Dose Effects of Recombinant Adenovirus Immunization in Rodents
Abstract
1. Introduction
2. Materials and Methods
2.1. Adenovirus Production
2.2. Animals
2.3. Immunizations
2.4. Statistical Analysis
2.5. Enzyme Linked Immunosorbent Assay (ELISA)
2.6. Enzyme-Linked Immune Spot (ELISpot) Assay
2.7. Influenza Virus Challenge
3. Results
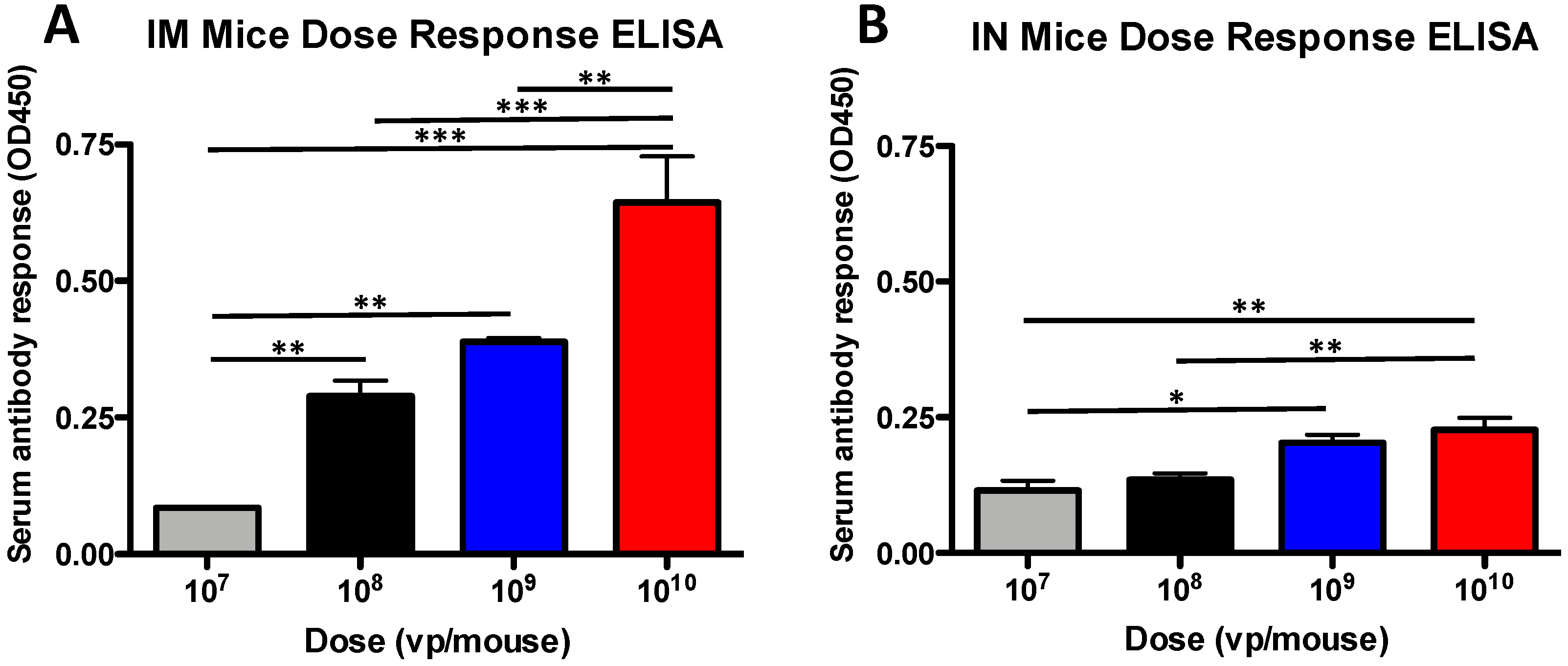
3.1. Antibody Dose-Dependent Responses in Mice
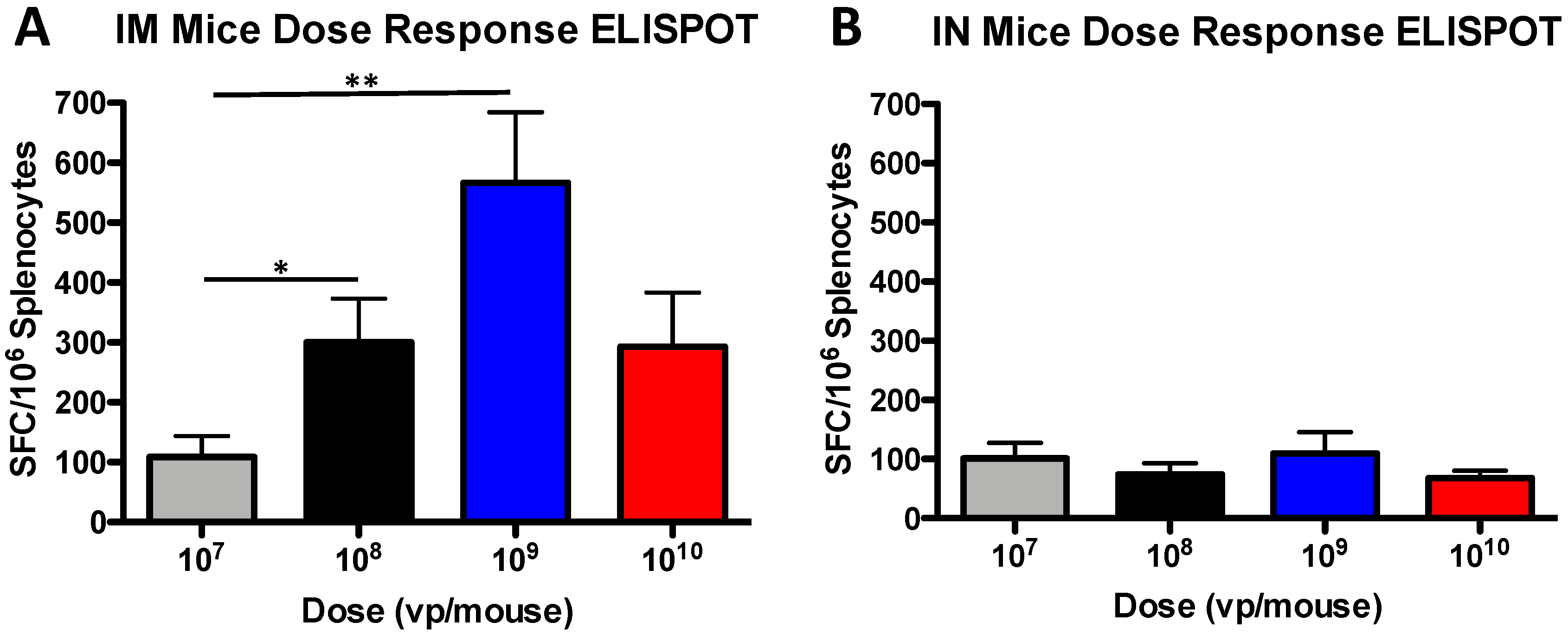
3.2. T Cell Dose-Dependent Responses in Mice
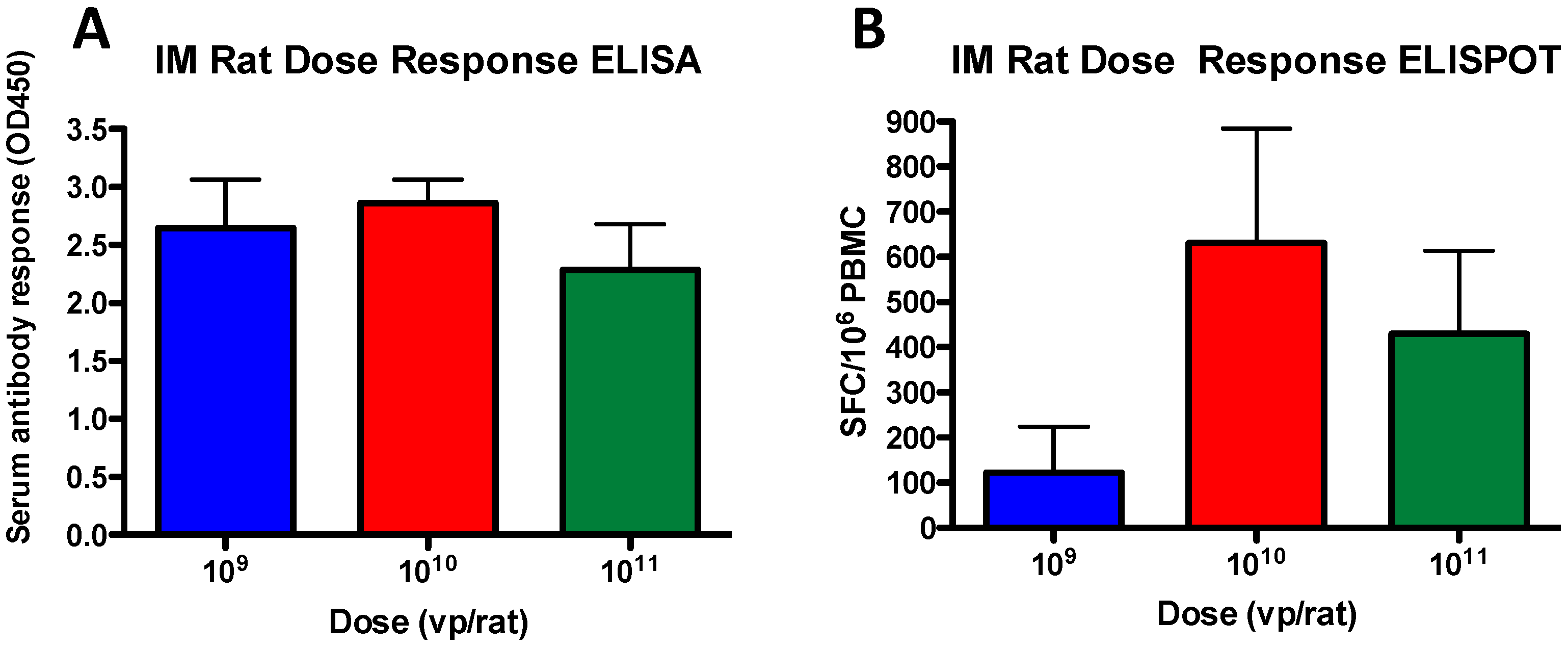
3.3. Antibody and T Cell Dose-Dependent Responses in Rats
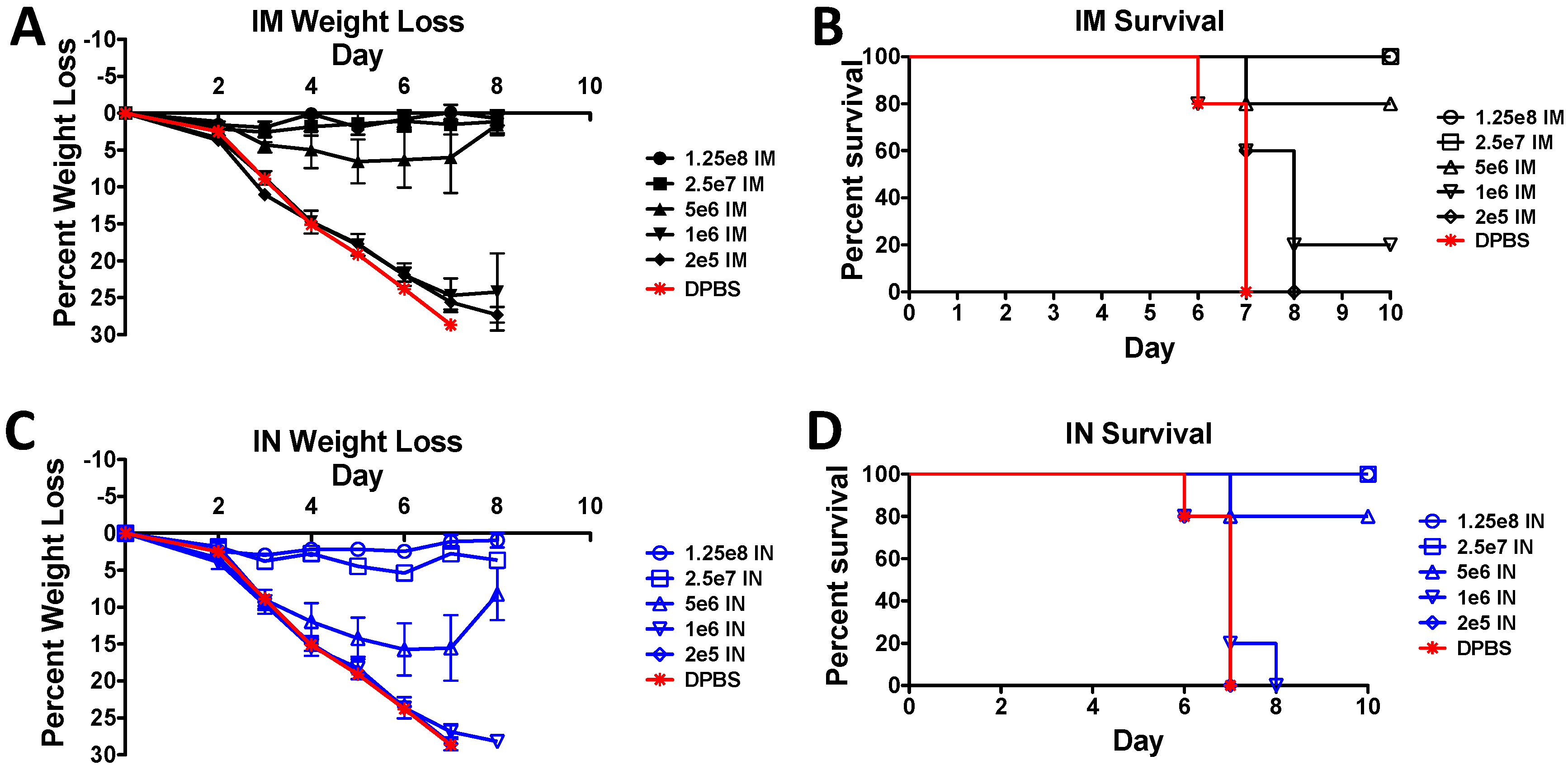
3.4. Protection against A Lethal Influenza Virus Challenge in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fields, B.N.; Knipe, D.M.; Howley, P.M. Fields Virology, 5th ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Clark, H.F.; Michalski, F.; Tweedell, K.S.; Yohn, D.; Zeigel, R.F. An adenovirus, FAV-1, isolated from the kidney of a frog (Rana pipiens). Virology 1973, 51, 392–400. [Google Scholar] [CrossRef]
- Larsen, S.H.; Nathans, D. Mouse adenovirus: Growth of plaque-purified FL virus in cell lines and characterization of viral DNA. Virology 1977, 82, 182–195. [Google Scholar] [CrossRef]
- Sutjipto, S.; Miller, S.E.; Simmons, D.G.; Dillman, R.C. Physicochemical characterization and pathogenicity studies of two turkey adenovirus isolants. Avian Dis. 1977, 21, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Abouhaidar, M.G.; Sira, S.; Campbell, J.B. Characterization of the genome of a vaccine strain of canine adenovirus type 1. Virus Genes 1988, 2, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Ledgerwood, J.E.; DeZure, A.D.; Stanley, D.A.; Coates, E.E.; Novik, L.; Enama, M.E.; Berkowitz, N.M.; Hu, Z.; Joshi, G.; Ploquin, A.; et al. Chimpanzee