Homologous Prime-Boost Vaccination with OVA Entrapped in Self-Adjuvanting Archaeosomes Induces High Numbers of OVA-Specific CD8+ T Cells that Protect Against Subcutaneous B16-OVA Melanoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vaccine Delivery Systems and Route of Immunization
2.2. Mouse Strains
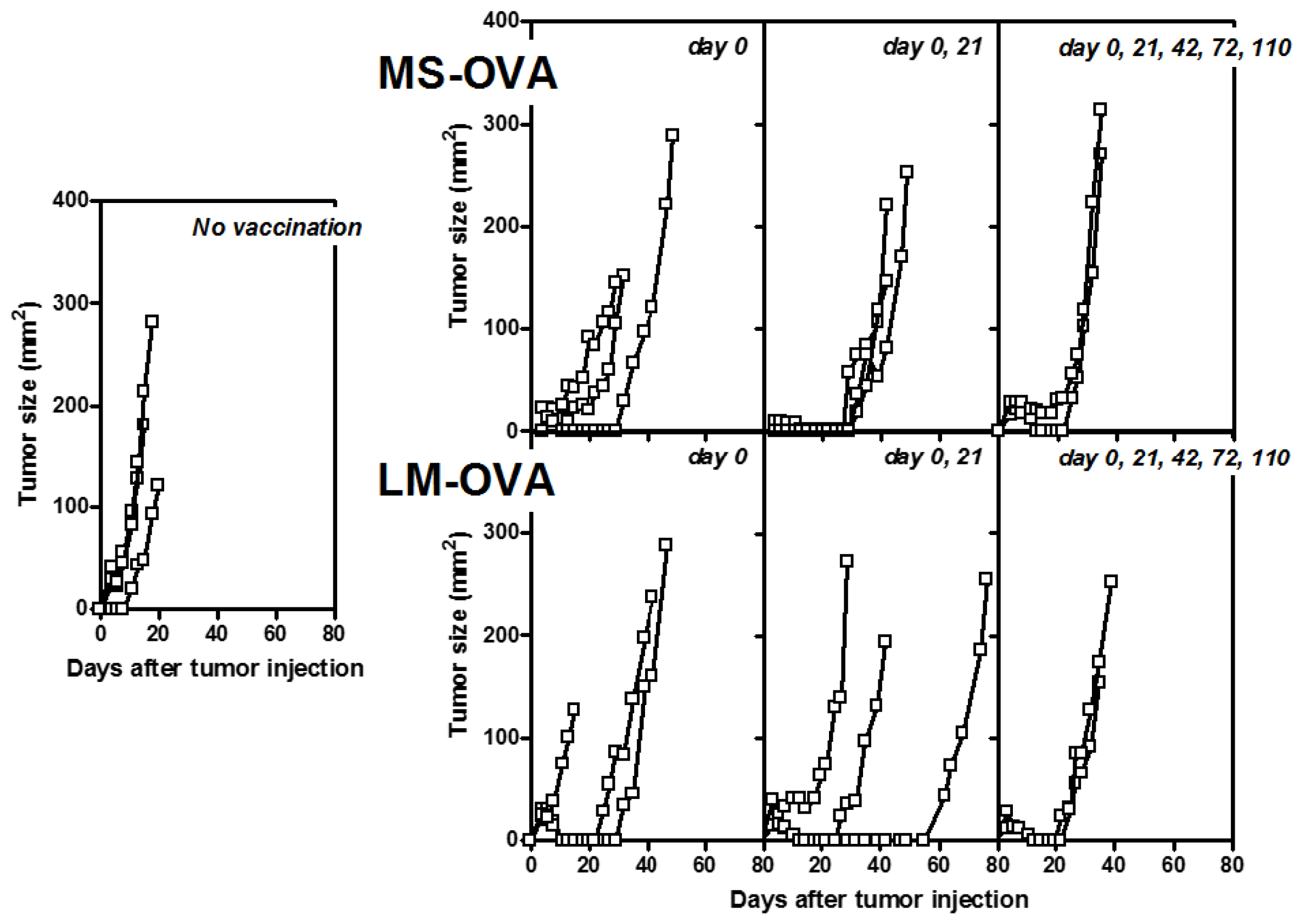
2.3. Tumor Model (B16-OVA, Melanoma)
2.4. Assessment of In Vivo Cytolytic Activity
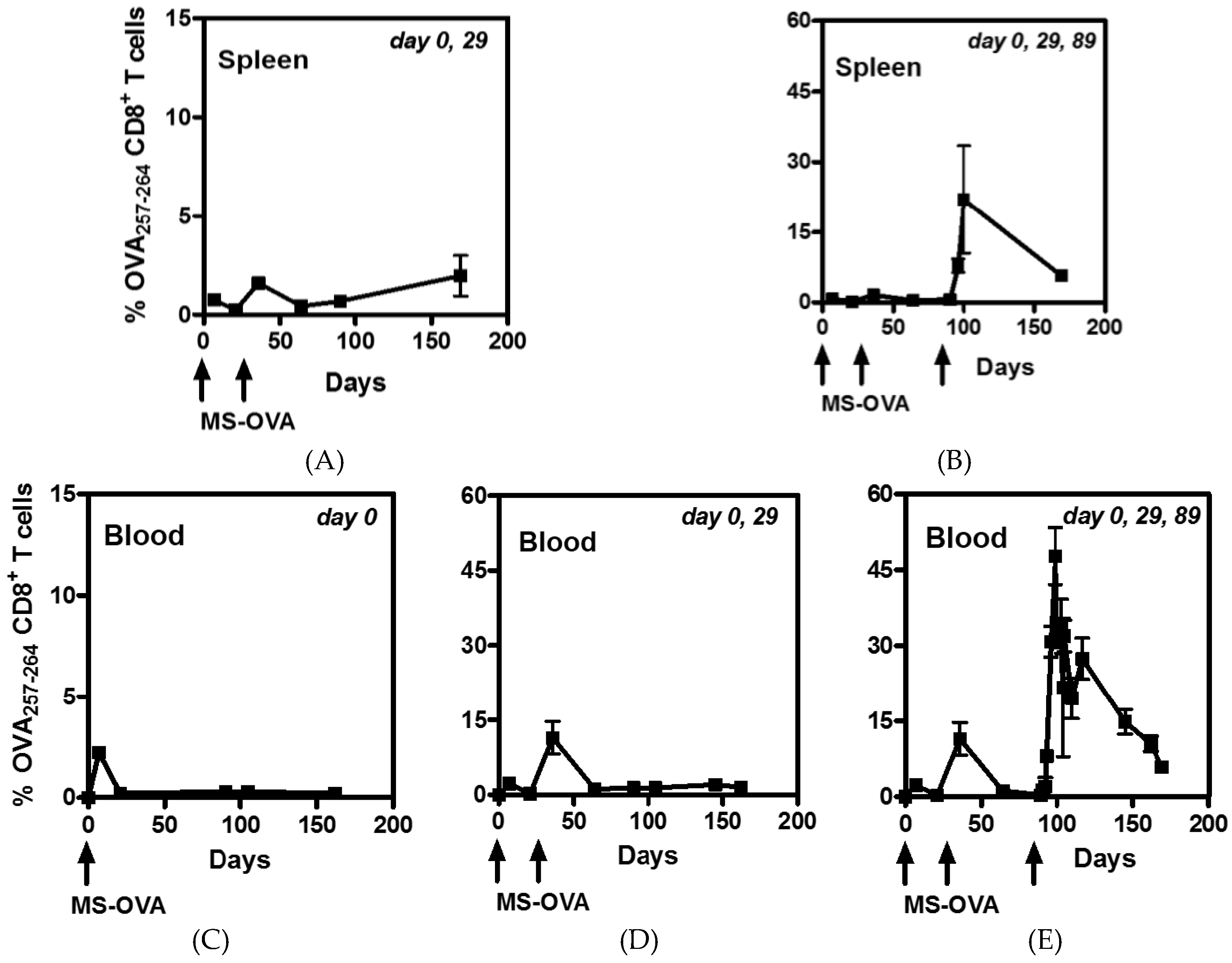
2.5. Detection of OVA-Specific CD8+ T Cells
3. Results
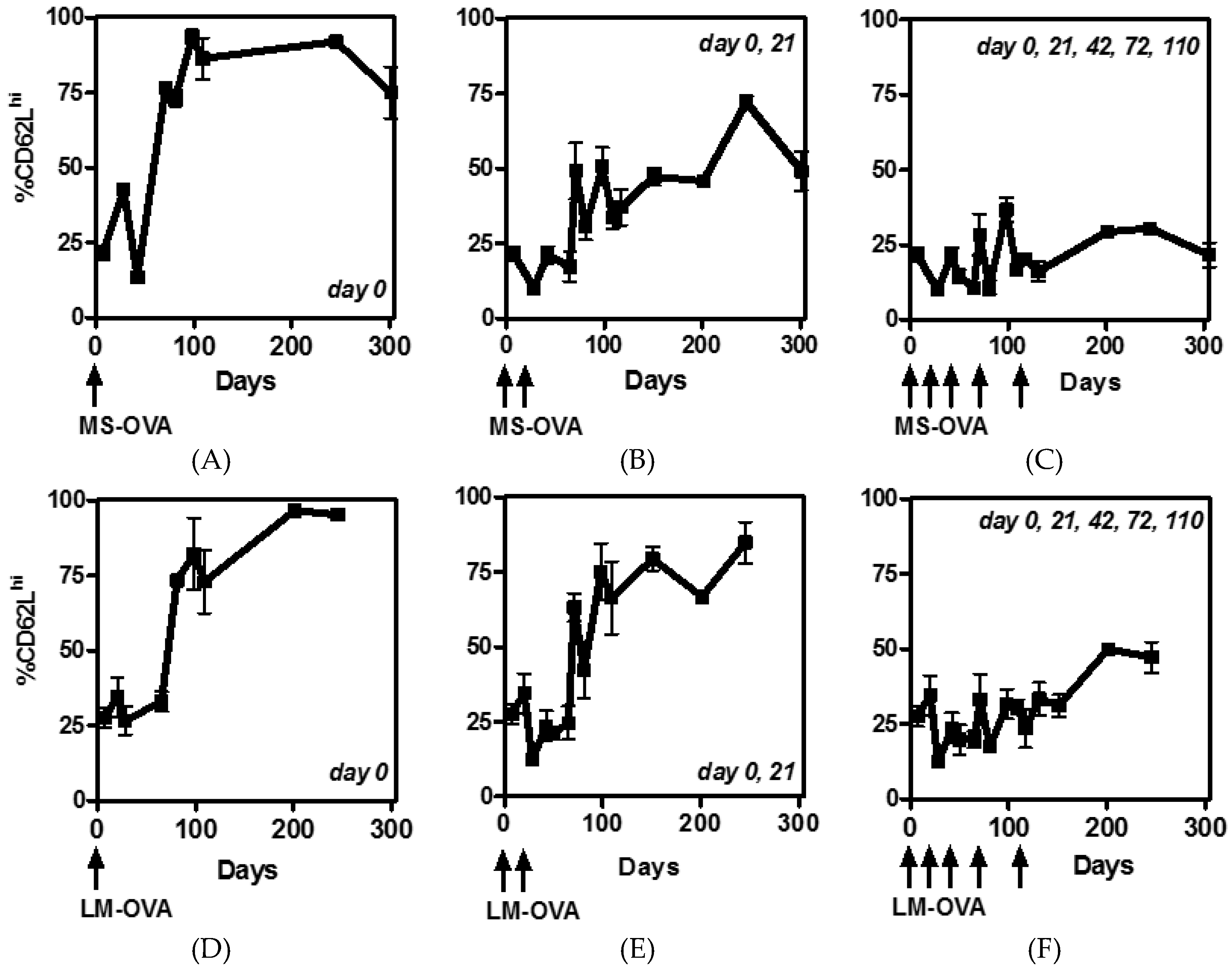
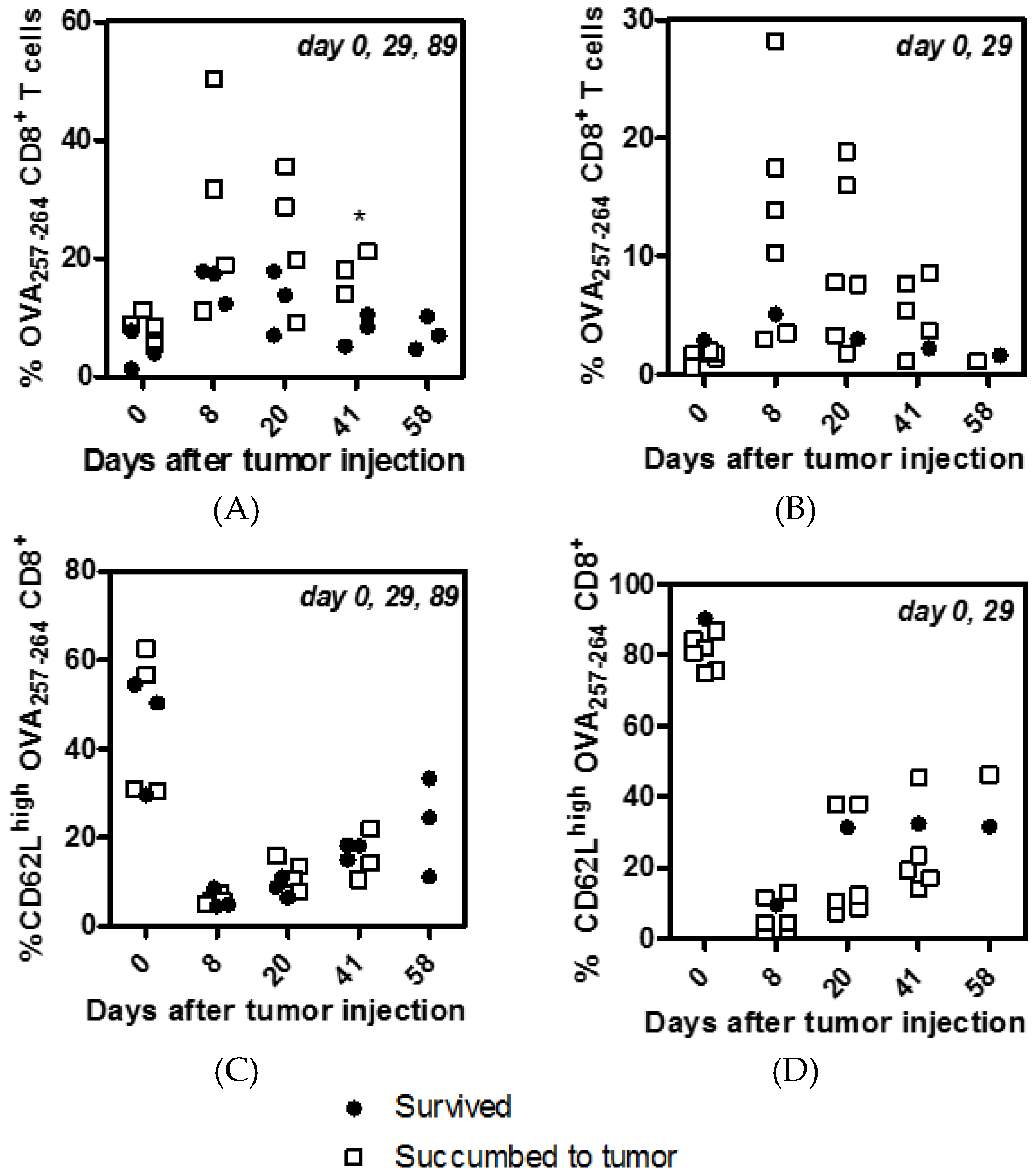
3.1. Multiple Boosting with MS-OVA Induced High Numbers of Circulating CD8+ T Cells
3.2. CD62L Expression on CD8+ T Cells Reduces with Multiple Boosting
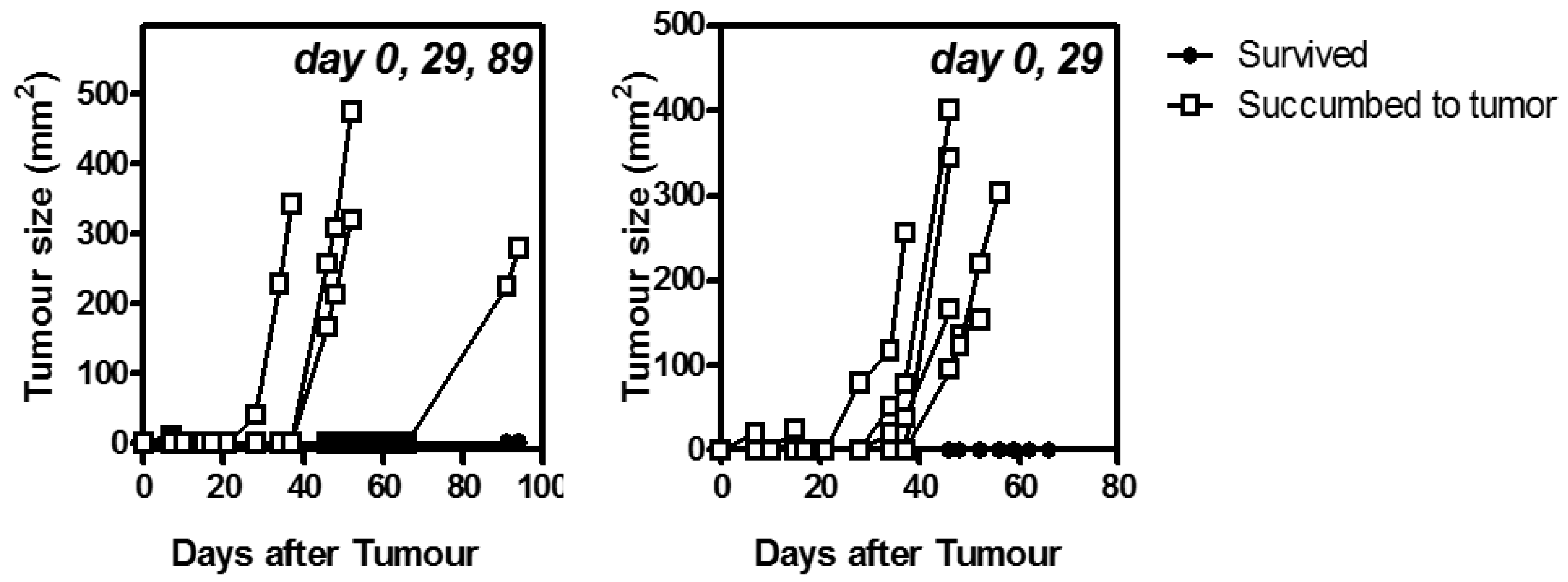
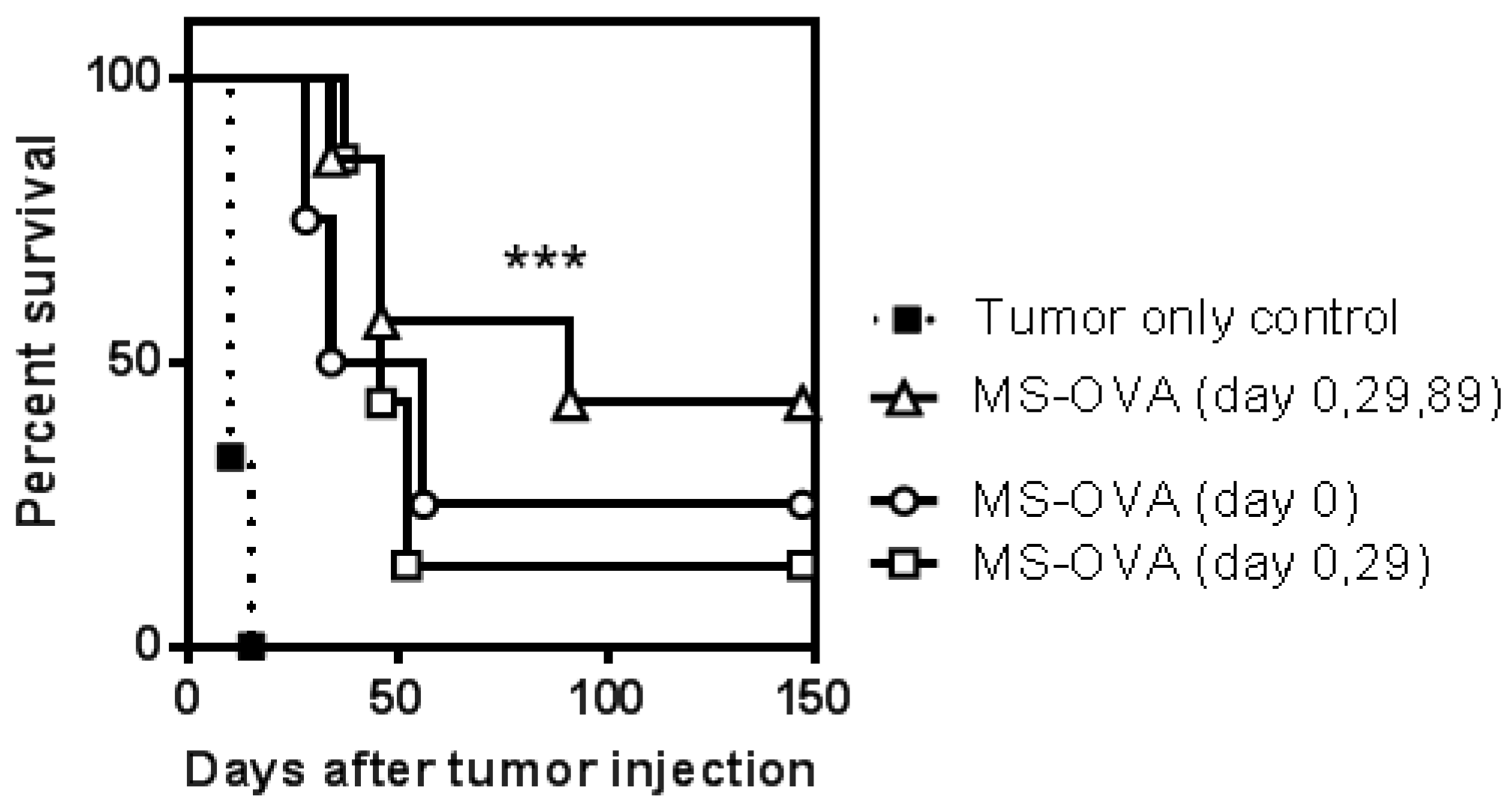
3.3. Staggered Boosting Results in a High Frequency of OVA-CD8+ T Cells and Long-Term Tumor Protection
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Liu, M.A. Immunologic basis of vaccine vectors. Immunity 2010, 33, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Yang, J.C.; Schwartzentruber, D.J.; Hwu, P.; Topalian, S.L.; Sherry, R.M.; Restifo, N.P.; Wunderlich, J.R.; Seipp, C.A.; Rogers-Freezer, L.; et al. Recombinant fowlpox viruses encoding the anchor-modified gp100 melanoma antigen can generate antitumor immune responses in patients with metastatic melanoma. Clin. Cancer Res. 2003, 9, 2973–2980. [Google Scholar] [PubMed]
- Overwijk, W.W.; Theoret, M.R.; Finkelstein, S.E.; Surman, D.R.; de Jong, L.A.; Vyth-Dreese, F.A.; Dellemijn, T.A.; Antony, P.A.; Spiess, P.J.; Palmer, D.C.; et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J. Exp. Med. 2003, 198, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Odegard, J.M.; Kelley-Clarke, B.; Tareen, S.U.; Campbell, D.J.; Flynn, P.A.; Nicolai, C.J.; Slough, M.M.; Vin, C.D.; McGowan, P.J.; Nelson, L.T.; et al. Virological and preclinical characterization of a dendritic cell targeting, integration-deficient lentiviral vector for cancer immunotherapy. J. Immunother. 