The Role of Serotype-Specific Immunological Memory in Pneumococcal Vaccination: Current Knowledge and Future Prospects
Abstract
1. Introduction
2. Generation of Memory B Cells in Response to Vaccination: Current Knowledge
3. Enumeration of Human Antigen-Specific Memory B Cells in Peripheral Blood
4. Memory B Cells Response to Pneumococcal Vaccination
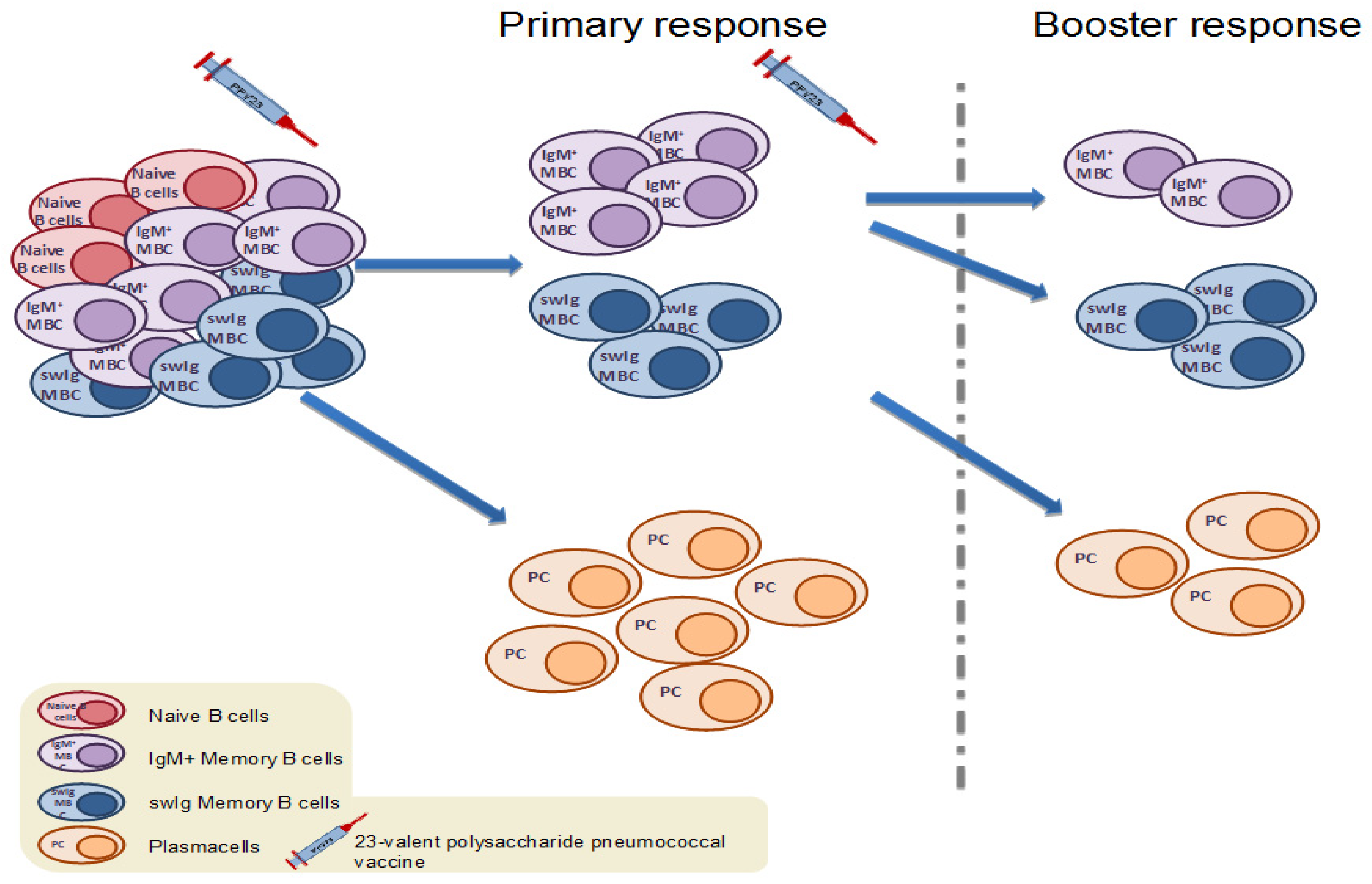
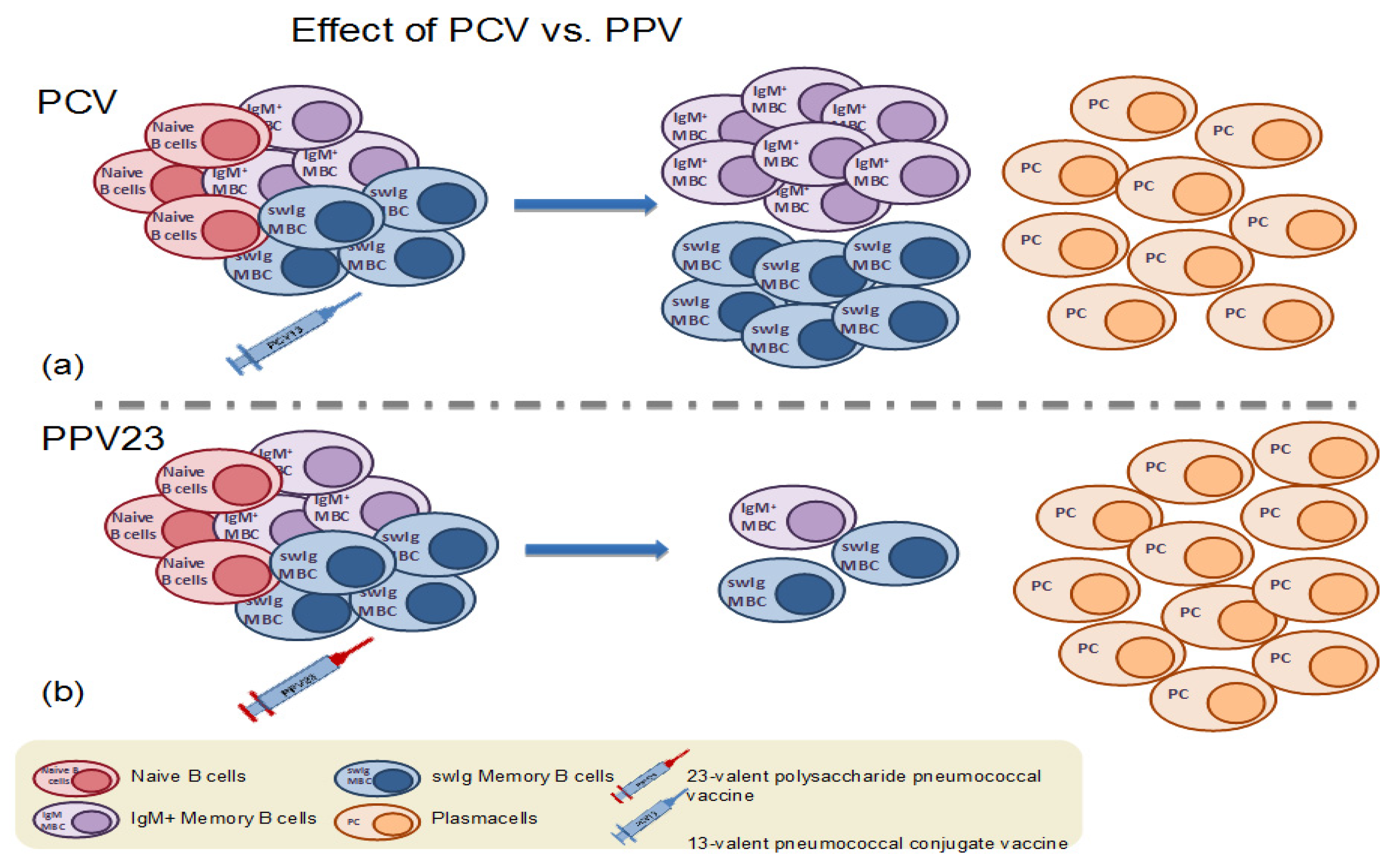
4.1. Memory B Cell Response to Immunization with the 23-Valent Plain Polysaccharide Pneumococcal Vaccine
4.2. Memory B Cell Response to Primary Immunization with Pneumococcal Conjugate Vaccines in Infants and Children
4.3. Memory B Cell Response to Primary Immunization with Pneumococcal Conjugate Vaccines in Adults
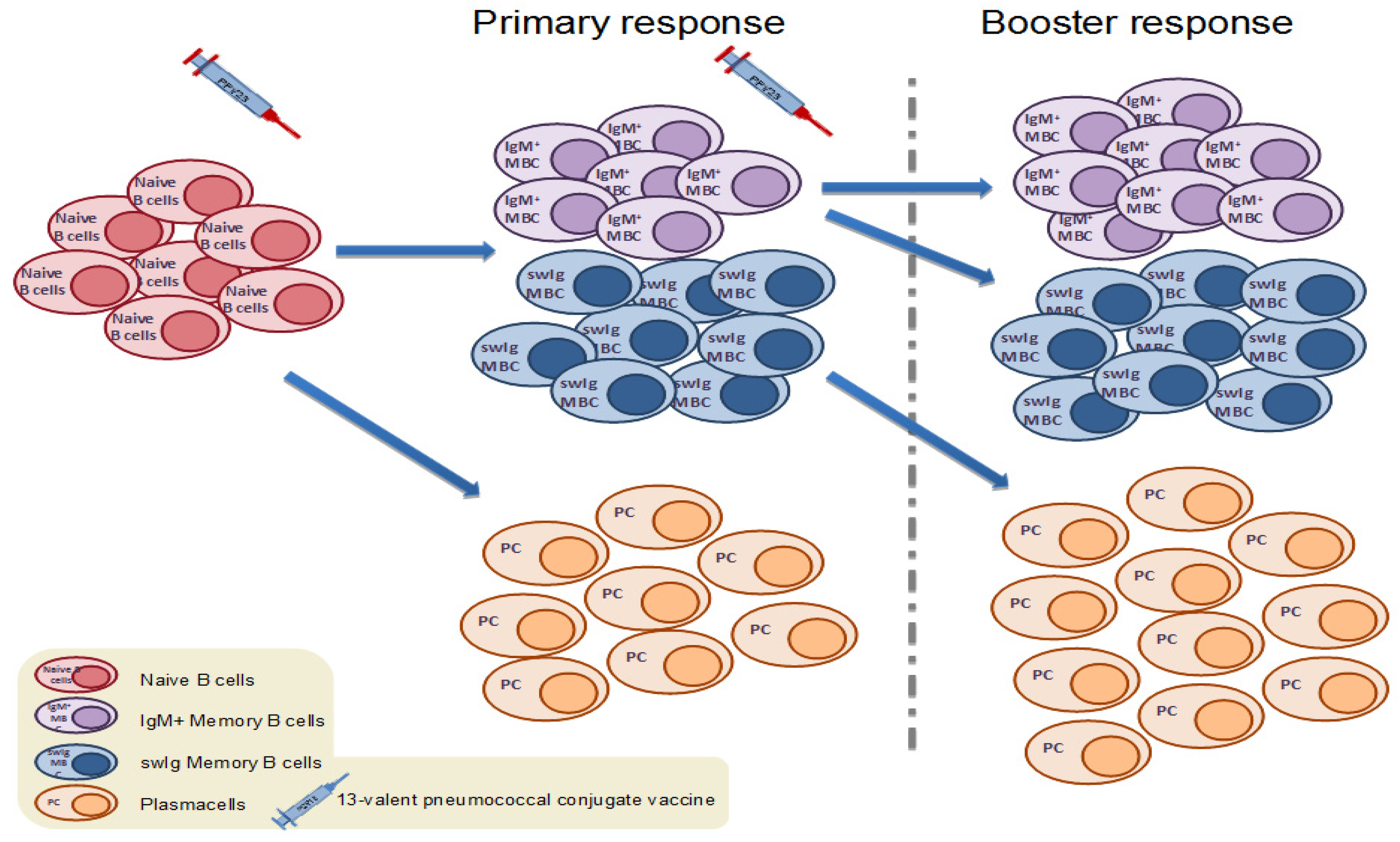
4.4. Memory B Cell Response to Booster Immunization with Pneumococcal Conjugate Vaccines in Children
4.5. Memory B Cell Response to Booster Immunization with Pneumococcal Conjugate Vaccines in Adults
