.; Deal, C.D.; Jerse, A.E. Experimental Gonococcal Infection in Male Volunteers: Cumulative Experience with Neisseria gonorrhoeae Strains FA1090 and MS11mkC. Front. Microbiol. 2011, 2, 123. [Google Scholar] [CrossRef]
- Greenberg, L.; Diena, B.B.; Ashton, F.A.; Wallace, R.; Kenny, C.P.; Znamirowski, R.; Ferrari, H.; Atkinson, J. Gonococcal vaccine studies in Inuvik. Can. J. Public Health 1974, 65, 29–33. [Google Scholar]
- Boslego, J.W.; Tramont, E.C.; Chung, R.C.; McChesney, D.G.; Ciak, J.; Sadoff, J.C.; Piziak, M.V.; Brown, J.D.; Brinton, C.C., Jr.; Wood, S.W.; et al. Efficacy trial of a parenteral gonococcal pilus vaccine in men. Vaccine 1991, 9, 154–162. [Google Scholar] [CrossRef]
- Lenz, J.D.; Dillard, J.P. Pathogenesis of Neisseria gonorrhoeae and the Host Defense in Ascending Infections of Human Fallopian Tube. Front. Immunol. 2018, 9, 2710. [Google Scholar] [CrossRef]
- Seifert, H.S.; Wright, C.J.; Jerse, A.E.; Cohen, M.S.; Cannon, J.G. Multiple gonococcal pilin antigenic variants are produced during experimental human infections. J. Clin. Investig. 1994, 93, 2744–2749. [Google Scholar] [CrossRef]
- Swanson, J.L.; Robbins, K.; Barrera, O.; Corwin, D.; Boslego, J.; Ciak, J.; Blake, M.S.; Koomey, J.M. Gonococcal pilin variants in experimental gonorrhea. J. Exp. Med. 1987, 165, 1344–1357. [Google Scholar] [CrossRef]
- Rice, P.A.; Vayo, H.E.; Tam, M.R.; Blake, M.S. Immunoglobin G antibodies directed against protein III block killing of serum resistant Neisseria gonorrhoeae by immune sera. J. Exp. Med. 1986, 164, 1735–1748. [Google Scholar] [CrossRef]
- Rosenqvist, E.; Musacchio, A.; Aase, A.; Hoiby, E.A.; Namork, E.; Kolberg, J.; Wedege, E.; Delvig, A.; Dalseg, R.; Michaelsen, T.E.; et al. Functional activities and epitope specificity of human and murine antibodies against the class 4 outer membrane protein (Rmp) of Neisseria meningitidis. Infect. Immun. 1999, 67, 1267–1276. [Google Scholar]
- Plummer, F.A.; Chubb, H.; Simonsen, J.N.; Bosire, M.; Slaney, L.; Maclean, I.; Ndinya-Achola, J.O.; Waiyaki, P.; Brunham, R.C. Antibody to Rmp (outer membrane protein 3) increases susceptibility to gonococcal infection. J. Clin. Investig. 1993, 91, 339–343. [Google Scholar] [CrossRef]
- Wetzler, L.M.; Blake, M.S.; Gotschlich, E.C. Protein I (Por) of Neisseria gonorrhoeae as an immunogen: Liposomes, proteosomes and the lack of blocking antibodies. In Transactions of the American Association of Physicians, 102nd ed.; Waverly Press: Baltimore, MD, USA, 1989; pp. 78–90. [Google Scholar]
- Derrick, J.P.; Urwin, R.; Suker, J.; Feavers, I.M.; Maiden, M.C. Structural and evolutionary inference from molecular variation in neisseria porins. Infect. Immun. 1999, 67, 2406–2413. [Google Scholar]
- Hobbs, M.M.; Alcorn, T.M.; Davis, R.H.; Fischer, W.; Thomas, J.C.; Martin, I.; Ison, C.; Sparling, P.F.; Cohen, M.S. Molecular typing of Neisseria gonorrhoeae causing repeated infections: Evolution of porin during passage within a community. J. Infect. Dis. 1999, 179, 371–381. [Google Scholar] [CrossRef][Green Version]
- Ram, S.; Cullinane, M.; Blom, A.M.; Gulati, S.; McQuillen, D.P.; Monks, B.G.; O’Connell, C.; Boden, R.; Elkins, C.; Pangburn, M.K.; et al. Binding of C4b-binding protein to porin: A molecular mechanism of serum resistance of Neisseria gonorrhoeae. J. Exp. Med. 2001, 193, 281–295. [Google Scholar] [CrossRef]
