Utility of the Neonatal Calf Model for Testing Vaccines and Intervention Strategies for Use against Human RSV Infection
Abstract
:1. Introduction
2. Age and Seasonal Affects
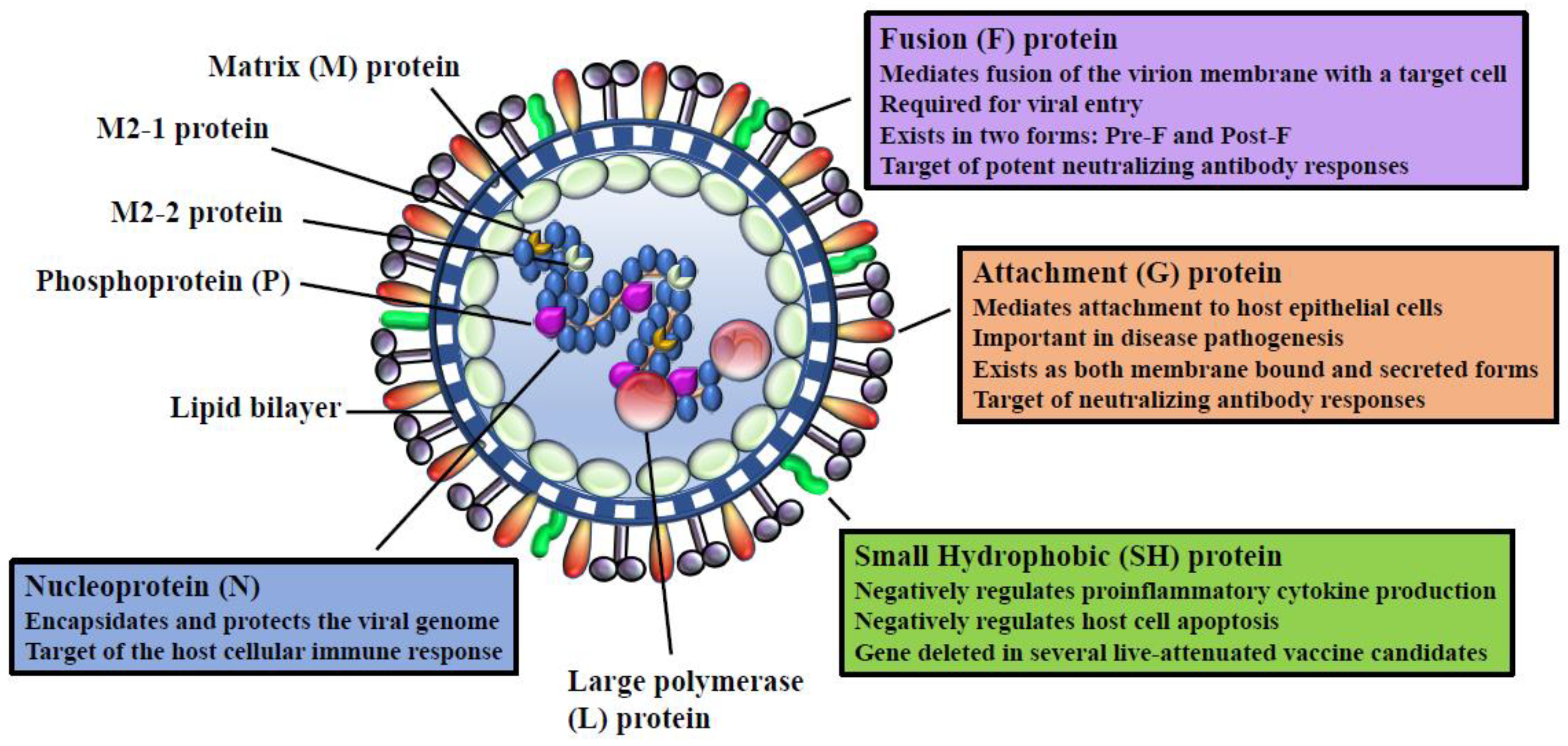
3. RSV Viral Proteins
4. RSV Life Cycle
5. Experimental bRSV Infection in Calves
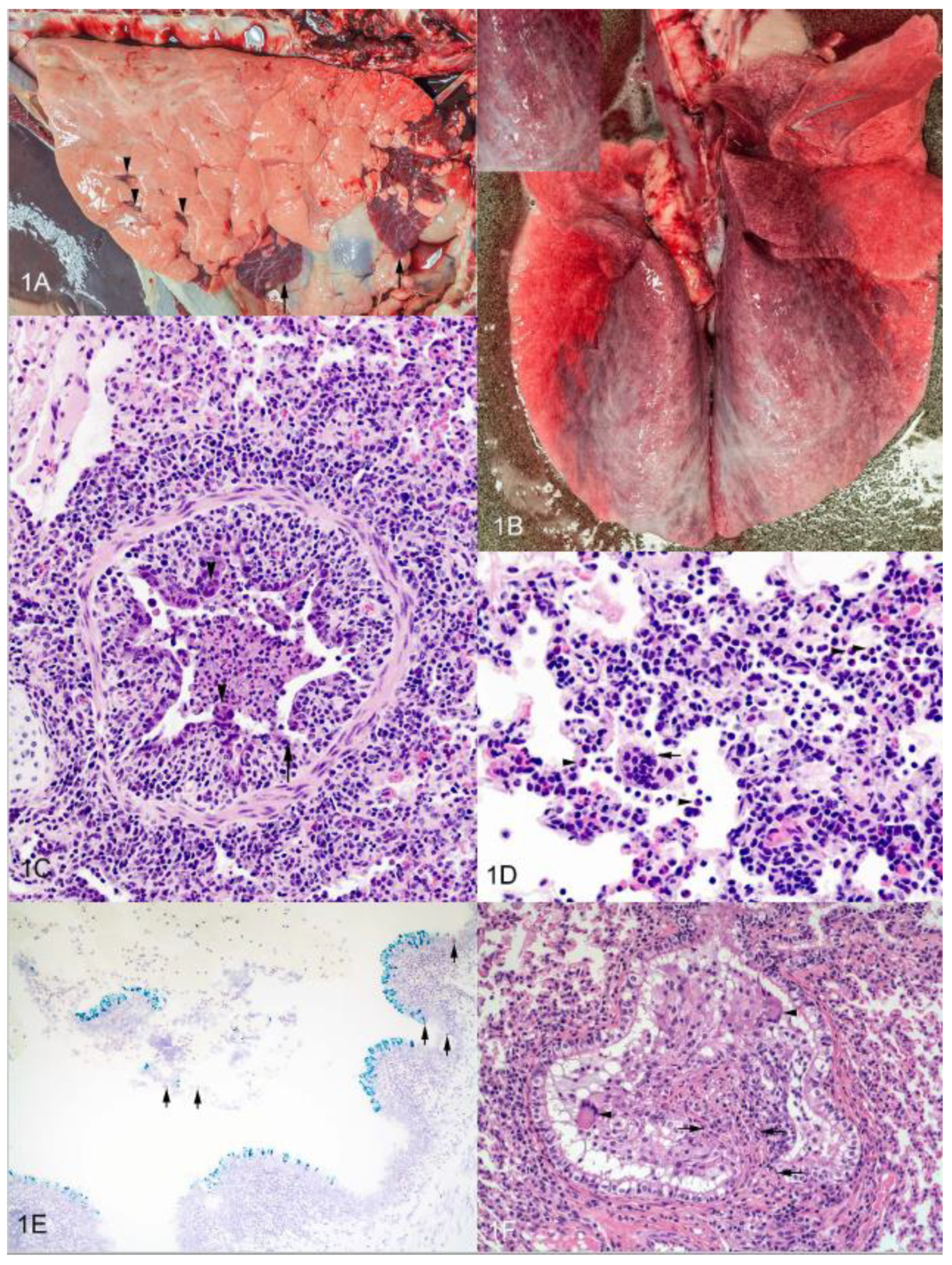
6. Histopathology of Experimental bRSV Infection and Similarities to Lesions in Human Infants
7. Innate Immune Response to bRSV and hRSV
8. Adaptive Immune Response
8.1. Humoral Immunity to hRSV and bRSV Infection
8.2. Cellular Immunity to hRSV and bRSV Infection
9. Genetic Influence in the Host’s Immune Response to bRSV
10. hRSV and bRSV Vaccine Development
10.1. General Considerations for RSV Vaccine Development
10.2. Vector-Based Vaccines
10.3. Live Attenuated Vaccines
10.4. Subunit and Nanoparticle-Based Vaccines
10.5. The Importance of Immunization Route
11. Utility of the Calf Model for Testing Antiviral and Therapeutic Compounds
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Nair, H.; Nokes, D.J.; Gessner, B.D.; Dherani, M.; Madhi, S.A.; Singleton, R.J.; O’Brien, K.L.; Roca, A.; Wright, P.F.; Bruce, N.; et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef]
- Glezen, W.P.; Taber, L.H.; Frank, A.L.; Kasel, J.A. Risk of primary infection and reinfection with respiratory syncytial virus. Am. J. Dis. Child 1986, 140, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R. Respiratory syncytial virus infection in elderly and high-risk adults. Exp. Lung Res. 2005, 31, 77. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.K.; Pendell, D.L. Market Impacts of Reducing the Prevalence of Bovine Respiratory Disease in United States Beef Cattle Feedlots. Front. Vet. Sci. 2017, 4, 189. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R. Respiratory syncytial virus infection in adults. Semin. Respir. Crit. Care Med. 2007, 28, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Valarcher, J.F.; Schelcher, F.; Bourhy, H. Evolution of bovine respiratory syncytial virus. J. Virol. 2000, 74, 10714–10728. [Google Scholar] [CrossRef] [PubMed]
- Van der Poel, W.H.; Brand, A.; Kramps, J.A.; Van Oirschot, J.T. Respiratory syncytial virus infections in human beings and in cattle. J. Infect. 1994, 29, 215–228. [Google Scholar] [CrossRef]
- Schmidt, M.E.; Varga, S.M. Modulation of the host immune response by respiratory syncytial virus proteins. J. Microbiol. 2017, 55, 161–171. [Google Scholar] [CrossRef]
- Anderson, L.J.; Hierholzer, J.C.; Tsou, C.; Hendry, R.M.; Fernie, B.F.; Stone, Y.; McIntosh, K. Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J. Infect. Dis. 1985, 151, 626–633. [Google Scholar] [CrossRef]
- Cristina, J.; Lopez, J.A.; Albo, C.; Garcia-Barreno, B.; Garcia, J.; Melero, J.A.; Portela, A. Analysis of genetic variability in human respiratory syncytial virus by the RNase A mismatch cleavage method: Subtype divergence and heterogeneity. Virology 1990, 174, 126–134. [Google Scholar] [CrossRef]
- Furze, J.M.; Roberts, S.R.; Wertz, G.W.; Taylor, G. Antigenically distinct G glycoproteins of BRSV strains share a high degree of genetic homogeneity. Virology 1997, 231, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Deplanche, M.; Lemaire, M.; Mirandette, C.; Bonnet, M.; Schelcher, F.; Meyer, G. In vivo evidence for quasispecies distributions in the bovine respiratory syncytial virus genome. J. Gen. Virol. 2007, 88, 1260–1265. [Google Scholar] [CrossRef] [Green Version]
- McLellan, J.S.; Yang, Y.; Graham, B.S.; Kwong, P.D. Structure of respiratory syncytial virus fusion glycoprotein in the postfusion conformation reveals preservation of neutralizing epitopes. J. Virol. 2011, 85, 7788–7796. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.R.; Spriggs, M.K.; Olmsted, R.A.; Collins, P.L. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: Extensive sequence divergence between antigenically related proteins. Proc. Natl. Acad. Sci. USA 1987, 84, 5625–5629. [Google Scholar] [CrossRef] [PubMed]
- Garcia, O.; Martin, M.; Dopazo, J.; Arbiza, J.; Frabasile, S.; Russi, J.; Hortal, M.; Perez-Brena, P.; Martinez, I.; Garcia-Barreno, B.; et al. Evolutionary pattern of human respiratory syncytial virus (subgroup A): Cocirculating lineages and correlation of genetic and antigenic changes in the G glycoprotein. J. Virol. 1994, 68, 5448–5459. [Google Scholar]
- Woelk, C.H.; Holmes, E.C. Variable immune-driven natural selection in the attachment (G) glycoprotein of respiratory syncytial virus (RSV). J. Mol. Evol. 2001, 52, 182–192. [Google Scholar] [CrossRef]
- Prozzi, D.; Walravens, K.; Langedijk, J.P.; Daus, F.; Kramps, J.A.; Letesson, J.J. Antigenic and molecular analyses of the variability of bovine respiratory syncytial virus G glycoprotein. J. Gen. Virol. 1997, 78, 359–366. [Google Scholar] [CrossRef]
- Lukens, M.V.; Claassen, E.A.; de Graaff, P.M.; van Dijk, M.E.; Hoogerhout, P.; Toebes, M.; Schumacher, T.N.; van der Most, R.G.; Kimpen, J.L.; van Bleek, G.M. Characterization of the CD8+ T cell responses directed against respiratory syncytial virus during primary and secondary infection in C57BL/6 mice. Virology 2006, 352, 157–168. [Google Scholar] [CrossRef] [Green Version]
- Meyer, G.; Deplanche, M.; Schelcher, F. Human and bovine respiratory syncytial virus vaccine research and development. Comp. Immunol. Microbiol. Infect. Dis. 2008, 31, 191–225. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, C.; Drews, S.J.; Marchant, D.J. Respiratory Syncytial Virus: Infection, Detection, and New Options for Prevention and Treatment. Clin. Microbiol. Rev. 2017, 30, 277–319. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.L.; Graham, B.S. Viral and host factors in human respiratory syncytial virus pathogenesis. J. Virol. 2008, 82, 2040–2055. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B.; Simőes, E.A.; Anderson, L.J. Clinical and epidemiologic features of respiratory syncytial virus. Curr. Top. Microbiol. Immunol. 2013, 372, 39–57. [Google Scholar] [PubMed]
- Sacco, R.E.; McGill, J.L.; Pillatzki, A.E.; Palmer, M.V.; Ackermann, M.R. Respiratory syncytial virus infection in cattle. Vet. Pathol. 2014, 51, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Antonis, A.F.; Schrijver, R.S.; Daus, F.; Steverink, P.J.; Stockhofe, N.; Hensen, E.J.; Langedijk, J.P.; van der Most, R.G. Vaccine-induced immunopathology during bovine respiratory syncytial virus infection: Exploring the parameters of pathogenesis. J. Virol. 2003, 77, 12067–12073. [Google Scholar] [CrossRef] [PubMed]
