Serotype-Specific IgG Antibody Waning after Pneumococcal Conjugate Primary Series Vaccinations with either the 10-Valent or the 13-Valent Vaccine
Abstract
1. Introduction
2. Materials and Methods
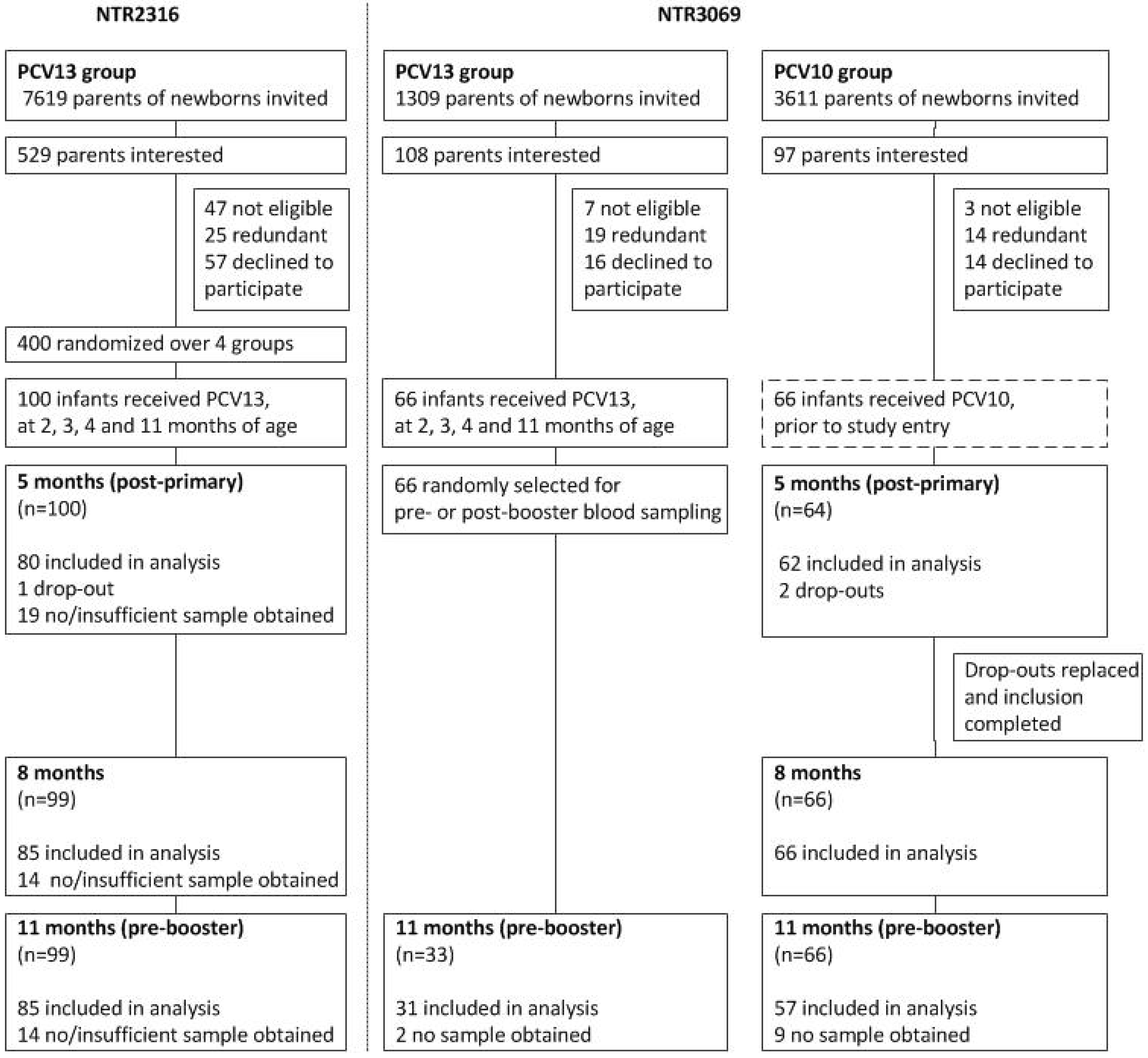
2.1. Study Design
2.2. Vaccines
2.3. Blood Collection and Laboratory Analyses
2.4. Statistical Analyses
3. Results
3.1. Study Population
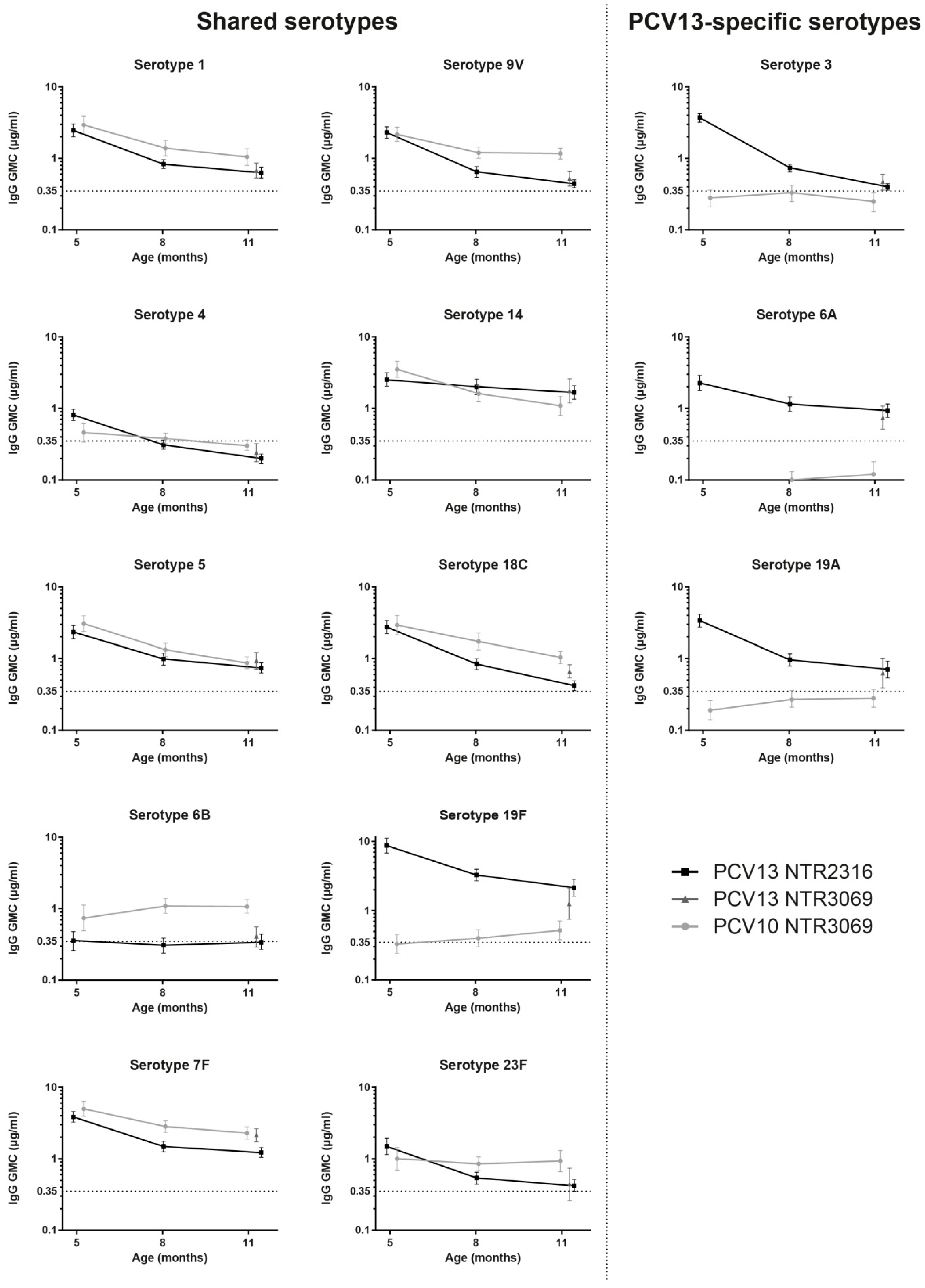
3.2. Comparison of IgG Levels at 11 Months of Age, between PCV13 Groups from Both Trials
3.3. Kinetics of IgG Responses to the 10 Shared-Serotypes Induced by PCV10 and PCV13
3.4. PCV13-Specific Serotypes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Deursen, A.M.; van Mens, S.P.; Sanders, E.A.; Vlaminckx, B.J.; de Melker, H.E.; Schouls, L.M.; de Greeff, S.C.; van der Ende, A.; the Invasive Pneumococcal Disease Sentinel Surveillance Laboratory Group. Invasive pneumococcal disease and 7-valent pneumococcal conjugate vaccine, The Netherlands. Emerg. Infect. Dis. 2012, 18, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Elberse, K.E.; van der Heide, H.G.; Witteveen, S.; van de Pol, I.; Schot, C.S.; van der Ende, A.; Berbers, G.A.; Schouls, L.M. Changes in the composition of the pneumococcal population and in IPD incidence in The Netherlands after the implementation of the 7-valent pneumococcal conjugate vaccine. Vaccine 2012, 30, 7644–7651. [Google Scholar] [CrossRef]
- Spijkerman, J.; van Gils, E.J.; Veenhoven, R.H.; Hak, E.; Yzerman, E.P.; van der Ende, A.; Wijmenga-Monsuur, A.J.; van den Dobbelsteen, G.P.; Sanders, E.A. Carriage of Streptococcus pneumoniae 3 years after start of vaccination program, The Netherlands. Emerg. Infect. Dis. 2011, 17, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Dagan, R.; Patterson, S.; Juergens, C.; Greenberg, D.; Givon-Lavi, N.; Porat, N.; Gurtman, A.; Gruber, W.C.; Scott, D.A. Comparative immunogenicity and efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines in reducing nasopharyngeal colonization: A randomized double-blind trial. Clin. Infect. Dis. 2013, 57, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shimol, S.; Greenberg, D.; Givon-Lavi, N.; Schlesinger, Y.; Somekh, E.; Aviner, S.; Miron, D.; Dagan, R. Early impact of sequential introduction of 7-valent and 13-valent pneumococcal conjugate vaccine on IPD in Israeli children <5 years: An active prospective nationwide surveillance. Vaccine 2014, 32, 3452–3459. [Google Scholar] [CrossRef] [PubMed]
