Simultaneous Administration of Recombinant Measles Viruses Expressing Respiratory Syncytial Virus Fusion (F) and Nucleo (N) Proteins Induced Humoral and Cellular Immune Responses in Cotton Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses and Cells
2.2. Animals and Immunization
2.3. Intracellular Cytokine Staining (ICS)
2.4. Serology
2.5. Detection of Infectious RSV
2.6. HE Stains and Immunostaining of RSV
2.7. Statistical Analysis
3. Results
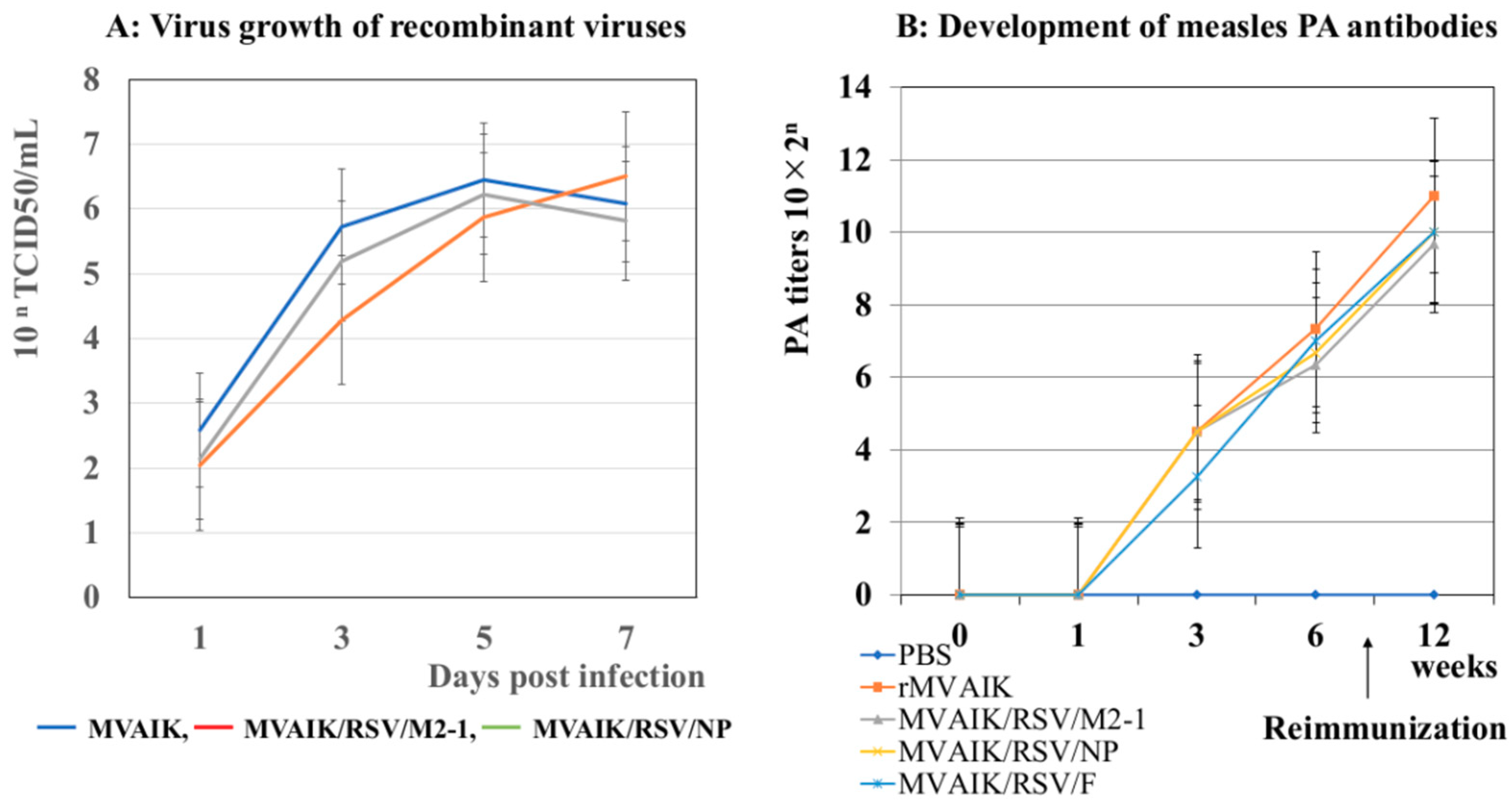
3.1. Virus Growth and the Development of PA Antibodies
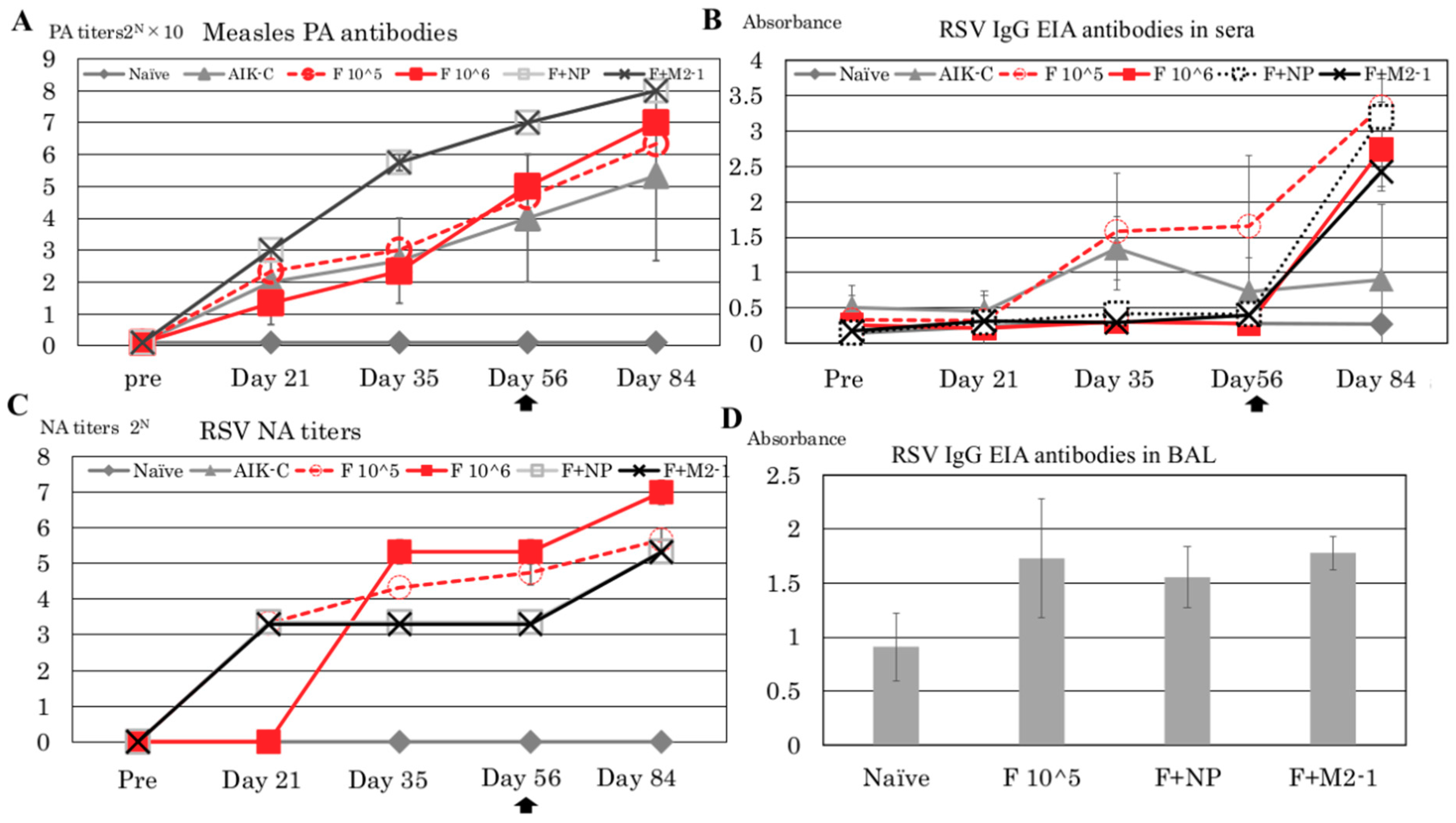
3.2. Detection of PA Antibodies Against Measles Virus and NA Against RSV
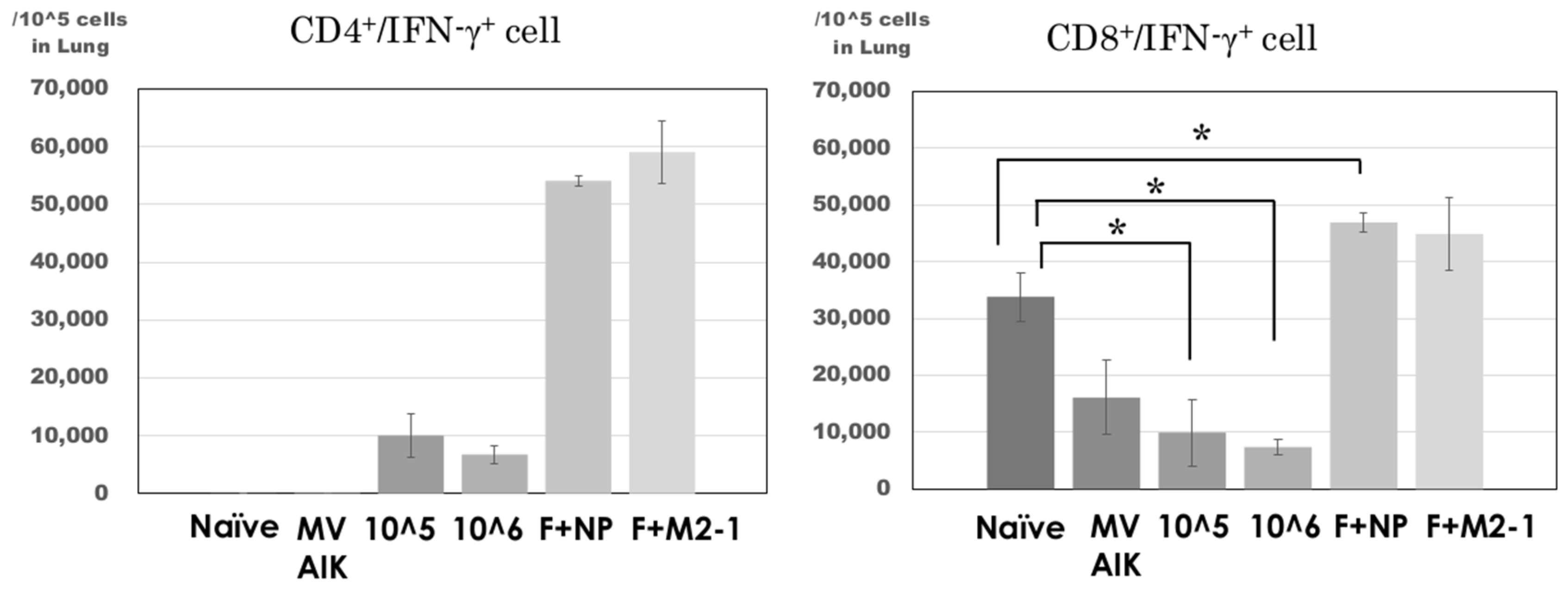
3.3. RSV-Specific CD8+/IFN-γ+ Cells in the Spleen
3.4. Analysis of CD4+/IFN-γ+ and CD8+/IFN-γ+ Cells in the Lung
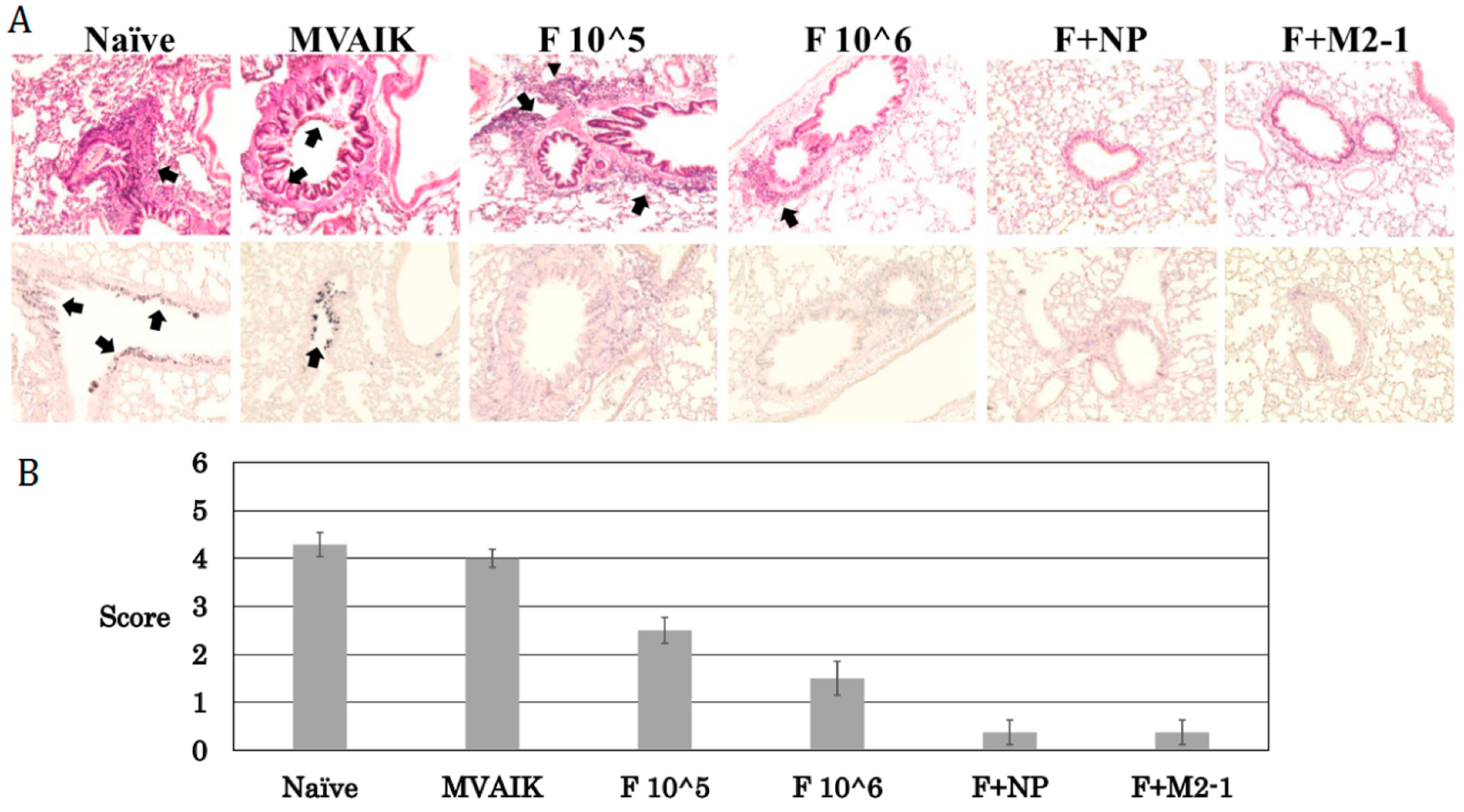
3.5. Histopathological Scoring
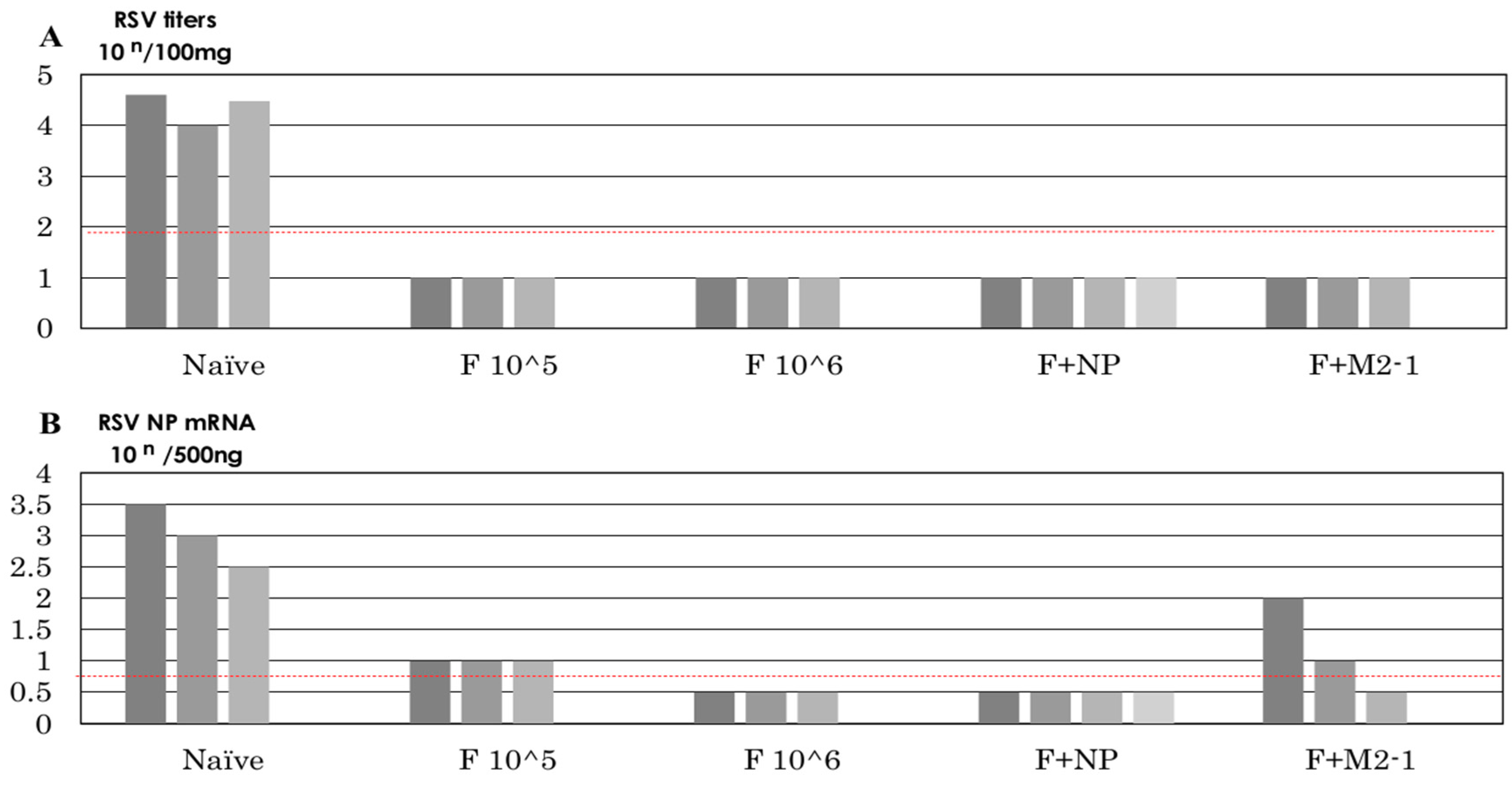
3.6. Recovery of Infectious Virus after the Challenge
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BAL | Bronchoalveolar lavage |
| CTL | cytotoxic T lymphocytes |
| FI-RSV | formalin-inactivated RSV |
| MV | measles virus |
| NA | neutralizing antibody |
| NP | nucleoprotein |
| PA | particle agglutination |
| RSV | respiratory syncytial virus |
| RSV-F | RSV-fusion protein |
References
- Cherukuri, A.; Patton, K.; Gasser, R.A., Jr.; Zuo, F.; Woo, J.; Esser, M.T. Adults 65 years old and older have reduced numbers of functional memory T cells to respiratory syncytial virus fusion protein. Clin. Vaccine Immunol. 2013, 20, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.; Nokes, D.J.; Gessner, B.D.; Dherani, M.; Madhi, S.A.; Singleton, R.J.; O′Brien, K.L.; Roca, A.; Wright, P.F.; Bruce, N.; et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef]
