Evaluation of Pneumococcal Surface Protein A as a Vaccine Antigen against Secondary Streptococcus pneumoniae Challenge during Influenza A Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Anti-Pneumococcal Vaccination of Mice
2.2. Antibody ELISA
2.3. Influenza-S. pneumoniae Co-Infection Model
2.4. Statistical Analyses
3. Results
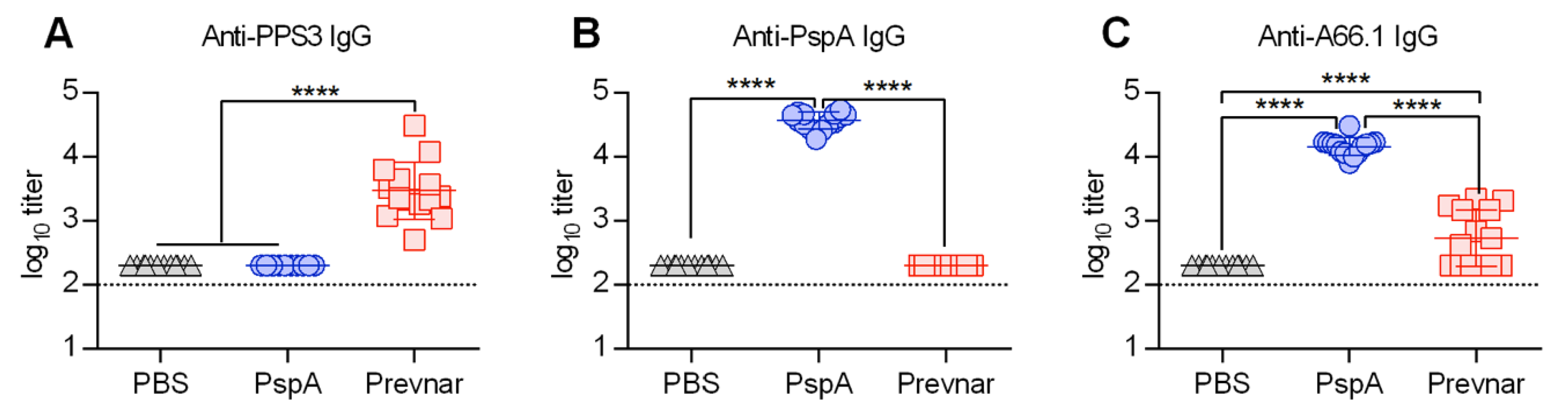
3.1. PspA Protein-Based Vaccination Generates Greater Anti-Pneumococcal IgG Antibody Levels Compared to Prevnar
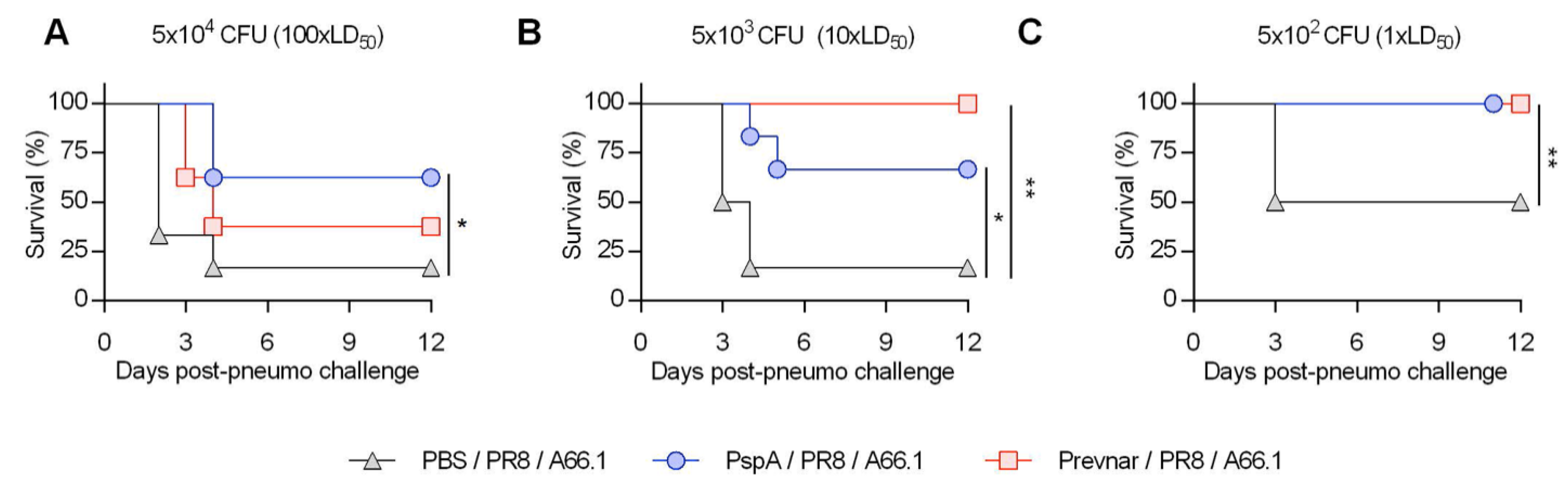
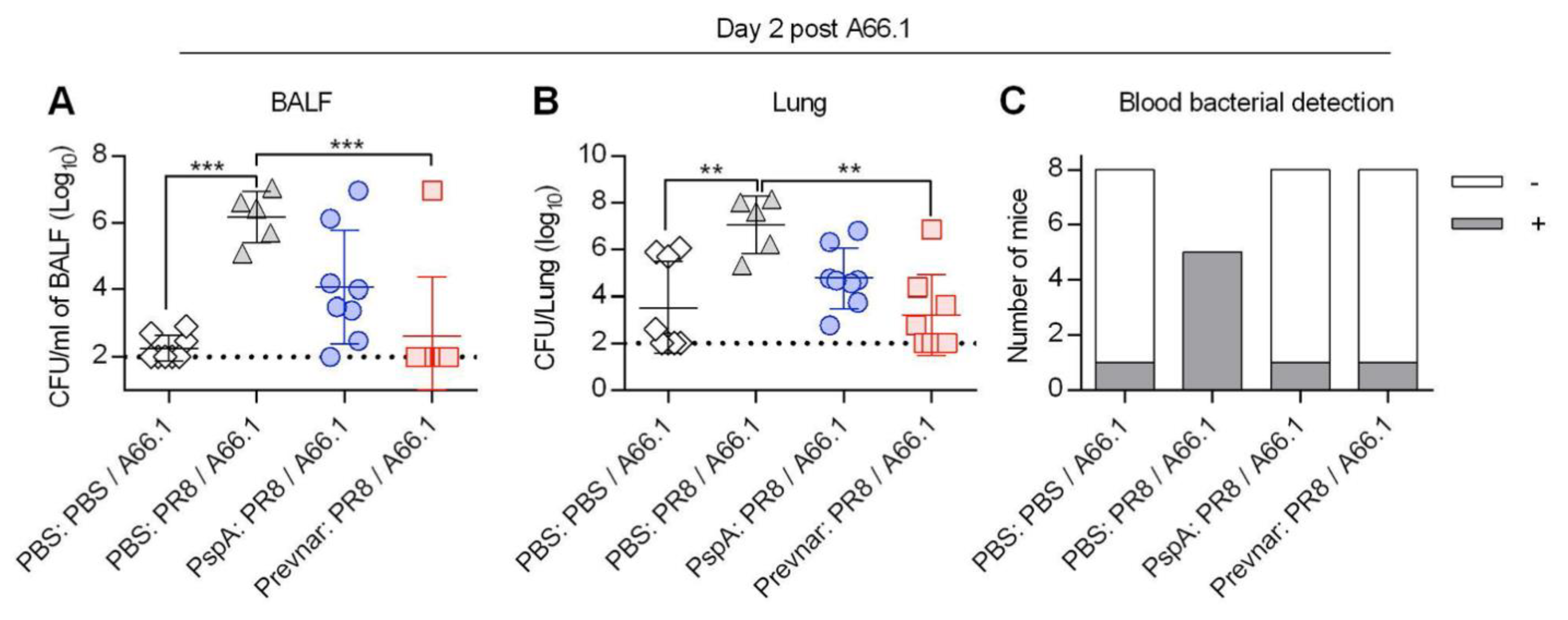
3.2. Vaccine-Induced Protection against Co-Infection Is Bacterial Dose Dependent
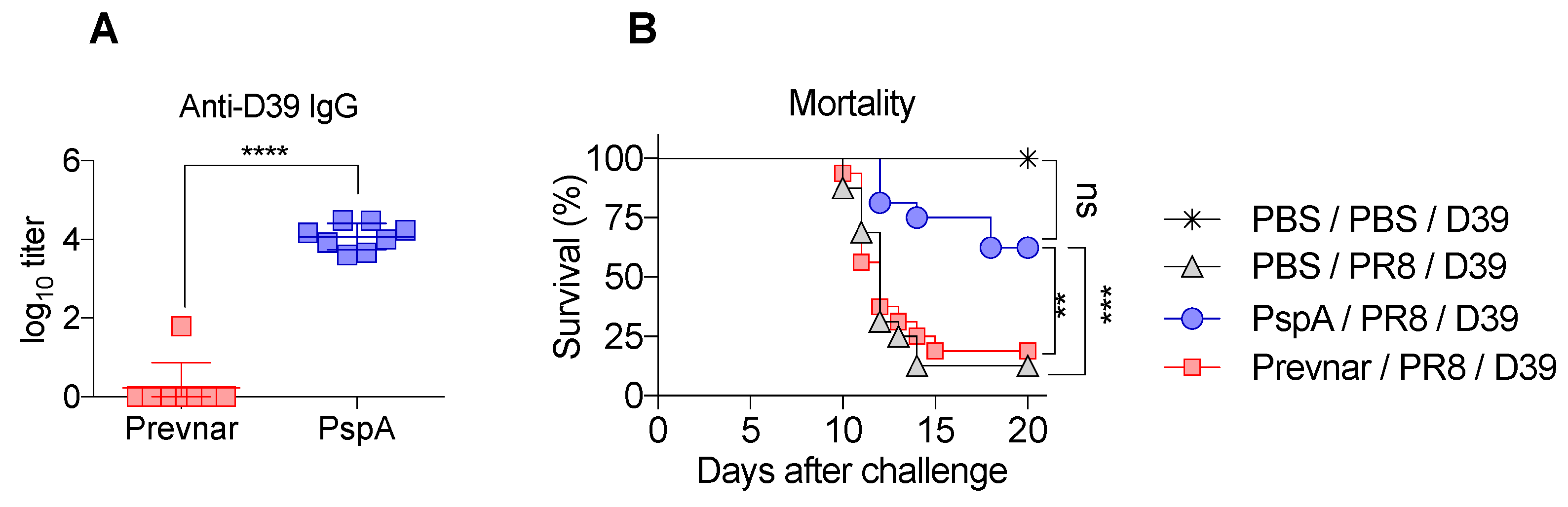
3.3. PspA Immunization Is Protective against Serotype 2 Bacterial Challenge during Co-Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morens, D.M.; Fauci, A.S. The 1918 influenza pandemic: Insights for the 21st century. J. Infect. Dis. 2007, 195, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Cilloniz, C.; Ewig, S.; Menendez, R.; Ferrer, M.; Polverino, E.; Reyes, S.; Gabarrus, A.; Marcos, M.A.; Cordoba, J.; Mensa, J.; et al. Bacterial co-infection with H1N1 infection in patients admitted with community acquired pneumonia. J. Infect. 2012, 65, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Koon, K.; Sanders, C.M.; Green, J.; Malone, L.; White, H.; Zayas, D.; Miller, R.; Lu, S.; Han, J. Co-detection of pandemic (H1N1) 2009 virus and other respiratory pathogens. Emerg. Infect. Dis. 2010, 16, 1976–1978. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Metzger, D.W. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat. Med. 2008, 14, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Shahangian, A.; Chow, E.K.; Tian, X.; Kang, J.R.; Ghaffari, A.; Liu, S.Y.; Belperio, J.A.; Cheng, G.; Deng, J.C. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J. Clin. Investig. 2009, 119, 1910–1920. [Google Scholar] [CrossRef] [PubMed]
- Kudva, A.; Scheller, E.V.; Robinson, K.M.; Crowe, C.R.; Choi, S.M.; Slight, S.R.; Khader, S.A.; Dubin, P.J.; Enelow, R.I.; Kolls, J.K.; et al. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J. Immunol. 2011, 186, 1666–1674. [Google Scholar] [CrossRef]
