Anti-Tat Immunity in HIV-1 Infection: Effects of Naturally Occurring and Vaccine-Induced Antibodies Against Tat on the Course of the Disease
Abstract
:1. Introduction
2. Role of Tat in the Virus Life Cycle
3. Role of Extracellular Tat
4. Role of Extracellular Tat in HIV Reservoir Maintenance and Residual Disease upon Effective cART
5. Anti-Tat Antibodies Protect from Disease Progression
5.1. Naturally Occurring Anti-Tat Antibodies
5.2. Vaccine-Induced Anti-Tat Abs
5.2.1. Vaccination of Nonhuman Primates with Tat or Tat/Env
5.2.2. Phase I Preventive Clinical Trials with Tat or Tat/Env
5.2.3. Phase I Therapeutic Clinical Trials with Tat
5.2.4. Phase II Therapeutic Clinical Trials with Tat in Italy and South Africa
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fauci, A.S. An HIV Vaccine Is Essential for Ending the HIV/AIDS Pandemic. JAMA. 2017, 318, 1535–1536. [Google Scholar] [CrossRef] [PubMed]
- Ringel, O.; Vieillard, V.; Debré, P.; Eichler, J.; Büning, H.; Dietrich, U. The Hard Way towards an Antibody-Based HIV-1 Env Vaccine: Lessons from Other Viruses. Viruses 2018, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- López-Huertas, M.R.; Callejas, S.; Abia, D.; Mateos, E.; Dopazo, A.; Alcamí, J.; Coiras, M. Modifications in host cell cytoskeleton structure and function mediated by intracellular HIV-1 Tat protein are greatly dependent on the second coding exon. Nucleic Acids Res. 2010, 38, 3287–3307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Huertas, M.R.; Mateos, E.; Sánchez Del Cojo, M.; Gómez-Esquer, F.; Díaz-Gil, G.; Rodríguez-Mora, S.; López, J.A.; Calvo, E.; López-Campos, G.; Alcamí, J.; et al. The presence of HIV-1 Tat protein second exon delays fas protein-mediated apoptosis in CD4+ T lymphocytes: A potential mechanism for persistent viral production. J. Biol. Chem. 2013, 288, 7626–7644. [Google Scholar] [CrossRef] [PubMed]
- Kukkonen, S.; Martinez-Viedma Mdel, P.; Kim, N.; Manrique, M.; Aldovini, A. HIV-1 Tat second exon limits the extent of Tat-mediated modulation of interferon-stimulated genes in antigen presenting cells. Retrovirology 2014, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Howcroft, T.K.; Strebel, K.; Martin, M.A.; Singer, D.S. Repression of MHC class I gene promoter activity by two-exon Tat of HIV. Science 1993, 260, 1320–1322. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, S.; Okamoto, T.; Peterlin, B.M. Tat competes with CIITA for the binding to P-TEFb and blocks the expression of MHC class II genes in HIV infection. Immunity 2000, 12, 61–70. [Google Scholar] [CrossRef]
- Clark, E.; Nava, B.; Caputi, M. Tat is a multifunctional viral protein that modulates cellular gene expression and functions. Oncotarget 2017, 8, 27569–27581. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Sanders-Beer, B.E.; Katz, K.S.; Maglott, D.R.; Pruitt, K.D.; Ptak, R.G. Human immunodeficiency virus type 1, human protein interaction database at NCBI. Nucleic Acids Res. 2009, 37, D417–D422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reeder, J.E.; Kwak, Y.T.; McNamara, R.P.; Forst, C.V.; D’Orso, I. HIV Tat controls RNA Polymerase II and the epigenetic landscape to transcriptionally reprogram target immune cells. Elife 2015, 4, e08955. [Google Scholar] [CrossRef]
- Knoener, R.A.; Becker, J.T.; Scalf, M.; Sherer, N.M.; Smith, L.M. Elucidating the in vivo interactome of HIV-1 RNA by hybridization capture and mass spectrometry. Sci. Rep. 2017, 7, 16965. [Google Scholar] [CrossRef] [PubMed]
- Jean, M.J.; Power, D.; Kong, W.; Huang, H.; Santoso, N.; Zhu, J. Identification of HIV-1 Tat-Associated Proteins Contributing to HIV-1 Transcription and Latency. Viruses 2017, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Razooky, B.S.; Pai, A.; Aull, K.; Rouzine, I.M.; Weinberger, L.S. A hardwired HIV latency program. Cell 2015, 160, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Defechereux, P.; Verdin, E. The site of HIV-1 integration in the human genome determines basal transcriptional activity and response to Tat transactivation. EMBO J. 2001, 20, 1726–1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dar, R.D.; Razooky, B.S.; Singh, A.; Trimeloni, T.V.; McCollum, J.M.; Cox, C.D.; Simpson, M.L.; Weinberger, L.S. Transcriptional burst frequency and burst size are equally modulated across the human genome. Proc. Natl. Acad. Sci. USA 2012, 109, 17454–17459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raj, A.; van Oudenaarden, A. Nature, nurture, or chance: Stochastic gene expression and its consequences. Cell 2008, 135, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, L.S.; Burnett, J.C.; Toettcher, J.E.; Arkin, A.P.; Schaffer, D.V. Stochastic gene expression in a lentiviral positive-feedback loop: HIV-1 Tat fluctuations drive phenotypic diversity. Cell 2005, 122, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Chertova, E.; Chertov, O.; Coren, L.V.; Roser, J.D.; Trubey, C.M.; Bess, J.W., Jr.; Sowder, R.C., 2nd; Barsov, E.; Hood, B.L.; Fisher, R.J.; et al. Proteomic and biochemical analysis of purified human immunodeficiency virus type 1 produced from infected monocyte-derived macrophages. J. Virol. 2006, 80, 9039–9052. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Marsh, J.W. Selective transcription and modulation of resting T cell activity by preintegrated HIV DNA. Science 2001, 293, 1503–1506. [Google Scholar] [CrossRef]
- Ensoli, B.; Gendelman, R.; Markham, P.; Fiorelli, V.; Colombini, S.; Raffeld, M.; Cafaro, A.; Chang, H.K.; Brady, J.N.; Gallo, R.C. Synergy between basic fibroblast growth factor and HIV-1 Tat protein in induction of Kaposi’s sarcoma. Nature 1994, 371, 674–680. [Google Scholar] [CrossRef]
- Chang, H.C.; Samaniego, F.; Nair, B.C.; Buonaguro, L.; Ensoli, B. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS 1997, 11, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Harrich, D.; Ulich, C.; García-Martínez, L.F.; Gaynor, R.B. Tat is required for efficient HIV-1 reverse transcription. EMBO J. 1997, 16, 1224–1235. [Google Scholar] [CrossRef] [PubMed]