- Murphy, B.R.; Graham, B.S.; Prince, G.A.; Walsh, E.E.; Chanock, R.M.; Karzon, D.T.; Wright, P.F. Serum and nasal-wash immunoglobulin G and A antibody response of infants and children to respiratory syncytial virus F and G glycoproteins following primary infection. J. Clin. Microbiol. 1986, 23, 1009–1014. [Google Scholar] [PubMed]
- Yamaji, Y.; Yasui, Y.; Nakayama, T. Development of Acquired Immunity following Repeated Respiratory Syncytial Virus Infections in Cotton Rats. PLoS ONE 2016, 11, e0155777. [Google Scholar] [CrossRef] [PubMed]
- Prince, G.A.; Horswood, R.L.; Chanock, R.M. Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. J. Virol. 1985, 55, 517–520. [Google Scholar] [PubMed]
- Kapikian, A.Z.; Mitchell, R.H.; Chanock, R.M.; Shvedoff, R.A.; Stewart, C.E. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with and inactivated RS virus vaccine. Am. J. Epidemiol. 1969, 89, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Canchola, J.G.; Brandt, C.D.; Pyles, G.; Chanock, R.M.; Jensen, K.; Parrott, R.H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 1969, 89, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Prince, G.A.; Jenson, A.B.; Hemming, V.G.; Murphy, B.R.; Walsh, E.E.; Horswood, R.L.; Chanock, R.M. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactivated virus. J. Virol. 1986, 57, 721–728. [Google Scholar] [PubMed]
- Murphy, B.R.; Walsh, E.E. Formalin-inactivated respiratory syncytial virus vaccine induces antibodies to the fusion glycoprotein that are deficient in fusion-inhibiting activity. J. Clin. Microbiol. 1988, 26, 1595–1597. [Google Scholar] [PubMed]