- McCullers, J.A.; Rehg, J.E. Lethal synergism between influenza virus and Streptococcus pneumoniae: Characterization of a mouse model and the role of platelet-activating factor receptor. J. Infect. Dis. 2002, 186, 341–350. [Google Scholar] [CrossRef]
- Esposito, S.; Principi, N. Impacts of the 13-Valent Pneumococcal Conjugate Vaccine in Children. J. Immunol. Res. 2015, 2015, 591580. [Google Scholar] [CrossRef]
- Sun, K. Limited Efficacy of Antibacterial Vaccination Against Secondary Serotype 3 Pneumococcal Pneumonia Following Influenza Infection. J. Infect. Dis. 2015, 212, 445–452. [Google Scholar]
- Madhi, S.A.; Klugman, K.P.; Vaccine Trialist, G. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat. Med. 2004, 10, 811–813. [Google Scholar] [CrossRef]
- Dominguez, A.; Castilla, J.; Godoy, P.; Delgado-Rodriguez, M.; Saez, M.; Soldevila, N.; Astray, J.; Mayoral, J.M.; Martin, V.; Quintana, J.M.; et al. Effectiveness of vaccination with 23-valent pneumococcal polysaccharide vaccine in preventing hospitalization with laboratory confirmed influenza during the 2009–2010 and 2010–2011 seasons. Hum. Vaccin. Immunother. 2013, 9, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Croney, C.M.; Coats, M.T.; Nahm, M.H.; Briles, D.E.; Crain, M.J. PspA family distribution, unlike capsular serotype, remains unaltered following introduction of the heptavalent pneumococcal conjugate vaccine. Clin. Vaccine Immunol. 2012, 19, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Hotomi, M.; Togawa, A.; Kono, M.; Ikeda, Y.; Takei, S.; Hollingshead, S.K.; Briles, D.E.; Suzuki, K.; Yamanaka, N. PspA family distribution, antimicrobial resistance and serotype of Streptococcus pneumoniae isolated from upper respiratory tract infections in Japan. PLoS ONE 2013, 8, e58124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hollingshead, S.K.; Becker, R.; Briles, D.E. Diversity of PspA: Mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect. Immun. 2000, 68, 5889–5900. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, L.S.; Sheffield, J.S.; Delucchi, P.; Briles, D.E. PspA, a surface protein of Streptococcus pneumoniae, is capable of eliciting protection against pneumococci of more than one capsular type. Infect. Immun. 1991, 59, 222–228. [Google Scholar] [PubMed]