- Bohan, C.A.; Kashanchi, F.; Ensoli, B.; Buonaguro, L.; Boris-Lawrie, K.A.; Brady, J.N. Analysis of Tat transactivation of human immunodeficiency virus transcription in vitro. Gene Expr. 1992, 2, 391–407. [Google Scholar] [PubMed]
- Feinberg, M.B.; Baltimore, D.; Frankel, A.D. The role of Tat in the human immunodeficiency virus life cycle indicates a primary effect on transcriptional elongation. Proc. Natl. Acad. Sci. USA 1991, 88, 4045–4049. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.; Pasternak, A.O.; Klaver, B.; Cornelissen, M.; Berkhout, B.; Das, A.T. The HIV-1 Tat Protein Enhances Splicing at the Major Splice Donor Site. J. Virol. 2018, 92, e01855-17. [Google Scholar] [CrossRef]
- Trono, D. Partial reverse transcripts in virions from human immunodeficiency and murine leukemia viruses. J. Virol. 1992, 66, 4893–4900. [Google Scholar] [Green Version]
- Zhang, H.; Dornadula, G.; Pomerantz, R.J. Endogenous reverse transcription of human immunodeficiency virus type 1 in physiological microenviroments: An important stage for viral infection of nondividing cells. J. Virol. 1996, 70, 2809–2824. [Google Scholar]
- Ho, Y.C.; Shan, L.; Hosmane, N.N.; Wang, J.; Laskey, S.B.; Rosenbloom, D.I.; Lai, J.; Blankson, J.N.; Siliciano, J.D.; Siliciano, R.F. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013, 155, 540–551. [Google Scholar] [CrossRef]
- Hosmane, N.N.; Kwon, K.J.; Bruner, K.M.; Capoferri, A.A.; Beg, S.; Rosenbloom, D.I.; Keele, B.F.; Ho, Y.C.; Siliciano, J.D.; Siliciano, R.F. Proliferation of latently infected CD4+ T cells carrying replication-competent HIV-1: Potential role in latent reservoir dynamics. J. Exp. Med. 2017, 214, 959–972. [Google Scholar] [CrossRef]
- Weinberger, A.D.; Weinberger, L.S. Stochastic Fate Selection in HIV-Infected Patients. Cell 2013, 155, 497–499. [Google Scholar] [CrossRef] [Green Version]
- Mediouni, S.; Chinthalapudi, K.; Ekka, M.K.; Usui, I.; Jablonski, J.A.; Clementz, M.A.; Mousseau, G.; Nowak, J.; Macherla, V.R.; Beverage, J.N.; et al. Didehydro-Cortistatin A Inhibits HIV-1 by Specifically Binding to the Unstructured Basic Region of Tat. mBio 2019, 10, e02662-18. [Google Scholar] [CrossRef] [Green Version]
- Mousseau, G.; Kessing, C.F.; Fromentin, R.; Trautmann, L.; Chomont, N.; Valente, S.T. The Tat Inhibitor Didehydro-Cortistatin A Prevents HIV-1 Reactivation from Latency. mBio 2015, 6, e00465-15. [Google Scholar] [CrossRef] [Green Version]
- Mediouni, S.; Kessing, C.F.; Jablonski, J.A.; Thenin-Houssier, S.; Clementz, M.; Kovach, M.D.; Mousseau, G.; de Vera, I.M.S.; Li, C.; Kojetin, D.J.; et al. The Tat inhibitor didehydro-cortistatin A suppresses SIV replication and reactivation. FASEB J. 2019, 33, 8280–8293. [Google Scholar] [CrossRef]
- Ensoli, B.; Barillari, G.; Salahuddin, S.Z.; Gallo, R.C.; Wong-Staal, F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi’s sarcoma lesions of AIDS patients. Nature 1990, 345, 84–86. [Google Scholar] [CrossRef]
- Ensoli, B.; Buonaguro, L.; Barillari, G.; Fiorelli, V.; Gendelman, R.; Morgan, R.A.; Wingfield, P.; Gallo, R.C. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J. Virol. 1993, 67, 277–287. [Google Scholar] [Green Version]
- Rayne, F.; Debaisieux, S.; Yezid, H.; Lin, Y.L.; Mettling, C.; Konate, K.; Chazal, N.; Arold, S.T.; Pugnière, M.; Sanchez, F.; et al. Phosphatidylinositol-(4,5)-bisphosphate enables efficient secretion of HIV-1 Tat by infected T-cells. EMBO J. 2010, 29, 1348–1362. [Google Scholar] [CrossRef]
- Mele, A.R.; Marino, J.; Chen, K.; Pirrone, V.; Janetopoulos, C.; Wigdahl, B.; Klase, Z.; Nonnemacher, M.R. Defining the molecular mechanisms of HIV-1 Tat secretion: PtdIns(4,5)P2 at the epicenter. Traffic 2018, 19, 655–665. [Google Scholar] [CrossRef]
- Jost, M.; Simpson, F.; Kavran, J.M.; Lemmon, M.A.; Schmid, S.L. Phosphatidylinositol-4,5-bisphosphate is required for endocytic coated vesicle formation. Curr. Biol. 1998, 8, 1399–1402. [Google Scholar] [CrossRef]
- Debaisieux, S.; Lachambre, S.; Gross, A.; Mettling, C.; Besteiro, S.; Yezid, H.; Henaff, D.; Chopard, C.; Mesnard, J.M.; Beaumelle, B. HIV-1 Tat inhibits phagocytosis by preventing the recruitment of Cdc42 to the phagocytic cup. Nat. Commun. 2015, 6, 6211. [Google Scholar] [CrossRef]
- Tryoen-Tóth, P.; Chasserot-Golaz, S.; Tu, A.; Gherib, P.; Bader, M.F.; Beaumelle, B.; Vitale, N. HIV-1 Tat protein inhibits neurosecretion by binding to phosphatidylinositol 4,5-bisphosphate. J. Cell Sci. 2013, 126, 454–463. [Google Scholar] [CrossRef]
- Rahimian, P.; He, J.J. Exosome-associated release, uptake, and neurotoxicity of HIV-1 Tat protein. J. Neurovirol. 2016, 22, 774–788. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, A.; Iordanskiy, S.; Das, R.; Van Duyne, R.; Santos, S.; Jaworski, E.; Guendel, I.; Sampey, G.; Dalby, E.; Iglesias-Ussel, M.; et al. Exosomes derived from HIV-1-infected cells contain trans-activation response element RNA. J. Biol. Chem. 2013, 288, 20014–20033. [Google Scholar] [CrossRef]
- Sampey, G.C.; Saifuddin, M.; Schwab, A.; Barclay, R.; Punya, S.; Chung, M.C.; Hakami, R.M.; Zadeh, M.A.; Lepene, B.; Klase, Z.A.; et al. Exosomes from HIV-1-infected Cells Stimulate Production of Pro-inflammatory Cytokines through Trans-activating Response (TAR) RNA. J. Biol. Chem. 2016, 291, 1251–1266. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Feng, Z.; Yue, H.; Bazdar, D.; Mbonye, U.; Zender, C.; Harding, C.V.; Bruggeman, L.; Karn, J.; Sieg, S.F.; et al. Exosomes derived from HIV-1-infected cells promote growth and progression of cancer via HIV TAR RNA. Nat. Commun. 2018, 9, 4585. [Google Scholar] [CrossRef]
- Poggi, A.; Zocchi, M.R. HIV-1 Tat triggers TGF-beta production and NK cell apoptosis that is prevented by pertussis toxin B. Clin. Dev. Immunol. 2006, 13, 369–372. [Google Scholar] [CrossRef]
- Westendorp, M.O.; Frank, R.; Ochsenbauer, C.; Stricker, K.; Dhein, J.; Walczak, H.; Debatin, K.M.; Krammer, P.H. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 1995, 375, 497–500. [Google Scholar] [CrossRef]