- Antonis, A.F.; de Jong, M.C.; van der Poel, W.H.; van der Most, R.G.; Stockhofe-Zurwieden, N.; Kimman, T.; Schrijver, R.S. Age-dependent differences in the pathogenesis of bovine respiratory syncytial virus infections related to the development of natural immunocompetence. J. Gen. Virol. 2010, 91, 2497–2506. [Google Scholar] [CrossRef] [Green Version]
- Gershwin, L.J.; Schelegle, E.S.; Gunther, R.A.; Anderson, M.L.; Woolums, A.R.; Larochelle, D.R.; Boyle, G.A.; Friebertshauser, K.E.; Singer, R.S. A bovine model of vaccine enhanced respiratory syncytial virus pathophysiology. Vaccine 1998, 16, 1225–1236. [Google Scholar] [CrossRef]
- Welliver, T.P.; Garofalo, R.P.; Hosakote, Y.; Hintz, K.H.; Avendano, L.; Sanchez, K.; Velozo, L.; Jafri, H.; Chavez-Bueno, S.; Ogra, P.L.; et al. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J. Infect. Dis. 2007, 195, 1126–1136. [Google Scholar] [CrossRef]
- Welliver, T.P.; Reed, J.L.; Welliver, R.C., Sr. Respiratory syncytial virus and influenza virus infections: Observations from tissues of fatal infant cases. Pediatr. Infect. Dis. J. 2008, 27, S92–S96. [Google Scholar] [CrossRef]
- Mcinnes, E.; Sopp, P.; Howard, C.J.; Taylor, G. Phenotypic analysis of local cellular responses in calves infected with bovine respiratory syncytial virus. Immunology 1999, 96, 396–403. [Google Scholar] [CrossRef] [Green Version]
- Røntved, C.M.; Tjørnehøj, K.; Viuff, B.; Larsen, L.E.; Godson, D.L.; Rønsholt, L.; Alexandersen, S. Increased pulmonary secretion of tumor necrosis factor-alpha in calves experimentally infected with bovine respiratory syncytial virus. Vet. Immunol. Immunopathol. 2000, 76, 199–214. [Google Scholar] [CrossRef]
- Sacco, R.E.; Nonnecke, B.J.; Palmer, M.V.; Waters, W.R.; Lippolis, J.D.; Reinhardt, T.A. Differential expression of cytokines in response to respiratory syncytial virus infection of calves with high or low circulating 25-hydroxyvitamin D3. PLoS ONE 2012, 7, e33074. [Google Scholar] [CrossRef] [PubMed]
- Heidema, J.; Lukens, M.V.; van Maren, W.W.; van Dijk, M.E.; Otten, H.G.; van Vught, A.J.; van der Werff, D.B.; van Gestel, S.J.; Semple, M.G.; Smyth, R.L.; et al. CD8+ T cell responses in bronchoalveolar lavage fluid and peripheral blood mononuclear cells of infants with severe primary respiratory syncytial virus infections. J. Immunol. 2007, 179, 8410–8417. [Google Scholar] [CrossRef] [PubMed]
- McNamara, P.S.; Ritson, P.; Selby, A.; Hart, C.A.; Smyth, R.L. Bronchoalveolar lavage cellularity in infants with severe respiratory syncytial virus bronchiolitis. Arch. Dis. Child 2003, 88, 922–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jozwik, A.; Habibi, M.S.; Paras, A.; Zhu, J.; Guvenel, A.; Dhariwal, J.; Almond, M.; Wong, E.H.; Sykes, A.; Maybeno, M.; et al. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat. Commun. 2015, 6, 10224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, C.D.; Unger, S.A.; Walton, M.; Schwarze, J. The Human Immune Response to Respiratory Syncytial Virus Infection. Clin. Microbiol. Rev. 2017, 30, 481–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNamara, P.S.; Flanagan, B.F.; Selby, A.M.; Hart, C.A.; Smyth, R.L. Pro- and anti-inflammatory responses in respiratory syncytial virus bronchiolitis. Eur. Respir. J. 2004, 23, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Taylor, G.; Thomas, L.H.; Stott, E.J. Effect of vaccination on cell populations in lung washes from calves after infection with respiratory syncytial virus. Res. Vet. Sci. 1989, 47, 231–235. [Google Scholar] [CrossRef]
- Blodörn, K.; Hägglund, S.; Gavier-Widen, D.; Eléouët, J.F.; Riffault, S.; Pringle, J.; Taylor, G.; Valarcher, J.F. A bovine respiratory syncytial virus model with high clinical expression in calves with specific passive immunity. BMC Vet. Res. 2015, 11, 76. [Google Scholar] [CrossRef]
- Kimman, T.G.; Westenbrink, F.; Schreuder, B.E.; Straver, P.J. Local and systemic antibody response to bovine respiratory syncytial virus infection and reinfection in calves with and without maternal antibodies. J. Clin. Microbiol. 1987, 25, 1097–1106. [Google Scholar]
- Laham, F.R.; Israele, V.; Casellas, J.M.; Garcia, A.M.; Lac Prugent, C.M.; Hoffman, S.J.; Hauer, D.; Thumar, B.; Name, M.I.; Pascual, A.; et al. Differential production of inflammatory cytokines in primary infection with human metapneumovirus and with other common respiratory viruses of infancy. J. Infect. Dis. 2004, 189, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.R.; Graham, B.S.; Prince, G.A.; Walsh, E.E.; Chanock, R.M.; Karzon, D.T.; Wright, P.F. Serum and nasal-wash immunoglobulin G and A antibody response of infants and children to respiratory syncytial virus F and G glycoproteins following primary infection. J. Clin. Microbiol. 1986, 23, 1009–1014. [Google Scholar] [PubMed]
- Heidema, J.; Rossen, J.W.; Lukens, M.V.; Ketel, M.S.; Scheltens, E.; Kranendonk, M.E.; van Maren, W.W.; van Loon, A.M.; Otten, H.G.; Kimpen, J.L.; et al. Dynamics of human respiratory virus-specific CD8+ T cell responses in blood and airways during episodes of common cold. J. Immunol. 2008, 181, 5551–5559. [Google Scholar] [CrossRef] [PubMed]
- Sheeran, P.; Jafri, H.; Carubelli, C.; Saavedra, J.; Johnson, C.; Krisher, K.; Sanchez, P.J.; Ramilo, O. Elevated cytokine concentrations in the nasopharyngeal and tracheal secretions of children with respiratory syncytial virus disease. Pediatr. Infect. Dis. J. 1999, 18, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Bont, L.; Heijnen, C.J.; Kavelaars, A.; van Aalderen, W.M.; Brus, F.; Draaisma, J.T.; Geelen, S.M.; van Vught, H.J.; Kimpen, J.L. Peripheral blood cytokine responses and disease severity in respiratory syncytial virus bronchiolitis. Eur. Respir. J. 1999, 14, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Hornsleth, A.; Klug, B.; Nir, M.; Johansen, J.; Hansen, K.S.; Christensen, L.S.; Larsen, L.B. Severity of respiratory syncytial virus disease related to type and genotype of virus and to cytokine values in nasopharyngeal secretions. Pediatr. Infect. Dis. J. 1998, 17, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Fach, S.J.; Meyerholz, D.K.; Gallup, J.M.; Ackermann, M.R.; Lehmkuhl, H.D.; Sacco, R.E. Neonatal ovine pulmonary dendritic cells support bovine respiratory syncytial virus replication with enhanced interleukin (IL)-4 And IL-10 gene transcripts. Viral Immunol. 2007, 20, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Meyerholz, D.K.; Grubor, B.; Fach, S.J.; Sacco, R.E.; Lehmkuhl, H.D.; Gallup, J.M.; Ackermann, M.R. Reduced clearance of respiratory syncytial virus infection in a preterm lamb model. Microbes Infect. Inst. Pasteur 2004, 6, 1312–1319. [Google Scholar] [CrossRef] [Green Version]