- Siber, G.R.; Chang, I.; Baker, S.; Fernsten, P.; O’Brien, K.L.; Santosham, M.; Klugman, K.P.; Madhi, S.A.; Paradiso, P.; Kohberger, R. Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies. Vaccine 2007, 25, 3816–3826. [Google Scholar] [CrossRef]
- Andrews, N.J.; Waight, P.A.; Burbidge, P.; Pearce, E.; Roalfe, L.; Zancolli, M.; Slack, M.; Ladhani, S.N.; Miller, E.; Goldblatt, D. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: A postlicensure indirect cohort study. Lancet Infect. Dis. 2014, 14, 839–846. [Google Scholar] [CrossRef]
- Dagan, R.; Juergens, C.; Trammel, J.; Patterson, S.; Greenberg, D.; Givon-Lavi, N.; Porat, N.; Gruber, W.C.; Scott, D.A. Modeling pneumococcal nasopharyngeal acquisition as a function of anticapsular serum antibody concentrations after pneumococcal conjugate vaccine administration. Vaccine 2016, 34, 4313–4320. [Google Scholar] [CrossRef]
- Dagan, R.; Givon-Lavi, N.; Fraser, D.; Lipsitch, M.; Siber, G.R.; Kohberger, R. Serum serotype-specific pneumococcal anticapsular immunoglobulin G concentrations after immunization with a 9-valent conjugate pneumococcal vaccine correlate with nasopharyngeal acquisition of pneumococcus. J. Infect. Dis. 2005, 192, 367–376. [Google Scholar] [CrossRef]
- Mitsi, E.; Roche, A.M.; Reine, J.; Zangari, T.; Owugha, J.T.; Pennington, S.H.; Gritzfeld, J.F.; Wright, A.D.; Collins, A.M.; van Selm, S.; et al. Agglutination by anti-capsular polysaccharide antibody is associated with protection against experimental human pneumococcal carriage. Mucosal Immunol. 2017, 10, 385–394. [Google Scholar] [CrossRef]
- Ojal, J.; Hammitt, L.L.; Gaitho, J.; Scott, J.A.G.; Goldblatt, D. Pneumococcal conjugate vaccine induced IgG and nasopharyngeal carriage of pneumococci: Hyporesponsiveness and immune correlates of protection for carriage. Vaccine 2017, 35, 4652–4657. [Google Scholar] [CrossRef] [PubMed]
- Dicko, A.; Santara, G.; Mahamar, A.; Sidibe, Y.; Barry, A.; Dicko, Y.; Diallo, A.; Dolo, A.; Doumbo, O.; Shafi, F.; et al. Safety, reactogenicity and immunogenicity of a booster dose of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in Malian children. Hum. Vaccines Immunother. 2013, 9, 382–388. [Google Scholar] [CrossRef]
- Vesikari, T.; Wysocki, J.; Chevallier, B.; Karvonen, A.; Czajka, H.; Arsene, J.P.; Lommel, P.; Dieussaert, I.; Schuerman, L. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. Pediatr. Infect. Dis. J. 2009, 28, S66–S76. [Google Scholar] [CrossRef] [PubMed]