- Marchiò, S.; Alfano, M.; Primo, L.; Gramaglia, D.; Butini, L.; Gennero, L.; De Vivo, E.; Arap, W.; Giacca, M.; Pasqualini, R.; et al. Cell surface-associated Tat modulates HIV-1 infection and spreading through a specific interaction with gp120 viral envelope protein. Blood 2005, 105, 2802–2811. [Google Scholar] [CrossRef]
- Buonaguro, L.; Buonaguro, F.M.; Giraldo, G.; Ensoli, B. The human immunodeficiency virus type 1 Tat protein transactivates tumor necrosis factor beta gene expression through a TAR-like structure. J. Virol. 1994, 68, 2677–2682. [Google Scholar] [Green Version]
- Nappi, F.; Chiozzini, C.; Bordignon, V.; Borsetti, A.; Bellino, S.; Cippitelli, M.; Barillari, G.; Caputo, A.; Tyagi, M.; Giacca, M.; et al. Immobilized HIV-1 Tat protein promotes gene transfer via a transactivation-independent mechanism which requires binding of Tat to viral particles. J. Gene Med. 2009, 11, 955–965. [Google Scholar] [CrossRef]
- Ott, M.; Emiliani, S.; Van Lint, C.; Herbein, G.; Lovett, J.; Chirmule, N.; McCloskey, T.; Pahwa, S.; Verdin, E. Immune hyperactivation of HIV-1-infected T cells mediated by Tat and the CD28 pathway. Science 1997, 275, 1481–1485. [Google Scholar] [CrossRef]
- Li, C.J.; Ueda, Y.; Shi, B.; Borodyansky, L.; Huang, L.; Li, Y.Z.; Pardee, A.B. Tat protein induces self-perpetuating permissivity for productive HIV-1 infection. Proc. Natl. Acad. Sci. USA 1997, 94, 8116–8120. [Google Scholar] [CrossRef] [Green Version]
- Gavioli, R.; Gallerani, E.; Fortini, C.; Fabris, M.; Bottoni, A.; Canella, A.; Bonaccorsi, A.; Marastoni, M.; Micheletti, F.; Cafaro, A.; et al. HIV-1 tat protein modulates the generation of cytotoxic T cell epitopes by modifying proteasome composition and enzymatic activity. J. Immunol. 2004, 173, 3838–3843. [Google Scholar] [CrossRef]
- Campbell, G.R.; Loret, E.P. What does the structure-function relationship of the HIV-1 Tat protein teach us about developing an AIDS vaccine? Retrovirology 2009, 6, 50. [Google Scholar] [CrossRef]
- Fanales-Belasio, E.; Moretti, S.; Nappi, F.; Barillari, G.; Micheletti, F.; Cafaro, A.; Ensoli, B. Native HIV-1 Tat protein targets monocyte-derived dendritic cells and enhances their maturation, function, and antigen-specific T cell responses. J. Immunol. 2002, 168, 197–206. [Google Scholar] [CrossRef]
- Fanales-Belasio, E.; Moretti, S.; Fiorelli, V.; Tripiciano, A.; Pavone Cossut, M.R.; Scoglio, A.; Collacchi, B.; Nappi, F.; Macchia, I.; Bellino, S.; et al. HIV-1 Tat addresses dendritic cells to induce a predominant Th1-type adaptive immune response that appears prevalent in the asymptomatic stage of infection. J. Immunol. 2009, 182, 2888–2897. [Google Scholar] [CrossRef]
- Li, J.C.; Yim, H.C.; Lau, A.S. Role of HIV-1 Tat in AIDS pathogenesis: Its effects on cytokine dysregulation and contributions to the pathogenesis of opportunistic infection. AIDS 2010, 24, 1609–1623. [Google Scholar] [CrossRef]
- Herbein, G.; Gras, G.; Khan, K.A.; Abbas, W. Macrophage signaling in HIV-1 infection. Retrovirology 2010, 7, 34. [Google Scholar] [CrossRef]
- Torre, D.; Ferrario, G.; Issi, M.; Pugliese, A.; Speranza, F. Expression of the alpha 5 beta 1 fibronectin receptor on T lymphocytes of patients with HIV-1 infection. J. Clin. Pathol. 1996, 49, 733–736. [Google Scholar] [CrossRef]
- Johnson, T.P.; Patel, K.; Johnson, K.R.; Maric, D.; Calabresi, P.A.; Hasbun, R.; Nath, A. Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc. Natl. Acad. Sci. USA 2013, 110, 13588–13593. [Google Scholar] [CrossRef] [Green Version]
- Remoli, A.L.; Marsili, G.; Perrotti, E.; Gallerani, E.; Ilari, R.; Nappi, F.; Cafaro, A.; Ensoli, B.; Gavioli, R.; Battistini, A. Intracellular HIV-1 Tat protein represses constitutive LMP2 transcription increasing proteasome activity by interfering with the binding of IRF-1 to STAT1. Biochem. J. 2006, 396, 371–380. [Google Scholar] [CrossRef]
- Gavioli, R.; Cellini, S.; Castaldello, A.; Voltan, R.; Gallerani, E.; Gagliardoni, F.; Fortini, C.; Cofano, E.B.; Triulzi, C.; Cafaro, A.; et al. The Tat protein broadens T cell responses directed to the HIV-1 antigens Gag and Env: Implications for the design of new vaccination strategies against AIDS. Vaccine 2008, 26, 727–737. [Google Scholar] [CrossRef]
- Nicoli, F.; Finessi, V.; Sicurella, M.; Rizzotto, L.; Gallerani, E.; Destro, F.; Cafaro, A.; Marconi, P.; Caputo, A.; Ensoli, B.; et al. The HIV-1 Tat protein induces the activation of CD8+ T cells and affects in vivo the magnitude and kinetics of antiviral responses. PLoS ONE 2013, 8, e77746. [Google Scholar] [CrossRef]
- Sforza, F.; Nicoli, F.; Gallerani, E.; Finessi, V.; Reali, E.; Cafaro, A.; Caputo, A.; Ensoli, B.; Gavioli, R. HIV-1 Tat affects the programming and functionality of human CD8⁺ T cells by modulating the expression of T-box transcription factors. AIDS 2014, 28, 1729–1738. [Google Scholar] [CrossRef]
- Chiozzini, C.; Collacchi, B.; Nappi, F.; Bauer, T.; Arenaccio, C.; Tripiciano, A.; Longo, O.; Ensoli, F.; Cafaro, A.; Ensoli, B.; et al. Surface-bound Tat inhibits antigen-specific CD8+ T-cell activation in an integrin-dependent manner. AIDS 2014, 28, 2189–2200. [Google Scholar] [CrossRef]
- Gutheil, W.G.; Subramanyam, M.; Flentke, G.R.; Sanford, D.G.; Muñoz, E.; Huber, B.T.; Bachovchin, W.W. Human immunodeficiency virus 1 Tat binds to dipeptidyl aminopeptidase IV (CD26): A possible mechanism for Tat’s immunosuppressive activity. Proc. Natl. Acad. Sci. USA 1994, 91, 6594–6598. [Google Scholar] [CrossRef]
- Schmitz, T.; Underwood, R.; Khiroya, R.; Bachovchin, W.W.; Huber, B.T. Potentiation of the immune response in HIV-1+ individuals. J. Clin. Investig. 1996, 97, 1545–1549. [Google Scholar] [CrossRef]
- Planès, R.; Bahraoui, E. HIV-1 Tat Protein Induces the Production of IDO in Human Monocyte Derived-Dendritic Cells through a Direct Mechanism: Effect on T Cells Proliferation. PLoS ONE 2013, 8, e74551. [Google Scholar] [CrossRef]
- Serrano-Villar, S.; Sainz, T.; Lee, S.A.; Hunt, P.W.; Sinclair, E.; Shacklett, B.L.; Ferre, A.L.; Hayes, T.L.; Somsouk, M.; Hsue, P.Y.; et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog. 2014, 10, e1004078. [Google Scholar] [CrossRef]