2015, 38, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Shahabi, V.; Maciag, P.C.; Rivera, S.; Wallecha, A. Live, attenuated strains of Listeria and Salmonella as vaccine vectors in cancer treatment. Bioeng. Bugs 2010, 1, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Paterson, Y.; Guirnalda, P.D.; Wood, L.M. Listeria and Salmonella bacterial vectors of tumor-associated antigens for cancer immunotherapy. Semin. Immunol. 2010, 22, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Gunn, G.R.; Zubair, A.; Peters, C.; Pan, Z.K.; Wu, T.C.; Paterson, Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J. Immunol. 2001, 167, 6471–6479. [Google Scholar] [PubMed]
- Tangney, M.; Gahan, C.G.M. Listeria monocytogenes as a vector for anti-cancer therapies. Curr. Gene Ther. 2010, 10, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Fensterle, J.; Bergmann, B.; Yone, C.L.R.P.; Hotz, C.; Meyer, S.R.; Spreng, S.; Goebel, W.; Rapp, U.R.; Gentschev, I. Cancer immunotherapy based on recombinant Salmonella enterica serovar Typhimurium aroA strains secreting prostate-specific antigen and cholera toxin subunit B. Cancer Gene Ther. 2008, 15, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Dudani, R.; Chapdelaine, Y.; van Faassen, H.; Smith, D.K.; Shen, H.; Krishnan, L.; Sad, S. Multiple mechanisms compensate to enhance tumor-protective CD8(+) T cell response in the long-term despite poor CD8(+) T cell priming initially: Comparison between an acute versus a chronic intracellular bacterium expressing a model antigen. J. Immunol. 2002, 168, 5737–5745. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.M.; Guirnalda, P.D.; Seavey, M.M.; Paterson, Y. Cancer immunotherapy using Listeria monocytogenes and listerial virulence factors. Immunol. Res. 2008, 42, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Ambrosch, F.; Wiedermann, G.; Jonas, S.; Althaus, B.; Finkel, B.; Glück, R.; Herzog, C. Immunogenicity and protectivity of a new liposomal hepatitis A vaccine. Vaccine 1997, 15, 1209–1213. [Google Scholar] [CrossRef]