adenovirus vector Ebola vaccine. N. Engl. J. Med. 2017, 376, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Santra, S.; Sun, Y.; Korioth-Schmitz, B.; Fitzgerald, J.; Charbonneau, C.; Santos, G.; Seaman, M.S.; Ratcliffe, S.J.; Montefiori, D.C.; Nabel, G.J.; et al. Heterologous prime/boost immunizations of rhesus monkeys using chimpanzee adenovirus vectors. Vaccine 2009, 27, 5837–5845. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.S.; Chen, Y.; Whale, T.; Babiuk, L.A.; Mehtali, M.; Tikoo, S.K. Replication-defective bovine adenovirus type 3 as an expression vector. J. Virol. 1999, 73, 9137–9144. [Google Scholar] [PubMed]
- Warnock, J.N.; Daigre, C.; Al-Rubeai, M. Introduction to viral vectors. Methods Mol. Biol. 2011, 737, 1–25. [Google Scholar]
- Weaver, E.A.; Nehete, P.N.; Buchl, S.S.; Senac, J.S.; Palmer, D.; Ng, P.; Sastry, K.J.; Barry, M.A. Comparison of replication-competent, first generation, and helper-dependent adenoviral vaccines. PLoS ONE 2009, 4, e5059. [Google Scholar] [CrossRef]
- Wold, W.S.; Toth, K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther. 2013, 13, 421–433. [Google Scholar] [CrossRef]
- Weaver, E.A.; Nehete, P.N.; Buchl, S.S.; Palmer, D.; Montefiori, D.; Ng, P.; Sastry, K.J.; Barry, M.A. Protection against mucosal SHIV challenge by peptide and helper-dependent adenovirus vaccines. Viruses 2009, 1, 920–938. [Google Scholar] [CrossRef] [PubMed]
- Weaver, E.A.; Rubrum, A.M.; Webby, R.J.; Barry, M.A. Protection against divergent influenza H1N1 virus by a centralized influenza hemagglutinin. PLoS ONE 2011, 6, e18314. [Google Scholar] [CrossRef] [PubMed]