- Barillari, G.; Buonaguro, L.; Fiorelli, V.; Hoffman, J.; Michaels, F.; Gallo, R.C.; Ensoli, B. Effects of cytokines from activated immune cells on vascular cell growth and HIV-1 gene expression. Implications for AIDS-Kaposi’s sarcoma pathogenesis. J. Immunol. 1992, 149, 3727–3734. [Google Scholar]
- Buonaguro, L.; Barillari, G.; Chang, H.K.; Bohan, C.A.; Kao, V.; Morgan, R.; Gallo, R.C.; Ensoli, B. Effects of the human immunodeficiency virus type 1 Tat protein on the expression of inflammatory cytokines. J. Virol. 1992, 66, 7159–7167. [Google Scholar]
- Scala, G.; Ruocco, M.R.; Ambrosino, C.; Mallardo, M.; Giordano, V.; Baldassarre, F.; Dragonetti, E.; Quinto, I.; Venuta, S. The expression of the interleukin 6 gene is induced by the human immunodeficiency virus 1 TAT protein. J. Exp. Med. 1994, 179, 961–971. [Google Scholar] [CrossRef]
- Lafrenie, R.M.; Wahl, L.M.; Epstein, J.S.; Yamada, K.M.; Dhawan, S. Activation of monocytes by HIV-Tat treatment is mediated by cytokine expression. J. Immunol. 1997, 159, 4077–4083. [Google Scholar]
- Ben Haij, N.; Planès, R.; Leghmari, K.; Serrero, M.; Delobel, P.; Izopet, J.; BenMohamed, L.; Bahraoui, E. HIV-1 Tat Protein Induces Production of Proinflammatory Cytokines by Human Dendritic Cells and Monocytes/Macrophages through Engagement of TLR4-MD2-CD14 Complex and Activation of NF-κB Pathway. PLoS ONE 2015, 10, e0129425. [Google Scholar] [CrossRef]
- Planès, R.; Ben Haij, N.; Leghmari, K.; Serrero, M.; BenMohamed, L.; Bahraoui, E. HIV-1 Tat Protein Activates both the MyD88 and TRIF Pathways To Induce Tumor Necrosis Factor Alpha and Interleukin-10 in Human Monocytes. J. Virol. 2016, 90, 5886–5898. [Google Scholar] [CrossRef] [Green Version]
- Albini, A.; Ferrini, S.; Benelli, R.; Sforzini, S.; Giunciuglio, D.; Aluigi, M.G.; Proudfoot, A.E.; Alouani, S.; Wells, T.N.; Mariani, G.; et al. HIV-1 Tat protein mimicry of chemokines. Proc. Natl. Acad. Sci. USA 1998, 95, 13153–13158. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Bosch, I.; Hofmann, W.; Sodroski, J.; Pardee, A.B. Tat protein induces human immunodeficiency virus type 1 (HIV-1) coreceptors and promotes infection with both macrophage-tropic and T-lymphotropic HIV-1 strains. J. Virol. 1998, 72, 8952–8960. [Google Scholar]
- Ghezzi, S.; Noonan, D.M.; Aluigi, M.G.; Vallanti, G.; Cota, M.; Benelli, R.; Morini, M.; Reeves, J.D.; Vicenzi, E.; Poli, G.; et al. Inhibition of CXCR4-dependent HIV-1 infection by extracellular HIV-1 Tat. Biochem. Biophys. Res. Commun. 2000, 270, 992–996. [Google Scholar] [CrossRef]
- Monini, P.; Cafaro, A.; Srivastava, I.K.; Moretti, S.; Sharma, V.A.; Andreini, C.; Chiozzini, C.; Ferrantelli, F.; Cossut, M.R.; Tripiciano, A.; et al. HIV-1 tat promotes integrin-mediated HIV transmission to dendritic cells by binding Env spikes and competes neutralization by anti-HIV antibodies. PLoS ONE 2012, 7, e48781. [Google Scholar] [CrossRef]
- Palmer, S.; Maldarelli, F.; Wiegand, A.; Bernstein, B.; Hanna, G.J.; Brun, S.C.; Kempf, D.J.; Mellors, J.W.; Coffin, J.M.; King, M.S. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc. Natl. Acad. Sci. USA 2008, 105, 3879–3884. [Google Scholar] [CrossRef] [Green Version]
- Hatano, H.; Delwart, E.L.; Norris, P.J.; Lee, T.H.; Neilands, T.B.; Kelley, C.F.; Hunt, P.W.; Hoh, R.; Linnen, J.M.; Martin, J.N.; et al. Evidence of persistent low-level viremia in long-term HAART-suppressed, HIV-infected individuals. AIDS 2010, 24, 2535–2539. [Google Scholar] [CrossRef] [Green Version]
- Álvarez Estévez, M.; Chueca Porcuna, N.; Guillot Suay, V.; Peña Monge, A.; García García, F.; Muñoz Medina, L.; Vinuesa García, D.; Parra Ruiz, J.; Hernández-Quero, J.; García García, F. Quantification of viral loads lower than 50 copies per milliliter by use of the Cobas AmpliPrep/Cobas TaqMan HIV-1 test, version 2.0, can predict the likelihood of subsequent virological rebound to >50 copies per milliliter. J. Clin. Microbiol. 2013, 51, 1555–1557. [Google Scholar] [CrossRef]
- Doyle, T.; Smith, C.; Vitiello, P.; Cambiano, V.; Johnson, M.; Owen, A.; Phillips, A.N.; Geretti, A.M. Plasma HIV-1 RNA detection below 50 copies/mL and risk of virologic rebound in patients receiving highly active antiretroviral therapy. Clin. Infect. Dis. 2012, 54, 724–732. [Google Scholar] [CrossRef]
- Sedaghat, A.R.; Siliciano, J.D.; Brennan, T.P.; Wilke, C.O.; Siliciano, R.F. Limits on replenishment of the resting CD4+ T cell reservoir for HIV in patients on HAART. PLoS Pathog. 2007, 3, e122. [Google Scholar] [CrossRef]
- Tobin, N.H.; Learn, G.H.; Holte, S.E.; Wang, Y.; Melvin, A.J.; McKernan, J.L.; Pawluk, D.M.; Mohan, K.M.; Lewis, P.F.; Mullins, J.I.; et al. Evidence that low-level viremias during effective highly active antiretroviral therapy result from two processes: Expression of archival virus and replication of virus. J. Virol. 2005, 79, 9625–9634. [Google Scholar] [CrossRef]
- Lorenzo-Redondo, R.; Fryer, H.R.; Bedford, T.; Kim, E.Y.; Archer, J.; Pond, S.L.K.; Chung, Y.S.; Penugonda, S.; Chipman, J.; Fletcher, C.V.; et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 2016, 530, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, C.V.; Staskus, K.; Wietgrefe, S.W.; Rothenberger, M.; Reilly, C.; Chipman, J.G.; Beilman, G.J.; Khoruts, A.; Thorkelson, A.; Schmidt, T.E.; et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc. Natl. Acad. Sci. USA 2014, 111, 2307–2312. [Google Scholar] [CrossRef] [Green Version]
- Sigal, A.; Kim, J.T.; Balazs, A.B.; Dekel, E.; Mayo, A.; Milo, R.; Baltimore, D. Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature 2011, 477, 95–98. [Google Scholar] [CrossRef]
- Furtado, M.R.; Callaway, D.S.; Phair, J.P.; Kunstman, K.J.; Stanton, J.L.; Macken, C.A.; Perelson, A.S.; Wolinsky, S.M. Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. N. Engl. J. Med. 1999, 340, 1614–1622. [Google Scholar] [CrossRef]