- Woolums, A.R.; Anderson, M.L.; Gunther, R.A.; Schelegle, E.S.; LaRochelle, D.R.; Singer, R.S.; Boyle, G.A.; Friebertshauser, K.E.; Gershwin, L.J. Evaluation of severe disease induced by aerosol inoculation of calves with bovine respiratory syncytial virus. Am. J. Vet. Res. 1999, 60, 473–480. [Google Scholar]
- Gershwin, L.J. Bovine respiratory syncytial virus infection: Immunopathogenic mechanisms. Anim. Health Res. Rev. 2007, 8, 207–213. [Google Scholar] [CrossRef]
- Caswell, J.L.; Williams, K.J. Respiratory System. In Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals, 5th ed.; Maxie, M.G., Ed.; Saunders: Edinburgh, UK, 2008. [Google Scholar]
- Kimman, T.G.; Straver, P.J.; Zimmer, G.M. Pathogenesis of naturally acquired bovine respiratory syncytial virus infection in calves: Morphologic and serologic findings. Am. J. Vet. Res. 1989, 50, 684–693. [Google Scholar] [PubMed]
- Aherne, W.; Bird, T.; Court, S.D.; Gardner, P.S.; McQuillin, J. Pathological changes in virus infections of the lower respiratory tract in children. J. Clin. Pathol. 1970, 23, 7–18. [Google Scholar] [CrossRef] [Green Version]
- Bem, R.A.; Domachowske, J.B.; Rosenberg, H.F. Animal models of human respiratory syncytial virus disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L148–L156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.E.; Gonzales, R.A.; Olson, S.J.; Wright, P.F.; Graham, B.S. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod. Pathol. 2007, 20, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Viuff, B.; Tjornehoj, K.; Larsen, L.E.; Rontved, C.M.; Uttenthal, A.; Ronsholt, L.; Alexandersen, S. Replication and clearance of respiratory syncytial virus: Apoptosis is an important pathway of virus clearance after experimental infection with bovine respiratory syncytial virus. Am. J. Pathol. 2002, 161, 2195–2207. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, H.K. Innate immune recognition of respiratory syncytial virus infection. BMB Rep. 2014, 47, 184–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valarcher, J.F.; Taylor, G. Bovine respiratory syncytial virus infection. Vet. Res. 2007, 38, 153–180. [Google Scholar] [CrossRef] [Green Version]
- Werling, D.; Koss, M.; Howard, C.J.; Taylor, G.; Langhans, W.; Hope, J.C. Role of bovine chemokines produced by dendritic cells in respiratory syncytial virus-induced T cell proliferation. Vet. Immunol. Immunopathol. 2002, 87, 225–233. [Google Scholar] [CrossRef]
- Lukens, M.V.; van de Pol, A.C.; Coenjaerts, F.E.; Jansen, N.J.; Kamp, V.M.; Kimpen, J.L.; Rossen, J.W.; Ulfman, L.H.; Tacke, C.E.; Viveen, M.C.; et al. A systemic neutrophil response precedes robust CD8(+) T-cell activation during natural respiratory syncytial virus infection in infants. J. Virol. 2010, 84, 2374–2383. [Google Scholar] [CrossRef]
- Smyth, R.L.; Mobbs, K.J.; O’Hea, U.; Ashby, D.; Hart, C.A. Respiratory syncytial virus bronchiolitis: Disease severity, interleukin-8, and virus genotype. Pediatr. Pulmonol. 2002, 33, 339–346. [Google Scholar] [CrossRef]
- Haeberle, H.A.; Takizawa, R.; Casola, A.; Brasier, A.R.; Dieterich, H.J.; Van Rooijen, N.; Gatalica, Z.; Garofalo, R.P. Respiratory syncytial virus-induced activation of nuclear factor-kappaB in the lung involves alveolar macrophages and toll-like receptor 4-dependent pathways. J. Infect. Dis. 2002, 186, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Lizundia, R.; Sauter, K.S.; Taylor, G.; Werling, D. Host species-specific usage of the TLR4-LPS receptor complex. Innate Immun. 2008, 14, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Scagnolari, C.; Midulla, F.; Pierangeli, A.; Moretti, C.; Bonci, E.; Berardi, R.; De Angelis, D.; Selvaggi, C.; Di Marco, P.; Girardi, E.; et al. Gene expression of nucleic acid-sensing pattern recognition receptors in children hospitalized for respiratory syncytial virus-associated acute bronchiolitis. Clin. Vaccine Immunol. 2009, 16, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, A.; Chang, T.H.; Harnack, R.; Frohlich, V.; Tominaga, K.; Dube, P.H.; Xiang, Y.; Bose, S. Activation of innate immune antiviral responses by Nod2. Nat. Immunol. 2009, 10, 1073–1080. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Jamaluddin, M.; Li, K.; Garofalo, R.P.; Casola, A.; Brasier, A.R. Retinoic acid-inducible gene I mediates early antiviral response and Toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J. Virol. 2007, 81, 1401–1411. [Google Scholar] [CrossRef] [PubMed]
- Marr, N.; Wang, T.I.; Kam, S.H.; Hu, Y.S.; Sharma, A.A.; Lam, A.; Markowski, J.; Solimano, A.; Lavoie, P.M.; Turvey, S.E. Attenuation of respiratory syncytial virus-induced and RIG-I-dependent type I IFN responses in human neonates and very young children. J. Immunol. 2014, 192, 948–957. [Google Scholar] [CrossRef]
- Bossert, B.; Conzelmann, K.K. Respiratory syncytial virus (RSV) nonstructural (NS) proteins as host range determinants: A chimeric bovine RSV with NS genes from human RSV is attenuated in interferon-competent bovine cells. J. Virol. 2002, 76, 4287–4293. [Google Scholar] [CrossRef]
- Schlender, J.; Bossert, B.; Buchholz, U.; Conzelmann, K.K. Bovine respiratory syncytial virus nonstructural proteins NS1 and NS2 cooperatively antagonize alpha/beta interferon-induced antiviral response. J. Virol. 2000, 74, 8234–8242. [Google Scholar] [CrossRef]
- Lo, M.S.; Brazas, R.M.; Holtzman, M.J. Respiratory syncytial virus nonstructural proteins NS1 and NS2 mediate inhibition of Stat2 expression and alpha/beta interferon responsiveness. J. Virol. 2005, 79, 9315–9319. [Google Scholar] [CrossRef]
- Spann, K.M.; Tran, K.C.; Collins, P.L. Effects of nonstructural proteins NS1 and NS2 of human respiratory syncytial virus on interferon regulatory factor 3, NF-kappaB, and proinflammatory cytokines. J. Virol. 2005, 79, 5353–5362. [Google Scholar] [CrossRef]
- Bossert, B.; Marozin, S.; Conzelmann, K.K. Nonstructural proteins NS1 and NS2 of bovine respiratory syncytial virus block activation of interferon regulatory factor 3. J. Virol. 2003, 77, 8661–8668. [Google Scholar] [CrossRef] [PubMed]
- Valarcher, J.F.; Furze, J.; Wyld, S.; Cook, R.; Conzelmann, K.K.; Taylor, G. Role of alpha/beta interferons in the attenuation and immunogenicity of recombinant bovine respiratory syncytial viruses lacking NS proteins. J. Virol. 2003, 77, 8426–8439. [Google Scholar] [CrossRef]
- Taylor, G.; Wyld, S.; Valarcher, J.F.; Guzman, E.; Thom, M.; Widdison, S.; Buchholz, U.J. Recombinant bovine respiratory syncytial virus with deletion of the SH gene induces increased apoptosis and pro-inflammatory cytokines in vitro, and is attenuated and induces protective immunity in calves. J. Gen. Virol. 2014, 95, 1244–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitehead, S.S.; Bukreyev, A.; Teng, M.N.; Firestone, C.Y.; St Claire, M.; Elkins, W.R.; Collins, P.L.; Murphy, B.R. Recombinant respiratory syncytial virus bearing a deletion of either the NS2 or SH gene is attenuated in chimpanzees. J. Virol. 1999, 73, 3438–3442. [Google Scholar] [PubMed]