- Lemckert, A.A.; Sumida, S.M.; Holterman, L.; Vogels, R.; Truitt, D.M.; Lynch, D.M.; Nanda, A.; Ewald, B.A.; Gorgone, D.A.; Lifton, M.A.; et al. Immunogenicity of heterologous prime-boost regimens involving recombinant adenovirus serotype 11 (Ad11) and Ad35 vaccine vectors in the presence of anti-ad5 immunity. J. Virol. 2005, 79, 9694–9701. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.G.; Jin, H.T.; West, E.E.; Penaloza-Macmaster, P.; Wieland, A.; Zilliox, M.J.; McElrath, M.J.; Barouch, D.H.; Ahmed, R. Comparative analysis of simian immunodeficiency virus gag-specific effector and memory CD8+ T cells induced by different adenovirus vectors. J. Virol. 2013, 87, 1359–1372. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Bishop, E.S.; Zhang, R.; Yu, X.; Farina, E.M.; Yan, S.; Zhao, C.; Zheng, Z.; Shu, Y.; Wu, X.; et al. Adenovirus-mediated gene delivery: Potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes Dis. 2017, 4, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Cerullo, V.; Capasso, C.; Vaha-Koskela, M.; Hemminki, O.; Hemminki, A. Cancer-targeted oncolytic adenoviruses for modulation of the immune system. Curr. Cancer Drug Targets 2018, 18, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, D. Adenoviral vector-based strategies against infectious disease and cancer. Hum. Vaccin. Immunother. 2016, 12, 2064–2074. [Google Scholar] [CrossRef] [PubMed]
- Appaiahgari, M.B.; Vrati, S. Adenoviruses as gene/vaccine delivery vectors: Promises and pitfalls. Expert Opin. Biol. Ther. 2015, 15, 337–351. [Google Scholar] [CrossRef] [PubMed]
- van Zyl-Smit, R.N.; Esmail, A.; Bateman, M.E.; Dawson, R.; Goldin, J.; van Rikxoort, E.; Douoguih, M.; Pau, M.G.; Sadoff, J.C.; McClain, J.B.; et al. Safety and Immunogenicity of Adenovirus 35 Tuberculosis Vaccine Candidate in Adults with Active or Previous Tuberculosis. A Randomized Trial. Am. J. Respir. Crit. Care Med. 2017, 195, 1171–1180. [Google Scholar] [CrossRef]