- Zhang, L.; Ramratnam, B.; Tenner-Racz, K.; He, Y.; Vesanen, M.; Lewin, S.; Talal, A.; Racz, P.; Perelson, A.S.; Korber, B.T.; et al. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N. Engl. J. Med. 1999, 340, 1605–1613. [Google Scholar] [CrossRef]
- Chun, T.W.; Justement, J.S.; Lempicki, R.A.; Yang, J.; Dennis, G., Jr.; Hallahan, C.W.; Sanford, C.; Pandya, P.; Liu, S.; McLaughlin, M.; et al. Gene expression and viral prodution in latently infected, resting CD4+ T cells in viremic versus aviremic HIV-infected individuals. Proc. Natl. Acad. Sci. USA 2003, 100, 1908–1913. [Google Scholar] [CrossRef]
- Lassen, K.G.; Ramyar, K.X.; Bailey, J.R.; Zhou, Y.; Siliciano, R.F. Nuclear retention of multiply spliced HIV-1 RNA in resting CD4+ T cells. PLoS Pathog. 2006, 2, e68. [Google Scholar] [CrossRef]
- Ensoli, B.; Bellino, S.; Tripiciano, A.; Longo, O.; Francavilla, V.; Marcotullio, S.; Cafaro, A.; Picconi, O.; Paniccia, G.; Scoglio, A.; et al. Therapeutic immunization with HIV-1 Tat reduces immune activation and loss of regulatory T-cells and improves immune function in subjects on HAART. PLoS ONE 2010, 5, e13540. [Google Scholar] [CrossRef]
- Mediouni, S.; Darque, A.; Baillat, G.; Ravaux, I.; Dhiver, C.; Tissot-Dupont, H.; Mokhtari, M.; Moreau, H.; Tamalet, C.; Brunet, C.; et al. Antiretroviral therapy does not block the secretion of the human immunodeficiency virus tat protein. Infect. Disord. Drug Targets 2012, 12, 81–86. [Google Scholar] [CrossRef]
- Dahl, V.; Lee, E.; Peterson, J.; Spudich, S.S.; Leppla, I.; Sinclair, E.; Fuchs, D.; Palmer, S.; Price, R.W. Raltegravir treatment intensification does not alter cerebrospinal fluid HIV-1 infection or immunoactivation in subjects on suppressive therapy. J. Infect. Dis. 2011, 204, 1936–1945. [Google Scholar] [CrossRef]
- Frankel, A.D.; Pabo, C.O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 1988, 55, 1189–1193. [Google Scholar] [CrossRef]
- Lin, X.; Irwin, D.; Kanazawa, S.; Huang, L.; Romeo, J.; Yen, T.S.; Peterlin, B.M. Transcriptional profiles of latent human immunodeficiency virus in infected individuals: Effects of Tat on the host and reservoir. J. Virol. 2003, 77, 8227–8236. [Google Scholar] [CrossRef]
- Murray, A.J.; Kwon, K.J.; Farber, D.L.; Siliciano, R.F. The Latent Reservoir for HIV-1: How Immunologic Memory and Clonal Expansion Contribute to HIV-1 Persistence. J. Immunol. 2016, 197, 407–417. [Google Scholar] [CrossRef] [Green Version]
- Lewis, G.K.; Pazgier, M.; Evans, D.T.; Ferrari, G.; Bournazos, S.; Parsons, M.S.; Bernard, N.F.; Finzi, A. Beyond Viral Neutralization. AIDS Res. Hum. Retrovir. 2017, 33, 760–764. [Google Scholar] [CrossRef]
- Finzi, D.; Blankson, J.; Siliciano, J.D.; Margolick, J.B.; Chadwick, K.; Pierson, T.; Smith, K.; Lisziewicz, J.; Lori, F.; Flexner, C.; et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 1999, 5, 512–517. [Google Scholar] [CrossRef]
- Crooks, A.M.; Bateson, R.; Cope, A.B.; Dahl, N.P.; Griggs, M.K.; Kuruc, J.D.; Gay, C.L.; Eron, J.J.; Margolis, D.M.; Bosch, R.J.; et al. Precise Quantitation of the Latent HIV-1 Reservoir: Implications for Eradication Strategies. J. Infect. Dis. 2015, 212, 1361–1365. [Google Scholar] [CrossRef]
- Siliciano, J.M.; Siliciano, R.F. The Remarkable Stability of the Latent Reservoir for HIV-1 in Resting Memory CD4+ T Cells. J. Infect. Dis. 2015, 212, 1345–1347. [Google Scholar] [CrossRef]
- Ensoli, F.; Cafaro, A.; Casabianca, A.; Tripiciano, A.; Bellino, S.; Longo, O.; Francavilla, V.; Picconi, O.; Sgadari, C.; Moretti, S.; et al. HIV-1 Tat immunization restores immune homeostasis and attacks the HAART-resistant blood HIV DNA: Results of a randomized phase II exploratory clinical trial. Retrovirology 2015, 12, 33. [Google Scholar] [CrossRef]
- Besson, G.J.; Lalama, C.M.; Bosch, R.J.; Gandhi, R.T.; Bedison, M.A.; Aga, E.; Riddler, S.A.; McMahon, D.K.; Hong, F.; Mellors, J.W. HIV-1 DNA decay dynamics in blood during more than a decade of suppressive antiretroviral therapy. Clin. Infect. Dis. 2014, 59, 1312–1321. [Google Scholar] [CrossRef]
- Arsenio, J.; Metz, P.J.; Chang, J.T. Asymmetric Cell Division in T Lymphocyte Fate Diversification. Trends Immunol. 2015, 36, 670–683. [Google Scholar] [CrossRef]
- Simonetti, F.R.; Sobolewski, M.D.; Fyne, E.; Shao, W.; Spindler, J.; Hattori, J.; Anderson, E.M.; Watters, S.A.; Hill, S.; Wu, X.; et al. Clonally expanded CD4+ T cells can produce infectious HIV-1 in vivo. Proc. Natl. Acad. Sci. USA 2016, 113, 1883–1888. [Google Scholar] [CrossRef]
- Reeves, D.B.; Duke, E.R.; Wagner, T.A.; Palmer, S.E.; Spivak, A.M.; Schiffer, J.T. A majority of HIV persistence during antiretroviral therapy is due to infected cell proliferation. Nat. Commun. 2018, 9, 4811. [Google Scholar] [CrossRef]
- Shan, L.; Deng, K.; Shroff, N.S.; Durand, C.M.; Rabi, S.A.; Yang, H.C.; Zhang, H.; Margolick, J.B.; Blankson, J.N.; Siliciano, R.F. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 2012, 36, 491–501. [Google Scholar] [CrossRef]
- Shan, L.; Deng, K.; Gao, H.; Xing, S.; Capoferri, A.A.; Durand, C.M.; Rabi, S.A.; Laird, G.M.; Kim, M.; Hosmane, N.N.; et al. Transcriptional Reprogramming during Effector-to-Memory Transition Renders CD4+ T Cells Permissive for Latent HIV-1 Infection. Immunity 2017, 47, 766–775. [Google Scholar] [CrossRef]
- Zauli, G.; Gibellini, D.; Caputo, A.; Bassini, A.; Negrini, M.; Monne, M.; Mazzoni, M.; Capitani, S. The human immunodeficiency virus type-1 Tat protein upregulates Bcl-2 gene expression in Jurkat T-cell lines and primary peripheral blood mononuclear cells. Blood 1995, 86, 3823–3834. [Google Scholar]
- Krone, W.J.; Debouck, C.; Epstein, L.G.; Heutink, P.; Meloen, R.; Goudsmit, J. Natural antibodies to HIV-tat epitopes and expression of HIV-1 genes in vivo. J. Med. Virol. 1988, 26, 261–270. [Google Scholar] [CrossRef]
- Reiss, P.; Lange, J.M.; de Ronde, A.; de Wolf, F.; Dekker, J.; Debouck, C.; Goudsmit, J. Speed of progression to AIDS and degree of antibody response to accessory gene products of HIV-1. J. Med. Virol. 1990, 30, 163–168. [Google Scholar] [CrossRef]
