Should Pneumococcal Serotype 3 Be Included in Serotype-Specific Immunoassays?
Abstract
1. Introduction
2. PCV13 Efficacy against Serotype 3
2.1. Efficacy against Invasive Pneumococcal Disease
2.2. Efficacy against Otitis Media
2.3. Efficacy against Carriage
3. Efficacy of Other Vaccines against Serotype 3
4. Reasons for Lack of Vaccine Efficacy
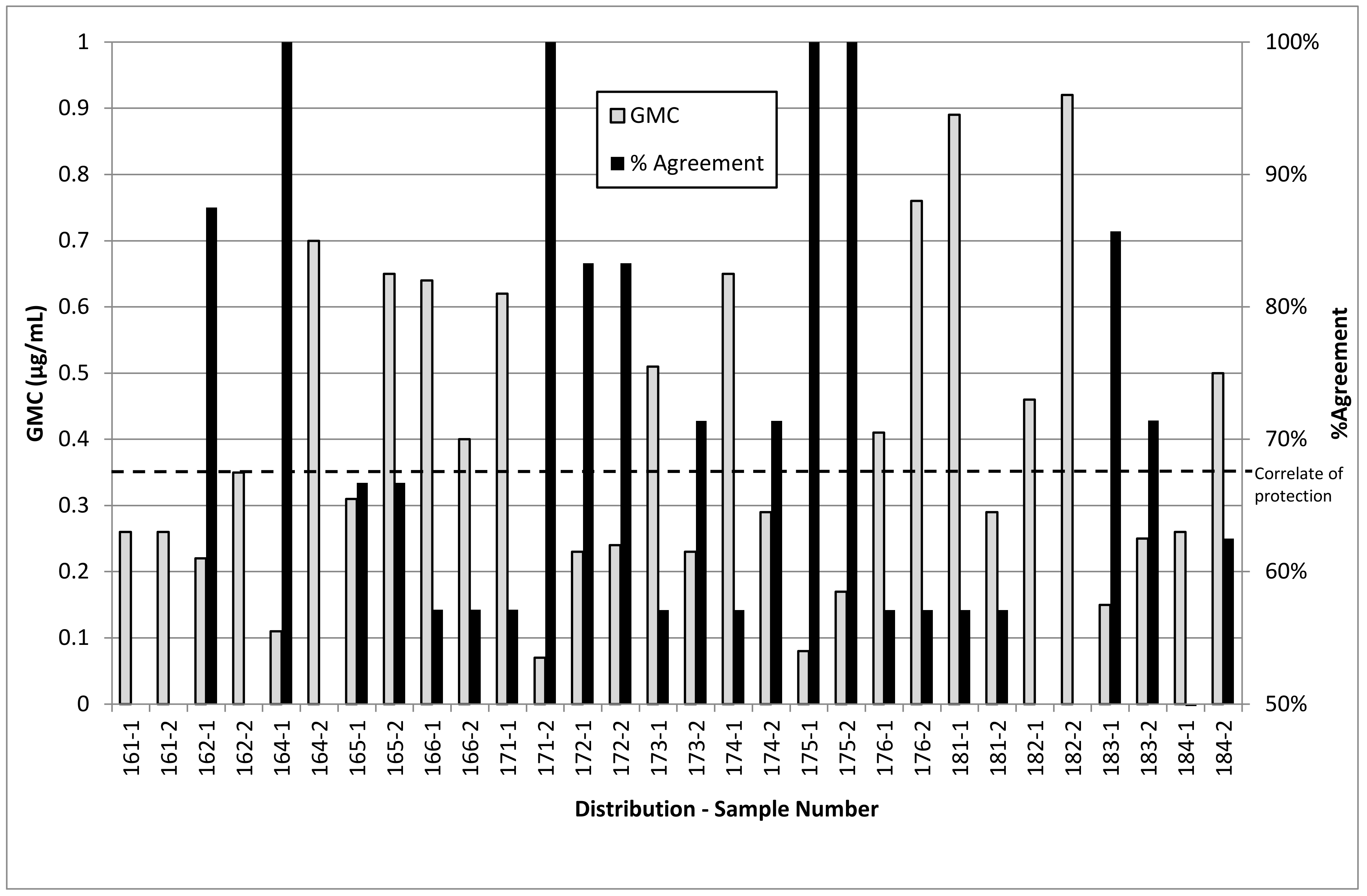
5. Luminex Assay Reproducibility
6. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hulten, K.G. The Changing Epidemiology of Pneumococcal Diseases. Lancet Infect. Dis. 2018, 18, 929–930. [Google Scholar] [CrossRef]
- Balsells, E.; Guillot, L.; Nair, H.; Kyaw, M.H. Serotype Distribution of Streptococcus Pneumoniae Causing Invasive Disease in Children in the Post-PCV Era: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0177113. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, M.A.; Schmitt, H.-J.; Syrochkina, M.; Sylvester, G. Pneumococcal Empyema and Complicated Pneumonias: Global Trends in Incidence, Prevalence, and Serotype Epidemiology. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 879–910. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.; Marimon, J.M.; Ercibengoa, M.; Pérez-Yarza, E.G.; Pérez-Trallero, E. Dynamics of Streptococcus Pneumoniae Serotypes Causing Acute Otitis Media Isolated from Children with Spontaneous Middle-Ear Drainage over a 12-Year Period (1999–2010) in a Region of Northern Spain. PLoS ONE 2013, 8, e54333. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, W.P.; Bryant, J.; Paradiso, P.R.; Siber, G.R. Which Pneumococcal Serogroups Cause the Most Invasive Disease: Implications for Conjugate Vaccine Formulation and Use, Part I. Clin. Infect. Dis. 2000, 30, 100–121. [Google Scholar] [CrossRef] [PubMed]
- Daly, T.M.; Hill, H.R. Use and Clinical Interpretation of Pneumococcal Antibody Measurements in the Evaluation of Humoral Immune Function. Clin. Vaccine Immunol. 2015, 22, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Pickering, J.W.; Martins, T.B.; Greer, R.W.; Schroder, M.C.; Astill, M.E.; Litwin, C.M.; Hildreth, S.W.; Hill, H.R. A Multiplexed Fluorescent Microsphere Immunoassay for Antibodies to Pneumococcal Capsular Polysaccharides. Am. J. Clin. Pathol. 2002, 117, 589–596. [Google Scholar] [CrossRef]
- Slotved, H.-C.; Dalby, T.; Harboe, Z.B.; Valentiner-Branth, P.; Fernandez de Casadevante, V.; Espenhain, L.; Fuursted, K.; Konradsen, H.B. The Incidence of Invasive Pneumococcal Serotype 3 Disease in the Danish Population Is Not Reduced by PCV-13 Vaccination. Heliyon 2016, 2, e00198. [Google Scholar] [CrossRef]