- Pollock, N.; Taylor, G.; Jobe, F.; Guzman, E. Modulation of the transcription factor NF-kappaB in antigen-presenting cells by bovine respiratory syncytial virus small hydrophobic protein. J. Gen. Virol. 2017, 98, 1587–1599. [Google Scholar] [PubMed]
- Hagglund, S.; Blodorn, K.; Naslund, K.; Vargmar, K.; Lind, S.B.; Mi, J.; Arainga, M.; Riffault, S.; Taylor, G.; Pringle, J.; et al. Proteome analysis of bronchoalveolar lavage from calves infected with bovine respiratory syncytial virus-Insights in pathogenesis and perspectives for new treatments. PLoS ONE 2017, 12, e0186594. [Google Scholar] [CrossRef] [PubMed]
- Funchal, G.A.; Jaeger, N.; Czepielewski, R.S.; Machado, M.S.; Muraro, S.P.; Stein, R.T.; Bonorino, C.B.; Porto, B.N. Respiratory syncytial virus fusion protein promotes TLR-4-dependent neutrophil extracellular trap formation by human neutrophils. PLoS ONE 2015, 10, e0124082. [Google Scholar] [CrossRef] [PubMed]
- Cortjens, B.; de Boer, O.J.; de Jong, R.; Antonis, A.F.; Sabogal Pineros, Y.S.; Lutter, R.; van Woensel, J.B.; Bem, R.A. Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease. J. Pathol. 2016, 238, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Sigurs, N.; Aljassim, F.; Kjellman, B.; Robinson, P.D.; Sigurbergsson, F.; Bjarnason, R.; Gustafsson, P.M. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010, 65, 1045–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics 1998, 102, 531–537. [CrossRef]
- Kimman, T.G.; Westenbrink, F. Immunity to human and bovine respiratory syncytial virus. Arch. Virol. 1990, 112, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.; Ahout, I.M.; de Jonge, M.I.; Ferwerda, G. Mucosal IgG Levels Correlate Better with Respiratory Syncytial Virus Load and Inflammation than Plasma IgG Levels. Clin. Vaccine Immunol. 2015, 23, 243–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowe, J.E., Jr.; Williams, J.V. Immunology of viral respiratory tract infection in infancy. Paediatr. Respir. Rev. 2003, 4, 112–119. [Google Scholar] [CrossRef]
- Taylor, G.; Thomas, L.H.; Furze, J.M.; Cook, R.S.; Wyld, S.G.; Lerch, R.; Hardy, R.; Wertz, G.W. Recombinant vaccinia viruses expressing the F, G or N, but not the M2, protein of bovine respiratory syncytial virus (BRSV) induce resistance to BRSV challenge in the calf and protect against the development of pneumonic lesions. J. Gen. Virol. 1997, 78, 3195–3206. [Google Scholar] [CrossRef]
- Jorquera, P.A.; Choi, Y.; Oakley, K.E.; Powell, T.J.; Boyd, J.G.; Palath, N.; Haynes, L.M.; Anderson, L.J.; Tripp, R.A. Nanoparticle vaccines encompassing the respiratory syncytial virus (RSV) G protein CX3C chemokine motif induce robust immunity protecting from challenge and disease. PLoS ONE 2013, 8, e74905. [Google Scholar] [CrossRef] [PubMed]
- Schrijver, R.S.; Langedijk, J.P.; Keil, G.M.; Middel, W.G.; Maris-Veldhuis, M.; Van Oirschot, J.T.; Rijsewijk, F.A. Immunization of cattle with a BHV1 vector vaccine or a DNA vaccine both coding for the G protein of BRSV. Vaccine 1997, 15, 1908–1916. [Google Scholar] [CrossRef]
- Taylor, G.; Rijsewijk, F.A.; Thomas, L.H.; Wyld, S.G.; Gaddum, R.M.; Cook, R.S.; Morrison, W.I.; Hensen, E.; van Oirschot, J.T.; Keil, G. Resistance to bovine respiratory syncytial virus (BRSV) induced in calves by a recombinant bovine herpesvirus-1 expressing the attachment glycoprotein of BRSV. J. Gen. Virol. 1998, 79, 1759–1767. [Google Scholar] [CrossRef]
- McGill, J.L.; Kelly, S.M.; Kumar, P.; Speckhart, S.; Haughney, S.L.; Henningson, J.; Narasimhan, B.; Sacco, R.E. Efficacy of mucosal polyanhydride nanovaccine against respiratory syncytial virus infection in the neonatal calf. Sci. Rep. 2018, 8, 3021. [Google Scholar] [CrossRef]
- Swanson, K.A.; Settembre, E.C.; Shaw, C.A.; Dey, A.K.; Rappuoli, R.; Mandl, C.W.; Dormitzer, P.R.; Carfi, A. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc. Natl. Acad. Sci. USA 2011, 108, 9619–9624. [Google Scholar] [CrossRef] [Green Version]
- Raghunandan, R.; Lu, H.; Zhou, B.; Xabier, M.G.; Massare, M.J.; Flyer, D.C.; Fries, L.F.; Smith, G.E.; Glenn, G.M. An insect cell derived respiratory syncytial virus (RSV) F nanoparticle vaccine induces antigenic site II antibodies and protects against RSV challenge in cotton rats by active and passive immunization. Vaccine 2014, 32, 6485–6492. [Google Scholar] [CrossRef] [Green Version]
- Ngwuta, J.O.; Chen, M.; Modjarrad, K.; Joyce, M.G.; Kanekiyo, M.; Kumar, A.; Yassine, H.M.; Moin, S.M.; Killikelly, A.M.; Chuang, G.Y.; et al. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci. Transl. Med. 2015, 7, 309ra162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, L.; Silacci, C.; Thom, M.; Boyington, J.C.; Druz, A.; Joyce, M.G.; Guzman, E.; Kong, W.P.; Lai, Y.T.; et al. Protection of calves by a prefusion-stabilized bovine RSV F vaccine. NPJ Vaccines 2017, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Steff, A.M.; Monroe, J.; Friedrich, K.; Chandramouli, S.; Nguyen, T.L.; Tian, S.; Vandepaer, S.; Toussaint, J.F.; Carfi, A. Pre-fusion RSV F strongly boosts pre-fusion specific neutralizing responses in cattle pre-exposed to bovine RSV. Nat. Commun. 2017, 8, 1085. [Google Scholar] [CrossRef] [PubMed]
- Boxus, M.; Tignon, M.; Roels, S.; Toussaint, J.F.; Walravens, K.; Benoit, M.A.; Coppe, P.; Letesson, J.J.; Letellier, C.; Kerkhofs, P. DNA immunization with plasmids encoding fusion and nucleocapsid proteins of bovine respiratory syncytial virus induces a strong cell-mediated immunity and protects calves against challenge. J. Virol. 2007, 81, 6879–6889. [Google Scholar] [CrossRef] [PubMed]
- Habibi, M.S.; Jozwik, A.; Makris, S.; Dunning, J.; Paras, A.; DeVincenzo, J.P.; de Haan, C.A.; Wrammert, J.; Openshaw, P.J.; Chiu, C.; et al. Impaired Antibody-mediated Protection and Defective IgA B-Cell Memory in Experimental Infection of Adults with Respiratory Syncytial Virus. Am. J. Respir. Crit. Care Med. 2015, 191, 1040–1049. [Google Scholar] [CrossRef] [Green Version]
- Kalina, W.V.; Woolums, A.R.; Gershwin, L.J. Formalin-inactivated bovine RSV vaccine influences antibody levels in bronchoalveolar lavage fluid and disease outcome in experimentally infected calves. Vaccine 2005, 23, 4625–4630. [Google Scholar] [CrossRef]
