Microcrystalline Tyrosine (MCT®): A Depot Adjuvant in Licensed Allergy Immunotherapy Offers New Opportunities in Malaria
Abstract
:1. Introduction
2. Material and Methods
2.1. Procedures Involving Animals
2.2. Construction and Expression of rPvCSP-c
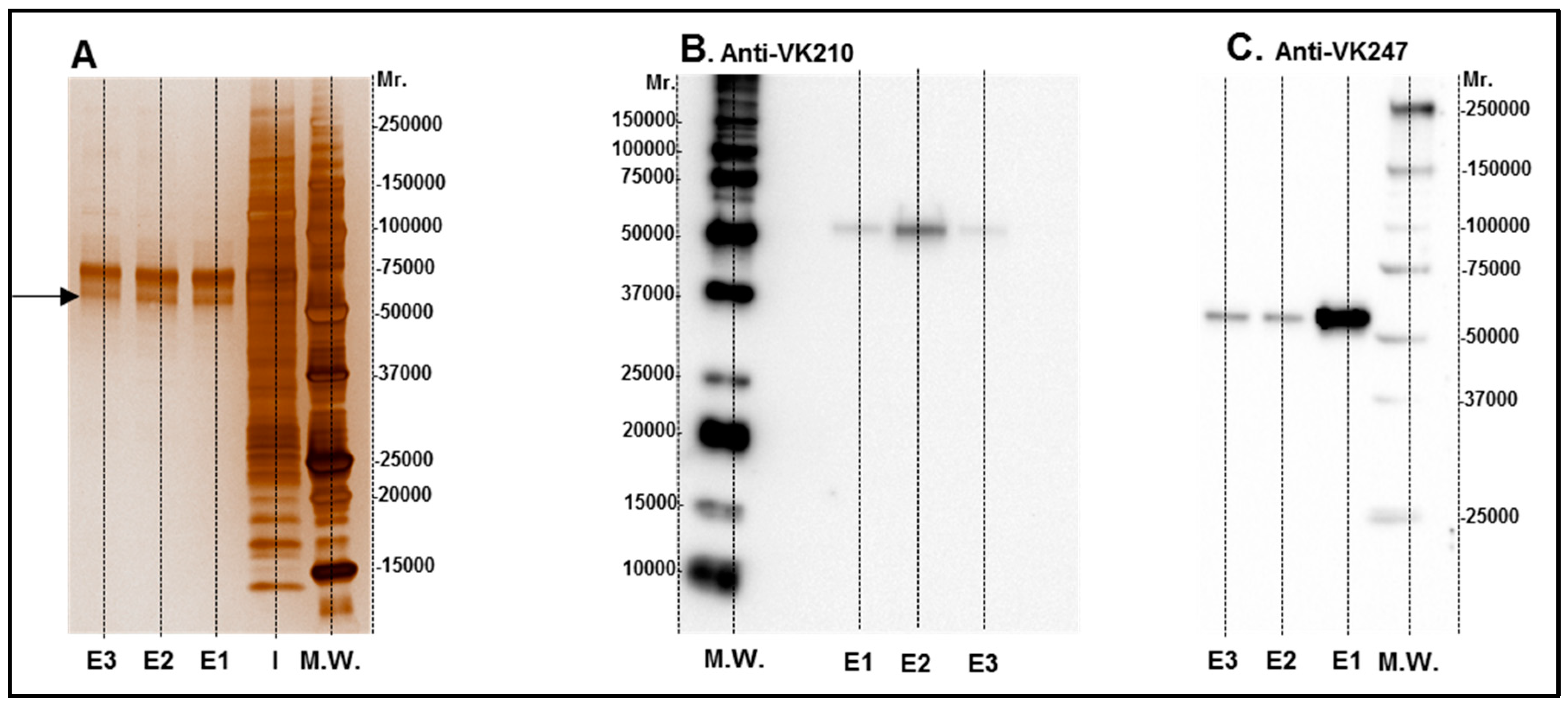
2.3. Purification of rPvCSP-c
2.4. Vaccine Formulation Using MCT and Aluminum Hydroxide Adjuvant (Alum) as Adjuvant Plus rPvCSP-c Protein
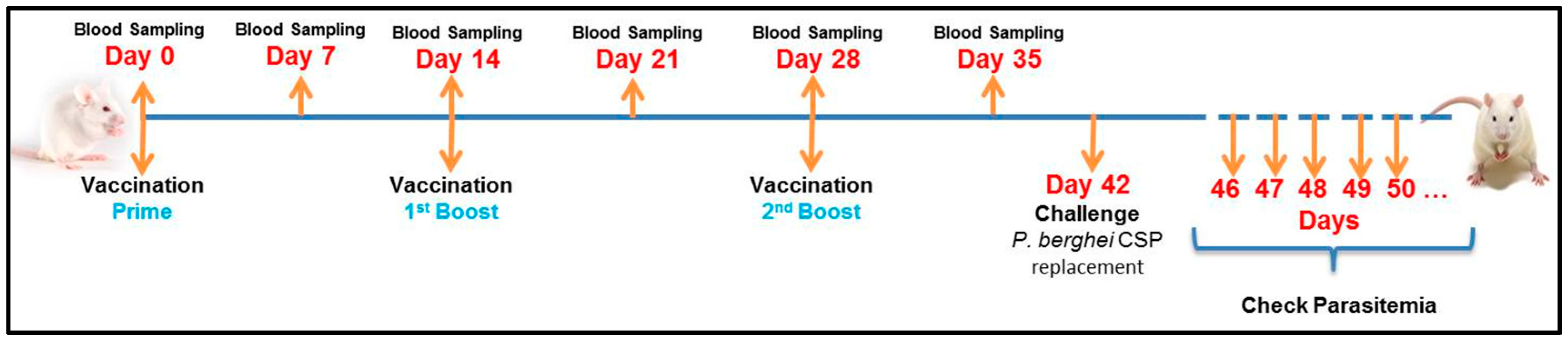
2.5. First Experimental Design to Test the Immunogenic Capacity of MCT for Malaria Vaccine Development Using rPvCSP-c as Antigen
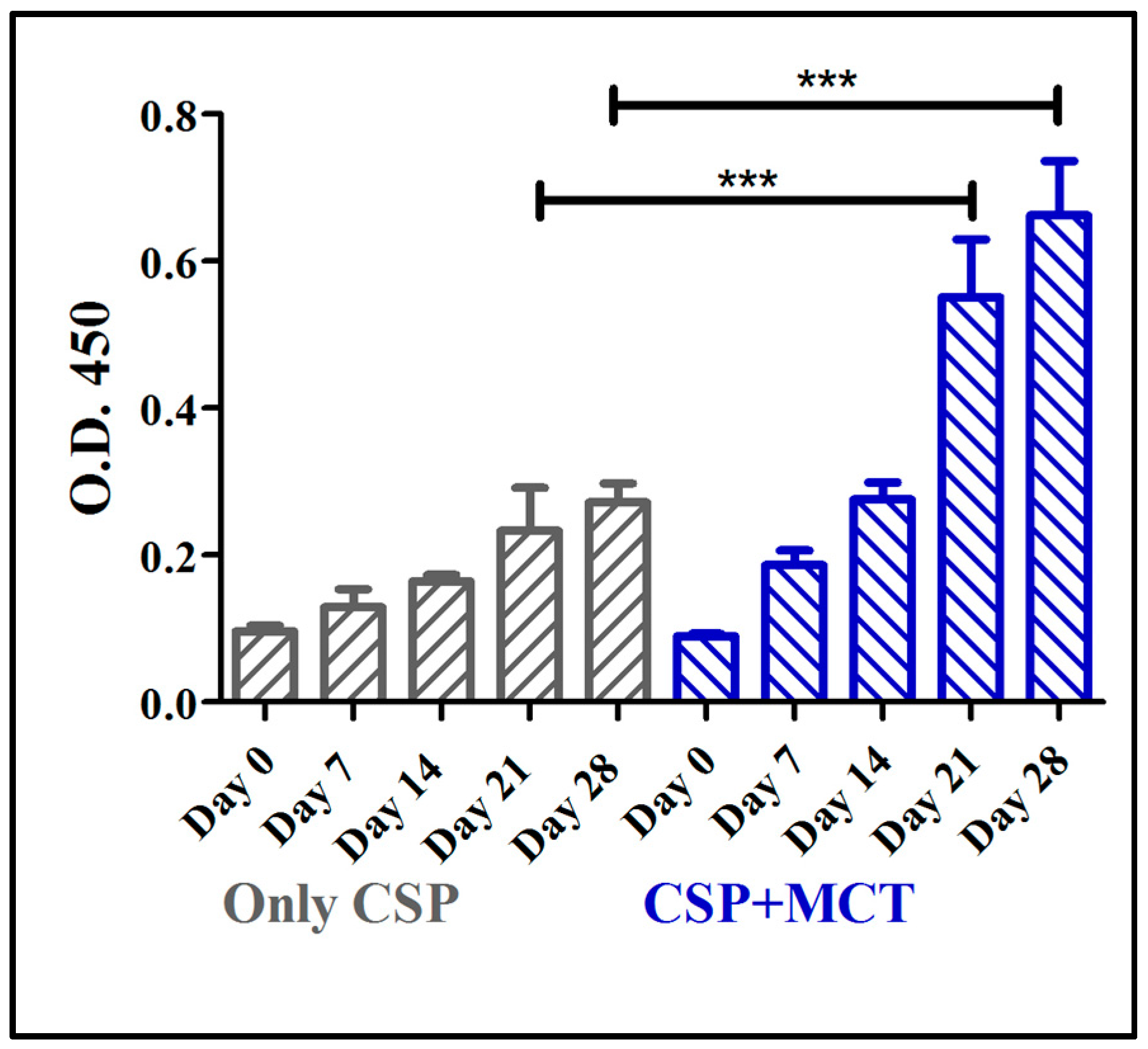
2.6. Assessment of Antibody Production (IgG total) of C57BL/6 Mice Vaccinated with rPvCSP-c with or without Adjuvant (MCT)
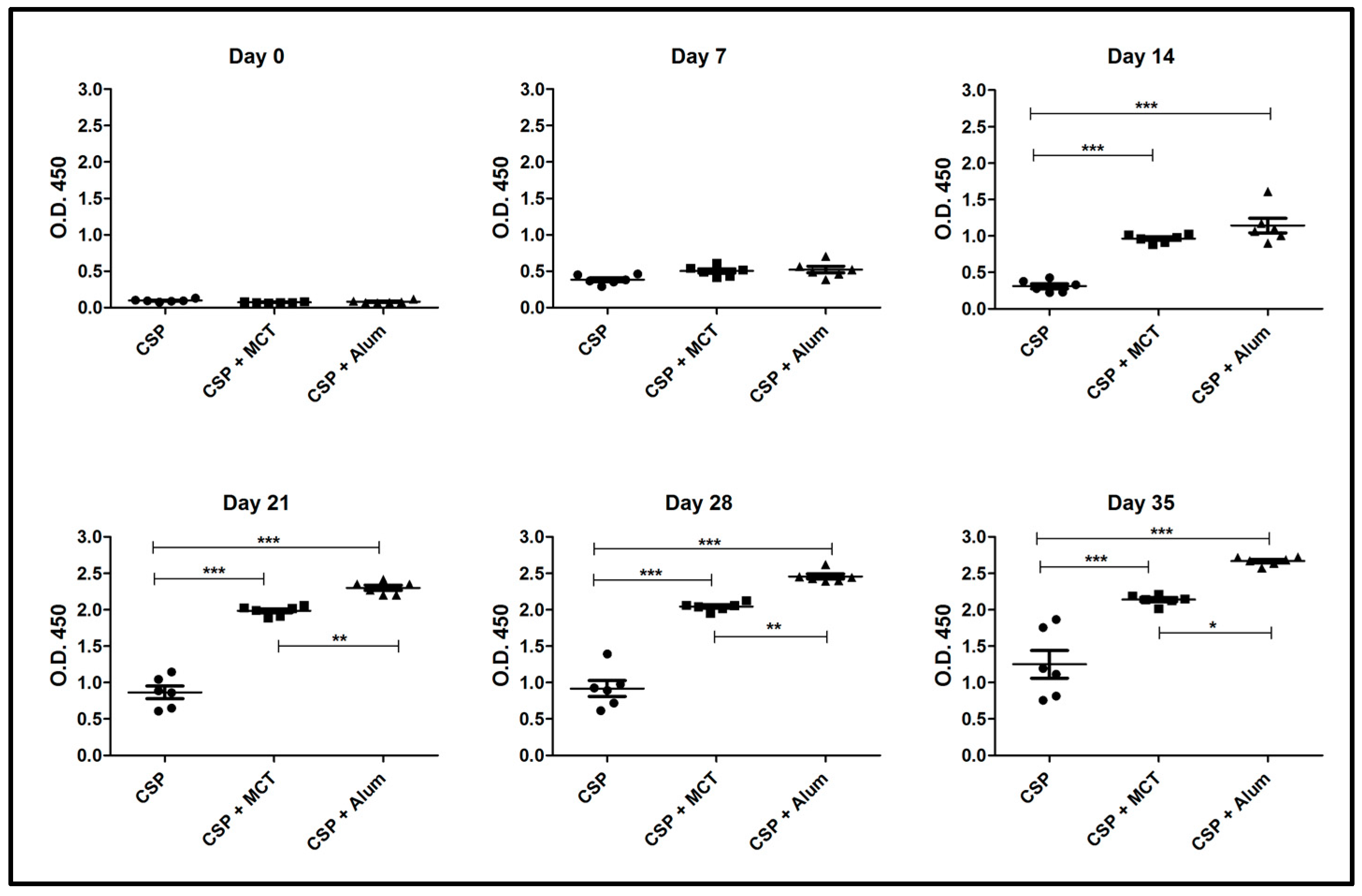