4.6. Differences in Memory B Cell Response to Pneumococcal Vaccination Depending on the Recipient’s Health Condition
5. Correlation between Serotype-Specific MBCs and Antibody Responses
6. The Potential of MBCs as Correlates of Protection
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Centers for Disease Control and Prevention (CDC). Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Streptococcus Pneumoniae, 2010; CDC: Atlanta, GA, USA, 2011.
- CDC. Updated Recommendations for Prevention of Invasive Pneumococcal Disease among Adults Using the 23-Valent Pneumococcal Polysaccharide Vaccine (PPSV23); CDC: Atlanta, GA, USA, 2010.
- Austrian, R.; Douglas, R.M.; Schiffman, G.; Coetzee, A.M.; Koornhof, H.J.; Hayden-Smith, S.; Reid, R.D. Prevention of pneumococcal pneumonia by vaccination. Trans. Assoc. Am. Physicians 1976, 89, 184–194. [Google Scholar] [PubMed]
- Riley, I.D.; Tarr, P.I.; Andrews, M.; Pfeiffer, M.; Howard, R.; Challands, P.; Jennison, G. Immunisation with a polyvalent pneumococcal vaccine. Reduction of adult respiratory mortality in a New Guinea Highlands community. Lancet (Lond. Engl.) 1977, 1, 1338–1341. [Google Scholar] [CrossRef]
- Stein, K.E. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J. Infect. Dis. 1992, 165 (Suppl. 1), S49–S52. [Google Scholar] [CrossRef]
- Black, S.; Shinefield, H.; Fireman, B.; Lewis, E.; Ray, P.; Hansen, J.R.; Elvin, L.; Ensor, K.M.; Hackell, J.; Siber, G.; et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr. Infect. Dis. J. 2000, 19, 187–195. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.L.; Moulton, L.H.; Reid, R.; Weatherholtz, R.; Oski, J.; Brown, L.; Kumar, G.; Parkinson, A.; Hu, D.; Hackell, J.; et al. Efficacy and safety of seven-valent conjugate pneumococcal vaccine in American Indian children: Group randomised trial. Lancet 2003, 362, 355–361. [Google Scholar] [CrossRef]
- WHO Weekly epidemiological record Relevé épidémiologique hebdomadaire advisory group of experts. Wkly. Epidemiol. Rec. 2012, 88, 1–16.