- Kieninger, D.M.; Kueper, K.; Steul, K.; Juergens, C.; Ahlers, N.; Baker, S.; Jansen, K.U.; Devlin, C.; Gruber, W.C.; Emini, E.A.; et al. Safety, tolerability, and immunologic noninferiority of a 13-valent pneumococcal conjugate vaccine compared to a 7-valent pneumococcal conjugate vaccine given with routine pediatric vaccinations in Germany. Vaccine 2010, 28, 4192–4203. [Google Scholar] [CrossRef] [PubMed]
- Jochems, S.P.; Weiser, J.N.; Malley, R.; Ferreira, D.M. The immunological mechanisms that control pneumococcal carriage. PLoS Pathog. 2017, 13, e1006665. [Google Scholar] [CrossRef] [PubMed]
- Ekstrom, N.; Ahman, H.; Verho, J.; Jokinen, J.; Vakevainen, M.; Kilpi, T.; Kayhty, H. Kinetics and avidity of antibodies evoked by heptavalent pneumococcal conjugate vaccines PncCRM and PncOMPC in the Finnish Otitis Media Vaccine Trial. Infect. Immun. 2005, 73, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Van Westen, E.; Wijmenga-Monsuur, A.J.; van Dijken, H.H.; van Gaans-van den Brink, J.A.; Kuipers, B.; Knol, M.J.; Berbers, G.A.; Sanders, E.A.; Rots, N.Y.; van Els, C.A. Differential B-cell memory around the 11-month booster in children vaccinated with a 10- or 13-valent pneumococcal conjugate vaccine. Clin. Infect. Dis. 2015, 61, 342–349. [Google Scholar] [CrossRef]
- Wijmenga-Monsuur, A.J.; van Westen, E.; Knol, M.J.; Jongerius, R.M.; Zancolli, M.; Goldblatt, D.; van Gageldonk, P.G.; Tcherniaeva, I.; Berbers, G.A.; Rots, N.Y. Direct comparison of immunogenicity induced by 10- or 13-valent pneumococcal conjugate vaccine around the 11-month booster in Dutch infants. PLoS ONE 2015, 10, e0144739. [Google Scholar] [CrossRef]
- Spijkerman, J.; Veenhoven, R.H.; Wijmenga-Monsuur, A.J.; Elberse, K.E.; van Gageldonk, P.G.; Knol, M.J.; de Melker, H.E.; Sanders, E.A.; Schouls, L.M.; Berbers, G.A. Immunogenicity of 13-valent pneumococcal conjugate vaccine administered according to 4 different primary immunization schedules in infants: A randomized clinical trial. JAMA 2013, 310, 930–937. [Google Scholar] [CrossRef]
- Elberse, K.E.; Tcherniaeva, I.; Berbers, G.A.; Schouls, L.M. Optimization and application of a multiplex bead-based assay to quantify serotype-specific IgG against Streptococcus pneumoniae polysaccharides: Response to the booster vaccine after immunization with the pneumococcal 7-valent conjugate vaccine. Clin. Vaccine Immunol. CVI 2010, 17, 674–682. [Google Scholar] [CrossRef]