- Steers, N.J.; Peachman, K.K.; McClain, S.; Alving, C.R.; Rao, M. Liposome-encapsulated HIV-1 Gag p24 containing lipid A induces effector CD4+ T-cells, memory CD8+ T-cells, and pro-inflammatory cytokines. Vaccine 2009, 27, 6939–6949. [Google Scholar] [CrossRef] [PubMed]
- Hamzah, J.; Altin, J.G.; Herringson, T.; Parish, C.R.; Hämmerling, G.J.; O′Donoghue, H.; Ganss, R. Targeted liposomal delivery of TLR9 ligands activates spontaneous antitumor immunity in an autochthonous cancer model. J. Immunol. 2009, 183, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Jung, J.; Lee, Y.; Kwon, H.J. Novel immunostimulatory phosphodiester oligodeoxynucleotides with CpT sequences instead of CpG motifs. Mol. Immunol. 2011, 48, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Kates, M. The phytanyl ether-linked polar lipids and isoprenoid neutral lipids of extremely halophilic bacteria. Prog. Chem. Fats Other Lipids 1978, 15, 301–342. [Google Scholar] [CrossRef]
- Krishnan, L.; Dicaire, C.J.; Patel, G.B.; Sprott, G.D. Archaeosome vaccine adjuvants induce strong humoral, cell-mediated, and memory responses: Comparison to conventional liposomes and alum. Infect. Immun. 2000, 68, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Sprott, G.D.; Sad, S.; Fleming, L.P.; Dicaire, C.J.; Patel, G.B.; Krishnan, L. Archaeosomes varying in lipid composition differ in receptor-mediated endocytosis and differentially adjuvant immune responses to entrapped antigen. Archaea 2003, 1, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Sprott, G.D.; Brisson, J.; Dicaire, C.J.; Pelletier, A.K.; Deschatelets, L.A.; Krishnan, L.; Patel, G.B. A structural comparison of the total polar lipids from the human archaea Methanobrevibacter smithii and Methanosphaera stadtmanae and its relevance to the adjuvant activities of their liposomes. Biochim. Biophys. Acta 1999, 1440, 275–288. [Google Scholar] [CrossRef]
- Sprott, G.D.; Tolson, D.L.; Patel, G.B. Archaeosomes as novel antigen delivery systems. FEMS Microbiol. Lett. 1997, 154, 17–22. [Google Scholar] [CrossRef]