- Deloria Knoll, M.; Park, D.E.; Johnson, T.S.; Chandir, S.; Nonyane, B.A.S.; Conklin, L.; Fleming-Dutra, K.E.; Loo, J.D.; Goldblatt, D.; Whitney, C.G.; et al. Systematic Review of the Effect of Pneumococcal Conjugate Vaccine Dosing Schedules on Immunogenicity. Pediatr. Infect. Dis. J. 2014, 33, S119–S129. [Google Scholar] [CrossRef]
- Rückinger, S.; Dagan, R.; Albers, L.; Schönberger, K.; von Kries, R. Immunogenicity of pneumococcal conjugate vaccines in infants after two or three primary vaccinations: A systematic review and meta-analysis. Vaccine 2011, 29, 9600–9606. [Google Scholar] [CrossRef]
- Scott, P.; Rutjes, A.W.S.; Bermetz, L.; Robert, N.; Scott, S.; Lourenço, T.; Egger, M.; Low, N. Comparing pneumococcal conjugate vaccine schedules based on 3 and 2 primary doses: Systematic review and meta-analysis. Vaccine 2011, 29, 9711–9721. [Google Scholar] [CrossRef]
- Whitney, C.G.; Goldblatt, D.; O’Brien, K.L. Dosing schedules for pneumococcal conjugate vaccine: Considerations for policy makers. Pediatr. Infect. Dis. J. 2014, 33, 172–181. [Google Scholar] [CrossRef]
- Mueller, J.E.; Yaro, S.; Ouédraogo, M.S.; Levina, N.; Njanpop-Lafourcade, B.-M.; Tall, H.; Idohou, R.S.; Sanou, O.; Kroman, S.S.; Drabo, A.; et al. Pneumococci in the African meningitis belt: Meningitis incidence and carriage prevalence in children and adults. PLoS ONE 2012, 7, e52464. [Google Scholar] [CrossRef] [PubMed]
- Kwambana-Adams, B.A.; Asiedu-Bekoe, F.; Sarkodie, B.; Afreh, O.K.; Kuma, G.K.; Owusu-Okyere, G.; Foster-Nyarko, E.; Ohene, S.-A.; Okot, C.; Worwui, A.K.; et al. An outbreak of pneumococcal meningitis among older children (≥5 years) and adults after the implementation of an infant vaccination programme with the 13-valent pneumococcal conjugate vaccine in Ghana. BMC Infect. Dis. 2016, 16, 575. [Google Scholar] [CrossRef] [PubMed]
- Joint Committee on Vaccination and immunisation (JCVI). Minute of the Meeting on 4 October 2017; JCVI: London, UK, 2017.
- Goldblatt, D.; Southern, J.; Andrews, N.J.; Burbidge, P.; Partington, J.; Roalfe, L.; Valente Pinto, M.; Thalasselis, V.; Plested, E.; Richardson, H.; et al. Pneumococcal conjugate vaccine 13 delivered as one primary and one booster dose (1 + 1) compared with two primary doses and a booster (2 + 1) in UK infants: A multicentre, parallel group randomised controlled trial. Lancet Infect. Dis. 2018, 18, 171–179. [Google Scholar] [CrossRef]
- Perniciaro, S.; van der Linden, M. Reassessing the 1 + 1 pneumococcal conjugate vaccine schedule. Lancet Infect. Dis. 2018, 18, 381–382. [Google Scholar] [CrossRef]
- Isturiz, R.; Gessner, B.D.; Madhava, H.; Paradiso, P.; Jodar, L. Reassessing the 1 + 1 pneumococcal conjugate vaccine schedule. Lancet Infect. Dis. 2018, 18, 382–383. [Google Scholar] [CrossRef]
- Schwickert, T.A.; Lindquist, R.L.; Shakhar, G.; Livshits, G.; Skokos, D.; Kosco-Vilbois, M.H.; Dustin, M.L.; Nussenzweig, M.C. In vivo imaging of germinal centres reveals a dynamic open structure. Nature 2007, 446, 83–87. [Google Scholar] [CrossRef] [PubMed]
- De Silva, N.S.; Klein, U. Dynamics of B cells in germinal centres. Nat. Rev. Immunol. 2015, 15, 137–148. [Google Scholar] [CrossRef]
- Corcoran, L.M.; Tarlinton, D.M. Regulation of germinal center responses, memory B cells and plasma cell formation-an update. Curr. Opin. Immunol. 2016, 39, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Mesin, L.; Ersching, J.; Victora, G.D. Germinal Center B Cell Dynamics. Immunity 2016, 45, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, T.; Kometani, K.; Ise, W. Memory B cells. Nat. Publ. Gr. 2015, 15, 149–159. [Google Scholar] [CrossRef]
- Seifert, M.; Przekopowitz, M.; Taudien, S.; Lollies, A.; Ronge, V.; Drees, B.; Lindemann, M.; Hillen, U.; Engler, H.; Singer, B.B.; et al. Functional capacities of human IgM memory B cells in early inflammatory responses and secondary germinal center reactions. Proc. Natl. Acad. Sci. USA 2015, 112, E546–E555. [Google Scholar] [CrossRef] [PubMed]