- Vissers, M.; Wijmenga-Monsuur, A.J.; Knol, M.J.; Badoux, P.; van Houten, M.A.; van der Ende, A.; Sanders, E.A.M.; Rots, N.Y. Increased carriage of non-vaccine serotypes with low invasive disease potential four years after switching to the 10-valent pneumococcal conjugate vaccine in The Netherlands. PLoS ONE 2018, 13, e0194823. [Google Scholar] [CrossRef] [PubMed]
- Bosch, A.A.; van Houten, M.A.; Bruin, J.P.; Wijmenga-Monsuur, A.J.; Trzcinski, K.; Bogaert, D.; Rots, N.Y.; Sanders, E.A. Nasopharyngeal carriage of Streptococcus pneumoniae and other bacteria in the 7th year after implementation of the pneumococcal conjugate vaccine in the Netherlands. Vaccine 2016, 34, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Tansey, S.; Thompson, A.; Razmpour, A.; Liang, J.; Jones, T.R.; Ferrera, G.; Maida, A.; Bona, G.; Sabatini, C.; et al. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine compared to those of a 7-valent pneumococcal conjugate vaccine given as a three-dose series with routine vaccines in healthy infants and toddlers. Clin. Vaccine Immunol. CVI 2010, 17, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Goldblatt, D.; Southern, J.; Ashton, L.; Andrews, N.; Woodgate, S.; Burbidge, P.; Waight, P.; Miller, E. Immunogenicity of a reduced schedule of pneumococcal conjugate vaccine in healthy infants and correlates of protection for serotype 6B in the United Kingdom. Pediatr. Infect. Dis. J. 2010, 29, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Poolman, J.; Frasch, C.; Nurkka, A.; Kayhty, H.; Biemans, R.; Schuerman, L. Impact of the conjugation method on the immunogenicity of Streptococcus pneumoniae serotype 19F polysaccharide in conjugate vaccines. Clin. Vaccine Immunol. CVI 2011, 18, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, M.R.; Spijkerman, J.; Francois, N.; Swinnen, K.; Borys, D.; Schuerman, L.; Veenhoven, R.H.; Sanders, E.A. Immunogenicity, safety, and reactogenicity of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine and DTPa-IPV-Hib when coadministered as a 3-dose primary vaccination schedule in The Netherlands: A randomized controlled trial. Pediatr. Infect. Dis. J. 2011, 30, e170–e178. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Cohen, J.F.; Chalumeau, M.; Levy, C. Impact of pneumococcal conjugate vaccines for children in high- and non-high-income countries. Expert Rev. Vaccines 2017, 16, 625–640. [Google Scholar] [CrossRef] [PubMed]
| Serotype | Trend Over Time | Interaction | ||||