- Lingel, A.; Bullard, B.L.; Weaver, E.A. Efficacy of an Adenoviral Vectored Multivalent Centralized Influenza Vaccine. Sci. Rep. 2017, 7, 14912. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, J.; Kong, Y.; Hou, L.; Li, Y. Immunogenicity of recombinant adenovirus type 5 vector-based ebola vaccine expressing glycoprotein from the 2014 epidemic strain in mice. Hum. Gene Ther. 2018, 29, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, J.A.; McCaffery, J.N.; Kashentseva, E.; Singh, B.; Dmitriev, I.P.; Curiel, D.T.; Moreno, A. A prime-boost immunization regimen based on a simian adenovirus 36 vectored multi-stage malaria vaccine induces protective immunity in mice. Vaccine 2017, 35, 3239–3248. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Tikoo, S.; Kobinger, G. A porcine adenovirus with low human seroprevalence is a promising alternative vaccine vector to human adenovirus 5 in an H5N1 virus disease model. PLoS ONE 2010, 5, e15301. [Google Scholar] [CrossRef] [PubMed]
- Abbink, P.; Lemckert, A.A.; Ewald, B.A.; Lynch, D.M.; Denholtz, M.; Smits, S.; Holterman, L.; Damen, I.; Vogels, R.; Thorner, A.R.; et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 2007, 81, 4654–4663. [Google Scholar] [CrossRef] [PubMed]
- Kostense, S.; Koudstaal, W.; Sprangers, M.; Weverling, G.J.; Penders, G.; Helmus, N.; Vogels, R.; Bakker, M.; Berkhout, B.; Havenga, M.; et al. Adenovirus types 5 and 35 seroprevalence in AIDS risk groups supports type 35 as a vaccine vector. AIDS 2004, 18, 1213–1216. [Google Scholar] [CrossRef] [PubMed]
- Privatt, S.R.; Bullard, B.L.; Weaver, E.A.; Wood, C.; West, J.T. Longitudinal quantification of adenovirus neutralizing responses in Zambian mother-infant pairs: Impact of HIV-1 infection and its treatment. Vaccine 2019, 37, 5177–5184. [Google Scholar] [CrossRef] [PubMed]
- Horton, R. STEP study: Disappointing, but not a failure. Lancet 2008, 370, 1665. [Google Scholar]