- Re, M.C.; Furlini, G.; Vignoli, M.; Ramazzotti, E.; Roderigo, G.; De Rosa, V.; Zauli, G.; Lolli, S.; Capitani, S.; La Placa, M. Effect of antibody to HIV-1 Tat protein on viral replication in vitro and progression of HIV-1 disease in vivo. J. Acquir. Immune. Defic. Syndr. Hum. Retrovirol. 1995, 10, 408–416. [Google Scholar] [CrossRef]
- Zagury, J.F.; Sill, A.; Blattner, W.; Lachgar, A.; Le Buanec, H.; Richardson, M.; Rappaport, J.; Hendel, H.; Bizzini, B.; Gringeri, A.; et al. Antibodies to the HIV-1 Tat protein correlated with nonprogression to AIDS: A rationale for the use of Tat toxoid as an HIV-1 vaccine. J. Hum. Virol. 1998, 1, 282–292. [Google Scholar]
- Re, M.C.; Vignoli, M.; Furlini, G.; Gibellini, D.; Colangeli, V.; Vitone, F.; La Placa, M. Antibodies against full-length Tat protein and some low-molecular-weight Tat-peptides correlate with low or undetectable viral load in HIV-1 seropositive patients. J. Clin. Virol. 2001, 21, 81–89. [Google Scholar] [CrossRef]
- Richardson, M.W.; Mirchandani, J.; Duong, J.; Grimaldo, S.; Kocieda, V.; Hendel, H.; Khalili, K.; Zagury, J.F.; Rappaport, J. Antibodies to Tat and Vpr in the GRIV cohort: Differential association with maintenance of long-term non-progression status in HIV-1 infection. Biomed. Pharmacother. 2003, 57, 4–14. [Google Scholar] [CrossRef]
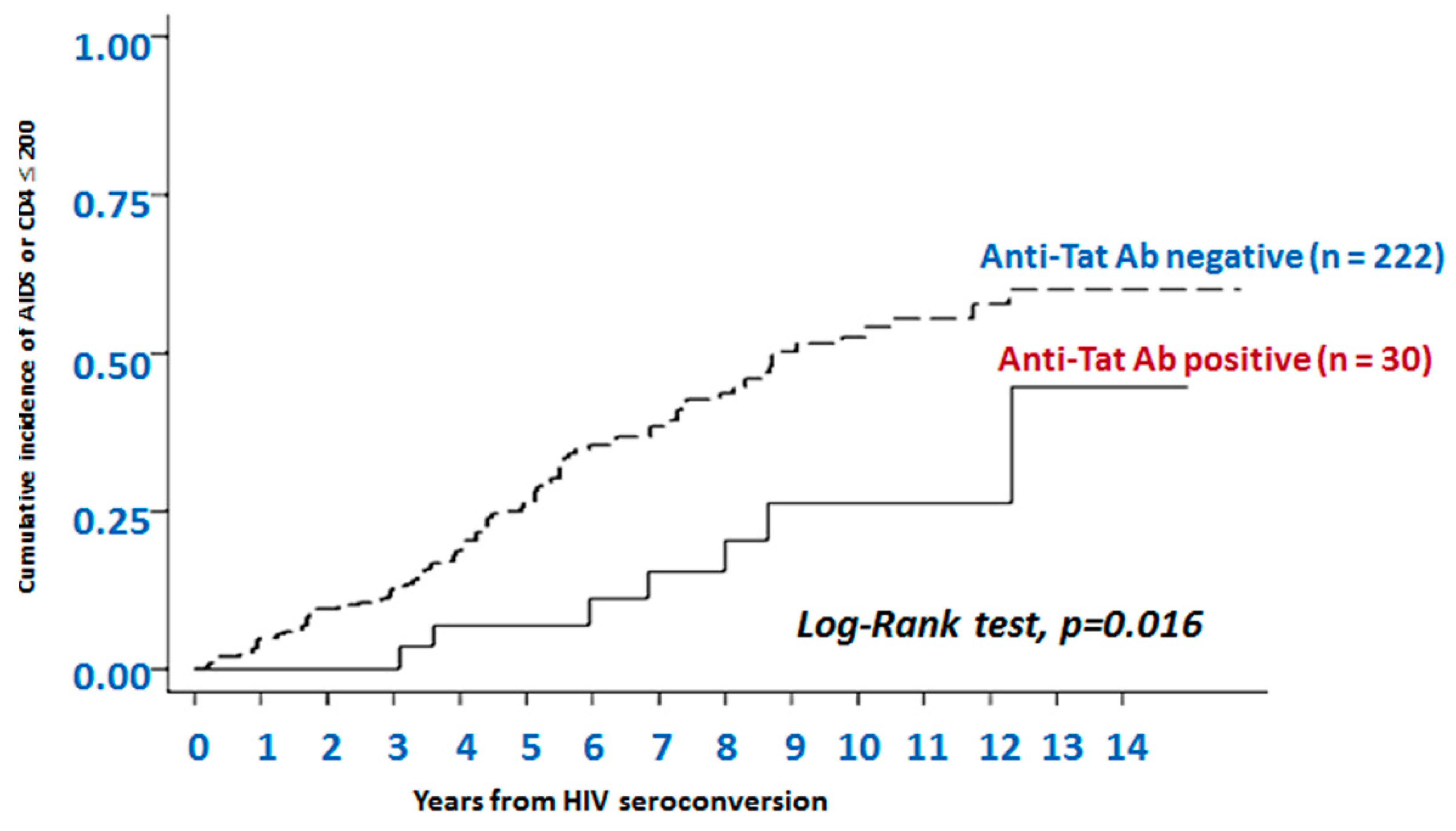
- Rezza, G.; Fiorelli, V.; Dorrucci, M.; Ciccozzi, M.; Tripiciano, A.; Scoglio, A.; Collacchi, B.; Ruiz-Alvarez, M.; Giannetto, C.; Caputo, A.; et al. The presence of anti-Tat antibodies is predictive of long-term nonprogression to AIDS or severe immunodeficiency: Findings in a cohort of HIV-1 seroconverters. J. Infect. Dis. 2005, 191, 1321–1324. [Google Scholar] [CrossRef]
- Bellino, S.; Tripiciano, A.; Picconi, O.; Francavilla, V.; Longo, O.; Sgadari, C.; Paniccia, G.; Arancio, A.; Angarano, G.; Ladisa, N.; et al. The presence of anti-Tat antibodies in HIV-infected individuals is associated with containment of CD4+ T-cell decay and viral load, and with delay of disease progression: Results of a 3-year cohort study. Retrovirology 2014, 11, 49. [Google Scholar] [CrossRef]
- Cafaro, A.; Tripiciano, A.; Sgadari, C.; Bellino, S.; Picconi, O.; Longo, O.; Francavilla, V.; Buttò, S.; Titti, F.; Monini, P.; et al. Development of a novel AIDS vaccine: The HIV-1 transactivator of transcription protein vaccine. Expert. Opin. Biol. Ther. 2015, 15 (Suppl. 1), S13–S29. [Google Scholar] [CrossRef]
- Buttò, S.; Fiorelli, V.; Tripiciano, A.; Ruiz-Alvarez, M.J.; Scoglio, A.; Ensoli, F.; Ciccozzi, M.; Collacchi, B.; Sabbatucci, M.; Tat Multicentric Study Group; et al. Sequence conservation and antibody cross-recognition of clade B human immunodeficiency virus (HIV) type 1 Tat protein in HIV-1-infected Italians, Ugandans, and South Africans. J. Infect. Dis. 2003, 188, 1171–1180. [Google Scholar]
- Pauza, C.D.; Trivedi, P.; Wallace, M.; Ruckwardt, T.J.; Le Buanec, H.; Lu, W.; Bizzini, B.; Burny, A.; Zagury, D.; Gallo, R.C. Vaccination with tat toxoid attenuates disease in simian/HIV-challenged macaques. Proc. Natl. Acad. Sci. USA 2000, 97, 3515–3519. [Google Scholar] [CrossRef]
- Silvera, P.; Richardson, M.W.; Greenhouse, J.; Yalley-Ogunro, J.; Shaw, N.; Mirchandani, J.; Khalili, K.; Zagury, J.F.; Lewis, M.G.; Rappaport, J. Outcome of simian-human immunodeficiency virus strain 89.6p challenge following vaccination of rhesus macaques with human immunodeficiency virus Tat protein. J. Virol. 2002, 76, 3800–3809. [Google Scholar] [CrossRef]
- Demberg, T.; Brocca-Cofano, E.; Kuate, S.; Aladi, S.; Vargas-Inchaustegui, D.A.; Venzon, D.; Kalisz, I.; Kalyanaraman, V.S.; Lee, E.M.; Pal, R.; et al. Impact of antibody quality and anamnestic response on viremia control post-challenge in a combined Tat/Env vaccine regimen in rhesus macaques. Virology 2013, 440, 210–221. [Google Scholar] [CrossRef] [Green Version]
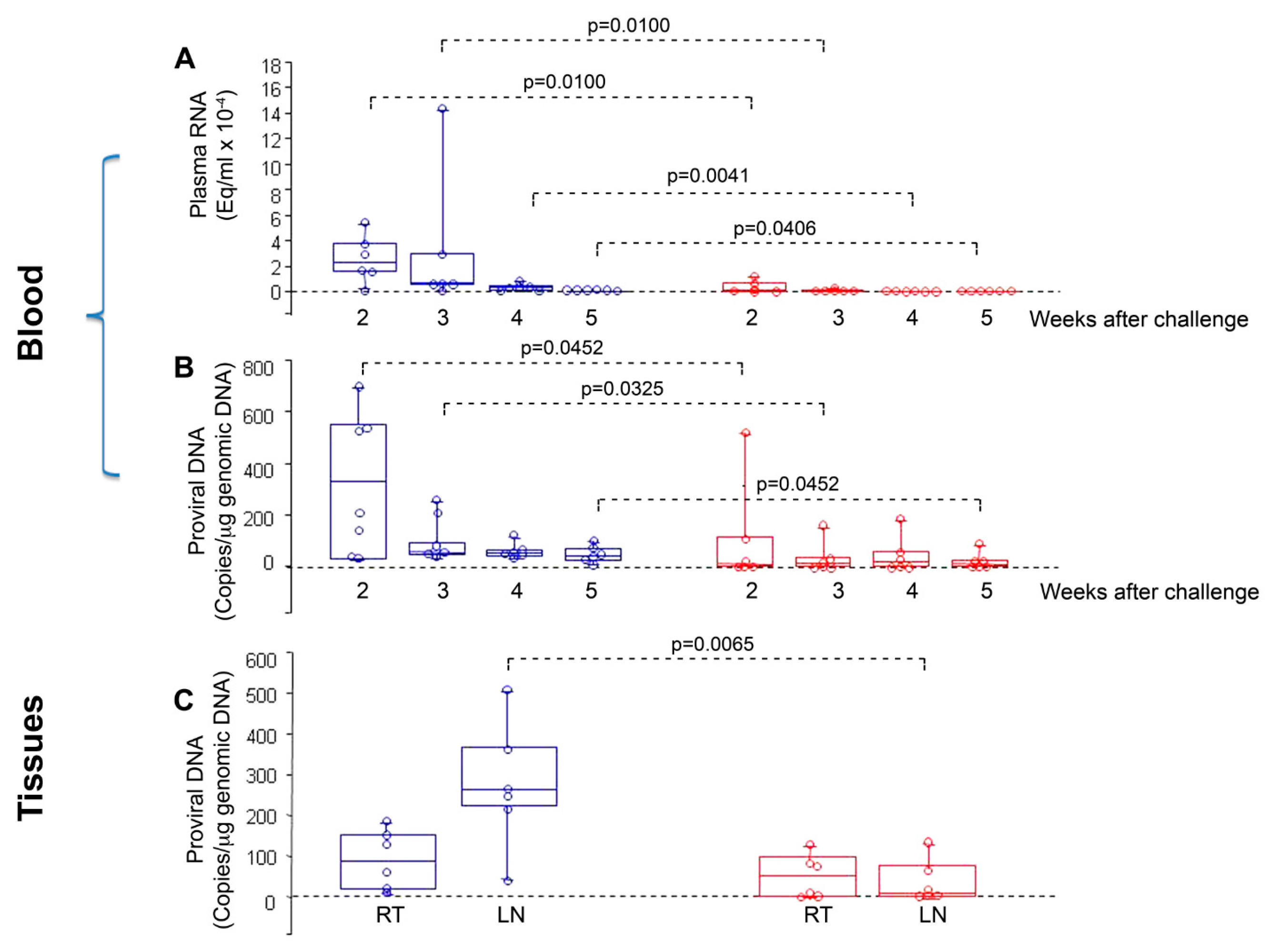
- Cafaro, A.; Caputo, A.; Fracasso, C.; Maggiorella, M.T.; Goletti, D.; Baroncelli, S.; Pace, M.; Sernicola, L.; Koanga-Mogtomo, M.L.; Betti, M.; et al. Control of SHIV-89.6P-infection of cynomolgus monkeys by HIV-1 Tat protein vaccine. Nat. Med. 1999, 5, 643–650. [Google Scholar] [CrossRef]
- Cafaro, A.; Caputo, A.; Maggiorella, M.T.; Baroncelli, S.; Fracasso, C.; Pace, M.; Borsetti, A.; Sernicola, L.; Negri, D.R.; Ten Haaft, P.; et al. SHIV89.6P pathogenicity in cynomolgus monkeys and control of viral replication and disease onset by human immunodeficiency virus type 1 Tat vaccine. J. Med. Primatol. 2000, 29, 193–208. [Google Scholar] [CrossRef]
- Cafaro, A.; Titti, F.; Fracasso, C.; Maggiorella, M.T.; Baroncelli, S.; Caputo, A.; Goletti, D.; Borsetti, A.; Pace, M.; Fanales-Belasio, E.; et al. Vaccination with DNA containing tat coding sequences and unmethylated CpG motifs protects cynomolgus monkeys upon infection with simian/human immunodeficiency virus (SHIV89.6P). Vaccine 2001, 19, 2862–2877. [Google Scholar] [CrossRef]
- Maggiorella, M.T.; Baroncelli, S.; Michelini, Z.; Fanales-Belasio, E.; Moretti, S.; Sernicola, L.; Cara, A.; Negri, D.R.; Buttò, S.; Fiorelli, V.; et al. Long-term protection against SHIV89.6P replication in HIV-1 Tat vaccinated cynomolgus monkeys. Vaccine 2004, 22, 3258–3269. [Google Scholar] [CrossRef]
- Borsetti, A.; Baroncelli, S.; Maggiorella, M.T.; Moretti, S.; Fanales-Belasio, E.; Sernicola, L.; Tripiciano, A.; Macchia, I.; Michelini, Z.; Belli, R.; et al. Containment of infection in tat vaccinated monkeys after rechallenge with a higher dose of SHIV89.6P(cy243). Viral. Immunol. 2009, 22, 117–124. [Google Scholar] [CrossRef]
- Cafaro, A.; Bellino, S.; Titti, F.; Maggiorella, M.T.; Sernicola, L.; Wiseman, R.W.; Venzon, D.; Karl, J.A.; O’Connor, D.; Monini, P.; et al. Impact of viral dose and major histocompatibility complex class IB haplotype on viral outcome in mauritian cynomolgus monkeys vaccinated with Tat upon challenge with simian/human immunodeficiency virus SHIV89.6P. J. Virol. 2010, 84, 8953–8958. [Google Scholar] [CrossRef]