- Jerse, A.E.; Wu, H.; Packiam, M.; Vonck, R.A.; Begum, A.A.; Garvin, L.E. Estradiol-Treated Female Mice as Surrogate Hosts for Neisseria gonorrhoeae Genital Tract Infections. Front. Microbiol. 2011, 2, 107. [Google Scholar] [CrossRef]
- Islam, E.A.; Shaik-Dasthagirisaheb, Y.; Kaushic, C.; Wetzler, L.M.; Gray-Owen, S.D. The reproductive cycle is a pathogenic determinant during gonococcal pelvic inflammatory disease in mice. Mucosal Immunol. 2016, 9, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Shaughnessy, J.; Ram, S.; Rice, P.A. Targeting Lipooligosaccharide (LOS) for a Gonococcal Vaccine. Front. Immunol. 2019, 10, 321. [Google Scholar] [CrossRef] [PubMed]
- Fegan, J.E.; Calmettes, C.; Islam, E.A.; Ahn, S.K.; Chaudhuri, S.; Yu, R.H.; Gray-Owen, S.D.; Moraes, T.F.; Schryvers, A.B. Utility of Hybrid Transferrin Binding Protein Antigens for Protection Against Pathogenic Neisseria Species. Front. Immunol. 2019, 10, 247. [Google Scholar] [CrossRef]
- Price, G.A.; Masri, H.P.; Hollander, A.M.; Russell, M.W.; Cornelissen, C.N. Gonococcal transferrin binding protein chimeras induce bactericidal and growth inhibitory antibodies in mice. Vaccine 2007, 25, 7247–7260. [Google Scholar] [CrossRef]
- Pizza, M.; Scarlato, V.; Masignani, V.; Giuliani, M.M.; Arico, B.; Comanducci, M.; Jennings, G.T.; Baldi, L.; Bartolini, E.; Capecchi, B.; et al. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing [see comments]. Science 2000, 287, 1816–1820. [Google Scholar] [CrossRef]
- Rappuoli, R.; Pizza, M.; Masignani, V.; Vadivelu, K. Meningococcal B vaccine (4CMenB): The journey from research to real world experience. Expert Rev. Vaccines 2018, 17, 1111–1121. [Google Scholar] [CrossRef]
- Jain, R.; Sonkar, S.C.; Chaudhry, U.; Bala, M.; Saluja, D. In-silico Hierarchical Approach for the Identification of Potential Universal Vaccine Candidates (PUVCs) from Neisseria gonorrhoeae. J. Theor. Biol. 2016, 410, 36–43. [Google Scholar] [CrossRef]
- Zielke, R.A.; Wierzbicki, I.H.; Baarda, B.I.; Gafken, P.R.; Soge, O.O.; Holmes, K.K.; Jerse, A.E.; Unemo, M.; Sikora, A.E. Proteomics-driven Antigen Discovery for Development of Vaccines Against Gonorrhea. Mol. Cell. Proteom. 2016, 15, 2338–2355. [Google Scholar] [CrossRef]
- Connor, D.O.; Zantow, J.; Hust, M.; Bier, F.F.; von Nickisch-Rosenegk, M. Identification of Novel Immunogenic Proteins of Neisseria gonorrhoeae by Phage Display. PLoS ONE 2016, 11, e0148986. [Google Scholar] [CrossRef]
- Baarda, B.I.; Martinez, F.G.; Sikora, A.E. Proteomics, Bioinformatics and Structure-Function Antigen Mining For Gonorrhea Vaccines. Front. Immunol. 2018, 9, 2793. [Google Scholar] [CrossRef]
- El-Rami, F.E.; Zielke, R.A.; Wi, T.; Sikora, A.E.; Unemo, M. Quantitative Proteomics of the 2016 WHO Neisseria gonorrhoeae Reference Strains Surveys Vaccine Candidates and Antimicrobial Resistance Determinants. Mol. Cell. Proteom. 2019, 18, 127–150. [Google Scholar] [CrossRef] [PubMed]
- Semchenko, E.A.; Day, C.J.; Seib, K.L. MetQ of Neisseria gonorrhoeae Is a Surface-Expressed Antigen That Elicits Bactericidal and Functional Blocking Antibodies. Infect. Immun. 2017, 85, e00898-16. [Google Scholar] [CrossRef] [PubMed]
- Almonacid-Mendoza, H.L.; Humbert, M.V.; Dijokaite, A.; Cleary, D.W.; Soo, Y.; Hung, M.C.; Orr, C.M.; Machelett, M.M.; Tews, I.; Christodoulides, M. Structure of the Recombinant Neisseria gonorrhoeae Adhesin Complex Protein (rNg-ACP) and Generation of Murine Antibodies with Bactericidal Activity against Gonococci. mSphere 2018, 3, e00331-18. [Google Scholar] [CrossRef] [PubMed]