- Krishnan, L.; Sad, S.; Patel, G.B.; Sprott, G.D. Archaeosomes induce long-term CD8+ cytotoxic T cell response to entrapped soluble protein by the exogenous cytosolic pathway, in the absence of CD4+ T cell help. J. Immunol. 2000, 165, 5177–5185. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, L.; Sprott, G.D. Archaeosome adjuvants: Immunological capabilities and mechanism(s) of action. Vaccine 2008, 26, 2043–2055. [Google Scholar] [CrossRef] [PubMed]
- Gurnani, K.; Kennedy, J.; Sad, S.; Sprott, G.D.; Krishnan, L. Phosphatidylserine receptor-mediated recognition of archaeosome adjuvant promotes endocytosis and MHC class I cross-presentation of the entrapped antigen by phagosome-to-cytosol transport and classical processing. J. Immunol. 2004, 173, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, L.; Deschatelets, L.; Stark, F.C.; Gurnani, K.; Sprott, G.D. Archaeosome adjuvant overcomes tolerance to tumor-associated melanoma antigens inducing protective CD8 T cell responses. Clin. Dev. Immunol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Sprott, G.D.; Yeung, A.; Dicaire, C.J.; Yu, S.H.; Whitfield, D.M. Synthetic archaeosome vaccines containing triglycosylarchaeols can provide additive and long-lasting immune responses that are enhanced by archaetidylserine. Archaea 2012. [Google Scholar] [CrossRef] [PubMed]
- Stark, F.C.; Sad, S.; Krishnan, L. Intracellular bacterial vectors that induce CD8(+) T cells with similar cytolytic abilities but disparate memory phenotypes provide contrasting tumor protection. Cancer Res. 2009, 69, 4327–4334. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, L.; Gurnani, K.; Dicaire, C.J.; van Faassen, H.; Zafer, A.; Kirschning, C.J.; Sad, S.; Sprott, G.D. Rapid clonal expansion and prolonged maintenance of memory CD8+ T cells of the effector (CD44highCD62Llow) and central (CD44highCD62Lhigh) phenotype by an archaeosome adjuvant independent of TLR2. J. Immunol. 2007, 178, 2396–2406. [Google Scholar] [CrossRef] [PubMed]