- Wagner, D.K.; Muelenaer, P.; Henderson, F.W.; Snyder, M.H.; Reimer, C.B.; Walsh, E.E.; Anderson, L.J.; Nelson, D.L.; Murphy, B.R. Serum immunoglobulin G antibody subclass response to respiratory syncytial virus F and G glycoproteins after first, second, and third infections. J. Clin. Microbiol. 1989, 27, 589–592. [Google Scholar]
- Antonis, A.F.; Claassen, E.A.; Hensen, E.J.; de Groot, R.J.; de Groot-Mijnes, J.D.; Schrijver, R.S.; van der Most, R.G. Kinetics of antiviral CD8 T cell responses during primary and post-vaccination secondary bovine respiratory syncytial virus infection. Vaccine 2006, 24, 1551–1561. [Google Scholar] [CrossRef] [Green Version]
- Gaddum, R.M.; Cook, R.S.; Furze, J.M.; Ellis, S.A.; Taylor, G. Recognition of bovine respiratory syncytial virus proteins by bovine CD8+ T lymphocytes. Immunology 2003, 108, 220–229. [Google Scholar] [CrossRef] [Green Version]
- Taylor, G.; Thomas, L.H.; Wyld, S.G.; Furze, J.; Sopp, P.; Howard, C.J. Role of T-lymphocyte subsets in recovery from respiratory syncytial virus infection in calves. J. Virol. 1995, 69, 6658–6664. [Google Scholar]
- West, K.; Petrie, L.; Konoby, C.; Haines, D.M.; Cortese, V.; Ellis, J.A. The efficacy of modified-live bovine respiratory syncytial virus vaccines in experimentally infected calves. Vaccine 1999, 18, 907–919. [Google Scholar] [CrossRef]
- Gaddum, R.M.; Cook, R.S.; Thomas, L.H.; Taylor, G. Primary cytotoxic T-cell responses to bovine respiratory syncytial virus in calves. Immunology 1996, 88, 421–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Openshaw, P.J.; Chiu, C. Protective and dysregulated T cell immunity in RSV infection. Curr. Opin. Virol. 2013, 3, 468–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, D.L.; Bickham, K.L.; Thome, J.J.; Kim, C.Y.; D’Ovidio, F.; Wherry, E.J.; Farber, D.L. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 2014, 7, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Teijaro, J.R.; Turner, D.; Pham, Q.; Wherry, E.J.; Lefrancois, L.; Farber, D.L. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol. 2011, 187, 5510–5514. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hu, Y.; Lee, Y.T.; Bouchard, K.R.; Benechet, A.; Khanna, K.; Cauley, L.S. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J. Leukoc. Biol. 2014, 95, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Zens, K.D.; Chen, J.K.; Farber, D.L. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight 2016, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.V.; Ma, W.; Miron, M.; Granot, T.; Guyer, R.S.; Carpenter, D.J.; Senda, T.; Sun, X.; Ho, S.H.; Lerner, H.; et al. Human Tissue-Resident Memory T Cells Are Defined by Core Transcriptional and Functional Signatures in Lymphoid and Mucosal Sites. Cell Rep. 2017, 20, 2921–2934. [Google Scholar] [CrossRef] [PubMed]
- Pichyangkul, S.; Yongvanitchit, K.; Limsalakpetch, A.; Kum-Arb, U.; Im-Erbsin, R.; Boonnak, K.; Thitithayanont, A.; Jongkaewwattana, A.; Wiboon-ut, S.; Mongkolsirichaikul, D.; et al. Tissue Distribution of Memory T and B Cells in Rhesus Monkeys following Influenza A Infection. J. Immunol. 2015, 195, 4378–4386. [Google Scholar] [CrossRef] [PubMed]
- Fogg, M.H.; Parsons, K.R.; Thomas, L.H.; Taylor, G. Identification of CD4+ T cell epitopes on the fusion (F) and attachment (G) proteins of bovine respiratory syncytial virus (BRSV). Vaccine 2001, 19, 3226–3240. [Google Scholar] [CrossRef]
- Lambert, L.; Sagfors, A.M.; Openshaw, P.J.; Culley, F.J. Immunity to RSV in Early-Life. Front. Immunol. 2014, 5, 466. [Google Scholar] [CrossRef] [PubMed]
- Legg, J.P.; Hussain, I.R.; Warner, J.A.; Johnston, S.L.; Warner, J.O. Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am. J. Respir. Crit. Care Med. 2003, 168, 633–639. [Google Scholar] [CrossRef]
- Kristjansson, S.; Bjarnarson, S.P.; Wennergren, G.; Palsdottir, A.H.; Arnadottir, T.; Haraldsson, A.; Jonsdottir, I. Respiratory syncytial virus and other respiratory viruses during the first 3 months of life promote a local TH2-like response. J. Allergy Clin. Immunol. 2005, 116, 805–811. [Google Scholar] [CrossRef]
- Miao, C.; Woolums, A.R.; Zarlenga, D.S.; Brown, C.C.; Brown, J.C., Jr.; Williams, S.M.; Scott, M.A. Effects of a single intranasal dose of modified-live bovine respiratory syncytial virus vaccine on cytokine messenger RNA expression following viral challenge in calves. Am. J. Vet. Res. 2004, 65, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.S.; Gershwin, L.J. Detection of IgE antibodies to bovine respiratory syncytial virus. Vet. Immunol. Immunopathol. 1989, 20, 313–323. [Google Scholar] [CrossRef]
- Stewart, R.S.; Gershwin, L.J. Role of IgE in the pathogenesis of bovine respiratory syncytial virus in sequential infections in vaccinated and nonvaccinated calves. Am. J. Vet. Res. 1989, 50, 349–355. [Google Scholar] [PubMed]
- Green, C.A.; Sande, C.J.; de Lara, C.; Thompson, A.J.; Silva-Reyes, L.; Napolitano, F.; Pierantoni, A.; Capone, S.; Vitelli, A.; Klenerman, P.; et al. Humoral and cellular immunity to RSV in infants, children and adults. Vaccine 2018, 36, 6183–6190. [Google Scholar] [CrossRef]
- McGill, J.L.; Rusk, R.A.; Guerra-Maupome, M.; Briggs, R.E.; Sacco, R.E. Bovine Gamma Delta T Cells Contribute to Exacerbated IL-17 Production in Response to Co-Infection with Bovine RSV and Mannheimia haemolytica. PLoS ONE 2016, 11, e0151083. [Google Scholar] [CrossRef]
- Onishi, R.M.; Gaffen, S.L. Interleukin-17 and its target genes: Mechanisms of interleukin-17 function in disease. Immunology 2010, 129, 311–321. [Google Scholar] [CrossRef]
- Khader, S.A.; Gaffen, S.L.; Kolls, J.K. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009, 2, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Stoppelenburg, A.J.; Salimi, V.; Hennus, M.; Plantinga, M.; Huis in ’t Veld, R.; Walk, J.; Meerding, J.; Coenjaerts, F.; Bont, L.; Boes, M. Local IL-17A potentiates early neutrophil recruitment to the respiratory tract during severe RSV infection. PLoS ONE 2013, 8, e78461. [Google Scholar] [CrossRef] [PubMed]