- Moghaddam, A.; Olszewska, W.; Wang, B.; Tregoning, J.S.; Helson, R.; Sattentau, Q.J.; Openshaw, P.J. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat. Med. 2006, 12, 905–907. [Google Scholar] [CrossRef] [PubMed]
- Piedra, P.A.; Wyde, P.R.; Castleman, W.L.; Ambrose, M.W.; Jewell, A.M.; Speelman, D.J.; Hildreth, S.W. Enhanced pulmonary pathology associated with the use of formalin-inactivated respiratory syncytial virus vaccine in cotton rats is not a unique viral phenomenon. Vaccine 1993, 11, 1415–1423. [Google Scholar] [CrossRef]
- Olson, M.R.; Hartwig, S.M.; Varga, S.M. The number of respiratory syncytial virus (RSV)-specific memory CD8 T cells in the lung is critical for their ability to inhibit RSV vaccine-enhanced pulmonary eosinophilia. J. Immunol. 2008, 181, 7958–7968. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.R.; Varga, S.M. CD8 T cells inhibit respiratory syncytial virus (RSV) vaccine-enhanced disease. J. Immunol. 2007, 179, 5415–5424. [Google Scholar] [CrossRef] [PubMed]
- Hussell, T.; Baldwin, C.J.; O’Garra, A.; Openshaw, P.J. CD8+ T cells control Th2-driven pathology during pulmonary respiratory syncytial virus infection. Eur. J. Immunol. 1997, 27, 3341–3349. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Zhang, H.; Hai, Y.; Cui, Y.; Wei, L.; Li, N.; Liu, J.; Li, C.; Liu, Y. Interleukin-27 inhibits vaccine-enhanced pulmonary disease following respiratory syncytial virus infection by regulating cellular memory responses. J. Virol. 2012, 86, 4505–4517. [Google Scholar] [CrossRef] [PubMed]