- Petousis-Harris, H.; Paynter, J.; Morgan, J.; Saxton, P.; McArdle, B.; Goodyear-Smith, F.; Black, S. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: A retrospective case-control study. Lancet 2017, 390, 1603–1610. [Google Scholar] [CrossRef]
- Beernink, P.T.; Ispasanie, E.; Lewis, L.A.; Ram, S.; Moe, G.R.; Granoff, D.M. A Meningococcal Native Outer Membrane Vesicle Vaccine With Attenuated Endotoxin and Overexpressed Factor H Binding Protein Elicits Gonococcal Bactericidal Antibodies. J. Infect. Dis. 2019, 219, 1130–1137. [Google Scholar] [CrossRef]
- Paynter, J.; Goodyear-Smith, F.; Morgan, J.; Saxton, P.; Black, S.; Petousis-Harris, H. Effectiveness of a Group B Outer Membrane Vesicle Meningococcal Vaccine in Preventing Hospitalization from Gonorrhea in New Zealand: A Retrospective Cohort Study. Vaccines 2019, 7, 5. [Google Scholar] [CrossRef]
- Semchenko, E.A.; Tan, A.; Borrow, R.; Seib, K.L. The serogroup B meningococcal vaccine Bexsero elicits antibodies to Neisseria gonorrhoeae. Clin. Infect. Dis. 2019, 69, 1101–1111. [Google Scholar] [CrossRef]
- Gottlieb, S.L.; Johnston, C. Future prospects for new vaccines against sexually transmitted infections. Curr. Opin. Infect. Dis. 2017, 30, 77–86. [Google Scholar] [CrossRef]
- Bai, X.; Findlow, J.; Borrow, R. Recombinant protein meningococcal serogroup B vaccine combined with outer membrane vesicles. Expert Opin. Biol. Ther. 2011, 11, 969–985. [Google Scholar] [CrossRef]
- Plante, M.; Jerse, A.; Hamel, J.; Couture, F.; Rioux, C.R.; Brodeur, B.R.; Martin, D. Intranasal Immunization with Gonococcal Outer Membrane Preparations Reduces the Duration of Vaginal Colonization of Mice by Neisseria gonorrhoeae. J. Infect. Dis. 2000, 182, 848–855. [Google Scholar] [CrossRef][Green Version]
- McClure, R.; Nudel, K.; Massari, P.; Tjaden, B.; Su, X.; Rice, P.A.; Genco, C.A. The Gonococcal Transcriptome during Infection of the Lower Genital Tract in Women. PLoS ONE 2015, 10, e0133982. [Google Scholar] [CrossRef] [PubMed]
- Nudel, K.; McClure, R.; Moreau, M.; Briars, E.; Abrams, A.J.; Tjaden, B.; Su, X.H.; Trees, D.; Rice, P.A.; Massari, P.; et al. Transcriptome Analysis of Neisseria gonorrhoeae during Natural Infection Reveals Differential Expression of Antibiotic Resistance Determinants between Men and Women. mSphere 2018, 3, e00312-18. [Google Scholar] [CrossRef] [PubMed]
- Doytchinova, I.A.; Flower, D.R. VaxiJen: A server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinform. 2007, 8, 4. [Google Scholar] [CrossRef]
- Yu, N.Y.; Wagner, J.R.; Laird, M.R.; Melli, G.; Rey, S.; Lo, R.; Dao, P.; Sahinalp, S.C.; Ester, M.; Foster, L.J.; et al. PSORTb 3.0: Improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 2010, 26, 1608–1615. [Google Scholar] [CrossRef]
- Yachdav, G.; Kloppmann, E.; Kajan, L.; Hecht, M.; Goldberg, T.; Hamp, T.; Honigschmid, P.; Schafferhans, A.; Roos, M.; Bernhofer, M.; et al. PredictProtein7—An open resource for online prediction of protein structural and functional features. Nucleic Acids Res. 2014, 42, W337–W343. [Google Scholar] [CrossRef]
- Shen, H.B.; Chou, K.C. Gneg-mPLoc: A top-down strategy to enhance the quality of predicting subcellular localization of Gram-negative bacterial proteins. J. Theor. Biol. 2010, 264, 326–333. [Google Scholar] [CrossRef]
- Vaxign. Available online: http://www.violinet.org/vaxign/index.php (accessed on 1 January 2019).