- Briles, D.E.; Hollingshead, S.K.; King, J.; Swift, A.; Braun, P.A.; Park, M.K.; Ferguson, L.M.; Nahm, M.H.; Nabors, G.S. Immunization of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J. Infect. Dis. 2000, 182, 1694–1701. [Google Scholar] [CrossRef] [PubMed]
- Nabors, G.S.; Braun, P.A.; Herrmann, D.J.; Heise, M.L.; Pyle, D.J.; Gravenstein, S.; Schilling, M.; Ferguson, L.M.; Hollingshead, S.K.; Briles, D.E.; et al. Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine 2000, 18, 1743–1754. [Google Scholar] [CrossRef]
- Arulanandam, B.P.; Lynch, J.M.; Briles, D.E.; Hollingshead, S.; Metzger, D.W. Intranasal vaccination with pneumococcal surface protein A and interleukin-12 augments antibody-mediated opsonization and protective immunity against Streptococcus pneumoniae infection. Infect. Immun. 2001, 69, 6718–6724. [Google Scholar] [CrossRef]
- Fukuyama, Y.; Yuki, Y.; Katakai, Y.; Harada, N.; Takahashi, H.; Takeda, S.; Mejima, M.; Joo, S.; Kurokawa, S.; Sawada, S.; et al. Nanogel-based pneumococcal surface protein A nasal vaccine induces microRNA-associated Th17 cell responses with neutralizing antibodies against Streptococcus pneumoniae in macaques. Mucosal Immunol. 2015, 8, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Piao, Z.; Akeda, Y.; Takeuchi, D.; Ishii, K.J.; Ubukata, K.; Briles, D.E.; Tomono, K.; Oishi, K. Protective properties of a fusion pneumococcal surface protein A (PspA) vaccine against pneumococcal challenge by five different PspA clades in mice. Vaccine 2014, 32, 5607–5613. [Google Scholar] [CrossRef]
- Kong, I.G.; Sato, A.; Yuki, Y.; Nochi, T.; Takahashi, H.; Sawada, S.; Mejima, M.; Kurokawa, S.; Okada, K.; Sato, S.; et al. Nanogel-based PspA intranasal vaccine prevents invasive disease and nasal colonization by Streptococcus pneumoniae. Infect. Immun. 2013, 81, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Winter, C.; Herbold, W.; Maus, R.; Langer, F.; Briles, D.E.; Paton, J.C.; Welte, T.; Maus, U.A. Important role for CC chemokine ligand 2-dependent lung mononuclear phagocyte recruitment to inhibit sepsis in mice infected with Streptococcus pneumoniae. J. Immunol. 