- Dudani, R.; Chapdelaine, Y.; van Faassen, H.; Smith, D.K.; Shen, H.; Krishnan, L.; Sad, S. Preexisting inflammation due to Mycobacterium bovis BCG infection differentially modulates T-cell priming against a replicating or nonreplicating immunogen. Infect. Immun. 2002, 70, 1957–1964. [Google Scholar] [CrossRef] [PubMed]
- Luu, R.A.; Gurnani, K.; Dudani, R.; Kammara, R.; van Faassen, H.; Sirard, J.C.; Krishnan, L.; Sad, S. Delayed expansion and contraction of CD8+ T cell response during infection with virulent Salmonella typhimurium. J. Immunol. 2006, 177, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Fisher, T.L.; Wei, C.; Frelinger, J.G.; Lord, E.M. Tumours can act as adjuvants for humoral immunity. Immunology 2001, 102, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Barber, D.L.; Wherry, E.J.; Ahmed, R. Cutting edge: Rapid in vivo killing by memory CD8 T cells. J. Immunol. 2003, 171, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, C.A.; Gattinoni, L.; Restifo, N.P. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol. Rev. 2006, 211, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S. Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. Int. Immunol. 2016, 28, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Wick, D.A.; Martin, S.D.; Nelson, B.H.; Webb, J.R. Profound CD8+ T cell immunity elicited by sequential daily immunization with exogenous antigen plus the TLR3 agonist poly(I:C). Vaccine 2011, 29, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Stark, F.C.; Gurnani, K.; Sad, S.; Krishnan, L. Lack of functional selectin ligand interactions compromises long term tumor protection by CD8+ T cells. PLoS ONE 2012, 7, e32211. [Google Scholar] [CrossRef] [PubMed]
- Provinciali, M. Immunosenescence and cancer vaccines. Cancer Immunol. Immunother. 2009, 58, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Provinciali, M.; Smorlesi, A. Immunoprevention and immunotherapy of cancer in ageing. Cancer Immunol. Immunother. 2005, 54, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Dominguez, A.L.; Lustgarten, J. High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. J. Immunol. 2006, 177, 8348–8355. [Google Scholar] [CrossRef] [PubMed]