- BLAST. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins (accessed on 8 July 2019).
- He, Y.; Racz, R.; Sayers, S.; Lin, Y.; Todd, T.; Hur, J.; Li, X.; Patel, M.; Zhao, B.; Chung, M.; et al. Updates on the web-based VIOLIN vaccine database and analysis system. Nucleic Acids Res. 2014, 42, D1124–D1132. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, B.; von, H.G.; Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef]
- Kall, L.; Krogh, A.; Sonnhammer, E.L. Advantages of combined transmembrane topology and signal peptide prediction—The Phobius web server. Nucleic Acids Res. 2007, 35, W429–W432. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sonderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Bendtsen, J.D.; Jensen, L.J.; Blom, N.; Von Heijne, G.; Brunak, S. Feature-based prediction of non-classical and leaderless protein secretion. Protein Eng. Des. Sel. 2004, 17, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Juncker, A.S.; Willenbrock, H.; Von Heijne, G.; Brunak, S.; Nielsen, H.; Krogh, A. Prediction of lipoprotein signal peptides in Gram-negative bacteria. Protein Sci. 2003, 12, 1652–1662. [Google Scholar] [CrossRef] [PubMed]
- UniProt, C. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res 2019, 47, D506–D515. [Google Scholar]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- PubMLST. Available online: https://pubmlst.org/neisseria (accessed on 4 April 2019).
- Jolley, K.A.; Maiden, M.C. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinform. 2010, 11, 595. [Google Scholar] [CrossRef]
- Liu, X.; Wetzler, L.M.; Massari, P. The PorB porin from commensal Neisseria lactamica induces Th1 and Th2 immune responses to ovalbumin in mice and is a potential immune adjuvant. Vaccine 2008, 26, 786–796. [Google Scholar] [CrossRef]
- Ram, S.; Ngampasutadol, J.; Cox, A.D.; Blom, A.M.; Lewis, L.A.; St Michael, F.; Stupak, J.; Gulati, S.; Rice, P.A. Heptose I glycan substitutions on Neisseria gonorrhoeae lipooligosaccharide influence C4b-binding protein binding and serum resistance. Infect. Immun. 2007, 75, 4071–4081. [Google Scholar] [CrossRef]
- Seydel, A.; Gounon, P.; Pugsley, A.P. Testing the ‘+2 rule’ for lipoprotein sorting in the Escherichia coli cell envelope with a new genetic selection. Mol. Microbiol. 1999, 34, 810–821. [Google Scholar] [CrossRef]
- Andersen, C.; Rak, B.; Benz, R. The gene bglH present in the bgl operon of Escherichia coli, responsible for uptake and fermentation of beta-glucosides encodes for a carbohydrate-specific outer membrane porin. Mol. Microbiol. 1999, 31, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Sechman, E.V.; Rohrer, M.S.; Seifert, H.S. A genetic screen identifies genes and sites involved in pilin antigenic variation in Neisseria gonorrhoeae. Mol. Microbiol. 2005, 57, 468–483. [Google Scholar] [CrossRef] [PubMed]
- Plummer, A.M.; Fleming, K.G. From Chaperones to the Membrane with a BAM! Trends Biochem. Sci. 2016, 41, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Webb, C.T.; Selkrig, J.; Perry, A.J.; Noinaj, N.; Buchanan, S.K.; Lithgow, T. Dynamic association of BAM complex modules includes surface exposure of the lipoprotein BamC. J. Mol. Biol. 2012, 422, 545–555. [Google Scholar] [CrossRef]
- Delgado, M.; Yero, D.; Niebla, O.; Gonzalez, S.; Climent, Y.; Perez, Y.; Cobas, K.; Caballero, E.; Garcia, D.; Pajon, R. Lipoprotein NMB0928 from Neisseria meningitidis serogroup B as a novel vaccine candidate. Vaccine 2007, 25, 8420–8431. [Google Scholar] [CrossRef]