|---|---|---|---|---|---|---|
| PCV10 | PCV13 | |||||
| GMC Ratio per Month a | p-Value | GMC Ratio per Month a | p-Value | Direction b | p-Value | |
| 1 | 0.82 | <0.001 | 0.82 | <0.001 | Negative | 0.811 |
| 4 | 0.93 | <0.001 | 0.81 | <0.001 | Negative | <0.001 |
| 5 | 0.79 | <0.001 | 0.84 | <0.001 | Positive | 0.019 |
| 6B | 1.07 | 0.035 | 1.00 | 0.906 | Negative | 0.106 |
| 7F | 0.86 | <0.001 | 0.84 | <0.001 | Negative | 0.252 |
| 9V | 0.89 | <0.001 | 0.78 | <0.001 | Negative | <0.001 |
| 14 | 0.81 | <0.001 | 0.94 | 0.010 | Positive | <0.001 |
| 18C | 0.83 | <0.001 | 0.76 | <0.001 | Negative | <0.001 |
| 19F | 1.10 | 0.005 | 0.81 | <0.001 | Negative | <0.001 |
| 23F | 0.99 | 0.824 | 0.83 | <0.001 | Negative | <0.001 |
| 3 | 0.98 | 0.519 | 0.72 | <0.001 | Negative | <0.001 |
| 6A | 1.11 | 0.009 | 0.88 | <0.001 | Negative | <0.001 |
| 19A | 1.08 | 0.015 | 0.79 | <0.001 | Negative | <0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Westen, E.; Knol, M.J.; Wijmenga-Monsuur, A.J.; Tcherniaeva, I.; Schouls, L.M.; Sanders, E.A.M.; Van Els, C.A.C.M.; Berbers, G.A.M.; Rots, N.Y. Serotype-Specific IgG Antibody Waning after Pneumococcal Conjugate Primary Series Vaccinations with either the 10-Valent or the 13-Valent Vaccine. Vaccines 2018, 6, 82. https://doi.org/10.3390/vaccines6040082
Van Westen E, Knol MJ, Wijmenga-Monsuur AJ, Tcherniaeva I, Schouls LM, Sanders EAM, Van Els CACM, Berbers GAM, Rots NY. Serotype-Specific IgG Antibody Waning after Pneumococcal Conjugate Primary Series Vaccinations with either the 10-Valent or the 13-Valent Vaccine. Vaccines. 2018; 6(4):82. https://doi.org/10.3390/vaccines6040082
Chicago/Turabian StyleVan Westen, Els, Mirjam J. Knol, Alienke J. Wijmenga-Monsuur, Irina Tcherniaeva, Leo M. Schouls, Elisabeth A. M. Sanders, Cecile A. C. M. Van Els, Guy A. M. Berbers, and Nynke Y. Rots. 2018. "Serotype-Specific IgG Antibody Waning after Pneumococcal Conjugate Primary Series Vaccinations with either the 10-Valent or the 13-Valent Vaccine" Vaccines 6, no. 4: 82. https://doi.org/10.3390/vaccines6040082
APA StyleVan Westen, E., Knol, M. J., Wijmenga-Monsuur, A. J., Tcherniaeva, I., Schouls, L. M., Sanders, E. A. M., Van Els, C. A. C. M., Berbers, G. A. M., & Rots, N. Y. (2018). Serotype-Specific IgG Antibody Waning after Pneumococcal Conjugate Primary Series Vaccinations with either the 10-Valent or the 13-Valent Vaccine. Vaccines, 6(4), 82. https://doi.org/10.3390/vaccines6040082