- McGill, J.L.; Nonnecke, B.J.; Lippolis, J.D.; Reinhardt, T.A.; Sacco, R.E. Differential chemokine and cytokine production by neonatal bovine gammadelta T-cell subsets in response to viral toll-like receptor agonists and in vivo respiratory syncytial virus infection. Immunology 2013, 139, 227–244. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, M.; Shimojo, N.; Sekine, K.; Nishimuta, T.; Kohno, Y. Respiratory syncytial virus infection suppresses IFN-gamma production of gammadelta T cells. Clin. Exp. Immunol. 2003, 131, 312–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girardi, M.; Oppenheim, D.E.; Steele, C.R.; Lewis, J.M.; Glusac, E.; Filler, R.; Hobby, P.; Sutton, B.; Tigelaar, R.E.; Hayday, A.C. Regulation of cutaneous malignancy by gammadelta T cells. Science 2001, 294, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.E.; Marson, F.A.; Bertuzzo, C.S.; Arns, C.W.; Ribeiro, J.D. Epidemiological and genetic characteristics associated with the severity of acute viral bronchiolitis by respiratory syncytial virus. J. Pediatr. (Rio J) 2013, 89, 531–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, R.; Bont, L.; Siezen, C.L.; Hodemaekers, H.M.; Ermers, M.J.; Doornbos, G.; van ’t Slot, R.; Wijmenga, C.; Goeman, J.J.; Kimpen, J.L.; et al. Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes. J. Infect. Dis. 2007, 196, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Glass, E.J.; Baxter, R.; Leach, R.J.; Jann, O.C. Genes controlling vaccine responses and disease resistance to respiratory viral pathogens in cattle. Vet. Immunol. Immunopathol. 2012, 148, 90–99. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, R.G.; Woolliams, J.A.; Glass, E.J.; Williams, J.L.; Fitzpatrick, J.L. Quantitative evaluation of genetic and environmental parameters determining antibody response induced by vaccination against bovine respiratory syncytial virus. Vaccine 2006, 24, 4007–4016. [Google Scholar] [CrossRef]
- Kim, H.W.; Canchola, J.G.; Brandt, C.D.; Pyles, G.; Chanock, R.M.; Jensen, K.; Parrott, R.H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 1969, 89, 422–434. [Google Scholar] [CrossRef]
- Chin, J.; Magoffin, R.L.; Shearer, L.A.; Schieble, J.H.; Lennette, E.H. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am. J. Epidemiol. 1969, 89, 449–463. [Google Scholar] [CrossRef]
- Kapikian, A.Z.; Mitchell, R.H.; Chanock, R.M.; Shvedoff, R.A.; Stewart, C.E. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am. J. Epidemiol. 1969, 89, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Kimman, T.G.; Sol, J.; Westenbrink, F.; Straver, P.J. A severe outbreak of respiratory tract disease associated with bovine respiratory syncytial virus probably enhanced by vaccination with modified live vaccine. Vet. Q. 1989, 11, 250–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiber, P.; Matheise, J.P.; Dessy, F.; Heimann, M.; Letesson, J.J.; Coppe, P.; Collard, A. High mortality rate associated with bovine respiratory syncytial virus (BRSV) infection in Belgian white blue calves previously vaccinated with an inactivated BRSV vaccine. J. Vet. Med. B Infect. Dis. Vet. Public Health 2000, 47, 535–550. [Google Scholar] [CrossRef] [PubMed]
- West, K.; Petrie, L.; Haines, D.M.; Konoby, C.; Clark, E.G.; Martin, K.; Ellis, J.A. The effect of formalin-inactivated vaccine on respiratory disease associated with bovine respiratory syncytial virus infection in calves. Vaccine 1999, 17, 809–820. [Google Scholar] [CrossRef]
- Mohanty, S.B.; Rockemann, D.D.; Davidson, J.P.; Sharabrin, O.I.; Forst, S.M. Effect of vaccinal serum antibodies on bovine respiratory syncytial viral infection in calves. Am. J. Vet. Res. 1981, 42, 881–883. [Google Scholar] [PubMed]
- Mazur, N.I.; Higgins, D.; Nunes, M.C.; Melero, J.A.; Langedijk, A.C.; Horsley, N.; Buchholz, U.J.; Openshaw, P.J.; McLellan, J.S.; Englund, J.A.; et al. The respiratory syncytial virus vaccine landscape: Lessons from the graveyard and promising candidates. Lancet Infect. Dis. 2018, 18, e295–e311. [Google Scholar] [CrossRef]
- Blanco, J.C.G.; Boukhvalova, M.S.; Morrison, T.G.; Vogel, S.N. A multifaceted approach to RSV vaccination. Hum. Vaccin. Immunother. 2018, 14, 1734–1745. [Google Scholar] [CrossRef]
- Villafana, T.; Falloon, J.; Griffin, M.P.; Zhu, Q.; Esser, M.T. Passive and active immunization against respiratory syncytial virus for the young and old. Expert Rev. Vaccines 2017, 16, 1–13. [Google Scholar] [CrossRef]
- Hurwitz, J.L. Respiratory syncytial virus vaccine development. Expert Rev. Vaccines 2011, 10, 1415–1433. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, H.; Tsutsumi, H.; Matsuda, K.; Nagai, K.; Ogra, P.L.; Chiba, S. Effect of maternal antibody on IgA antibody response in nasopharyngeal secretion in infants and children during primary respiratory syncytial virus infection. J. Gen. Virol. 1994, 75, 2115–2119. [Google Scholar] [CrossRef] [Green Version]
- Stensballe, L.G.; Ravn, H.; Kristensen, K.; Agerskov, K.; Meakins, T.; Aaby, P.; Simoes, E.A. Respiratory syncytial virus neutralizing antibodies in cord blood, respiratory syncytial virus hospitalization, and recurrent wheeze. J. Allergy Clin. Immunol. 2009, 123, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Jans, J.; Wicht, O.; Widjaja, I.; Ahout, I.M.; de Groot, R.; Guichelaar, T.; Luytjes, W.; de Jonge, M.I.; de Haan, C.A.; Ferwerda, G. Characteristics of RSV-Specific Maternal Antibodies in Plasma of Hospitalized, Acute RSV Patients under Three Months of Age. PLoS ONE 2017, 12, e0170877. [Google Scholar] [CrossRef] [PubMed]
- Freitas, G.R.; Silva, D.A.; Yokosawa, J.; Paula, N.T.; Costa, L.F.; Carneiro, B.M.; Ribeiro, L.Z.; Oliveira, T.F.; Mineo, J.R.; Queiroz, D.A. Antibody response and avidity of respiratory syncytial virus-specific total IgG, IgG1, and IgG3 in young children. J. Med. Virol. 2011, 83, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Kimman, T.G.; Zimmer, G.M.; Westenbrink, F.; Mars, J.; van Leeuwen, E. Epidemiological study of bovine respiratory syncytial virus infections in calves: Influence of maternal antibodies on the outcome of disease. Vet. Rec. 1988, 123, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.; Thom, M.; Capone, S.; Pierantoni, A.; Guzman, E.; Herbert, R.; Scarselli, E.; Napolitano, F.; Giuliani, A.; Folgori, A.; et al. Efficacy of a virus-vectored vaccine against human and bovine respiratory syncytial virus infections. Sci. Transl. Med. 2015, 7, 300ra127. [Google Scholar] [CrossRef] [PubMed]
- Green, C.A.; Scarselli, E.; Sande, C.J.; Thompson, A.J.; de Lara, C.M.; Taylor, K.S.; Haworth, K.; Del Sorbo, M.; Angus, B.; Siani, L.; et al. Chimpanzee adenovirus- and MVA-vectored respiratory syncytial virus vaccine is safe and immunogenic in adults. Sci. Transl. Med. 2015, 7, 300ra126. [Google Scholar] [CrossRef]
- Malkin, E.; Yogev, R.; Abughali, N.; Sliman, J.; Wang, C.K.; Zuo, F.; Yang, C.F.; Eickhoff, M.; Esser, M.T.; Tang, R.S.; et al. Safety and immunogenicity of a live attenuated RSV vaccine in healthy RSV-seronegative children 5 to 24 months of age. PLoS ONE 2013, 8, e77104. [Google Scholar] [CrossRef]
- Karron, R.A.; Wright, P.F.; Belshe, R.B.; Thumar, B.; Casey, R.; Newman, F.; Polack, F.P.; Randolph, V.B.; Deatly, A.; Hackell, J.; et al. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J. Infect. Dis. 2005, 191, 1093–1104. [Google Scholar] [CrossRef]