2.7. Vaccination Schedules Using rPvCSP-c Conjugated with MCT and Comparing it with Aluminum Hydroxide
2.8. Isolation of Parasites and Challenge
2.9. Statistical Modeling to Predict Parasitaemia
2.10. Statistical Analysis
3. Results and Discussion
3.1. Production and Characterization of Purified Recombinant rPvCSP-c and Vaccine Formulation
3.2. Humoral Immune Response in C57BL/6 Mice Induced by rPvCSP-c Formulated in MCT
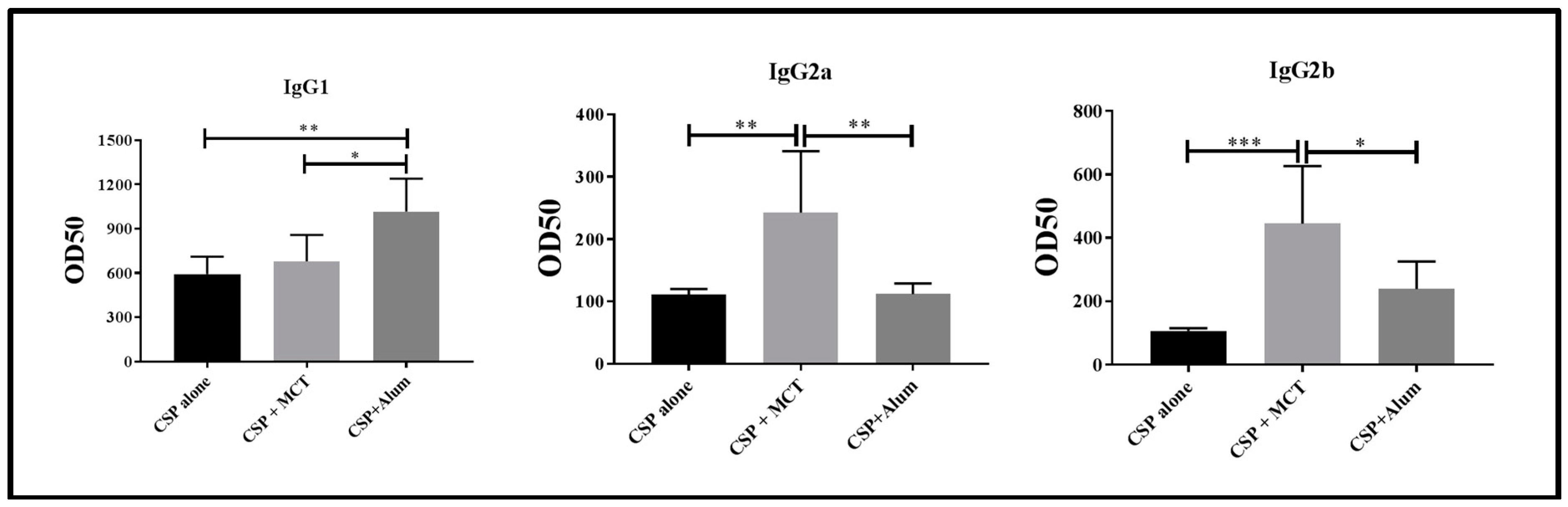
3.3. Humoral Immune Response of BALB/c Mice Vaccinated with rPvCSP-c Formulated in MCT, Alum or without Adjuvant
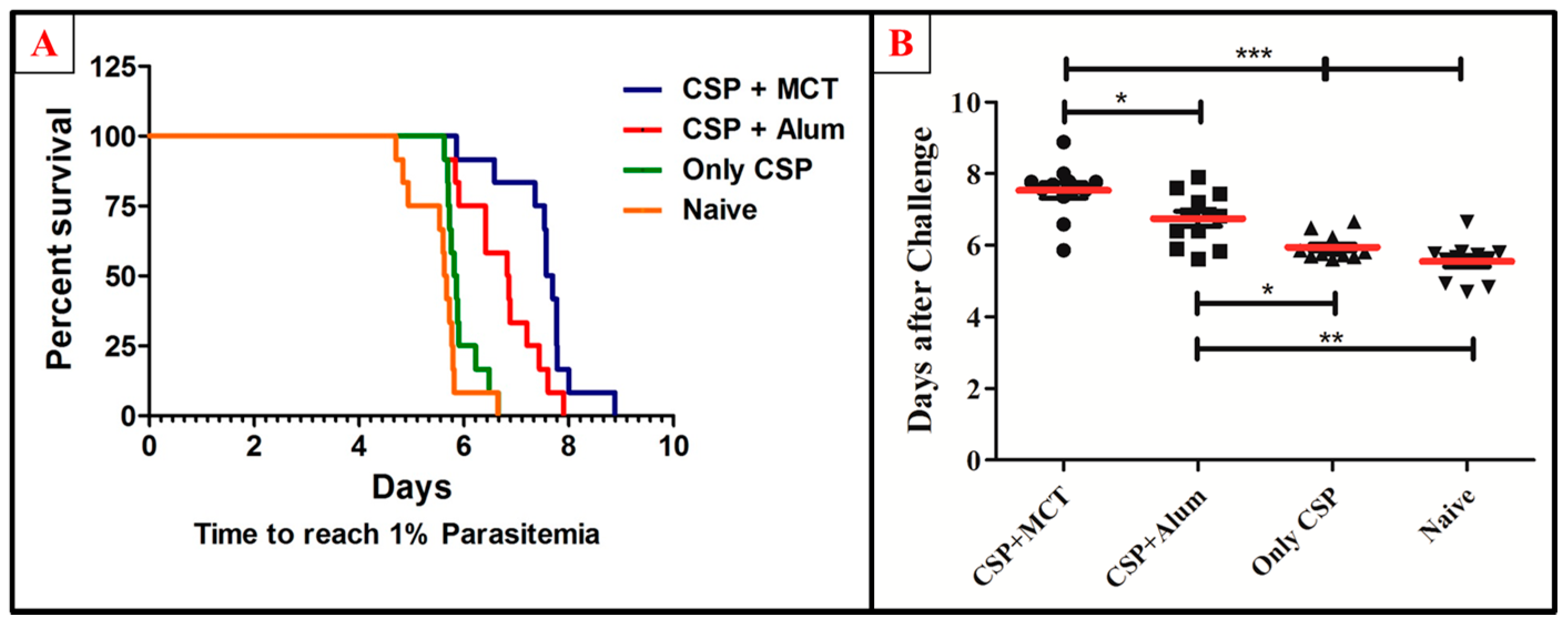
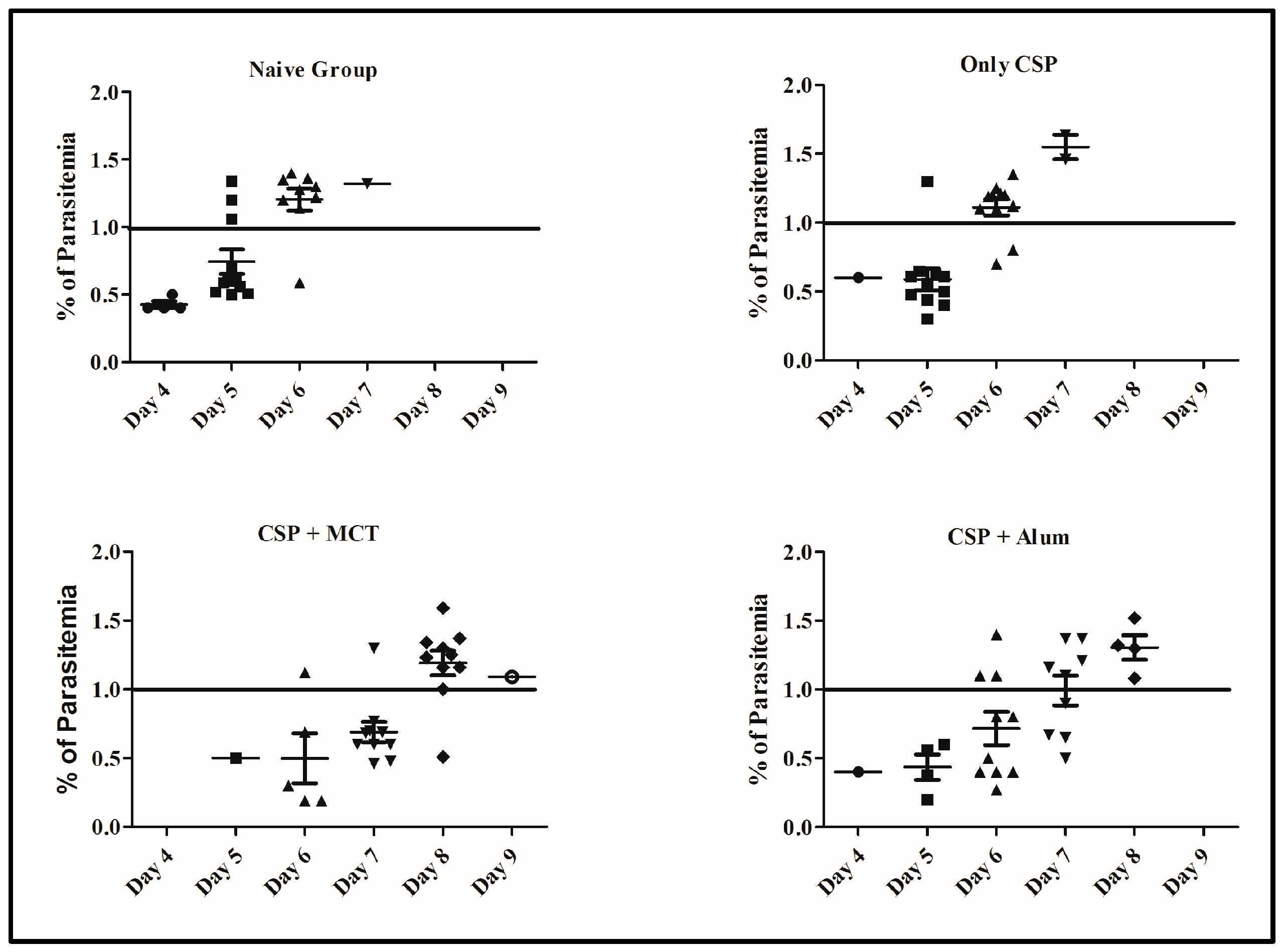
3.4. Protective Capacity of Vaccines Subsequent to Challenge with Recombinant P. berghei Expressing CSP of P. vivax