- Gregg, R.; Smith, C.M.; Clark, F.J.; Dunnion, D.; Khan, N.; Chakraverty, R.; Nayak, L.; Moss, P.A. The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clin. Exp. Immunol. 2005, 140, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Ostrand-Rosenberg, S. Myeloid-derived suppressor cells: More mechanisms for inhibiting antitumor immunity. Cancer Immunol. Immunother. 2010, 59, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J.; Teichgräber, V.; Becker, T.C.; Masopust, D.; Kaech, S.M.; Antia, R.; Von Andrian, U.H.; Ahmed, R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003, 4, 225–324. [Google Scholar] [CrossRef] [PubMed]
- Restifo, N.P.; Kawakami, Y.; Marincola, F.; Shamamian, P.; Taggarse, A.; Esquivel, F.; Rosenberg, S.A. Molecular Mechanisms Used by Tumors to Escape Immune Recognition: Immunogenetherapy and the Cell Biology of Major Histocompatibility Complex Class I. J. Immunother. Emphas Tumor Immunol. 1993, 14, 182–190. [Google Scholar] [CrossRef]
- Sojka, D.K.; Huang, Y.H.; Fowell, D.J. Mechanisms of regulatory T-cell suppression—A diverse arsenal for a moving target. Immunology 2008, 124, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Redmond, W.L.; Linch, S.N. Combinatorial immunotherapeutic approaches to restore the function of anergic tumor-reactive cytotoxic CD8+ T cells. Hum. Vaccines Immunother. 2016, 12, 2519–2522. [Google Scholar] [CrossRef] [PubMed]
- O′Hagan, D.T.; Valiante, N.M. Recent advances in the discovery and delivery of vaccine adjuvants. Nat. Rev. Drug. Discov. 2003, 2, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Alving, C.R. Design and selection of vaccine adjuvants: animal models and human trials. Vaccine 2002, 20, S56–S64. [Google Scholar] [CrossRef]
- Ben Ahmeida, E.T.; Gregoriadis, G.; Potter, C.W.; Jennings, R. Immunopotentiation of local and systemic humoral immune responses by ISCOMs, liposomes and FCA: Role in protection against influenza A in mice. Vaccine 1993, 11, 1302–1309. [Google Scholar] [CrossRef]