- Woolums, A.R.; Gunther, R.A.; McArthur-Vaughan, K.; Anderson, M.L.; Omlor, A.; Boyle, G.A.; Friebershauser, K.E.; Mclnturff, P.S.; Gershwin, L.J. Cytotoxic T lymphocyte activity and cytokine expression in calves vaccinated with formalin-inactivated bovine respiratory syncytial virus prior to challenge. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 57–74. [Google Scholar] [CrossRef]
- Brandenburg, A.H.; Kleinjan, A.; van Het Land, B.; Moll, H.A.; Timmerman, H.H.; de Swart, R.L.; Neijens, H.J.; Fokkens, W.; Osterhaus, A.D. Type 1-like immune response is found in children with respiratory syncytial virus infection regardless of clinical severity. J. Med. Virol. 2000, 62, 267–277. [Google Scholar] [CrossRef]
- Chang, J.; Srikiatkhachorn, A.; Braciale, T.J. Visualization and characterization of respiratory syncytial virus F-specific CD8(+) T cells during experimental virus infection. J. Immunol. 2001, 167, 4254–4260. [Google Scholar] [CrossRef] [PubMed]
- De Baets, S.; Schepens, B.; Sedeyn, K.; Schotsaert, M.; Roose, K.; Bogaert, P.; Fiers, P.; Saelens, X. Recombinant influenza virus carrying the respiratory syncytial virus (RSV) F85-93 CTL epitope reduces RSV replication in mice. J. Virol. 2013, 87, 3314–3323. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Qi, X.; Gong, W.; Mei, X.; Wei, L.; Ma, C.; Yin, X. Long-lasting balanced immunity and protective efficacy against respiratory syncytial virus in mice induced by a recombinant protein G1F/M2. Vaccine 2007, 25, 7422–7428. [Google Scholar] [CrossRef] [PubMed]
- Cherukuri, A.; Stokes, K.L.; Patton, K.; Kuo, H.; Sakamoto, K.; Lambert, S.; Stillman, E.; Moore, M.L.; Lee, S. An adjuvanted respiratory syncytial virus fusion protein induces protection in aged BALB/c mice. Immun. Ageing 2012, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.S. Vaccines against respiratory syncytial virus: the time has finally come. Vaccine 2016, 34, 3535–3541. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Sawada, A.; Yamaji, Y.; Ito, T. Recombinant measles AIK-C vaccine strain expressing heterologous virus antigens. Vaccine 2016, 34, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Sawada, A.; Komase, K.; Nakayama, T. AIK-C measles vaccine expressing fusion protein of respiratory syncytial virus induces protective antibodies in cotton rats. Vaccine 2011, 29, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, Y.; Nakayama, T. Recombinant measles viruses expressing respiratory syncytial virus proteins induced virus-specific CTL responses in cotton rats. Vaccine 2014, 32, 4529–4536. [Google Scholar] [CrossRef] [PubMed]
- Lukens, M.V.; Claassen, E.A.; de Graaff, P.M.; van Dijk, M.E.; Hoogerhout, P.; Toebes, M.; Schumacher, T.N.; van der Most, R.G.; Kimpen, J.L.; van Bleek, G.M. Characterization of the CD8+ T cell responses directed against respiratory syncytial virus during primary and secondary infection in C57BL/6 mice. Virology 2006, 352, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.R.; Varga, S.M. Pulmonary immunity and immunopathology: lessons from respiratory syncytial virus. Expert Rev. Vaccines 2008, 7, 1239–1255. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.A.; Galarneau, J.R.; Bowenkamp, K.E.; Swanson, K.A.; Palmer, G.A.; Palladino, G.; Markovits, J.E.; Valiante, N.M.; Dormitzer, P.R.; Otten, G.R. The role of non-viral antigens in the cotton rat model of respiratory syncytial virus vaccine-enhanced disease. Vaccine 2013, 31, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.L.; Hill, M.G.; Cristina, J.; Grosfeld, H. Transcription elongation