- Pajon, R.; Yero, D.; Niebla, O.; Climent, Y.; Sardinas, G.; Garcia, D.; Perera, Y.; Llanes, A.; Delgado, M.; Cobas, K.; et al. Identification of new meningococcal serogroup B surface antigens through a systematic analysis of neisserial genomes. Vaccine 2009, 28, 532–541. [Google Scholar] [CrossRef]
- Volokhina, E.B.; Beckers, F.; Tommassen, J.; Bos, M.P. The beta-barrel outer membrane protein assembly complex of Neisseria meningitidis. J. Bacteriol. 2009, 191, 7074–7085. [Google Scholar] [CrossRef]
- Awanye, A.M.; Chang, C.M.; Wheeler, J.X.; Chan, H.; Marsay, L.; Dold, C.; Rollier, C.S.; Bird, L.E.; Nettleship, J.E.; Owens, R.J.; et al. Immunogenicity profiling of protein antigens from capsular group B Neisseria meningitidis. Sci. Rep. 2019, 9, 6843. [Google Scholar] [CrossRef]
- Vik, A.; Aas, F.E.; Anonsen, J.H.; Bilsborough, S.; Schneider, A.; Egge-Jacobsen, W.; Koomey, M. Broad spectrum O-linked protein glycosylation in the human pathogen Neisseria gonorrhoeae. Proc. Natl. Acad. Sci. USA 2009, 106, 4447–4452. [Google Scholar] [CrossRef]
- Anonsen, J.H.; Egge-Jacobsen, W.; Aas, F.E.; Borud, B.; Koomey, M.; Vik, A. Novel protein substrates of the phospho-form modification system in Neisseria gonorrhoeae and their connection to O-linked protein glycosylation. Infect. Immun. 2012, 80, 22–30. [Google Scholar] [CrossRef]
- Chu, C.L.; Yu, Y.L.; Kung, Y.C.; Liao, P.Y.; Liu, K.J.; Tseng, Y.T.; Lin, Y.C.; Hsieh, S.S.; Chong, P.C.; Yang, C.Y. The immunomodulatory activity of meningococcal lipoprotein Ag473 depends on the conformation made up of the lipid and protein moieties. PLoS ONE 2012, 7, e40873. [Google Scholar] [CrossRef] [PubMed]
- Ladomersky, E.; Petris, M.J. Copper tolerance and virulence in bacteria. Metallomics 2015, 7, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Jen, F.E.; Djoko, K.Y.; Bent, S.J.; Day, C.J.; McEwan, A.G.; Jennings, M.P. A genetic screen reveals a periplasmic copper chaperone required for nitrite reductase activity in pathogenic Neisseria. FASEB J. 2015, 29, 3828–3838. [Google Scholar] [CrossRef] [PubMed]
- Gangaiah, D.; Raterman, E.L.; Wu, H.; Fortney, K.R.; Gao, H.; Liu, Y.; Jerse, A.E.; Spinola, S.M. Both MisR (CpxR) and MisS (CpxA) Are Required for Neisseria gonorrhoeae Infection in a Murine Model of Lower Genital Tract Infection. Infect. Immun. 2017, 85, e00307-17. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Genco, C.A. Fur-mediated global regulatory circuits in pathogenic Neisseria species. J. Bacteriol. 2012, 194, 6372–6381. [Google Scholar] [CrossRef] [PubMed]
- Quillin, S.J.; Hockenberry, A.J.; Jewett, M.C.; Seifert, H.S. Neisseria gonorrhoeae Exposed to Sublethal Levels of Hydrogen Peroxide Mounts a Complex Transcriptional Response. mSystems 2018, 3, e00156-18. [Google Scholar] [CrossRef]
- Velez Acevedo, R.N.; Ronpirin, C.; Kandler, J.L.; Shafer, W.M.; Cornelissen, C.N. Identification of regulatory elements that control expression of the tbpBA operon in Neisseria gonorrhoeae. J. Bacteriol. 2014, 196, 2762–2774. [Google Scholar] [CrossRef]
- McClure, R.; Sunkavalli, A.; Balzano, P.M.; Massari, P.; Cho, C.; Nauseef, W.M.; Apicella, M.A.; Genco, C.A. Global Network Analysis of Neisseria gonorrhoeae Identifies Coordination Between Pathways, Processes and Regulators Expressed During Human Infection. mSystems 2019. submitted. [Google Scholar]