- Buchholz, U.J.; Cunningham, C.K.; Muresan, P.; Gnanashanmugam, D.; Sato, P.; Siberry, G.K.; Rexroad, V.; Valentine, M.; Perlowski, C.; Schappell, E.; et al. Live Respiratory Syncytial Virus (RSV) Vaccine Candidate Containing Stabilized Temperature-Sensitivity Mutations Is Highly Attenuated in RSV-Seronegative Infants and Children. J. Infect. Dis. 2018, 217, 1338–1346. [Google Scholar] [CrossRef]
- Sastry, M.; Zhang, B.; Chen, M.; Joyce, M.G.; Kong, W.P.; Chuang, G.Y.; Ko, K.; Kumar, A.; Silacci, C.; Thom, M.; et al. Adjuvants and the vaccine response to the DS-Cav1-stabilized fusion glycoprotein of respiratory syncytial virus. PLoS ONE 2017, 12, e0186854. [Google Scholar] [CrossRef]
- McLellan, J.S.; Chen, M.; Joyce, M.G.; Sastry, M.; Stewart-Jones, G.B.; Yang, Y.; Zhang, B.; Chen, L.; Srivatsan, S.; Zheng, A.; et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 2013, 342, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Beran, J.; Lickliter, J.D.; Schwarz, T.F.; Johnson, C.; Chu, L.; Domachowske, J.B.; Van Damme, P.; Withanage, K.; Fissette, L.A.; David, M.P.; et al. Safety and Immunogenicity of 3 Formulations of an Investigational Respiratory Syncytial Virus Vaccine in Nonpregnant Women: Results From 2 Phase 2 Trials. J. Infect. Dis. 2018, 217, 1616–1625. [Google Scholar] [CrossRef] [Green Version]
- Kavanagh, O.V.; Adair, B.M.; Welsh, M.; Earley, B. Immunogenetic responses in calves to intranasal delivery of bovine respiratory syncytial virus (BRSV) epitopes encapsulated in poly (DL-lactide-co-glycolide) microparticles. Res. Vet. Sci. 2013, 95, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Riffault, S.; Meyer, G.; Deplanche, M.; Dubuquoy, C.; Durand, G.; Soulestin, M.; Castagné, N.; Bernard, J.; Bernardet, P.; Dubosclard, V.; et al. A new subunit vaccine based on nucleoprotein nanoparticles confers partial clinical and virological protection in calves against bovine respiratory syncytial virus. Vaccine 2010, 28, 3722–3734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- August, A.; Glenn, G.M.; Kpamegan, E.; Hickman, S.P.; Jani, D.; Lu, H.; Thomas, D.N.; Wen, J.; Piedra, P.A.; Fries, L.F. A Phase 2 randomized, observer-blind, placebo-controlled, dose-ranging trial of aluminum-adjuvanted respiratory syncytial virus F particle vaccine formulations in healthy women of childbearing age. Vaccine 2017, 35, 3749–3759. [Google Scholar] [CrossRef] [PubMed]
- Fries, L.; Shinde, V.; Stoddard, J.J.; Thomas, D.N.; Kpamegan, E.; Lu, H.; Smith, G.; Hickman, S.P.; Piedra, P.; Glenn, G.M. Immunogenicity and safety of a respiratory syncytial virus fusion protein (RSV F) nanoparticle vaccine in older adults. Immun. Ageing 2017, 14, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glenn, G.M.; Fries, L.F.; Thomas, D.N.; Smith, G.; Kpamegan, E.; Lu, H.; Flyer, D.; Jani, D.; Hickman, S.P.; Piedra, P.A. A Randomized, Blinded, Controlled, Dose-Ranging Study of a Respiratory Syncytial Virus Recombinant Fusion (F) Nanoparticle Vaccine in Healthy Women of Childbearing Age. J. Infect. Dis. 2016, 213, 411–422. [Google Scholar] [CrossRef]
- Blodorn, K.; Hagglund, S.; Fix, J.; Dubuquoy, C.; Makabi-Panzu, B.; Thom, M.; Karlsson, P.; Roque, J.L.; Karlstam, E.; Pringle, J.; et al. Vaccine safety and efficacy evaluation of a recombinant bovine respiratory syncytial virus (BRSV) with deletion of the SH gene and subunit vaccines based on recombinant human RSV proteins: N-nanorings, P and M2-1, in calves with maternal antibodies. PLoS ONE 2014, 9, e100392. [Google Scholar] [CrossRef]
- McLellan, J.S.; Chen, M.; Leung, S.; Graepel, K.W.; Du, X.; Yang, Y.; Zhou, T.; Baxa, U.; Yasuda, E.; Beaumont, T.; et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 2013, 340, 1113–1117. [Google Scholar] [CrossRef]
- Graham, B.S. Vaccine development for respiratory syncytial virus. Curr. Opin. Virol. 2017, 23, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Jorquera, P.A.; Tripp, R.A. Synthetic Biodegradable Microparticle and Nanoparticle Vaccines against the Respiratory Syncytial Virus. Vaccines 2016, 4. [Google Scholar] [CrossRef]
- Narasimhan, B.; Goodman, J.T.; Vela Ramirez, J.E. Rational Design of Targeted Next-Generation Carriers for Drug and Vaccine Delivery. Annu. Rev. Biomed. Eng. 2016, 18, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Renukaradhya, G.J.; Narasimhan, B.; Mallapragada, S.K. Respiratory nanoparticle-based vaccines and challenges associated with animal models and translation. J. Control. Release 2015, 219, 622–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, T.L.; Castagné, N.; Bhella, D.; Varela, P.F.; Bernard, J.; Chilmonczyk, S.; Berkenkamp, S.; Benhamo, V.; Grznarova, K.; Grosclaude, J.; et al. The nine C-terminal amino acids of the respiratory syncytial virus protein P are necessary and sufficient for binding to ribonucleoprotein complexes in which six ribonucleotides are contacted per N protein protomer. J. Gen. Virol. 2007, 88, 196–206. [Google Scholar] [CrossRef] [Green Version]
- Hervé, P.L.; Deloizy, C.; Descamps, D.; Rameix-Welti, M.A.; Fix, J.; McLellan, J.S.; Eléouët, J.F.; Riffault, S. RSV N-nanorings fused to palivizumab-targeted neutralizing epitope as a nanoparticle RSV vaccine. Nanomedicine 2017, 13, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Roux, X.; Dubuquoy, C.; Durand, G.; Tran-Tolla, T.L.; Castagné, N.; Bernard, J.; Petit-Camurdan, A.; Eléouët, J.F.; Riffault, S. Sub-nucleocapsid nanoparticles: A nasal vaccine against respiratory syncytial virus. PLoS ONE 2008, 3, e1766. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.; Raghunandan, R.; Wu, Y.; Liu, Y.; Massare, M.; Nathan, M.; Zhou, B.; Lu, H.; Boddapati, S.; Li, J.; et al. Respiratory syncytial virus fusion glycoprotein expressed in insect cells form protein nanoparticles that induce protective immunity in cotton rats. PLoS ONE 2012, 7, e50852. [Google Scholar] [CrossRef]
- Mills, J.; Van Kirk, J.E.; Wright, P.F.; Chanock, R.M. Experimental respiratory syncytial virus infection of adults. Possible mechanisms of resistance to infection and illness. J. Immunol. 1971, 107, 123–130. [Google Scholar]
- Tsutsumi, H.; Matsuda, K.; Yamazaki, H.; Ogra, P.L.; Chiba, S. Different kinetics of antibody responses between IgA and IgG classes in nasopharyngeal secretion in infants and children during primary respiratory syncytial virus infection. Acta. Paediatr. Jpn. 1995, 37, 464–468. [Google Scholar] [CrossRef]
- Ellis, J.A.; Gow, S.P.; Goji, N. Response to experimentally induced infection with bovine respiratory syncytial virus following intranasal vaccination of seropositive and seronegative calves. J. Am. Vet. Med. Assoc. 2010, 236, 991–999. [Google Scholar] [CrossRef]
