Neonatal Genetic Delivery of Anti-Respiratory Syncytial Virus (RSV) Antibody by Non-Human Primate-Based Adenoviral Vector to Provide Protection against RSV
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. RSV
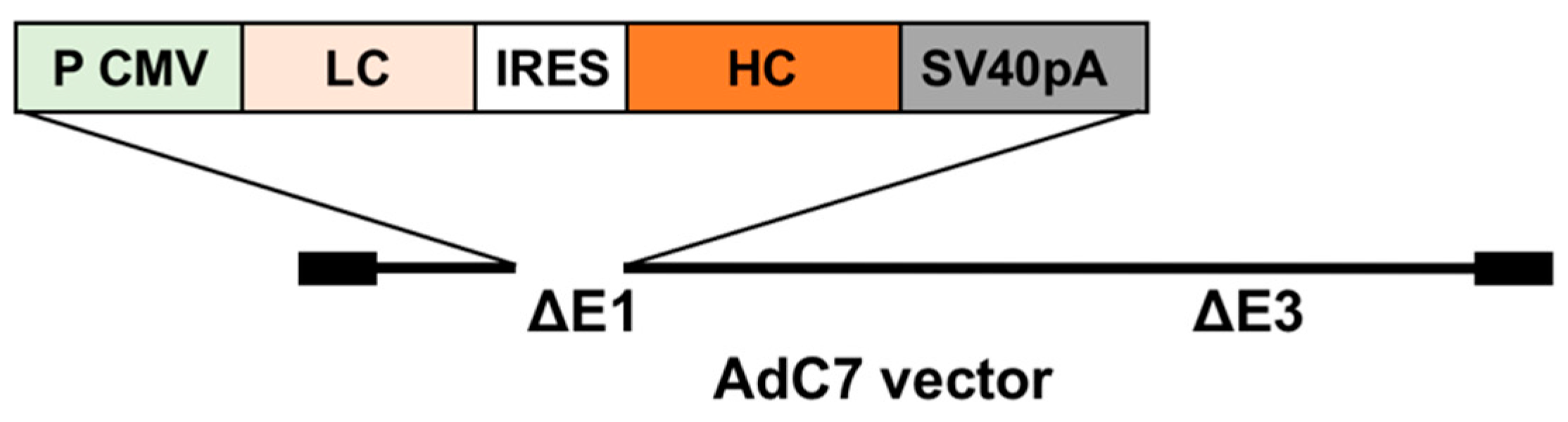
2.3. Generation of an AdC7 Vector Expressing Murine Anti-RSV IgG (AdC7αRSV)
2.4. Western Blot Analysis
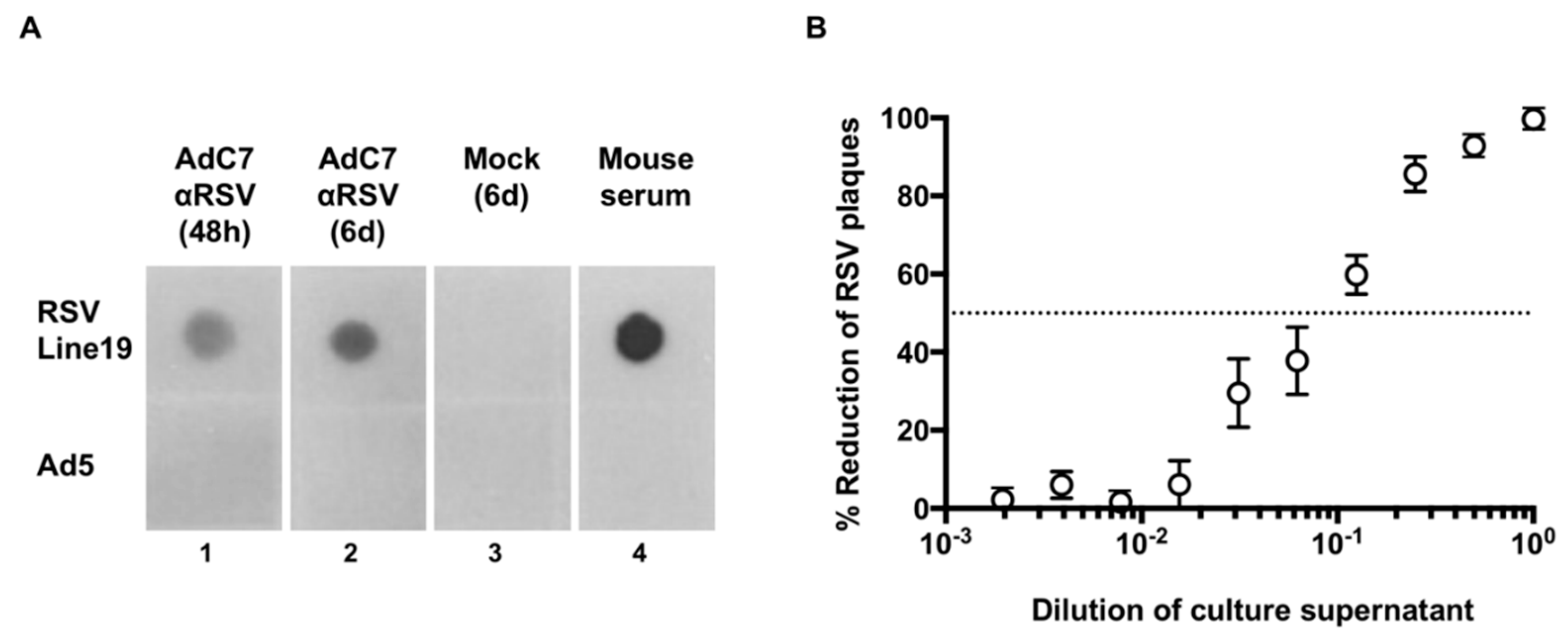
2.5. Dot Blot
2.6. Plaque Reduction Assay
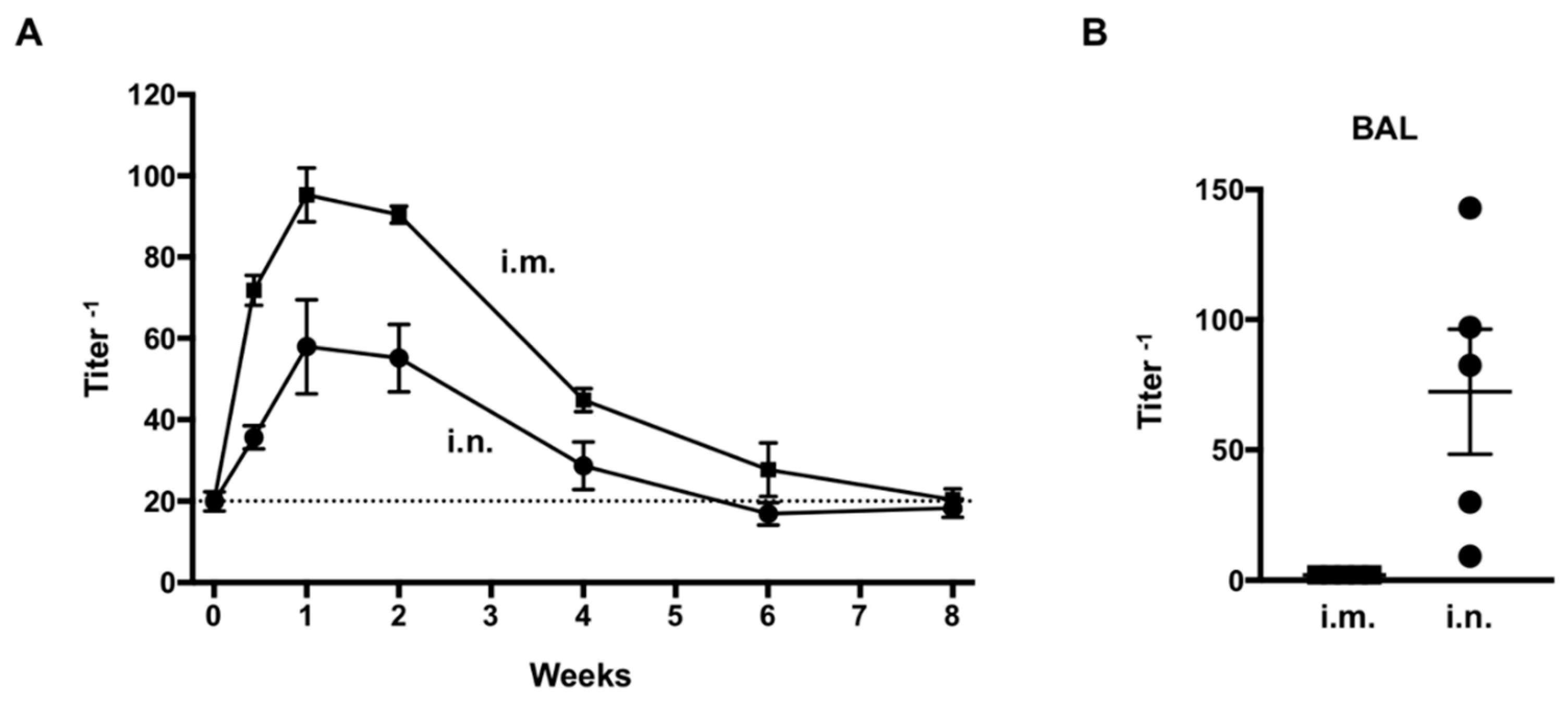
2.7. Expression of anti-RSV IgG In Vivo
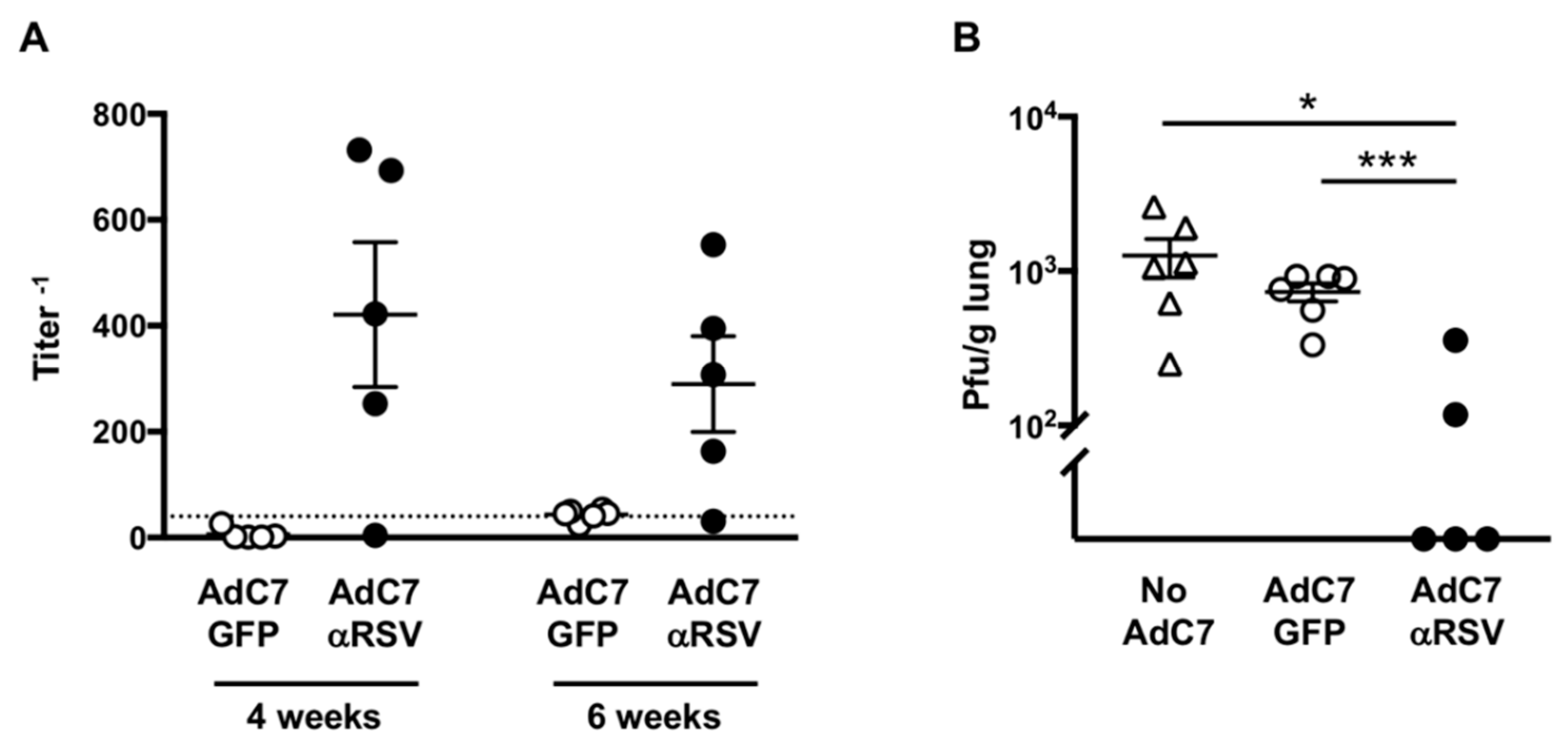
2.8. Protection Against RSV Infection Following AdC7αRSV Administration
2.9. Statistics
3. Results
3.1. Expression of Murine Anti-RSV In Vitro
3.2. Assessment of Anti-RSV IgG Delivered by AdC7αRSV In Vivo
3.3. Protection Against RSV Infection Following AdC7αRSV Administration in Adult Mice
3.4. Protection Against RSV Following AdC7αRSV Administration to Neonatal Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scheltema, N.M.; Gentile, A.; Lucion, F.; Nokes, D.J.; Munywoki, P.K.; Madhi, S.A.; Groome, M.J.; Cohen, C.; Moyes, J.; Thorburn, K.; et al. Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): A retrospective case series. Lancet Glob. Health 2017, 5, e984–e991. [Google Scholar] [CrossRef]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Nair, H.; Nokes, D.J.; Gessner, B.D.; Dherani, M.; Madhi, S.A.; Singleton, R.J.; O’Brien, K.L.; Roca, A.; Wright, P.F.; Bruce, N.; et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef]