- Sekaly, R.P. The failed HIV Merck vaccine study: A step back or a launching point for future vaccine development? J. Exp. Med. 2008, 205, 7–12. [Google Scholar] [CrossRef]
- Steinbrook, R. One step forward, two steps back—Will there ever be an AIDS vaccine? N. Engl. J. Med. 2007, 357, 2653–2655. [Google Scholar] [CrossRef]
- Hoelscher, M.A.; Garg, S.; Bangari, D.S.; Belser, J.A.; Lu, X.; Stephenson, I.; Bright, R.A.; Katz, J.M.; Mittal, S.K.; Sambhara, S. Development of adenoviral-vector-based pandemic influenza vaccine against antigenically distinct human H5N1 strains in mice. Lancet 2006, 367, 475–481. [Google Scholar] [CrossRef]
- Bangari, D.S.; Mittal, S.K. Development of nonhuman adenoviruses as vaccine vectors. Vaccine 2006, 24, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Abbink, P.; Kirilova, M.; Boyd, M.; Mercado, N.; Li, Z.; Nityanandam, R.; Nanayakkara, O.; Peterson, R.; Larocca, R.A.; Aid, M.; et al. Rapid cloning of novel rhesus adenoviral vaccine vectors. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; Walsh, S.R.; Seaman, M.S.; Tucker, R.P.; Krause, K.H.; Patel, A.; Johnson, J.A.; Kleinjan, J.; Yanosick, K.E.; Perry, J.; et al. First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001). J. Infect. Dis. 2013, 207, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Weaver, E.A.; Barry, M.A. Low seroprevalent species D adenovirus vectors as influenza vaccines. PLoS ONE 2013, 8, e73313. [Google Scholar] [CrossRef] [PubMed]
- Hartlage, A.S.; Murthy, S.; Kumar, A.; Trivedi, S.; Dravid, P.; Sharma, H.; Walker, C.M.; Kapoor, A. Vaccination to prevent T cell subversion can protect against persistent hepacivirus infection. Nat. Commun. 2019, 10, 1113. [Google Scholar] [CrossRef]