- McDermott, J.E.; Taylor, R.C.; Yoon, H.; Heffron, F. Bottlenecks and hubs in inferred networks are important for virulence in Salmonella typhimurium. J. Comput. Biol. 2009, 16, 169–180. [Google Scholar] [CrossRef]
- McDermott, J.E.; Diamond, D.L.; Corley, C.; Rasmussen, A.L.; Katze, M.G.; Waters, K.M. Topological analysis of protein co-abundance networks identifies novel host targets important for HCV infection and pathogenesis. BMC Syst. Biol. 2012, 6, 28. [Google Scholar] [CrossRef]
- Song, H.S.; McClure, R.S.; Bernstein, H.C.; Overall, C.C.; Hill, E.A.; Beliaev, A.S. Integrated in silico Analyses of Regulatory and Metabolic Networks of Synechococcus sp. PCC 7002 Reveal Relationships between Gene Centrality and Essentiality. Life 2015, 5, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yan, W.; Zheng, H.; Du, Q.; Zhang, L.; Ban, Y.; Li, N.; Wei, F. Regulation of IL-10 and IL-12 production and function in macrophages and dendritic cells. F1000Res 2015, 4, F1000 Faculty Rev-1465. [Google Scholar] [CrossRef] [PubMed]
- Pizza, M.; Rappuoli, R. Neisseria meningitidis: Pathogenesis and immunity. Curr. Opin. Microbiol. 2015, 23, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Del, T.E.; Bottini, S.; Muzzi, A.; Serruto, D. Analysis of the regulated transcriptome of Neisseria meningitidis in human blood using a tiling array. J. Bacteriol. 2012, 194, 6217–6232. [Google Scholar]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Madico, G.; Gursky, O.; Fairman, J.; Massari, P. Structural and Immunological Characterization of Novel Recombinant MOMP-Based Chlamydial Antigens. Vaccines 2017, 6, 2. [Google Scholar] [CrossRef]
- Giuliani, M.M.; Adu-Bobie, J.; Comanducci, M.; Arico, B.; Savino, S.; Santini, L.; Brunelli, B.; Bambini, S.; Biolchi, A.; Capecchi, B.; et al. A universal vaccine for serogroup B meningococcus. Proc. Natl. Acad. Sci. USA 2006, 103, 10834–10839. [Google Scholar] [CrossRef]
- Serruto, D.; Bottomley, M.J.; Ram, S.; Giuliani, M.M.; Rappuoli, R. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: Immunological, functional and structural characterization of the antigens. Vaccine 2012, 30 (Suppl. 2), B87–B97. [Google Scholar] [CrossRef]
- Vipond, C.; Suker, J.; Jones, C.; Tang, C.; Feavers, I.M.; Wheeler, J.X. Proteomic analysis of a meningococcal outer membrane vesicle vaccine prepared from the group B strain NZ98/254. Proteomics 2006, 6, 3400–3413. [Google Scholar] [CrossRef]
- Leng, C.H.; Liu, S.J.; Chen, H.W.; Chong, P. Recombinant bacterial lipoproteins as vaccine candidates. Expert Rev. Vaccines 2015, 14, 1623–1632. [Google Scholar] [CrossRef]
- Jiang, H.Q.; Hoiseth, S.K.; Harris, S.L.; McNeil, L.K.; Zhu, D.; Tan, C.; Scott, A.A.; Alexander, K.; Mason, K.; Miller, L.; et al. Broad vaccine coverage predicted for a bivalent recombinant factor H binding protein based vaccine to prevent serogroup B meningococcal disease. Vaccine 2010, 28, 6086–6093. [Google Scholar] [CrossRef] [PubMed]
- Hooda, Y.; Shin, H.E.; Bateman, T.J.; Moraes, T.F. Neisserial surface lipoproteins: Structure, function and biogenesis. Pathog. Dis. 2017, 75. [Google Scholar] [CrossRef] [PubMed]
- Nunez, G.; Sakamoto, K.; Soares, M.P. Innate Nutritional Immunity. J. Immunol. 2018, 201, 11–18. [Google Scholar] [CrossRef]
- Cornelissen, C.N. Subversion of nutritional immunity by the pathogenic Neisseriae. Pathog. Dis. 2018, 76. [Google Scholar] [CrossRef]
- Moreau, M.R.; Massari, P.; Genco, C.A. The ironclad truth: How in vivo transcriptomics and in vitro mechanistic studies shape our understanding of Neisseria gonorrhoeae gene regulation during mucosal infection. Pathog. Dis. 2017, 75. [Google Scholar] [CrossRef]