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Ethical Conduct of Research
References
- Garçon, N.; Leroux-Roels, G.; Wen-Fang, C. Vaccine adjuvants. In Understanding Modern Vaccines: Perspectives in Vaccinology; Garçon, N., Stern, P.L., Cunningham, A.L., Eds.; Elsevier: London, UK, 2011; Volume 1, pp. 89–113. [Google Scholar]
- Kramer, M.F.; Heath, M.D. Aluminium in allergen-specific subcutaneous immunotherapy—A German perspective. Vaccine 2014, 32, 4140–4148. [Google Scholar] [CrossRef] [PubMed]
- Bergfors, E.; Trollfors, B.; Inerot, A. Unexpectedly high incidence of persistent itching nodules and delayed hypersensitivity to aluminium in children after the use of adsorbed vaccines from a single manufacturer. Vaccine 2003, 22, 64–69. [Google Scholar] [CrossRef]
- Exley, C. Aluminium adjuvants and adverse events in sub-cutaneous allergy immunotherapy. Allergy Asthma Clin. Immunol. 2014, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, R.K.; Eidi, H.; Crépeaux, G.; Authier, F.J.; Cadusseau, J. Biopersistence and brain translocation of aluminum adjuvants of vaccines. Front. Neurol. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tleugabulova, D.; Falcón, V.; Pentón, E. Evidence for the denaturation of recombinant hepatitis B surface antigen on aluminium hydroxide gel. J. Chromatogr. B Biomed. Sci. Appl. 1998, 720, 153–163. [Google Scholar] [CrossRef]
- Bell, A.J.; Heath, M.D.; Hewings, S.J.; Skinner, M.A. The adsorption of allergoids and 3-O-desacyl-4′-monophosphoryl lipid A (MPL®) to microcrystalline tyrosine (MCT) in formulations for use in allergy immunotherapy. J. Inorg. Biochem. 2015, 152, 147–153. [Google Scholar] [CrossRef] [PubMed]