- Kugelberg, E. B cell memory: Making sense in humans. Nat. Rev. Immunol. 2015, 15, 3822. [Google Scholar] [CrossRef] [PubMed]
- Blanchard-Rohner, G.; Snape, M.D.; Kelly, D.F.; John, T.; Morant, A.; Yu, L.-M.; Borkowski, A.; Ceddia, F.; Borrow, R.; Siegrist, C.-A.; et al. The magnitude of the antibody and memory B cell responses during priming with a protein-polysaccharide conjugate vaccine in human infants is associated with the persistence of antibody and the intensity of booster response. J. Immunol. 2008, 180, 2165–2173. [Google Scholar] [CrossRef] [PubMed]
- Perrett, K.P.; Jin, C.; Clutterbuck, E.A.; John, T.M.; Winter, A.P.; Kibwana, E.; Yu, L.-M.; Curtis, N.; Pollard, A.J. B cell memory to a serogroup C meningococcal conjugate vaccine in childhood and response to booster: Little association with serum IgG antibody. J. Immunol. 2012, 189, 2673–2681. [Google Scholar] [CrossRef]
- Blanchard-Rohner, G.; Snape, M.D.; Kelly, D.F.; O’Connor, D.; John, T.; Clutterbuck, E.A.; Ohene-Kena, B.; Klinger, C.L.; Odrljin, T.; Pollard, A.J. The B-cell response to a primary and booster course of MenACWY-CRM197vaccine administered at 2, 4 and 12 months of age. Vaccine 2013, 31, 2441–2448. [Google Scholar] [CrossRef]
- Clutterbuck, E.A.; Oh, S.; Hamaluba, M.; Westcar, S.; Beverley, P.C.L.L.; Pollard, A.J. Serotype-specific and age-dependent generation of pneumococcal polysaccharide-speciflc memory B-cell and antibody responses to immunization with a pneumococcal conjugate vaccine. Clin. Vaccine Immunol. 2008, 15, 182–193. [Google Scholar] [CrossRef]
- Clutterbuck, E.A.; Lazarus, R.; Yu, L.-M.; Bowman, J.; Bateman, E.A.L.; Diggle, L.; Angus, B.; Peto, T.E.; Beverley, P.C.; Mant, D.; et al. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J. Infect. Dis. 2012, 205, 1408–1416. [Google Scholar] [CrossRef]
- Farmaki, P.F.; Chini, M.C.; Mangafas, N.M.; Tzanoudaki, M.T.; Piperi, C.P.; Lazanas, M.Z.; Spoulou, V.S. Immunogenicity and Immunological Memory Induced by the 13-Valent Pneumococcal Conjugate Followed by the 23-Valent Polysaccharide Vaccine in HIV-Infected Adults. J. Infect. Dis. 2018, 218, 26–34. [Google Scholar] [CrossRef]
- Ohtola, J.A. Quantitative and Functional Antibody Responses to the 13-Valent Conjugate and/or 23-Valent Purified Polysaccharide Vaccine in Aging HIV-Infected Adults. J. AIDS Clin. Res. 2016, 07, 617–630. [Google Scholar] [CrossRef]
- Trück, J.; Lazarus, R.; Clutterbuck, E.A.; Bowman, J.; Kibwana, E.; Bateman, E.A.L.L.; Pollard, A.J. The zwitterionic type I Streptococcus pneumoniae polysaccharide does not induce memory B cell formation in humans. Immunobiology 2013, 218, 368–372. [Google Scholar] [CrossRef]
- Clutterbuck, E.A.; Salt, P.; Oh, S.; Marchant, A.; Beverley, P.; Pollard, A.J. The kinetics and phenotype of the human B-cell response following immunization with a heptavalent pneumococcal-CRM conjugate vaccine. Immunology 2006, 119, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, K.K.; Kirchner, H.L.; Kimmel, R.; Greenspan, N.S.; Schreiber, J.R. Significant Variation in Serotype-Specific Immunogenicity of the Seven-Valent Streptococcus pneumoniae Capsular Polysaccharide–CRM197 Conjugate Vaccine Occurs Despite Vigorous T Cell Help Induced by the Carrier Protein. J. Infect. Dis. 2003, 187, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Baxendale, H.E.; Keating, S.M.; Johnson, M.; Southern, J.; Miller, E.; Goldblatt, D. The early kinetics of circulating pneumococcal-specific memory B cells following pneumococcal conjugate and plain polysaccharide vaccines in the elderly. Vaccine 2010, 28, 4763–4770. [Google Scholar] [CrossRef] [PubMed]
- Papadatou, I.; Piperi, C.; Alexandraki, K.; Kattamis, A.; Theodoridou, M.; Spoulou, V. Antigen-specific B-cell response to 13-valent pneumococcal conjugate vaccine in asplenic individuals with β-thalassemia previously immunized with 23-valent pneumococcal polysaccharide vaccine. Clin. Infect. Dis. 2014, 59, 862–865. [Google Scholar] [CrossRef] [PubMed]