2009, 182, 4931–4937. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Yang, J.; Eisele, L.E.; Underwood, A.J.; Koestler, B.J.; Waters, C.M.; Metzger, D.W.; Bai, G. Two DHH subfamily 1 proteins in Streptococcus pneumoniae possess cyclic di-AMP phosphodiesterase activity and affect bacterial growth and virulence. J. Bacteriol. 2013, 195, 5123–5132. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluijs, K.F.; van Elden, L.J.; Nijhuis, M.; Schuurman, R.; Pater, J.M.; Florquin, S.; Goldman, M.; Jansen, H.M.; Lutter, R.; van der Poll, T. IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. J. Immunol. 2004, 172, 7603–7609. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Davis, K.M.; Weiser, J.N. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J. Clin. Investig. 2011, 121, 3657–3665. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Moltedo, B.; Moran, T.M. Type I interferon induction during influenza virus infection increases susceptibility to secondary Streptococcus pneumoniae infection by negative regulation of gammadelta T cells. J. virology 2012, 86, 12304–12312. [Google Scholar] [CrossRef]
- Jakab, G.J. Immune impairment of alveolar macrophage phagocytosis during influenza virus pneumonia. Am. Rev. Respir. Dis. 1982, 126, 778–782. [Google Scholar]
- Kodihalli, S.; Sivanandan, V.; Nagaraja, K.V.; Shaw, D.; Halvorson, D.A. Effect of avian influenza virus infection on the phagocytic function of systemic phagocytes and pulmonary macrophages of turkeys. Avian Dis. 1994, 38, 93–102. [Google Scholar] [CrossRef]
- Rynda-Apple, A.; Harmsen, A.; Erickson, A.S.; Larson, K.; Morton, R.V.; Richert, L.E.; Harmsen, A.G. Regulation of IFN-gamma by IL-13 dictates susceptibility to secondary postinfluenza MRSA pneumonia. Eur. J. Immunol. 2014, 44, 3263–3272. [Google Scholar] [CrossRef]
- Lee, B.; Robinson, K.M.; McHugh, K.J.; Scheller, E.V.; Mandalapu, S.; Chen, C.; Di, Y.P.; Clay, M.E.; Enelow, R.I.; Dubin, P.J.; et al. Influenza-induced type I interferon enhances susceptibility to gram-negative and gram-positive bacterial pneumonia in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L158–L167. [Google Scholar] [CrossRef]