- Mothe, B.; Manzardo, C.; Sanchez-Bernabeu, A.; Coll, P.; Moron-Lopez, S.; Puertas, M.C.; Rosas-Umbert, M.; Cobarsi, P.; Escrig, R.; Perez-Alvarez, N.; et al. Therapeutic Vaccination Refocuses T-cell Responses Towards Conserved Regions of HIV-1 in Early Treated Individuals (BCN 01 study). EClinicalMedicine 2019, 11, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, S.; Lai, R.; D’Agostino, M.R.; Vaseghi-Shanjani, M.; Zganiacz, A.; Yao, Y.; Jeyanathan, M.; Xing, Z. Single-Dose Mucosal Immunotherapy With Chimpanzee Adenovirus-Based Vaccine Accelerates Tuberculosis Disease Control and Limits Its Rebound After Antibiotic Cessation. J. Infect. Dis. 2019, 220, 1355–1366. [Google Scholar] [CrossRef]
- Weaver, E.A.; Barry, M.A. Effects of Shielding Adenoviral Vectors with Polyethylene Glycol (PEG) on Vector-specific and Vaccine-mediated Immune Responses. Hum. Gene Ther. 2008, 19, 1369–1382. [Google Scholar] [CrossRef]
- Capasso, C.; Hirvinen, M.; Garofalo, M.; Romaniuk, D.; Kuryk, L.; Sarvela, T.; Vitale, A.; Antopolsky, M.; Magarkar, A.; Viitala, T.; et al. Oncolytic adenoviruses coated with MHC-I tumor epitopes increase the antitumor immunity and efficacy against melanoma. Oncoimmunology 2016, 5, e1105429. [Google Scholar] [CrossRef]
- D’Alise, A.M.; Leoni, G.; Cotugno, G.; Troise, F.; Langone, F.; Fichera, I.; De Lucia, M.; Avalle, L.; Vitale, R.; Leuzzi, A.; et al. Adenoviral vaccine targeting multiple neoantigens as strategy to eradicate large tumors combined with checkpoint blockade. Nat. Commun. 2019, 10, 2688. [Google Scholar] [CrossRef]
- Wu, W.H.; Alkutkar, T.; Karanam, B.; Roden, R.B.; Ketner, G.; Ibeanu, O.A. Capsid display of a conserved human papillomavirus L2 peptide in the adenovirus 5 hexon protein: A candidate prophylactic hpv vaccine approach. Virol. J. 2015, 12, 140. [Google Scholar] [CrossRef] [PubMed]