- Dennison, C.; David, S.; Lee, J. Bacterial copper storage proteins. J. Biol. Chem. 2018, 293, 4616–4627. [Google Scholar] [CrossRef]
- Stork, M.; Bos, M.P.; Jongerius, I.; de Kok, N.; Schilders, I.; Weynants, V.E.; Poolman, J.T.; Tommassen, J. An outer membrane receptor of Neisseria meningitidis involved in zinc acquisition with vaccine potential. PLoS Pathog. 2010, 6, e1000969. [Google Scholar] [CrossRef]
- Hecel, A.; Rowinska-Zyrek, M.; Kozlowski, H. Copper(II)-Induced Restructuring of ZnuD, a Zinc(II) Transporter from Neisseria meningitidis. Inorg. Chem. 2019, 58, 5932–5942. [Google Scholar] [CrossRef]
- Maurakis, S.; Keller, K.; Maxwell, C.N.; Pereira, K.; Chazin, W.J.; Criss, A.K.; Cornelissen, C.N. The novel interaction between Neisseria gonorrhoeae TdfJ and human S100A7 allows gonococci to subvert host zinc restriction. PLoS Pathog. 2019, 15, e1007937. [Google Scholar] [CrossRef]
- Djoko, K.Y.; Franiek, J.A.; Edwards, J.L.; Falsetta, M.L.; Kidd, S.P.; Potter, A.J.; Chen, N.H.; Apicella, M.A.; Jennings, M.P.; McEwan, A.G. Phenotypic characterization of a copA mutant of Neisseria gonorrhoeae identifies a link between copper and nitrosative stress. Infect. Immun. 2012, 80, 1065–1071. [Google Scholar] [CrossRef]
- Deasy, A.M.; Guccione, E.; Dale, A.P.; Andrews, N.; Evans, C.M.; Bennett, J.S.; Bratcher, H.B.; Maiden, M.C.; Gorringe, A.R.; Read, R.C. Nasal Inoculation of the Commensal Neisseria lactamica Inhibits Carriage of Neisseria meningitidis by Young Adults: A Controlled Human Infection Study. Clin. Infect. Dis. 2015, 60, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.C.; Cheng, A.L.; Brotto, M.; Chuang, C.Y. Visual gene-network analysis reveals the cancer gene co-expression in human endometrial cancer. BMC Genom. 2014, 15, 300. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, C.J.; Kohane, I.S.; Butte, A.J. Systematic survey reveals general applicability of “guilt-by-association” within gene coexpression networks. BMC Bioinform. 2005, 6, 227. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, W.; Russell, M.W. Suppression of host adaptive immune responses by Neisseria gonorrhoeae: Role of interleukin 10 and type 1 regulatory T cells. Mucosal Immunol. 2014, 7, 165–176. [Google Scholar] [CrossRef]
- Liu, Y.; Hammer, L.A.; Liu, W.; Hobbs, M.M.; Zielke, R.A.; Sikora, A.E.; Jerse, A.E.; Egilmez, N.K.; Russell, M.W. Experimental vaccine induces Th1-driven immune responses and resistance to Neisseria gonorrhoeae infection in a murine model. Mucosal Immunol. 2017, 10, 1594–1608. [Google Scholar] [CrossRef]
- Liu, Y.; Perez, J.; Hammer, L.A.; Gallagher, H.C.; De Jesus, M.; Egilmez, N.K.; Russell, M.W. Intravaginal Administration of Interleukin 12 during Genital Gonococcal Infection in Mice Induces Immunity to Heterologous Strains of Neisseria gonorrhoeae. mSphere 2018, 3, e00421-17. [Google Scholar] [CrossRef]
- Filip, C.; Fletcher, G.; Wulff, J.L.; Earhart, C.F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J. Bacteriol. 1973, 115, 717–722. [Google Scholar]


| Candidate | mRNA Expression In Vivo (RPKM) | PREDICTED | Core Genome 5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antigen Score 1 | Localization 2 | Structure 3 | Function 4 | ||||||||
| TM Domain | Signal Sequence | Size (kDa) | Annotation/Homology | ||||||||
| NGO0416 | 196 | 127 | 0.5 | P, non-cytosol | 0 | SP | 10.8 | hypothetical | NEIS0782NMB0844 | yes | |