- Lowell, G.H.; Kaminski, R.W.; Van Cott, T.C.; Slike, B.; Kersey, K.; Zawoznik, E.; Loomis-Price, L.; Smith, G.; Redfield, R.R.; Amselem, S.; et al. Proteosomes, emulsomes, and cholera toxin B improve nasal immunogenicity of human immunodeficiency virus gp160 in mice: Induction of serum, intestinal, vaginal, and lung IgA and IgG. J. Infect. Dis. 1997, 175, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Woodland, D.L. Jump-starting the immune system: Prime-boosting comes of age. Trends Immunol. 2004, 25, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.K.; Su, Y.; Feng, J.; Lan, X.; Zhu, X.; Liu, Y.; Gao, D.; Seo, Y.; VanBrocklin, H.F.; Broaddus, V.C.; et al. The effect of internalizing human single chain antibody fragment on liposome targeting to epithelioid and sarcomatoid mesothelioma. Biomaterials 2011, 32, 2605–2613. [Google Scholar] [CrossRef] [PubMed]
- Felnerova, D.; Viret, J.F.; Glück, R.; Moser, C. Liposomes and virosomes as delivery systems for antigens, nucleic acids and drugs. Curr. Opin. Biotechnol. 2004, 15, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Passero, F.C.; Grapsa, D.; Syrigos, K.N.; Saif, M.W. The safety and efficacy of Onivyde (irinotecan liposome injection) for the treatment of metastatic pancreatic cancer following gemcitabine-based therapy. Expert Rev. Anticancer Ther. 2016, 16, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.; Galipeau, J. GM-CSF-Based Fusion Cytokines as Ligands for Immune Modulation. J. Immunol. 2011, 186, 5527–5532. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.; Galipeau, J. GMCSF-interleukin fusion cytokines induce novel immune effectors that can serve as biopharmaceuticals for treatment of autoimmunity and cancer. J. Intern. Med. 2011, 269, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Carballido, E.; Fishman, M. Sipuleucel-T: Prototype for development of anti-tumor vaccines. Curr. Oncol. Rep. 2011, 13, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Cheever, M.A.; Higano, C. PROVENGE (Sipuleucel-T) in Prostate Cancer: The First FDA Approved Therapeutic Cancer Vaccine. Clin. Cancer Res. 2011, 17, 3520–3526. [Google Scholar] [CrossRef] [PubMed]