- Meissner, H.C. Viral Bronchiolitis in Children. N. Engl. J. Med. 2016, 374, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B.; Weinberg, G.A.; Iwane, M.K.; Blumkin, A.K.; Edwards, K.M.; Staat, M.A.; Auinger, P.; Griffin, M.R.; Poehling, K.A.; Erdman, D.; et al. The burden of respiratory syncytial virus infection in young children. N. Engl. J. Med. 2009, 360, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Gerretsen, H.E.; Sande, C.J. Development of respiratory syncytial virus (RSV) vaccines for infants. J. Infect. 2017, 74 (Suppl. 1), S143–S146. [Google Scholar] [CrossRef]
- Rezaee, F.; Linfield, D.T.; Harford, T.J.; Piedimonte, G. Ongoing developments in RSV prophylaxis: A clinician’s analysis. Curr. Opin. Virol. 2017, 24, 70–78. [Google Scholar] [CrossRef]
- Higgins, D.; Trujillo, C.; Keech, C. Advances in RSV vaccine research and development—A global agenda. Vaccine 2016, 34, 2870–2875. [Google Scholar] [CrossRef]
- Resch, B. Product review on the monoclonal antibody palivizumab for prevention of respiratory syncytial virus infection. Hum. Vaccines Immunother. 2017, 13, 2138–2149. [Google Scholar] [CrossRef]