- McDougall, S.A.; Heath, M.D.; Kramer, M.F.; Skinner, M.A. Analysis of aluminium in rat following administration of allergen immunotherapy using either aluminium or microcrystalline-tyrosine-based adjuvants. Bioanalysis 2016, 8, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Klimek, L.; Schmidt-Weber, C.B.; Kramer, M.F.; Skinner, M.A.; Heath, M.D. Clinical use of adjuvants in allergen-immunotherapy. Expert Rev. Clin. Immunol. 2017, 13, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.W.; Marshall, J.S.; Ulrich, J.T. A Th1-inducing adjuvant, MPL, enhances antibody profiles in experimental animals suggesting it has the potential to improve the efficacy of allergy vaccines. Int. Arch. Allergy Immunol. 2001, 126, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Garner, P.; Gelband, H.; Graves, P.; Jones, K.; Maclehose, H.; Olliaro, P. Systematic Reviews in Malaria: Global Policies Need Global Reviews. Infect. Dis. Clin. N. Am. 2009, 23, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Gething, P.W.; Elyazar, I.R.; Moyes, C.L.; Smith, D.L.; Battle, K.E.; Guerra, C.A.; Patil, A.P.; Tatem, A.J.; Howes, R.E.; Myers, M.F.; et al. A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Negl. Trop. Dis. 2012. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S. Epidemiological aspects of vivax and falciparum malaria: Global spectrum. Asian Pac. J. Trop. Dis. 2014, 4, S13–S26. [Google Scholar] [CrossRef]
- WHO. Malaria. Available online: http://www.who.int/mediacentre/factsheets/fs094/en/ (accessed on 1 December 2015).