- Parato, K.A.; Breitbach, C.J.; Le Boeuf, F.; Wang, J.; Storbeck, C.; Ilkow, C.; Diallo, J.S.; Falls, T.; Burns, J.; Garcia, V.; et al. The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol. Ther. 2012, 20, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Ten Hagen, T.L.M. Liposomal cytokines in the treatment of infectious diseases and cancer. Methods Enzymol. 2005, 391, 125–145. [Google Scholar] [PubMed]
- Kedar, E.; Palgi, O.; Golod, G.; Babai, I.; Barenholz, Y. Delivery of cytokines by liposomes. III. Liposome-encapsulated GM-CSF and TNF-alpha show improved pharmacokinetics and biological activity and reduced toxicity in mice. J. Immunother. 1997, 20, 180–193. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stark, F.C.; McCluskie, M.J.; Krishnan, L. Homologous Prime-Boost Vaccination with OVA Entrapped in Self-Adjuvanting Archaeosomes Induces High Numbers of OVA-Specific CD8+ T Cells that Protect Against Subcutaneous B16-OVA Melanoma. Vaccines 2016, 4, 44. https://doi.org/10.3390/vaccines4040044
Stark FC, McCluskie MJ, Krishnan L. Homologous Prime-Boost Vaccination with OVA Entrapped in Self-Adjuvanting Archaeosomes Induces High Numbers of OVA-Specific CD8+ T Cells that Protect Against Subcutaneous B16-OVA Melanoma. Vaccines. 2016; 4(4):44. https://doi.org/10.3390/vaccines4040044
Chicago/Turabian StyleStark, Felicity C., Michael J. McCluskie, and Lakshmi Krishnan. 2016. "Homologous Prime-Boost Vaccination with OVA Entrapped in Self-Adjuvanting Archaeosomes Induces High Numbers of OVA-Specific CD8+ T Cells that Protect Against Subcutaneous B16-OVA Melanoma" Vaccines 4, no. 4: 44. https://doi.org/10.3390/vaccines4040044
APA StyleStark, F. C., McCluskie, M. J., & Krishnan, L. (2016). Homologous Prime-Boost Vaccination with OVA Entrapped in Self-Adjuvanting Archaeosomes Induces High Numbers of OVA-Specific CD8+ T Cells that Protect Against Subcutaneous B16-OVA Melanoma. Vaccines, 4(4), 44. https://doi.org/10.3390/vaccines4040044