- Mok, H.; Palmer, D.J.; Ng, P.; Barry, M.A. Evaluation of polyethylene glycol modification of first-generation and helper-dependent adenoviral vectors to reduce innate immune responses. Mol. Ther. 2005, 11, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Mittereder, N.; March, K.L.; Trapnell, B.C. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J. Virol. 1996, 70, 7498–7509. [Google Scholar] [PubMed]
- Bullard, B.L.; Corder, B.N.; Gorman, M.J.; Diamond, M.S.; Weaver, E.A. Efficacy of a T Cell-Biased Adenovirus Vector as a Zika Virus Vaccine. Sci. Rep. 2018, 8, 18017. [Google Scholar] [CrossRef] [PubMed]
- Weaver, E.A.; Lu, Z.; Camacho, Z.T.; Moukdar, F.; Liao, H.X.; Ma, B.J.; Muldoon, M.; Theiler, J.; Nabel, G.J.; Letvin, N.L.; et al. Cross-subtype T-cell immune responses induced by a human immunodeficiency virus type 1 group m consensus env immunogen. J. Virol. 2006, 80, 6745–6756. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Hahn, M.; Coyle, E.M.; King, L.R.; Lin, T.L.; Treanor, J.; Sant, A.; Golding, H. Repeat vaccination reduces antibody affinity maturation across different influenza vaccine platforms in humans. Nat. Commun. 2019, 10, 3338. [Google Scholar] [CrossRef]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weaver, E.A. Dose Effects of Recombinant Adenovirus Immunization in Rodents. Vaccines 2019, 7, 144. https://doi.org/10.3390/vaccines7040144
Weaver EA. Dose Effects of Recombinant Adenovirus Immunization in Rodents. Vaccines. 2019; 7(4):144. https://doi.org/10.3390/vaccines7040144
Chicago/Turabian StyleWeaver, Eric A. 2019. "Dose Effects of Recombinant Adenovirus Immunization in Rodents" Vaccines 7, no. 4: 144. https://doi.org/10.3390/vaccines7040144
APA StyleWeaver, E. A. (2019). Dose Effects of Recombinant Adenovirus Immunization in Rodents. Vaccines, 7(4), 144. https://doi.org/10.3390/vaccines7040144