- Stresman, G.H. Beyond temperature and precipitation: Ecological risk factors that modify malaria transmission. Acta Trop. 2010, 116, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Sutcliffe, C.G.; Kobayashi, T.; Hamapumbu, H.; Shields, T.; Kamanga, A.; Mharakurwa, S.; Thuma, P.E.; Glass, G.; Moss, W.J. Changing individual-level risk factors for malaria with declining transmission in southern Zambia: A cross-sectional study. Malar. J. 2011, 10, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, V.R.; Queiroz, A.T.; Lopes, F.M.; Andrade, B.B.; Barral-Netto, M. Networking the host immune response in Plasmodium vivax malaria. Malar. J. 2013, 12, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Sandoval, A.; Bachmann, M.F. Plasmodium vivax malaria vaccines: Why are we where we are? Hum. Vaccines Immunother. 2013, 12, 2558–2565. [Google Scholar] [CrossRef] [PubMed]
- De Barra, E.; Hodgson, S.H.; Ewer, K.J.; Bliss, C.M.; Hennigan, K.; Collins, A.; Berrie, E.; Lawrie, A.M.; Gilbert, S.C.; Nicosia, A.; et al. A Phase Ia Study to Assess the Safety and Immunogenicity of New Malaria Vaccine Candidates ChAd63 CS Administered Alone and with MVA CS. PLoS ONE 2014, 9, 115–161. [Google Scholar] [CrossRef] [PubMed]
- Longley, R.J.; Hill, A.V.; Spencer, A.J. Malaria vaccines: Identifying Plasmodium falciparum liver-stage targets. Front. Microbiol. 2015, 6, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.S.; Lourenço, J.; Hill, A.V.S.; Gupta, S. Modeling Combinations of Pre-erythrocytic Plasmodium falciparum Malaria Vaccines. Am. J. Trop. Med. Hyg. 2015, 93, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Prudêncio, M.; Rodriguez, A.; Mota, M.M. The silent path to thousands of merozoites: The Plasmodium liver stage. Nat. Rev. Microbiol. 2006, 4, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Kazmin, D.; Nakaya, H.I.; Lee, E.K.; Johnson, M.J.; van der Most, R.; van den Berg, R.A.; Ballou, W.R.; Jongert, E.; Wille-Reece, U.; Ockenhouse, C.; et al. Systems analysis of protective immune responses to RTS,S malaria vaccination in humans. Proc. Natl. Acad. Sci. USA 2017, 114, 2425–2430. [Google Scholar] [CrossRef] [PubMed]
- Radin, K.; Clement, F.; Jongert, E.; Sterckx, Y.G.; Ockenhouse, C.; Regules, J.; Lemiale, F.; Leroux-Roels, G. A monoclonal antibody-based immunoassay to measure the antibody response against the repeat region of the circumsporozoite protein of Plasmodium falciparum. Malar. J. 2016, 15, 543. [Google Scholar] [CrossRef] [PubMed]
- Agnandji, S.T.; Fendel, R.; Mestré, M.; Janssens, M.; Vekemans, J.; Held, J.; Gnansounou, F.; Haertle, S.; von Glasenapp, I.; Oyakhirome, S.; et al. Induction of Plasmodium falciparum-specific CD4+ T cells and memory B cells in Gabonese children vaccinated with RTS,S/AS01(E) and RTS,S/AS02(D). PLoS ONE 2011, 6, e18559. [Google Scholar] [CrossRef] [PubMed]
- Salman, A.M.; Montoya-Díaz, E.; West, H.; Lall, A.; Atcheson, E.; Lopez-Camacho, C.; Ramesar, J.; Bauza, K.; Collins, K.; Reis, F.; et al. Rational development of a highly protective P. vivax vaccine evaluated using transgenic rodent parasite challenge models. Sci. Rep. 2017, 7, 46482. [Google Scholar] [CrossRef] [PubMed]