factor of respiratory syncytial virus, a nonsegmented negative-strand RNA virus. Proc. Natl. Acad. Sci. USA 1996, 93, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Fearns, R.; Collins, P.L. Role of the M2-1 transcription antitermination protein of respiratory syncytial virus in sequential transcription. J. Virol. 1999, 73, 5852–5864. [Google Scholar] [PubMed]
- Hardy, R.W.; Harmon, S.B.; Wertz, G.W. Diverse gene junctions of respiratory syncytial virus modulate the efficiency of transcription termination and respond differently to M2-mediated antitermination. J. Virol. 1999, 73, 170–176. [Google Scholar] [PubMed]
- Murphy, L.B.; Loney, C.; Murray, J.; Bhella, D.; Ashton, P.; Yeo, R.P. Investigations into the amino-terminal domain of the respiratory syncytial virus nucleocapsid protein reveal elements important for nucleocapsid formation and interaction with the phosphoprotein. Virology 2003, 307, 143–153. [Google Scholar] [CrossRef]
- Mcellan, J.S. Neutralizing epitopes on the respiratory syncytial virus fusion glycoprotein. Curr. Opin. Virol. 2015, 11, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Ngwuta, J.O.; Chen, M.; Modjarrad, K.; Joyce, M.G.; Kanekiyo, M.; Kumar, A.; Yassine, H.M.; Moin, S.M.; Killikelly, A.M.; Chuang, G.-Y.; et al. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci. Transl. Med. 2015, 7, 309. [Google Scholar] [CrossRef] [PubMed]
- Fahy, R.J.; Diaz, P.T.; Hart, J.; Wewers, M.D. BAL and serum IgG levels in healthy asymptomatic HIV-infected patients. Chest 2001, 119, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Peebles, R.S., Jr.; Liu, M.C.; Lichtenstein, L.M.; Hamilton, R.G. IgA, IgG and IgM quantification in bronchoalveolar lavage fluids from allergic rhinitics, allergic asthmatics, and normal subjects by monoclonal antibody-based immunoenzymetric assays. J. Immunol. Methods 1995, 179, 77–86. [Google Scholar] [CrossRef]