- Titti, F.; Maggiorella, M.T.; Ferrantelli, F.; Sernicola, L.; Bellino, S.; Collacchi, B.; Fanales Belasio, E.; Moretti, S.; Pavone Cossut, M.R.; Belli, R.; et al. Biocompatible anionic polymeric microspheres as priming delivery system for effetive HIV/AIDS Tat-based vaccines. PLoS ONE 2014, 9, e111360. [Google Scholar] [CrossRef]
- Demberg, T.; Florese, R.H.; Heath, M.J.; Larsen, K.; Kalisz, I.; Kalyanaraman, V.S.; Lee, E.M.; Pal, R.; Venzon, D.; Grant, R.; et al. A replication-competent adenovirus-human immunodeficiency virus (Ad-HIV) tat and Ad-HIV env priming/Tat and envelope protein boosting regimen elicits enhanced protective efficacy against simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J. Virol. 2007, 81, 3414–3427. [Google Scholar]
- Bachler, B.C.; Humbert, M.; Palikuqi, B.; Siddappa, N.B.; Lakhashe, S.K.; Rasmussen, R.A.; Ruprecht, R.M. Novel biopanning strategy to identify epitopes associated with vaccine protection. J. Virol. 2013, 87, 4403–4416. [Google Scholar] [CrossRef]
- Ensoli, B.; Fiorelli, V.; Ensoli, F.; Lazzarin, A.; Visintini, R.; Narciso, P.; Di Carlo, A.; Tripiciano, A.; Longo, O.; Bellino, S.; et al. The preventive phase I trial with the HIV-1 Tat-based vaccine. Vaccine 2009, 28, 371–378. [Google Scholar] [CrossRef]
- Bellino, S.; Francavilla, V.; Longo, O.; Tripiciano, A.; Paniccia, G.; Arancio, A.; Fiorelli, V.; Scoglio, A.; Collacchi, B.; Campagna, M.; et al. Parallel conduction of the phase I preventive and therapeutic trials based on the Tat vaccine candidate. Rev. Recent Clin. Trials 2009, 4, 195–204. [Google Scholar] [CrossRef]
- Ensoli, B.; Fiorelli, V.; Ensoli, F.; Lazzarin, A.; Visintini, R.; Narciso, P.; Di Carlo, A.; Monini, P.; Magnani, M.; Garaci, E. The therapeutic phase I trial of the recombinant native HIV-1 Tat protein. AIDS 2008, 22, 2207–2209. [Google Scholar] [CrossRef]
- Bbosa, N.; Kaleebu, P.; Ssemwanga, D. HIV subtype diversity worldwide. Curr. Opin. HIV AIDS 2019, 14, 153–160. [Google Scholar] [CrossRef]
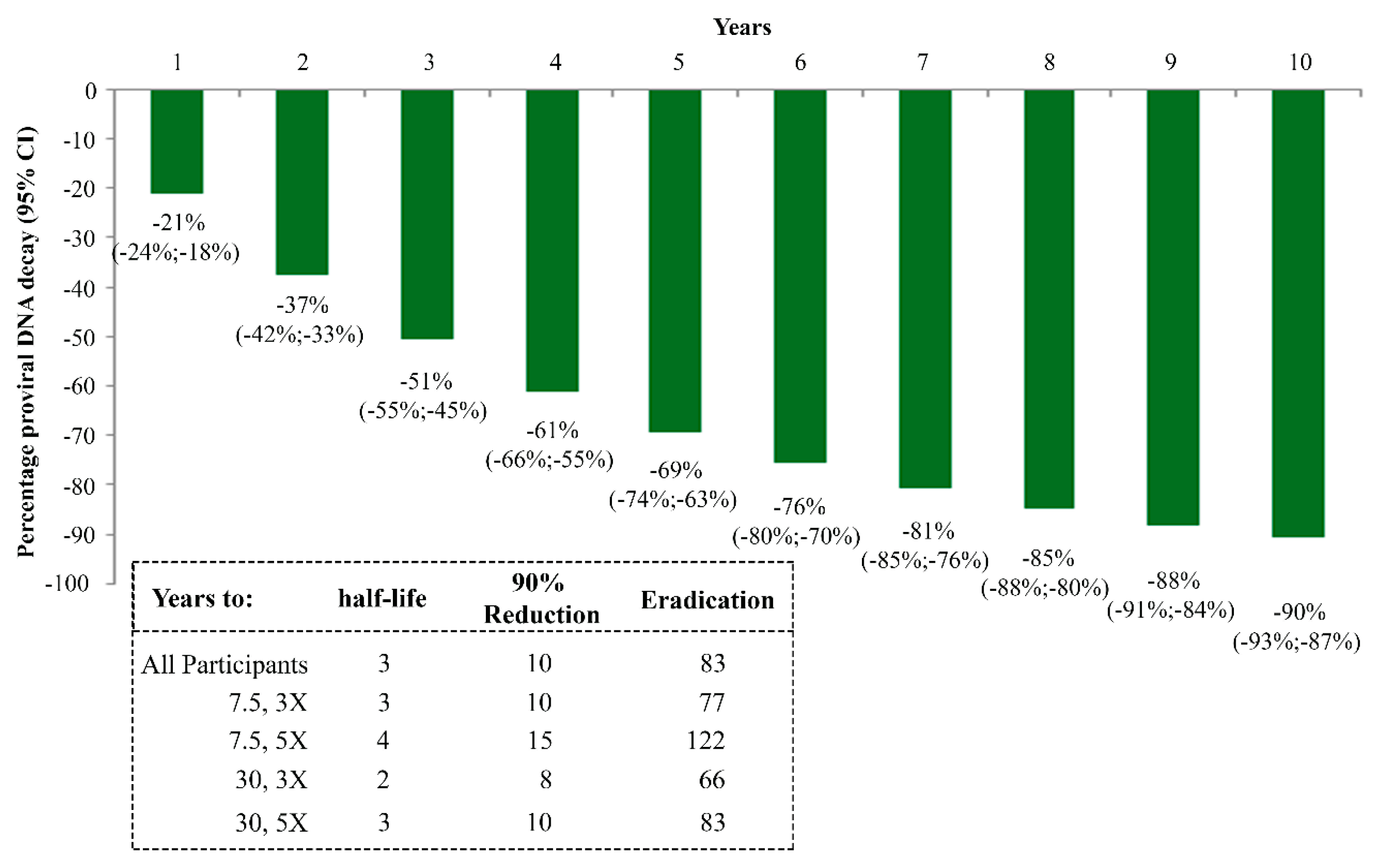
- Sgadari, C.; Monini, P.; Tripiciano, A.; Picconi, O.; Casabianca, A.; Orlandi, C.; Moretti, S.; Francavilla, V.; Arancio, A.; Paniccia, G.; et al. Continued Decay of HIV Proviral DNA Upon Vaccination With HIV-1 Tat of Subjects on Long-Term ART: An 8-Year Follow-Up Study. Front. Immunol. 2019, 10, 233. [Google Scholar] [CrossRef]
- Golob, J.L.; Stern, J.; Holte, S.; Kitahata, M.M.; Crane, H.M.; Coombs, R.W.; Goecker, E.; Woolfrey, A.E.; Harrington, R.D. HIV DNA levels and decay in a cohort of 111 long-term virally suppressed patients. AIDS 2018, 32, 2113–2118. [Google Scholar] [CrossRef]
- Jaafoura, S.; de Goër de Herve, M.G.; Hernandez-Vargas, E.A.; Hendel-Chavez, H.; Abdoh, M.; Mateo, M.C.; Krzysiek, R.; Merad, M.; Seng, R.; Tardieu, M.; et al. Progressive contraction of the latent HIV reservoir around a core of less-differentiated CD4⁺ memory T Cells. Nat. Commun. 2014, 5, 5407. [Google Scholar] [CrossRef]
- Ensoli, B.; Nchabeleng, M.; Ensoli, F.; Tripiciano, A.; Bellino, S.; Picconi, O.; Sgadari, C.; Longo, O.; Tavoschi, L.; Joffe, D.; et al. SMU-MeCRU study group. HIV-Tat immunization induces cross-clade neutralizing antibodies and CD4(+) T cell increases in antiretroviral-treated South African volunteers: A randomized phase II clinical trial. Retrovirology 2016, 13, 34. [Google Scholar] [CrossRef]
- Burgers, W.A.; Manrique, A.; Masopust, D.; McKinnon, L.R.; Reynolds, M.R.; Rolland, M.; Blish, C.; Chege, G.K.; Curran, R.; Fischer, W.; et al. Measurements of immune responses for establishing correlates of vaccine protection against HIV. AIDS Res. Hum. Retrovir. 2012, 28, 641–648. [Google Scholar] [CrossRef]
- Albert-Vega, C.; Tawfik, D.M.; Trouillet-Assant, S.; Vachot, L.; Mallet, F.; Textoris, J. Immune Functional Assays, From Custom to Standardized Tests for Precision Medicine. Front. Immunol. 2018, 9, 2367. [Google Scholar] [CrossRef] [Green Version]
- Goonetilleke, N.; Liu, M.K.; Salazar-Gonzalez, J.F.; Ferrari, G.; Giorgi, E.; Ganusov, V.V.; Keele, B.F.; Learn, G.H.; Turnbull, E.L.; Salazar, M.G.; et al. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J. Exp. Med. 2009, 206, 1253–1272. [Google Scholar] [CrossRef]
- Addo, M.M.; Altfeld, M.; Rosenberg, E.S.; Eldridge, R.L.; Philips, M.N.; Habeeb, K.; Khatri, A.; Brander, C.; Robbins, G.K.; Mazzara, G.P.; et al. HIV Controller Study Collaboration. The HIV-1 regulatory proteins Tat and Rev are frequently targeted by cytotoxic T lymphocytes derived from HIV-1-infected individuals. Proc. Natl. Acad. Sci. USA 2001, 98, 1781–1786. [Google Scholar] [CrossRef]
- Van Baalen, C.A.; Pontesilli, O.; Huisman, R.C.; Geretti, A.M.; Klein, M.R.; de Wolf, F.; Miedema, F.; Gruters, R.A.; Osterhaus, A.D. Human immunodeficiency virus type 1 Rev- and Tat-specific cytotoxic T lymphocyte frequencies inversely correlate with rapid progression to AIDS. J. Gen. Virol. 1997, 78, 1913–1918. [Google Scholar] [CrossRef]
- Cao, J.; McNevin, J.; Malhotra, U.; McElrath, M.J. Evolution of CD8+ T cell immunity and viral escape following acute HIV-1 infection. J. Immunol. 2003, 171, 3837–3846. [Google Scholar] [CrossRef]
- Allen, T.M.; O’Connor, D.H.; Jing, P.; Dzuris, J.L.; Mothé, B.R.; Vogel, T.U.; Dunphy, E.; Liebl, M.E.; Emerson, C.; Wilson, N.; et al. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 2000, 407, 386–390. [Google Scholar] [CrossRef]
- O’Connor, D.H.; Allen, T.M.; Vogel, T.U.; Jing, P.; DeSouza, I.P.; Dodds, E.; Dunphy, E.J.; Melsaether, C.; Mothé, B.; Yamamoto, H.; et al. Acute phase cytotoxic T lymphocyte escape is a hallmark of simian immunodeficiency virus infection. Nat. Med. 2002, 8, 493–499. [Google Scholar] [CrossRef]
- Guillon, C.; Stankovic, K.; Ataman-Onal, Y.; Biron, F.; Verrier, B. Evidence for CTL-mediated selection of Tat and Rev mutants after the onset of the asymptomatic period during HIV type 1 infection. AIDS Res. Hum. Retroviruses 2006, 22, 1283–1292. [Google Scholar] [CrossRef]