- Horácio, A.N.; Silva-Costa, C.; Lopes, J.P.; Ramirez, M.; Melo-Cristino, J.; Vaz, T. The Portuguese Group for the Study of Streptococcal Infections. Serotype 3 Remains the Leading Cause of Invasive Pneumococcal Disease in Adults in Portugal (2012–2014) Despite Continued Reductions in Other 13-Valent Conjugate Vaccine Serotypes. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Silva-Costa, C.; Brito, M.J.; Pinho, M.D.; Friães, A.; Aguiar, S.I.; Ramirez, M.; Melo-Cristino, J. Pediatric Complicated Pneumonia Caused by Streptococcus Pneumoniae Serotype 3 in 13-Valent Pneumococcal Conjugate Vaccinees, Portugal, 2010–2015. Emerg. Infect. Dis. 2018, 24, 1307–1314. [Google Scholar] [CrossRef]
- Moore, M.R.; Link-Gelles, R.; Schaffner, W.; Lynfield, R.; Lexau, C.; Bennett, N.M.; Petit, S.; Zansky, S.M.; Harrison, L.H.; Reingold, A.; et al. Effect of Use of 13-Valent Pneumococcal Conjugate Vaccine in Children on Invasive Pneumococcal Disease in Children and Adults in the USA: Analysis of Multisite, Population-Based Surveillance. Lancet Infect. Dis. 2015, 15, 301–309. [Google Scholar] [CrossRef]
- Andrews, N.J.; Waight, P.A.; Burbidge, P.; Pearce, E.; Roalfe, L.; Zancolli, M.; Slack, M.; Ladhani, S.N.; Miller, E.; Goldblatt, D. Serotype-Specific Effectiveness and Correlates of Protection for the 13-Valent Pneumococcal Conjugate Vaccine: A Postlicensure Indirect Cohort Study. Lancet Infect. Dis. 2014, 14, 839–846. [Google Scholar] [CrossRef]
- Oligbu, G.; Collins, S.; Andrews, N.; Sheppard, C.L.; Fry, N.K.; Slack, M.P.E.; Borrow, R.; Ladhani, S.N. Characteristics and Serotype Distribution of Childhood Cases of Invasive Pneumococcal Disease Following Pneumococcal Conjugate Vaccination in England and Wales, 2006–2014. Clin. Infect. Dis. 2017, 65, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Galanis, I.; Lindstrand, A.; Darenberg, J.; Browall, S.; Nannapaneni, P.; Sjöström, K.; Morfeldt, E.; Morfeldt, E.; Naucler, P.; Blennow, M.; et al. Effects of PCV7 and PCV13 on Invasive Pneumococcal Disease and Carriage in Stockholm, Sweden. Eur. Respir. J. 2016, 47, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, Á.; Ciruela, P.; Hernández, S.; García-García, J.J.; Soldevila, N.; Izquierdo, C.; Moraga-Llop, F.; Díaz, A.; de Sevilla, M.F.; González-Peris, S.; et al. Effectiveness of the 13-Valent Pneumococcal Conjugate Vaccine in Preventing Invasive Pneumococcal Disease in Children Aged 7–59 Months. A Matched Case-Control Study. PLoS ONE 2017, 12, e0183191. [Google Scholar]
- Cohen, R.; Levy, C.; Bingen, E.; Koskas, M.; Nave, I.; Varon, E. Impact of 13-valent pneumococcal conjugate vaccine on pneumococcal nasopharyngeal carriage in children with acute otitis media. Pediatr. Infect. Dis. J. 2012, 31, 297–301. [Google Scholar] [CrossRef]
- Cohen, R.; Varon, E.; Doit, C.; Schlemmer, C.; Romain, O.; Thollot, F.; Béchet, S.; Bonacorsi, S.; Levy, C. A 13-Year Survey of Pneumococcal Nasopharyngeal Carriage in Children with Acute Otitis Media Following PCV7 and PCV13 Implementation. Vaccine 2015, 33, 5118–5126. [Google Scholar] [CrossRef]