- Lee, C.S.; Bishop, E.S.; Zhang, R.; Yu, X.; Farina, E.M.; Yan, S.; Zhao, C.; Zheng, Z.; Shu, Y.; Wu, X.; et al. Adenovirus-Mediated Gene Delivery: Potential Applications for Gene and Cell-Based Therapies in the New Era of Personalized Medicine. Genes Dis. 2017, 4, 43–63. [Google Scholar] [CrossRef]
- Fausther-Bovendo, H.; Kobinger, G.P. Pre-existing immunity against Ad vectors: Humoral, cellular, and innate response, what’s important? Hum. Vaccines Immunother. 2014, 10, 2875–2884. [Google Scholar] [CrossRef] [PubMed]
- Wold, W.S.; Toth, K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther. 2013, 13, 421–433. [Google Scholar] [CrossRef]
- Sharma, A.; Wendland, R.; Sung, B.; Wu, W.; Grunwald, T.; Worgall, S. Maternal immunization with chimpanzee adenovirus expressing RSV fusion protein protects against neonatal RSV pulmonary infection. Vaccine 2014, 32, 5761–5768. [Google Scholar] [CrossRef] [PubMed]
- Adkins, B.; Leclerc, C.; Marshall-Clarke, S. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 2004, 4, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Waddington, S.N.; Kennea, N.L.; Buckley, S.M.; Gregory, L.G.; Themis, M.; Coutelle, C. Fetal and neonatal gene therapy: Benefits and pitfalls. Gene Ther. 2004, 11 (Suppl. 1), S92–S97. [Google Scholar] [CrossRef]