| NGO0690 | 142 | 157 | 0.7 | P/OM, non-cytosol | 1 | LP | 12.1 | hypothetical, lipoprotein | NEIS1164NMB1047 | yes | |
| NGO0948 | 125 | 105 | 0.43 | P/OM, non-cytosol | 0 | - | 44.8 | lipoprotein, NlpB/DapX/BamC | NEIS0906NMB0928 | yes | |
| NGO1043 | 1748 | 519 | 0.82 | P/OM, non-cytosol | 0 | LP | 10.2 | hypothetical, lipoprotein | NEIS2446NMB1468 | yes | |
| NGO1215 | 190 | 292 | 0.67 | P, non-cytosol | 0 | SP | 16.1 | hypothetical, PCu(A)C/, AccA | NEIS1474NMB1557 | yes | |
| NGO1701 | 127 | 121 | 0.41 | P, non-cytosol | 0 | SP | 14.4 | membrane, TAT_Cys_rich four helix bundle copper-binding protein | NEIS2720 | yes | |
| Gene | Total Alleles | Frequency 1 | Distribution 2 (% of 4198 Strains) | p-Distance | Polymorphic Sites (Total) | Polymorphic Sites 3 | ||
|---|---|---|---|---|---|---|---|---|
| Allele | # of Strains | Allele | Non-Synonimous Mutations | |||||
| ngo0416 (NEIS0782) | 6 | 17 | 3069 | 73.7 | 0.006 | 5 | - | - |
| 26 | 124 | 2.9 | - | - | ||||
| ngo0690 (NEIS1164) | 17 | 7 | 3332 | 79.3 | 0.01 | 27 | - | - |
| 32 | 815 | 19.4 | 32 | S37R | ||||
| ngo0948 (NEIS0906) | 55 | 12 | 2354 | 57 | 0.005 | 109 | - | - |
| 28 | 363 | 8.6 | 28 | A319R | ||||
| 32 | 324 | 7.7 | 32 | I18M, T19K | ||||
| 214 | 248 | 5.9 | 214 | Q49R | ||||
| ngo1043 (NEIS2446) | 27 | 57 | 2720 | 64.7 | 0.01 | 26 | - | - |
| 64 | 691 | 16.4 | 64 | V65A | ||||
| 58 | 283 | 6.7 | 58 | V65A, A116T | ||||
| 80 | 262 | 6.2 | 80 | V65A | ||||
| ngo1215 (NEIS1474) | 13 | 13 | 3817 | 91 | 0.012 | 27 | 13 | x143P |
| 16 | 273 | 7 | 16 | x143S | ||||
| ngo1701 (NEIS2720) | 11 | 1 | 3379 | 80.4 | 0.005 | 10 | - | - |
| 2 | 803 | 19.1 | 2 | E126A | ||||
| N. gonorrhoeae Strains | ||||
|---|---|---|---|---|
| Sera Groups | F62 | U08401 | U08402 | FA1090 c |
| NHS alone | 3% | 6% | 3% | 0% |
| Adjuvant control | 0% (1/10) | 14% (1/10) | 8% (1/10) | 0% (1/10) |
| NGO0416 | 9% (1/10) | 28% (1/10) | 8% (1/10) | 30% (1/5) |
| NGO0690 | 53% (1/40) | 50% (1/10) | 46% (1/20) | 45% (1/10) |
| NGO0948 | 50% (1/10) | 50% (1/10) | 52% (1/10) | 57% (1/5) |
| NGO1043 | 46% (1/10) | 55% (1/10) | 48% (1/10) | 13% (1/5) |
| NGO1215 | 17% (1/10) | 57% (1/10) | 23% (1/10) | 0% (1/5) |
| NGO1701 | 50% (1/40) | 55% (1/10) | 46% (1/20) | 52% (1/5) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, T.; McClure, R.; Harrison, O.B.; Genco, C.; Massari, P. Integrated Bioinformatic Analyses and Immune Characterization of New Neisseria gonorrhoeae Vaccine Antigens Expressed during Natural Mucosal Infection. Vaccines 2019, 7, 153. https://doi.org/10.3390/vaccines7040153
Zhu T, McClure R, Harrison OB, Genco C, Massari P. Integrated Bioinformatic Analyses and Immune Characterization of New Neisseria gonorrhoeae Vaccine Antigens Expressed during Natural Mucosal Infection. Vaccines. 2019; 7(4):153. https://doi.org/10.3390/vaccines7040153
Chicago/Turabian StyleZhu, Tianmou, Ryan McClure, Odile B. Harrison, Caroline Genco, and Paola Massari. 2019. "Integrated Bioinformatic Analyses and Immune Characterization of New Neisseria gonorrhoeae Vaccine Antigens Expressed during Natural Mucosal Infection" Vaccines 7, no. 4: 153. https://doi.org/10.3390/vaccines7040153
APA StyleZhu, T., McClure, R., Harrison, O. B., Genco, C., & Massari, P. (2019). Integrated Bioinformatic Analyses and Immune Characterization of New Neisseria gonorrhoeae Vaccine Antigens Expressed during Natural Mucosal Infection. Vaccines, 7(4), 153. https://doi.org/10.3390/vaccines7040153