- Aricescu, A.R.; Lu, W.; Jones, E.Y. A time-and cost-efficient system for high-level protein production in mammalian cells. Acta Crytallogr. Sect. D 2006, 62, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Longley, R.J.; Reyes-Sandoval, A.; Montoya-Díaz, E.; Dunachie, S.; Kumpitak, C.; Nguitragool, W.; Mueller, I.; Sattabongkot, J. Acquisition and Longevity of Antibodies to Plasmodium vivax Preerythrocytic Antigens in Western Thailand. Clin. Vaccine Immunol. 2015, 23, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Bauza, K.; Malinauskas, T.; Pfander, C.; Anar, B.; Jones, E.Y.; Billker, O.; Hill, A.V.; Reyes-Sandoval, A. Efficacy of a Plasmodium vivax Malaria Vaccine Using ChAd63 and Modified Vaccinia Ankara Expressing Thrombospondin-Related Anonymous Protein as Assessed with Transgenic Plasmodium berghei Parasites. Infect. Immun. 2014, 82, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Sandoval, A.; Wyllie, D.H.; Bauza, K.; Milicic, A.; Forbes, E.K.; Rollier, C.S.; Hill, A.V. CD8+ T effector memory cells protect against liver-stage malaria. J. Immunol. 2011, 187, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Miranda, G.; Heath, M.D.; Mohsen, M.O.; Gomes, A.C.; Engeroff, P.; Flaxman, A.; Leoratti, F.M.S.; El-Turabi, A.; Reyes-Sandoval, A.; Skinner, M.A.; et al. Virus-Like Particle (VLP) Plus Microcrystalline Tyrosine (MCT) Adjuvants Enhance Vaccine Efficacy Improving T and B Cell Immunogenicity and Protection against Plasmodium berghei/vivax. Vaccines 2017. [Google Scholar] [CrossRef] [PubMed]
- De Ribeiro, B.P.; Cassiano, G.C.; de Souza, R.M.; Cysne, D.N.; Grisotto, M.A.G.; de Azevedo dos, A.P.S.; Marinho, C.R.; Machado, R.L.; Nascimento, F.R. Polymorphisms in Plasmodium vivax Circumsporozoite Protein (CSP) Influence Parasite Burden and Cytokine Balance in a Pre-Amazon Endemic Area from Brazil. PLoS Negl. Trop. Dis. 2016, 10, e0004479. [Google Scholar] [CrossRef] [PubMed]
- De Souza, J.B. Protective immunity against malaria after vaccination. Parasite Immunol. 2014, 36, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Jegerlehner, A.; Zabel, F.; Langer, A.; Dietmeier, K.; Jennings, G.T.; Saudan, P.; Bachmann, M.F. Bacterially Produced Recombinant Influenza Vaccines Based on Virus-Like Particles. PLoS ONE 2013, 8, e78947. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabral-Miranda, G.; Heath, M.D.; Gomes, A.C.; Mohsen, M.O.; Montoya-Diaz, E.; Salman, A.M.; Atcheson, E.; Skinner, M.A.; Kramer, M.F.; Reyes-Sandoval, A.; et al. Microcrystalline Tyrosine (MCT®): A Depot Adjuvant in Licensed Allergy Immunotherapy Offers New Opportunities in Malaria. Vaccines 2017, 5, 32. https://doi.org/10.3390/vaccines5040032
Cabral-Miranda G, Heath MD, Gomes AC, Mohsen MO, Montoya-Diaz E, Salman AM, Atcheson E, Skinner MA, Kramer MF, Reyes-Sandoval A, et al. Microcrystalline Tyrosine (MCT®): A Depot Adjuvant in Licensed Allergy Immunotherapy Offers New Opportunities in Malaria. Vaccines. 2017; 5(4):32. https://doi.org/10.3390/vaccines5040032
Chicago/Turabian StyleCabral-Miranda, Gustavo, Matthew D. Heath, Ariane C. Gomes, Mona O. Mohsen, Eduardo Montoya-Diaz, Ahmed M. Salman, Erwan Atcheson, Murray A. Skinner, Matthias F. Kramer, Arturo Reyes-Sandoval, and et al. 2017. "Microcrystalline Tyrosine (MCT®): A Depot Adjuvant in Licensed Allergy Immunotherapy Offers New Opportunities in Malaria" Vaccines 5, no. 4: 32. https://doi.org/10.3390/vaccines5040032
APA StyleCabral-Miranda, G., Heath, M. D., Gomes, A. C., Mohsen, M. O., Montoya-Diaz, E., Salman, A. M., Atcheson, E., Skinner, M. A., Kramer, M. F., Reyes-Sandoval, A., & Bachmann, M. F. (2017). Microcrystalline Tyrosine (MCT®): A Depot Adjuvant in Licensed Allergy Immunotherapy Offers New Opportunities in Malaria. Vaccines, 5(4), 32. https://doi.org/10.3390/vaccines5040032