- Krause, A.; Xu, Y.; Ross, S.; Wu, W.; Joh, J.; Worgall, S. Absence of vaccine-enhanced RSV disease and changes in pulmonary dendritic cells with adenovirus-based RSV vaccine. Virol. J. 2011, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Skaricic, D.; Traube, C.; De, B.; Joh, J.; Boyer, J.; Crystal, R.G.; Worgall, S. Genetic delivery of an anti-RSV antibody to protect against pulmonary infection with RSV. Virology 2008, 378, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.A.; Yoshimura, K.; Trapnell, B.C.; Yoneyama, K.; Rosenthal, E.R.; Dalemans, W.; Fukayama, M.; Bargon, J.; Stier, L.E.; Stratford-Perricaudet, L.; et al. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell 1992, 68, 143–155. [Google Scholar] [CrossRef]
- Mittereder, N.; March, K.L.; Trapnell, B.C. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J. Virol. 1996, 70, 7498–7509. [Google Scholar]
- Johnson, S.; Oliver, C.; Prince, G.A.; Hemming, V.G.; Pfarr, D.S.; Wang, S.C.; Dormitzer, M.; O’Grady, J.; Koenig, S.; Tamura, J.K.; et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J. Infect. Dis. 1997, 176, 1215–1224. [Google Scholar] [CrossRef]
- Robbie, G.J.; Criste, R.; Dall’acqua, W.F.; Jensen, K.; Patel, N.K.; Losonsky, G.A.; Griffin, M.P. A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults. Antimicrob. Agents Chemother. 2013, 57, 6147–6153. [Google Scholar] [CrossRef] [PubMed]
- Synagis® (Palivizumab). Available online: https://www.synagis.com (accessed on 26 November 2018).