- Durant, L.R.; Makris, S.; Voorburg, C.M.; Loebbermann, J.; Johansson, C.; Openshaw, P.J. Regulatory T cells prevent Th2 immune responses and pulmonary eosinophilia during respiratory syncytial virus infection in mice. J. Virol. 2013, 87, 10946–10954. [Google Scholar] [CrossRef] [PubMed]
- Loebbermann, J.; Schnoeller, C.; Thornton, H.; Durant, L.; Sweeney, N.P.; Schuijs, M.; O′Garra, A.; Johansson, C. Openshaw, P.J. IL-10 regulates viral lung immunopathology during acute respiratory syncytial virus infection in mice. PLoS ONE 2012, 7, e32371. [Google Scholar] [CrossRef] [PubMed]
- Srikiatkhachorn, A.; Braciale, T.J. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J. Exp. Med. 1997, 186, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.S.; Modjarrad, K.; McLellan, J.S. Novel antigens for RSV vaccines. Curr Opin Immunol 2015, 35, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Tidjani, O.; Grunitsky, B.; Guérin, N.; Lévy-Bruhl, D.; Lecam, N.; Xuereff, C.; Tatagan, K. Serological effects of Edmonston-Zagreb, Schwarz, and AIK-C measles vaccine strains given at ages 4-5 or 8-10 months. Lancet 1989, 334, 1357–1360. [Google Scholar] [CrossRef]
- Bolotovski, V.M.; Grabowsky, M.; Clements, C.J.; Albrecht, P.; Brenner, E.R.; Zargaryantzs, A.I.; Litvinov, S.K.; Mikheyeva, I.V. Immunization of 6 and 9 month old infants with AIK-C, Edmonston-Zagreb, Leningrad-16 and Schwarz strains of measles vaccine. Int. J. Epidemiol. 1994, 23, 1069–1077. [Google Scholar] [CrossRef] [PubMed]

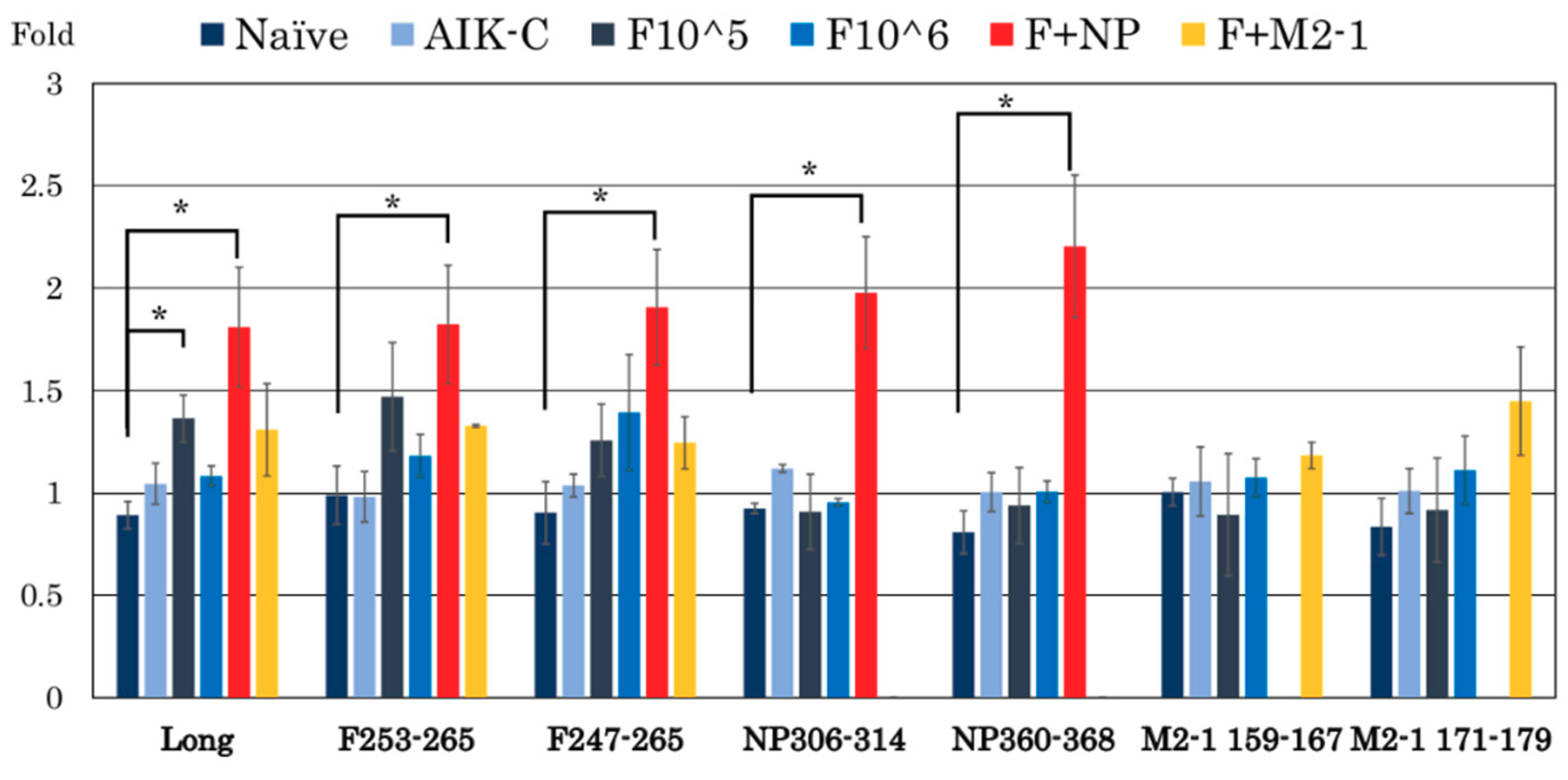