- Ellis, J.A.; Gow, S.P.; Mahan, S.; Leyh, R. Duration of immunity to experimental infection with bovine respiratory syncytial virus following intranasal vaccination of young passively immune calves. J. Am. Vet. Med. Assoc. 2013, 243, 1602–1608. [Google Scholar] [CrossRef] [PubMed]
- Tirabassi, R.S.; Ace, C.I.; Levchenko, T.; Torchilin, V.P.; Selin, L.K.; Nie, S.; Guberski, D.L.; Yang, K. A mucosal vaccination approach for herpes simplex virus type 2. Vaccine 2011, 29, 1090–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.; Whalen, B.J.; Tirabassi, R.S.; Selin, L.K.; Levchenko, T.S.; Torchilin, V.P.; Kislauskis, E.H.; Guberski, D.L. A DNA vaccine prime followed by a liposome-encapsulated protein boost confers enhanced mucosal immune responses and protection. J. Immunol. 2008, 180, 6159–6167. [Google Scholar] [CrossRef] [PubMed]
- Dean, G.S.; Clifford, D.; Whelan, A.O.; Tchilian, E.Z.; Beverley, P.C.; Salguero, F.J.; Xing, Z.; Vordermeier, H.M.; Villarreal-Ramos, B. Protection Induced by Simultaneous Subcutaneous and Endobronchial Vaccination with BCG/BCG and BCG/Adenovirus Expressing Antigen 85A against Mycobacterium bovis in Cattle. PLoS ONE 2015, 10, e0142270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.K.; Soike, K.; Giannasca, P.; Hill, J.; Weltzin, R.; Kleanthous, H.; Blanchard, J.; Monath, T.P. Immunization of rhesus monkeys with a mucosal prime, parenteral boost strategy protects against infection with Helicobacter pylori. Vaccine 1999, 17, 3072–3082. [Google Scholar] [CrossRef]
- Tchilian, E.Z.; Ronan, E.O.; de Lara, C.; Lee, L.N.; Franken, K.L.; Vordermeier, M.H.; Ottenhoff, T.H.; Beverley, P.C. Simultaneous immunization against tuberculosis. PLoS ONE 2011, 6, e27477. [Google Scholar] [CrossRef]
- Jordan, R.; Shao, M.; Mackman, R.L.; Perron, M.; Cihlar, T.; Lewis, S.A.; Eisenberg, E.J.; Carey, A.; Strickley, R.G.; Chien, J.W.; et al. Antiviral Efficacy of a Respiratory Syncytial Virus (RSV) Fusion Inhibitor in a Bovine Model of RSV Infection. Antimicrob. Agents Chemother. 2015, 59, 4889–4900. [Google Scholar] [CrossRef] [Green Version]
- Walsh, P.; Behrens, N.; Carvallo Chaigneau, F.R.; McEligot, H.; Agrawal, K.; Newman, J.W.; Anderson, M.; Gershwin, L.J. A Randomized Placebo Controlled Trial of Ibuprofen for Respiratory Syncytial Virus Infection in a Bovine Model. PLoS ONE 2016, 11, e0152913. [Google Scholar] [CrossRef]
- Cortjens, B.; de Jong, R.; Bonsing, J.G.; van Woensel, J.B.M.; Antonis, A.F.G.; Bem, R.A. Local dornase alfa treatment reduces NETs-induced airway obstruction during severe RSV infection. Thorax 2018, 73, 578–580. [Google Scholar] [CrossRef]
| Sample | Advantages | Disadvantages | hRSV | bRSV |
|---|---|---|---|---|
| Lung tissue | Allows complete diagnosis Assessment of gross pathology and histology Viral burden Cellular immunity | Requires post-mortem species Does not represent the whole lung Need to disrupt and digest specimens in order to isolate cells | ||
| BAL Pre and post mortem | Longitudinal sampling (kinetics) Allows frequent collection Analysis of immune response at site of infection (LRT) Viral burden Cellular immunity Humoral immunity | Requires trained staff and specific materials Invasive A histopathological study cannot be performed Does not represent the whole lung Dilution of secretions too high to preform humoral analysis ex vivo | ||
| Nasal fluid | Longitudinal sampling (kinetics) Non-invasive Allows frequent collection Analysis of immune response at site of infection (URT) Viral burden Cellular immunity Humoral immunity | Representation of the upper respiratory tract only | ||
| Nasopharyngeal or tracheal aspirate | Viral load Cellular immunity Humoral immunity | Requires trained staff and specific materials Representation of the upper respiratory tract only |
| |
| Peripheral blood | Easy to perform Non-invasive Allows frequent collection Longitudinal sampling (kinetics) Cellular immunity Humoral immunity | Representation of the peripheral response may underestimate the local response |
|
| Vaccine Candidate | Category | Antigen/Adjuvant | Host Species and Current Testing Phase a | Immunization Route | References |
|---|---|---|---|---|---|
| PanAd3-RSV prime/MVA-RSV boost | Vector | N, M2, F; no adjuvant | bovine | IM b/IM or IN c/IM | [146] |
| N, M2, F; no adjuvant | Human (Phase I) | IM/IM or IN/IM | [147] | ||
| MVA-BN | Vector | F, G, N, M2; no adjuvant | Human (Phase II) | IM | [137] |
| SH gene deletion | Live-attenuated | All native genes except SH | bovine | IN/IT d IN | [74] [39] |
| Medi559: Gene-deletion for SH plus additional point mutations | Human (Phase IIa) | IN | [148,149] | ||
| RSVcps2: Similar to Medi559 with additional stabilizing mutations | Human (Phase I) | IN | [150] | ||
| Pre-F | Subunit | Pre-F with Montanide ISA71 adjuvant | bovine | IM | [93,151] |
| Pre-F, no adjuvant | bovine | IM | [94] | ||
| Pre-F with Poly(I:C) adjuvant | Macaques | IM | [152] | ||
| Pre-F with alum | Human (Phase II) | IM | [153] | ||
| PLGA encapsulating post-F and G | Nanoparticle | Post-F and G encapsulated in PLGA, no additional adjuvant | bovine | IN | [154] |
| BRSV-F/G Nanovaccine | Nanoparticle | Post-F and G encapsulated in CPH:CPTEG particle, no additional adjuvant | bovine | IN | [89] |
| N nanorings (NSRS) | Nanoparticle | NSRS with Montanide ISA71 adjuvant | bovine | IM | [155] |
| NSRS with Montanide IMS4132 adjuvant | bovine | IN | [155] | ||
| RSV F nanoparticle | Nanoparticle | Near-full-length F (pre-F conformation) with aluminum hydroxide | Human (Phase III: infants via maternal immunization) | IM | [156,157,158] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra-Maupome, M.; Palmer, M.V.; McGill, J.L.; Sacco, R.E. Utility of the Neonatal Calf Model for Testing Vaccines and Intervention Strategies for Use against Human RSV Infection. Vaccines 2019, 7, 7. https://doi.org/10.3390/vaccines7010007
Guerra-Maupome M, Palmer MV, McGill JL, Sacco RE. Utility of the Neonatal Calf Model for Testing Vaccines and Intervention Strategies for Use against Human RSV Infection. Vaccines. 2019; 7(1):7. https://doi.org/10.3390/vaccines7010007
Chicago/Turabian StyleGuerra-Maupome, Mariana, Mitchell V. Palmer, Jodi L. McGill, and Randy E. Sacco. 2019. "Utility of the Neonatal Calf Model for Testing Vaccines and Intervention Strategies for Use against Human RSV Infection" Vaccines 7, no. 1: 7. https://doi.org/10.3390/vaccines7010007
APA StyleGuerra-Maupome, M., Palmer, M. V., McGill, J. L., & Sacco, R. E. (2019). Utility of the Neonatal Calf Model for Testing Vaccines and Intervention Strategies for Use against Human RSV Infection. Vaccines, 7(1), 7. https://doi.org/10.3390/vaccines7010007