- Dagan, R.; Patterson, S.; Juergens, C.; Greenberg, D.; Givon-Lavi, N.; Porat, N.; Gurtman, A.; Gruber, W.C.; Scott, D.A. Comparative Immunogenicity and Efficacy of 13-Valent and 7-Valent Pneumococcal Conjugate Vaccines in Reducing Nasopharyngeal Colonization: A Randomized Double-Blind Trial. Clin. Infect. Dis. 2013, 57, 952–962. [Google Scholar] [CrossRef]
- Prymula, R.; Peeters, P.; Chrobok, V.; Kriz, P.; Novakova, E.; Kaliskova, E.; Kohl, I.; Lommel, P.; Poolman, J.; Prieels, J.P.; et al. Pneumococcal Capsular Polysaccharides Conjugated to Protein D for Prevention of Acute Otitis Media Caused by Both Streptococcus Pneumoniae and Non-Typable Haemophilus Influenzae: A Randomised Double-Blind Efficacy Study. Lancet 2006, 367, 740–748. [Google Scholar] [CrossRef]
- Andrews, N.J.; Waight, P.A.; George, R.C.; Slack, M.P.E.; Miller, E. Impact and Effectiveness of 23-Valent Pneumococcal Polysaccharide Vaccine against Invasive Pneumococcal Disease in the Elderly in England and Wales. Vaccine 2012, 30, 6802–6808. [Google Scholar] [CrossRef]
- Moore, M.R.; Link-Gelles, R.; Schaffner, W.; Lynfield, R.; Holtzman, C.; Harrison, L.H.; Zansky, S.M.; Rosen, J.B.; Reingold, A.; Scherzinger, K.; et al. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: A matched case-control study. Lancet Respir. Med. 2016, 4, 399–406. [Google Scholar] [CrossRef]
- Van der Linden, M.; Falkenhorst, G.; Perniciaro, S.; Fitzner, C.; Imöhl, M. Effectiveness of Pneumococcal Conjugate Vaccines (PCV7 and PCV13) against Invasive Pneumococcal Disease among Children under Two Years of Age in Germany. PLoS ONE 2016, 11, e0161257. [Google Scholar] [CrossRef] [PubMed]
- De Wals, P. Commentary on paradoxical observations pertaining to the impact of the 13-valent pneumococcal conjugate vaccine on serotype 3 Streptococcus pneumoniae infections in children. Vaccine 2018, 36, 5495–5496. [Google Scholar] [CrossRef]
- Deceuninck, G.; De Wals, P.; Boulianne, N.; Lefebvre, B.; De Wals, P. Effectiveness of Three Pneumococcal Conjugate Vaccines to Prevent Invasive Pneumococcal Disease in Quebec, Canada. Vaccine 2015, 33, 2684–2689. [Google Scholar] [CrossRef] [PubMed]
- Grimprel, E.; Laudat, F.; Patterson, S.; Baker, S.A.; Sidhu, M.S.; Gruber, W.C.; Emini, E.A.; Scott, D.A. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine (PCV13) when given as a toddler dose to children immunized with PCV7 as infants. Vaccine 2011, 29, 9675–9683. [Google Scholar] [CrossRef] [PubMed]
- Urbancikova, I.; Prymula, R.; Goldblatt, D.; Roalfe, L.; Prymulova, K.; Kosina, P. Immunogenicity and safety of a booster dose of the 13-valent pneumococcal conjugate vaccine in children primed with the 10-valent or 13-valent pneumococcal conjugate vaccine in the Czech Republic and Slovakia. Vaccine 2017, 35, 5186–5193. [Google Scholar] [CrossRef]