- Licciardi, P.V.; Toh, Z.Q.; Clutterbuck, E.A.; Balloch, A.; Marimla, R.A.; Tikkanen, L.; Lamb, K.E.; Bright, K.J.; Rabuatoka, U.; Tikoduadua, L.; et al. No long-term evidence of hyporesponsiveness after use of pneumococcal conjugate vaccine in children previously immunized with pneumococcal polysaccharide vaccine. J. Allergy Clin. Immunol. 2016, 137, 1772–1779.e11. [Google Scholar] [CrossRef]
- Trück, J.; Thompson, A.; Morales-Aza, B.; Clutterbuck, E.A.; Voysey, M.; Clarke, E.; Snape, M.D.; Kelly, D.F.; Finn, A.; Pollard, A.J. Memory B cell response to a PCV-13 booster in 3.5 year old children primed with either PCV-7 or PCV-13. Vaccine 2017, 35, 2701–2708. [Google Scholar] [CrossRef] [PubMed]
- Trück, J.; Mitchell, R.; Jawad, S.; Clutterbuck, E.A.; Snape, M.D.; Kelly, D.F.; Voysey, M.; Pollard, A.J. Divergent Memory B Cell Responses in a Mixed Infant Pneumococcal Conjugate Vaccine Schedule. Pediatr. Infect. Dis. J. 2016, 36, 1. [Google Scholar] [CrossRef]
- Van Westen, E.; Wijmenga-Monsuur, A.J.; van Dijken, H.H.; van Gaans-van den Brink, J.A.M.; Kuipers, B.; Knol, M.J.; Berbers, G.A.M.; Sanders, E.A.M.; Rots, N.Y.; van Els, C.A.C.M. Differential B-cell memory around the 11-month booster in children vaccinated with a 10- or 13-valent pneumococcal conjugate vaccine. Clin. Infect. Dis. 2015, 61, 342–349. [Google Scholar] [CrossRef]
- Valentini, D.; Marcellini, V.; Bianchi, S.; Villani, A.; Facchini, M.; Donatelli, I.; Castrucci, M.R.; Marasco, E.; Farroni, C.; Carsetti, R. Generation of switched memory B cells in response to vaccination in Down syndrome children and their siblings. Vaccine 2015, 33, 6689–6696. [Google Scholar] [CrossRef]
- Licciardi, P.V.; Hoe, E.; Toh, Z.Q.; Balloch, A.; Moberley, S.; Binks, P.; Marimla, R.; Leach, A.; Skull, S.; Mulholland, K.; et al. Repeat pneumococcal polysaccharide vaccination does not impair functional immune responses among Indigenous Australians. Clin. Transl. Immunol. 2017, 6, e158. [Google Scholar] [CrossRef]
- Iyer, A.S.; Leggat, D.J.; Ohtola, J.A.; Duggan, J.M.; Georgescu, C.A.; Al Rizaiza, A.A.; Khuder, S.A.; Khaskhely, N.M.; Westerink, M.J. Response to Pneumococcal Polysaccharide Vaccination in HIV-Positive Individuals on Long Term Highly Active Antiretroviral Therapy. J. AIDS Clin. Res. 2015, 6, 421. [Google Scholar] [CrossRef] [PubMed]
- Leggat, D.J.; Iyer, A.S.; Ohtola, J.A.; Kommoori, S.; Georgescu, C.A.; Khuder, S.A.; Khaskhely, N.M.; Westerink, M.J. Response to Pneumococcal Polysaccharide Vaccination in Newly Diagnosed HIV-Positive Individuals. J. AIDS Clin. Res. 2015, 6, 419. [Google Scholar] [CrossRef] [PubMed]
- Leggat, D.J.; Khaskhely, N.M.; Iyer, A.S.; Mosakowski, J.; Thompson, R.S.; Weinandy, J.D.; Westerink, M.J.M.A.J. Pneumococcal polysaccharide vaccination induces polysaccharide-specific B cells in adult peripheral blood expressing CD19+CD20+CD3−CD70−CD27+IgM+CD43+CD5+/−. Vaccine 2013, 31, 4632–4640. [Google Scholar] [CrossRef] [PubMed]
- Leggat, D.J.; Thompson, R.S.; Khaskhely, N.M.; Iyer, A.S.; Westerink, M.A.J. The immune response to pneumococcal polysaccharides 14 and 23F among elderly individuals consists predominantly of switched memory B cells. J. Infect. Dis. 2013, 208, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Khaskhely, N.; Mosakowski, J.; Thompson, R.S.; Khuder, S.; Smithson, S.L.; Westerink, M.A.J. Phenotypic Analysis of Pneumococcal Polysaccharide-Specific B Cells. J. Immunol. 2012, 188, 2455–2463. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S.; Aubert, R.D.; Glidewell, J.; Ahmed, R. Tracking human antigen-specific memory B cells: A sensitive and generalized ELISPOT system. J. Immunol. Methods 2004, 286, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.; Kelly, D.F.; Pollard, A.J.; Trück, J. Polysaccharide-specific B cell responses to vaccination in humans. Hum. Vaccin. Immunother. 2014, 10, 1661–1668. [Google Scholar] [CrossRef]
- Ohtola, J.A.; Khaskhely, N.M.; Saul-Mcbeth, J.L.; Iyer, A.S.; Leggat, D.J.; Khuder, S.A.; Westerink, M.A.J. Alterations in serotype-specific B cell responses to the 13-valent pneumococcal conjugate vaccine in aging HIV-infected adults. Vaccine 2016, 34, 451–457. [Google Scholar] [CrossRef]