- Wissinger, E.; Goulding, J.; Hussell, T. Immune homeostasis in the respiratory tract and its impact on heterologous infection. Semin. Immunol. 2009, 21, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Walzl, G.; Tafuro, S.; Moss, P.; Openshaw, P.J.; Hussell, T. Influenza virus lung infection protects from respiratory syncytial virus-induced immunopathology. J. Exp. Med. 2000, 192, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Vijay, R.; Channappanavar, R.; Athmer, J.; Meyerholz, D.K.; Pagedar, N.; Tilley, S.; Perlman, S. Nasal priming by a murine coronavirus provides protective immunity against lethal heterologous virus pneumonia. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; You, Q.; Hagstrom, J.N.; Sands, M.; High, K.A. Successful expression of human factor IX following repeat administration of adenoviral vector in mice. Proc. Natl. Acad. Sci. USA 1996, 93, 3056–3061. [Google Scholar] [CrossRef] [PubMed]
- Huard, J.; Lochmuller, H.; Acsadi, G.; Jani, A.; Holland, P.; Guerin, C.; Massie, B.; Karpati, G. Differential short-term transduction efficiency of adult versus newborn mouse tissues by adenoviral recombinants. Exp. Mol. Pathol. 1995, 62, 131–143. [Google Scholar] [CrossRef]
- Domachowske, J.B.; Khan, A.A.; Esser, M.T.; Jensen, K.; Takas, T.; Villafana, T.; Dubovsky, F.; Griffin, M.P. Safety, Tolerability and Pharmacokinetics of MEDI8897, an Extended Half-life Single-dose Respiratory Syncytial Virus Prefusion F-targeting Monoclonal Antibody Administered as a Single Dose to Healthy Preterm Infants. Pediatr. Infect. Dis. J. 2018, 37, 886–892. [Google Scholar] [CrossRef]
- Griffin, M.P.; Khan, A.A.; Esser, M.T.; Jensen, K.; Takas, T.; Kankam, M.K.; Villafana, T.; Dubovsky, F. Safety, Tolerability, and Pharmacokinetics of MEDI8897, the Respiratory Syncytial Virus Prefusion F-Targeting Monoclonal Antibody with an Extended Half-Life, in Healthy Adults. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomi, R.; Sharma, A.; Wu, W.; Worgall, S. Neonatal Genetic Delivery of Anti-Respiratory Syncytial Virus (RSV) Antibody by Non-Human Primate-Based Adenoviral Vector to Provide Protection against RSV. Vaccines 2019, 7, 3. https://doi.org/10.3390/vaccines7010003
Gomi R, Sharma A, Wu W, Worgall S. Neonatal Genetic Delivery of Anti-Respiratory Syncytial Virus (RSV) Antibody by Non-Human Primate-Based Adenoviral Vector to Provide Protection against RSV. Vaccines. 2019; 7(1):3. https://doi.org/10.3390/vaccines7010003
Chicago/Turabian StyleGomi, Rika, Anurag Sharma, Wenzhu Wu, and Stefan Worgall. 2019. "Neonatal Genetic Delivery of Anti-Respiratory Syncytial Virus (RSV) Antibody by Non-Human Primate-Based Adenoviral Vector to Provide Protection against RSV" Vaccines 7, no. 1: 3. https://doi.org/10.3390/vaccines7010003
APA StyleGomi, R., Sharma, A., Wu, W., & Worgall, S. (2019). Neonatal Genetic Delivery of Anti-Respiratory Syncytial Virus (RSV) Antibody by Non-Human Primate-Based Adenoviral Vector to Provide Protection against RSV. Vaccines, 7(1), 3. https://doi.org/10.3390/vaccines7010003