- Allard, S.D.; De Keersmaecker, B.; de Goede, A.L.; Verschuren, E.J.; Koetsveld, J.; Reedijk, M.L.; Wylock, C.; De Bel, A.V.; Vandeloo, J.; Pistoor, F.; et al. A phase I/IIa immunotherapy trial of HIV-1-infected patients with Tat, Rev and Nef expressing dendritic cells followed by treatment interruption. Clin. Immunol. 2012, 142, 252–268. [Google Scholar] [CrossRef]
- Goldstein, G.; Damiano, E.; Donikyan, M.; Pasha, M.; Beckwith, E.; Chicca, J. HIV-1 Tat B-cell epitope vaccination was ineffectual in preventing viral rebound after ART cessation: HIV rebound with current ART appears to be due to infection with new endogenous founder virus and not to resurgence of pre-existing Tat-dependent viremia. Hum. Vaccines. Immunother. 2012, 8, 1425–1430. [Google Scholar] [CrossRef]
- Novitsky, V.; Rybak, N.; McLane, M.F.; Gilbert, P.; Chigwedere, P.; Klein, I.; Gaolekwe, S.; Chang, S.Y.; Peter, T.; Thior, I.; et al. Identification of human immunodeficiency virus type 1 subtype C Gag-, Tat-, Rev-, and Nef-specific elispot-based cytotoxic T-lymphocyte responses for AIDS vaccine design. J. Virol. 2001, 75, 9210–9228. [Google Scholar] [CrossRef]
- Kamori, D.; Ueno, T. HIV-1 Tat and Viral Latency: What We Can Learn from Naturally Occurring Sequence Variations. Front. Microbiol. 2017, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Dey, S.S.; Xue, Y.; Joachimiak, M.P.; Friedland, G.D.; Burnett, J.C.; Zhou, Q.; Arkin, A.P.; Schaffer, D.V. Mutual information analysis reveals coevolving residues in Tat that compensate for two distinct functions in HIV-1 gene expression. J. Biol. Chem. 2012, 287, 7945–7955. [Google Scholar] [CrossRef]
- Tahirov, T.H.; Babayeva, N.D.; Varzavand, K.; Cooper, J.J.; Sedore, S.C.; Price, D.H. Crystal structure of HIV-1 Tat complexed with human P-TEFb. Nature 2010, 465, 747–751. [Google Scholar] [CrossRef]
- D’Orso, I.; Frankel, A.D. HIV-1 Tat: Its Dependence on Host Factors is Crystal Clear. Viruses 2010, 2, 2226–2234. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.; Neuveut, C.; Tiffany, H.L.; Benkirane, M.; Rich, E.A.; Murphy, P.M.; Jeang, K.T. Selective CXCR4 antagonism by Tat: Implications for in vivo expansion of coreceptor use by HIV-1. Proc. Natl. Acad. Sci. USA 2000, 97, 11466–11471. [Google Scholar] [CrossRef]
- Hoogewerf, A.J.; Kuschert, G.S.; Proudfoot, A.E.; Borlat, F.; Clark-Lewis, I.; Power, C.A.; Wells, T.N. Glycosaminoglycans mediate cell surface oligomerization of chemokines. Biochemistry 1997, 36, 13570–13578. [Google Scholar] [CrossRef]
- Cafaro, A.; Sgadari, C.; Picconi, O.; Tripiciano, A.; Moretti, S.; Francavilla, V.; Pavone Cossut, M.R.; Buttò, S.; Cozzone, G.; Ensoli, F.; et al. cART intensification by the HIV-1 Tat B clade vaccine: Progress to phase III efficacy studies. Expert Rev. Vaccines 2018, 17, 115–126. [Google Scholar] [CrossRef]
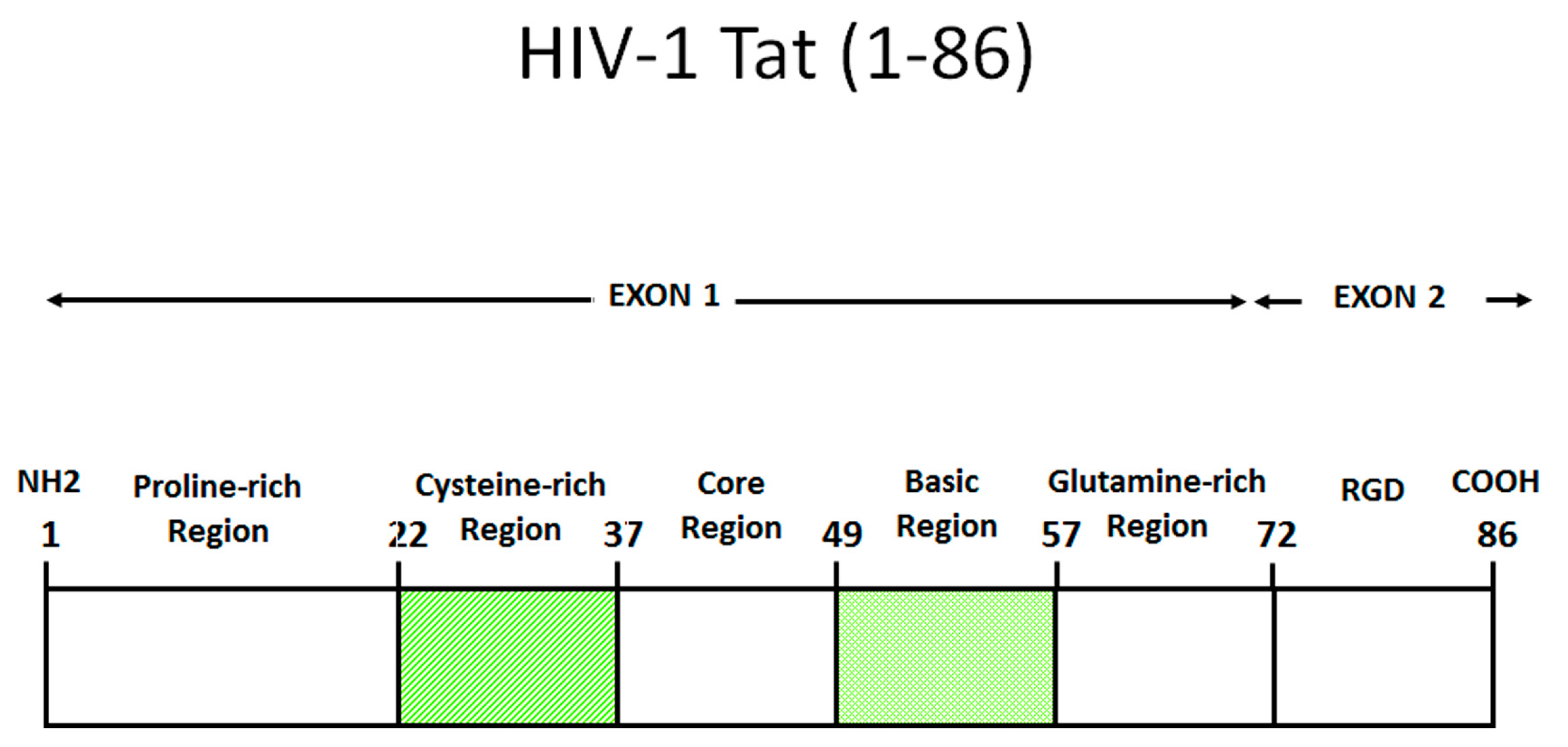
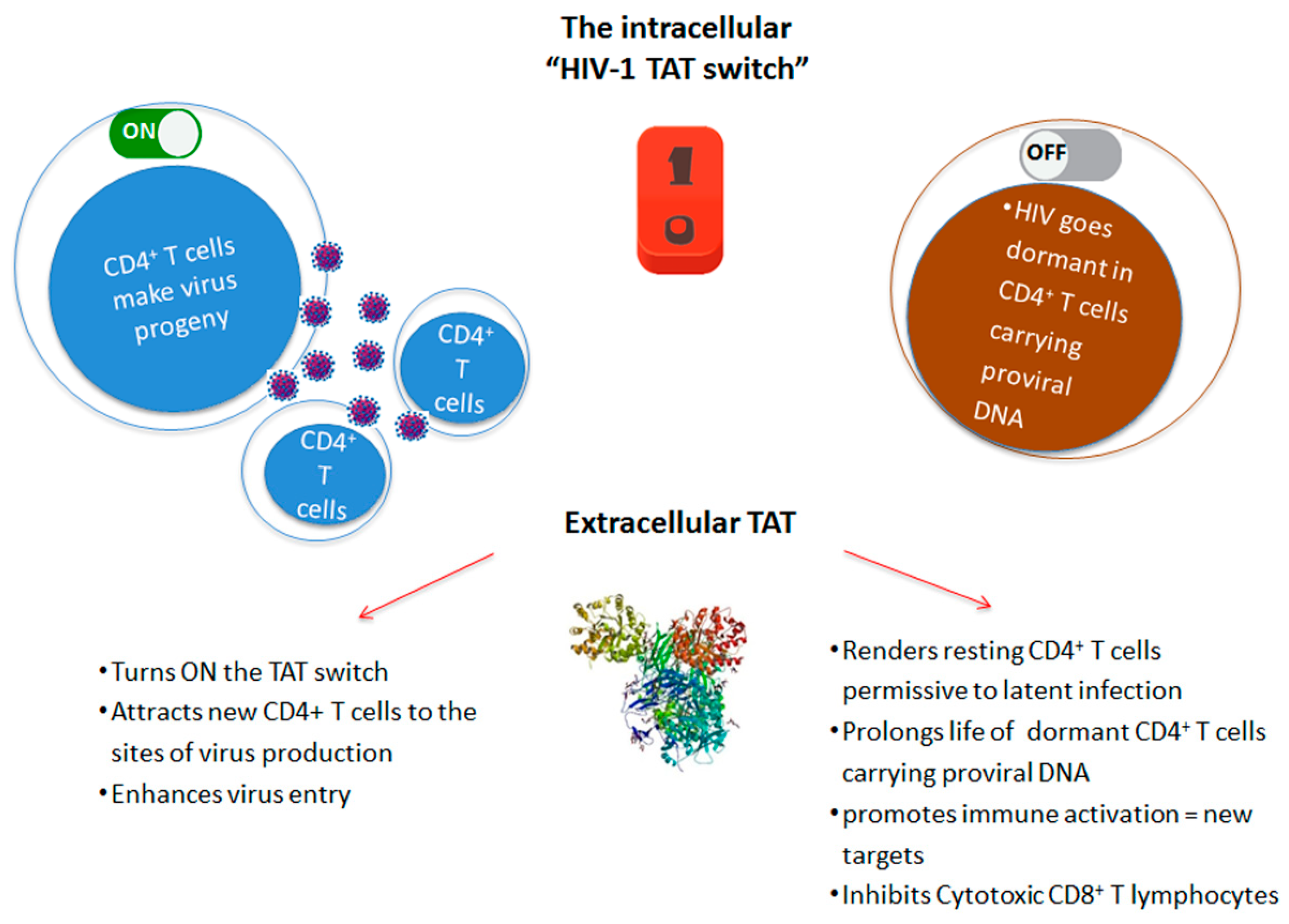
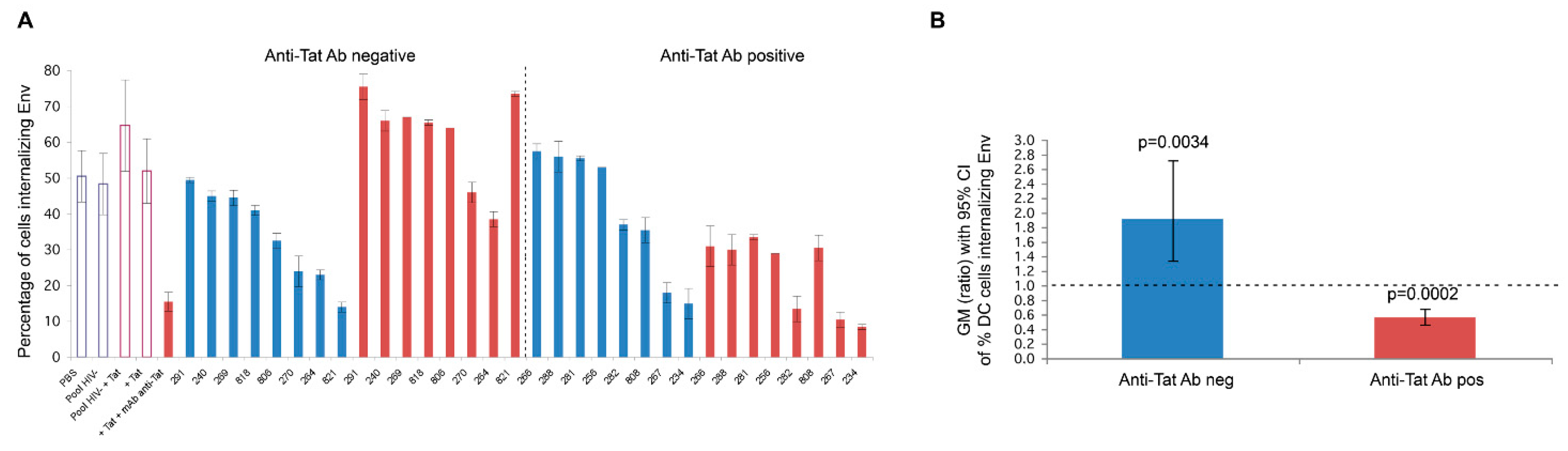
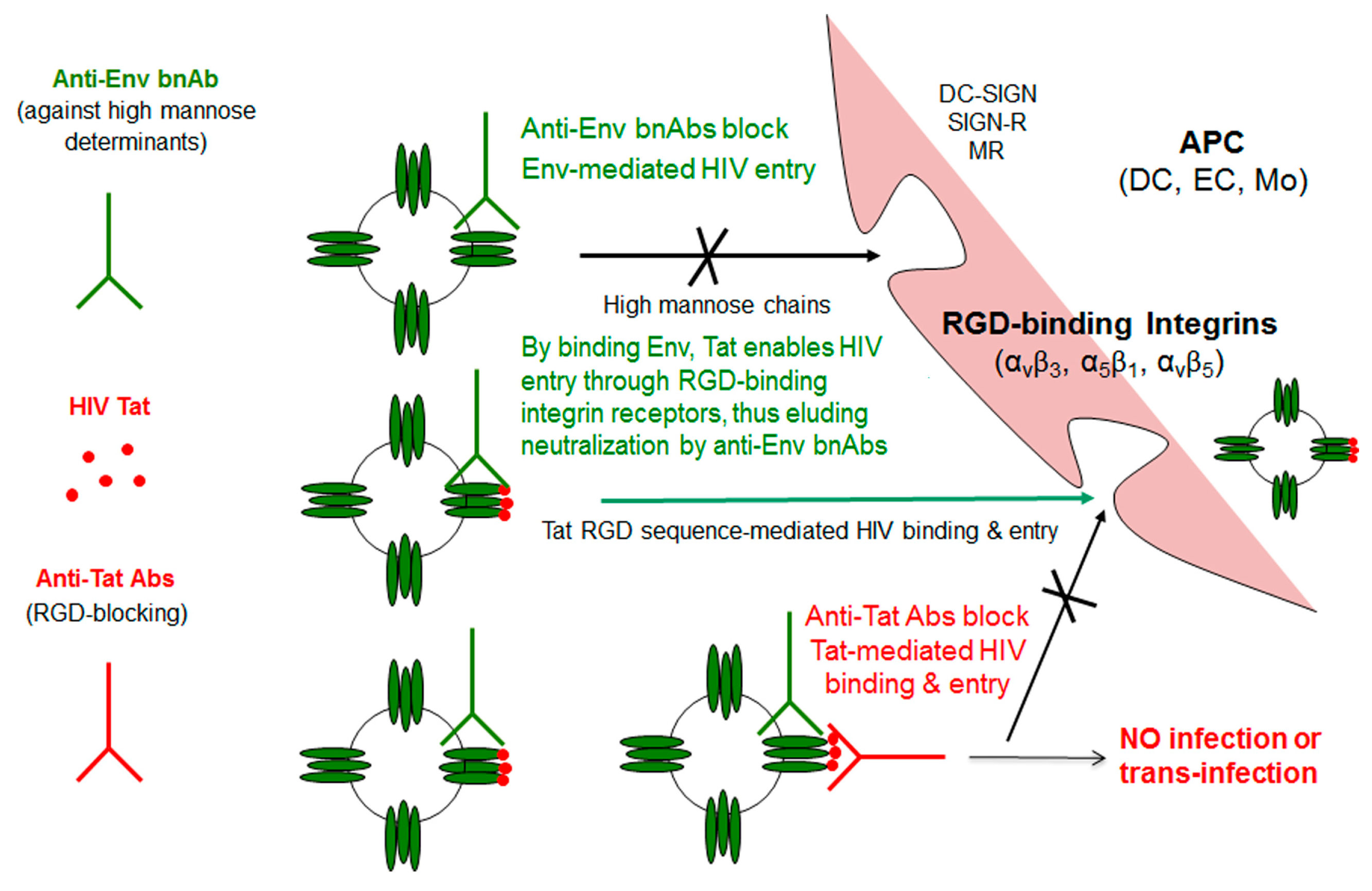
| Reference | |
|---|---|
| It is detected in cell-free virion | [18] |
| It is detected in infected cells prior to virus integration | [19] |
| It is released extracellularly in the absence of cell death or cell permeability changes | [20,21] |
| Initiates reverse transcription (RT) | [22] |
| Increases the rate of transcription | [23] |
| Promotes transcription elongation | [24] |
| Contributes in splicing regulation | [25] |
| Enhances virion infectivity | [26,27] |
| Amplifies stochastic basal transcriptional fluctuations at the HIV LTR promoter (the Tat circuitry), establishing active or latent infection, or the reactivation of latent HIV | [13,14,15,16,17] |
| Study Code | Volunteers Number | Status | Results | Potential Clinical Benefit |
|---|---|---|---|---|
| ISS OBS T-003 [117] | 73 | naïve to cART |
| Prevention of progression |
| OBS-IFO [118] | 29 | starting cART |
| Improved time-to-response to therapy |
| ISS OBS T-002 | 127 | on cART |
| Therapy intensification |
| Reference Study | [136] | [137] | [138] |
|---|---|---|---|
| HIV reservoir measure | Total HIV DNA | Total HIV DNA | Integrated DNA |
| cART duration before enrolment | 6 years (mean) | ≥ 5 years | ≥2 years * |
| Vaccination | Tat vaccine | None | None |
| cART continuation through the study | Yes | Yes | Yes |
| Proviral DNA decay: estimated half-life | |||
| All patients | 2–3 years | 12 years | NA |
| Persistent virological suppression (VL = 0) | 1 year | 7 years | >7 years |
| Residual viremia (≥1≤40 RNA copies/mL) | 3 years | 12 years | NA |
| Viremic blips (≥40 RNA copies/mL) | 4 years | 22 years | NA |
| Proviral DNA decay: estimated time to eradication | Total body reservoir | Total body reservoir | Total blood reservoir |
| Persistent virological suppression (VL = 0) | 31 years | NA | >200 years |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cafaro, A.; Tripiciano, A.; Picconi, O.; Sgadari, C.; Moretti, S.; Buttò, S.; Monini, P.; Ensoli, B. Anti-Tat Immunity in HIV-1 Infection: Effects of Naturally Occurring and Vaccine-Induced Antibodies Against Tat on the Course of the Disease. Vaccines 2019, 7, 99. https://doi.org/10.3390/vaccines7030099
Cafaro A, Tripiciano A, Picconi O, Sgadari C, Moretti S, Buttò S, Monini P, Ensoli B. Anti-Tat Immunity in HIV-1 Infection: Effects of Naturally Occurring and Vaccine-Induced Antibodies Against Tat on the Course of the Disease. Vaccines. 2019; 7(3):99. https://doi.org/10.3390/vaccines7030099
Chicago/Turabian StyleCafaro, Aurelio, Antonella Tripiciano, Orietta Picconi, Cecilia Sgadari, Sonia Moretti, Stefano Buttò, Paolo Monini, and Barbara Ensoli. 2019. "Anti-Tat Immunity in HIV-1 Infection: Effects of Naturally Occurring and Vaccine-Induced Antibodies Against Tat on the Course of the Disease" Vaccines 7, no. 3: 99. https://doi.org/10.3390/vaccines7030099
APA StyleCafaro, A., Tripiciano, A., Picconi, O., Sgadari, C., Moretti, S., Buttò, S., Monini, P., & Ensoli, B. (2019). Anti-Tat Immunity in HIV-1 Infection: Effects of Naturally Occurring and Vaccine-Induced Antibodies Against Tat on the Course of the Disease. Vaccines, 7(3), 99. https://doi.org/10.3390/vaccines7030099