- Defrance, T.; Taillardet, M.; Genestier, L. T cell-independent B cell memory. Curr. Opin. Immunol. 2011, 23, 330–336. [Google Scholar] [CrossRef]
- Obukhanych, T.V.; Nussenzweig, M.C. T-independent type II immune responses generate memory B cells. J. Exp. Med. 2006, 203, 305–310. [Google Scholar] [CrossRef]
- Haas, K.M.; Poe, J.C.; Steeber, D.A.; Tedder, T.F. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity 2005, 23, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Westerink, M.A.J.; Schroeder, H.W.; Nahm, M.H.; Nahm, M.H. Immune Responses to pneumococcal vaccines in children and adults: Rationale for age-specific vaccination. Aging Dis. 2012, 3, 51–67. [Google Scholar] [PubMed]
- Licciardi, P.V.; Van Phan, T.; Toh, Z.Q.; Balloch, A.; Hong, N.V.P.; Trung, K.V.; Trang, D.V.T.; Bright, K.; Temple, B.; Hoan, P.T.; et al. Immunogenicity and memory B cell response following alternative pneumococcal vaccination strategies in Vietnam. In Proceedings of the ISPPD10, Glasgow, Scotland, 26–30 June 2016. [Google Scholar]
- Goronzy, J.J.; Weyand, C.M. Understanding immunosenescence to improve responses to vaccines. Nat. Immunol. 2013, 14, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Gilks, C.F.; Ojoo, S.A.; Ojoo, J.C.; Brindle, R.J.; Paul, J.; Batchelor, B.I.; Kimari, J.N.; Newnham, R.; Bwayo, J.; Plummer, F.A. Invasive pneumococcal disease in a cohort of predominantly HIV-1 infected female sex-workers in Nairobi, Kenya. Lancet (Lond. Engl.) 1996, 347, 718–723. [Google Scholar] [CrossRef]
- Shatz, D. V Vaccination considerations in the asplenic patient. Expert Rev. Vaccines 2005, 4, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Klugman, K.P.; Madhi, S.A.; Feldman, C. HIV and pneumococcal disease. Curr. Opin. Infect. Dis. 2007, 20, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Cameron, P.U.; Jones, P.; Gorniak, M.; Dunster, K.; Paul, E.; Lewin, S.; Woolley, I.; Spelman, D. Splenectomy associated changes in IgM memory B cells in an adult spleen registry cohort. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Moir, S.; Ho, J.; Malaspina, A.; Wang, W.; DiPoto, A.C.; O’Shea, M.A.; Roby, G.; Kottilil, S.; Arthos, J.; Proschan, M.A.; et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J. Exp. Med. 2008, 205, 1797–1805. [Google Scholar] [CrossRef] [PubMed]
- Moir, S.; Fauci, A.S. B cells in HIV infection and disease. Nat. Rev. Immunol. 2009, 9, 235–245. [Google Scholar] [CrossRef]
- Verstegen, R.H.J.; Driessen, G.J.; Bartol, S.J.W.; van Noesel, C.J.M.; Boon, L.; van der Burg, M.; van Dongen, J.J.M.; de Vries, E.; van Zelm, M.C. Defective B-cell memory in patients with Down syndrome. J. Allergy Clin. Immunol. 2014, 134, 1346–1353. [Google Scholar] [CrossRef]
- Carsetti, R.; Valentini, D.; Marcellini, V.; Scarsella, M.; Marasco, E.; Giustini, F.; Bartuli, A.; Villani, A.; Ugazio, A.G. Reduced numbers of switched memory B cells with high terminal differentiation potential in Down syndrome. Eur. J. Immunol. 2015, 45, 903–914. [Google Scholar] [CrossRef] [PubMed]
| PCV10 | PCV13 | PPV23 | |
| Valency | 10-valent | 13-valent | 23-valent |
| Serotypes included | 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F and 23F | 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F | 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F |
| Type | Conjugated PS | Conjugated PS | Plain PS |
| Carrier protein (s) | TT with serotype 18C, DT with 19F, PD with all other serotypes | CRM with each serotype | none |
| Polysaccharide amount | 1 μg/serotype (*3 μg/serotype for 4, 18C, 19F) | 2.2 μg/serotype (*4.4 μg/serotype for 6B) | 25 μg/serotype |
| Administration route | IM | IM | IM |
| Target population | healthy children | healthy children and adults >50 years of age | at risk population >2 years of age and adults >65 years of age |
| Reference | Population | Sample Size | Vaccine(s) Schedule | Method of MBC Enumeration |
|---|---|---|---|---|
| (a) Response to primary immunization with PCVs | ||||