- Lewnard, J.A.; Givon-Lavi, N.; Weinberger, D.M.; Lipsitch, M.; Dagan, R. Pan-serotype Reduction in Progression of Streptococcus pneumoniae to Otitis Media After Rollout of Pneumococcal Conjugate Vaccines. Clin. Infect. Dis. 2017, 65, 1853–1861. [Google Scholar] [CrossRef]
- Ben-Shimol, S.; Givon-Lavi, N.; Leibovitz, E.; Raiz, S.; Greenberg, D.; Dagan, R. Impact of Widespread Introduction of Pneumococcal Conjugate Vaccines on Pneumococcal and Nonpneumococcal Otitis Media. Clin. Infect. Dis. 2016, 63, 611–618. [Google Scholar] [CrossRef]
- Poolman, J.; Kriz, P.; Feron, C.; Di-Paolo, E.; Henckaertsm, I.; Miseurm, A.; Wauters, D.; Prymula, R.; Schuerman, L. Pneumococcal serotype 3 otitis media, limited effect of polysaccharide conjugate immunisation and strain characteristics. Vaccine 2009, 27, 3213–3222. [Google Scholar] [CrossRef]
- Kim, J.O.; Romero-Steiner, S.; Sørensen, U.B.S.; Blom, J.; Carvalho, M.; Barnard, S.; Carlone, G.; Weiser, J.N. Relationship between Cell Surface Carbohydrates and Intrastrain Variation on Opsonophagocytosis of Streptococcus pneumoniae. Infect. Immun. 1999, 67, 2327–2333. [Google Scholar]
- Choi, E.H.; Zhang, F.; Lu, Y.-J.; Malley, R. Capsular Polysaccharide (CPS) Release by Serotype 3 Pneumococcal Strains Reduces the Protective Effect of Anti-Type 3 CPS Antibodies. Clin. Vaccine Immunol. 2016, 23, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Nurkka, A.; Joensuu, J.; Henckaerts, I.; Peeters, P.; Poolman, J.; Kilpi, T.; Käyhty, H. Immunogenicity and Safety of the Eleven Valent Pneumococcal Polysaccharide-Protein D Conjugate Vaccine in Infants. Pediatr. Infect. Dis. J. 2004, 23, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Poolman, J.; Borrow, R. Hyporesponsiveness and Its Clinical Implications after Vaccination with Polysaccharide or Glycoconjugate Vaccines. Expert Rev. Vaccines 2011, 10, 307–322. [Google Scholar] [CrossRef] [PubMed]
- UK NEQAS Immunology, Immunochemistry and Allergy. Available online: https://www.immqas.org.uk (accessed on 6 December 2018).
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linley, E.; Bell, A.; Gritzfeld, J.F.; Borrow, R. Should Pneumococcal Serotype 3 Be Included in Serotype-Specific Immunoassays? Vaccines 2019, 7, 4. https://doi.org/10.3390/vaccines7010004
Linley E, Bell A, Gritzfeld JF, Borrow R. Should Pneumococcal Serotype 3 Be Included in Serotype-Specific Immunoassays? Vaccines. 2019; 7(1):4. https://doi.org/10.3390/vaccines7010004
Chicago/Turabian StyleLinley, Ezra, Abigail Bell, Jenna F. Gritzfeld, and Ray Borrow. 2019. "Should Pneumococcal Serotype 3 Be Included in Serotype-Specific Immunoassays?" Vaccines 7, no. 1: 4. https://doi.org/10.3390/vaccines7010004
APA StyleLinley, E., Bell, A., Gritzfeld, J. F., & Borrow, R. (2019). Should Pneumococcal Serotype 3 Be Included in Serotype-Specific Immunoassays? Vaccines, 7(1), 4. https://doi.org/10.3390/vaccines7010004