- Robinson, K.M.; Lee, B.; Scheller, E.V.; Mandalapu, S.; Enelow, R.I.; Kolls, J.K.; Alcorn, J.F. The role of IL-27 in susceptibility to post-influenza Staphylococcus aureus pneumonia. Respiratory research 2015, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- King, Q.O.; Lei, B.; Harmsen, A.G. Pneumococcal surface protein A contributes to secondary Streptococcus pneumoniae infection after influenza virus infection. J. Infect. Dis. 2009, 200, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.J.; Marks, L.R.; Hu, J.C.; Reddinger, R.; Mandell, L.; Roche-Hakansson, H.; King-Lyons, N.D.; Connell, T.D.; Hakansson, A.P. Novel Strategy To Protect against Influenza Virus-Induced Pneumococcal Disease without Interfering with Commensal Colonization. Infect. Immun. 2016, 84, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Moffitt, K.; Malley, R. Rationale and prospects for novel pneumococcal vaccines. Hum. Vaccin. Immunother. 2016, 12, 383–392. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roberts, S.; Williams, C.M.; Salmon, S.L.; Bonin, J.L.; Metzger, D.W.; Furuya, Y. Evaluation of Pneumococcal Surface Protein A as a Vaccine Antigen against Secondary Streptococcus pneumoniae Challenge during Influenza A Infection. Vaccines 2019, 7, 146. https://doi.org/10.3390/vaccines7040146
Roberts S, Williams CM, Salmon SL, Bonin JL, Metzger DW, Furuya Y. Evaluation of Pneumococcal Surface Protein A as a Vaccine Antigen against Secondary Streptococcus pneumoniae Challenge during Influenza A Infection. Vaccines. 2019; 7(4):146. https://doi.org/10.3390/vaccines7040146
Chicago/Turabian StyleRoberts, Sean, Clare M. Williams, Sharon L. Salmon, Jesse L. Bonin, Dennis W. Metzger, and Yoichi Furuya. 2019. "Evaluation of Pneumococcal Surface Protein A as a Vaccine Antigen against Secondary Streptococcus pneumoniae Challenge during Influenza A Infection" Vaccines 7, no. 4: 146. https://doi.org/10.3390/vaccines7040146
APA StyleRoberts, S., Williams, C. M., Salmon, S. L., Bonin, J. L., Metzger, D. W., & Furuya, Y. (2019). Evaluation of Pneumococcal Surface Protein A as a Vaccine Antigen against Secondary Streptococcus pneumoniae Challenge during Influenza A Infection. Vaccines, 7(4), 146. https://doi.org/10.3390/vaccines7040146