| Peptides | Sequence | MHC |
|---|---|---|
| F247–261 | VSTYMLTNSELLSLI | unknown |
| F253–265 | TNSELLSLINDMP | unknown |
| NP306–314 | NPKASLLSL | HLA-B7 (Human) |
| NP360–368 | NGVINYSVL | H-2b (Mouse C57BL/6) |
| M2-1159–167 | KTIKNTLDI | H-2b (Mouse C57BL/6) |
| M2-1171–179 | ITINNPKEL | H-2b (Mouse C57BL/6) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaji, Y.; Sawada, A.; Yasui, Y.; Ito, T.; Nakayama, T. Simultaneous Administration of Recombinant Measles Viruses Expressing Respiratory Syncytial Virus Fusion (F) and Nucleo (N) Proteins Induced Humoral and Cellular Immune Responses in Cotton Rats. Vaccines 2019, 7, 27. https://doi.org/10.3390/vaccines7010027
Yamaji Y, Sawada A, Yasui Y, Ito T, Nakayama T. Simultaneous Administration of Recombinant Measles Viruses Expressing Respiratory Syncytial Virus Fusion (F) and Nucleo (N) Proteins Induced Humoral and Cellular Immune Responses in Cotton Rats. Vaccines. 2019; 7(1):27. https://doi.org/10.3390/vaccines7010027
Chicago/Turabian StyleYamaji, Yoshiaki, Akihito Sawada, Yosuke Yasui, Takashi Ito, and Tetsuo Nakayama. 2019. "Simultaneous Administration of Recombinant Measles Viruses Expressing Respiratory Syncytial Virus Fusion (F) and Nucleo (N) Proteins Induced Humoral and Cellular Immune Responses in Cotton Rats" Vaccines 7, no. 1: 27. https://doi.org/10.3390/vaccines7010027
APA StyleYamaji, Y., Sawada, A., Yasui, Y., Ito, T., & Nakayama, T. (2019). Simultaneous Administration of Recombinant Measles Viruses Expressing Respiratory Syncytial Virus Fusion (F) and Nucleo (N) Proteins Induced Humoral and Cellular Immune Responses in Cotton Rats. Vaccines, 7(1), 27. https://doi.org/10.3390/vaccines7010027