| Clutterbuck et al. 2008, Clin. and Vac. Immunology [29] | adults 20–50 years and children 12m | 60 | 1–2 PCV7 | cultured ELISPOT |
| Clutterbuck et al. 2012, JID [30] | adults 50–70 years | 150 | 2PCV7 + PPV23 or PPV23 + 2PCV7 or PCV7-PPV23-PCV7 | cultured ELISPOT and Flow Cytometry |
| Farmaki et al. 2018, JID [31] | HIV+ adults | 40 | PCV13 + PPV23 | Flow Cytometry |
| Ohtola et al. 2016, Vaccine [32] | HIV+ vs. healthy controls, 50–65 years old | 51 | PCV13 + PPV23 or only PPV23 | Flow Cytometry |
| Truck et al. 2013, Immunobiology [33] | healthy adults 5–70 years | 84 | PPV23 or PCV7 | cultured ELISPOT |
| Clutterbuck et al. 2006, Immunology [34] | healthy adults | 10 | 1–2 PCV7 | cultured ELISPOT |
| Kamboj et al. 2003, JID [35] | healthy adults, 22–35 years | 24 | PPV23 or PCV7 | cultured ELISPOT |
| Baxendale et al. 2010, Vaccine [36] | healthy adults 50–80 years | 37 | PPV23 or PCV7 | cultured ELISPOT |
| (b) Response to booster immunization with PCVs | ||||
| Baxendale et al. 2010, Vaccine [36] | healthy adults 50–80 years | 37 | PPV23 or PCV7 | cultured ELISPOT |
| Papadatou et al.2014, CID [37] | asplenic adults (β–thalassemia), 19–48 years old | 39 | PCV13 | cultured ELISPOT |
| Clutterbuck et al. 2012, JID [30] | adults 50–70 years | 150 | 2PCV7 + PPV23 or PPV23 + 2PCV7 or PCV7-PPV23-PCV7 | cultured ELISPOT and Flow Cytometry |
| Farmaki et al. 2018, JID [31] | HIV+ adults | 40 | PCV13 + PPV23 | Flow Cytometry |
| Licciardi et al. 2016, J. Allergy Clin.Immun. [38] | healthy Fijian children | 185 | PCV13 | cultured ELISPOT |
| Truck et al. 2017, Vaccine [39] | healthy children 3,5 years | 62 | PCV13 | cultured ELISPOT |
| Truck et al. 2016, Ped.Inf.Dis. J. [40] | healthy children 1,2 years old | 135 | PCV10 or 13 | cultured ELISPOT |
| van Westen et al. 2015, CID [41] | infants 1 year | 104 | PCV10 or PCV13 | cultured ELISPOT |
| Valentini et al. 2015, Vaccine [42] | children with Down Syndrome vs. controls, 3–12 years old | 30 | PCV13 | cultured ELISPOT |
| (c) Response to immunization with PPV23 | ||||
| Licciardi et al. 2017, Clin. & Transl. Immun. [43] | Indigenous vs. non-indigenous Australians | 60 | PPV23 | cultured ELISPOT |
| Iyer et al. 2015, J AIDS Clin. Res. [44] | HIV+ adults on HAART vs. HIV- controls | 65 | PPV23 | Flow Cytometry |
| Leggat et al.2015, J AIDS Clin. Res. [45] | HIV+ newly diagnosed vs. HIV- controls | 65 | PPV23 | Flow Cytometry |
| Leggat et al. 2013, Vaccine [46] | healthy adults, 24–30 years | 17 | PPV23 | Flow Cytometry |
| Leggat et al.2013, JID [47] | elderly adults 64–88 years | 14 | PPV23 | Flow Cytometry |
| Khaskhely et. al. 2012, J Immunol. [48] | healthy adults 18–30 years | 22 | PPV23 | Flow Cytometry |
| Truck et al. 2013, Immunobiology [33] | healthy adults 5–70 years | 84 | PPV23 or PCV7 | cultured ELISPOT |
| Kamboj et al. 2003, JID [35] | healthy adults, 22–35 years | 24 | PPV23 or PCV7 | cultured ELISPOT |
| Baxendale et al. 2010, Vaccine [36] | healthy adults 50–80 years | 37 | PPV23 or PCV7 | cultured ELISPOT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadatou, I.; Tzovara, I.; Licciardi, P.V. The Role of Serotype-Specific Immunological Memory in Pneumococcal Vaccination: Current Knowledge and Future Prospects. Vaccines 2019, 7, 13. https://doi.org/10.3390/vaccines7010013
Papadatou I, Tzovara I, Licciardi PV. The Role of Serotype-Specific Immunological Memory in Pneumococcal Vaccination: Current Knowledge and Future Prospects. Vaccines. 2019; 7(1):13. https://doi.org/10.3390/vaccines7010013
Chicago/Turabian StylePapadatou, Ioanna, Irene Tzovara, and Paul V. Licciardi. 2019. "The Role of Serotype-Specific Immunological Memory in Pneumococcal Vaccination: Current Knowledge and Future Prospects" Vaccines 7, no. 1: 13. https://doi.org/10.3390/vaccines7010013
APA StylePapadatou, I., Tzovara, I., & Licciardi, P. V. (2019). The Role of Serotype-Specific Immunological Memory in Pneumococcal Vaccination: Current Knowledge and Future Prospects. Vaccines, 7(1), 13. https://doi.org/10.3390/vaccines7010013





