Edwardsiella tarda OmpA Encapsulated in Chitosan Nanoparticles Shows Superior Protection over Inactivated Whole Cell Vaccine in Orally Vaccinated Fringed-Lipped Peninsula Carp (Labeo fimbriatus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Expression and Purification of OmpA of E. tarda
2.2. Preparation of Chitosan Nanoparticles
2.3. Characterization and Encapsulation Efficiency of Chitosan Nanoparticles
2.4. In Vitro Release Test
2.5. Vaccine Preparation for Oral Immunization
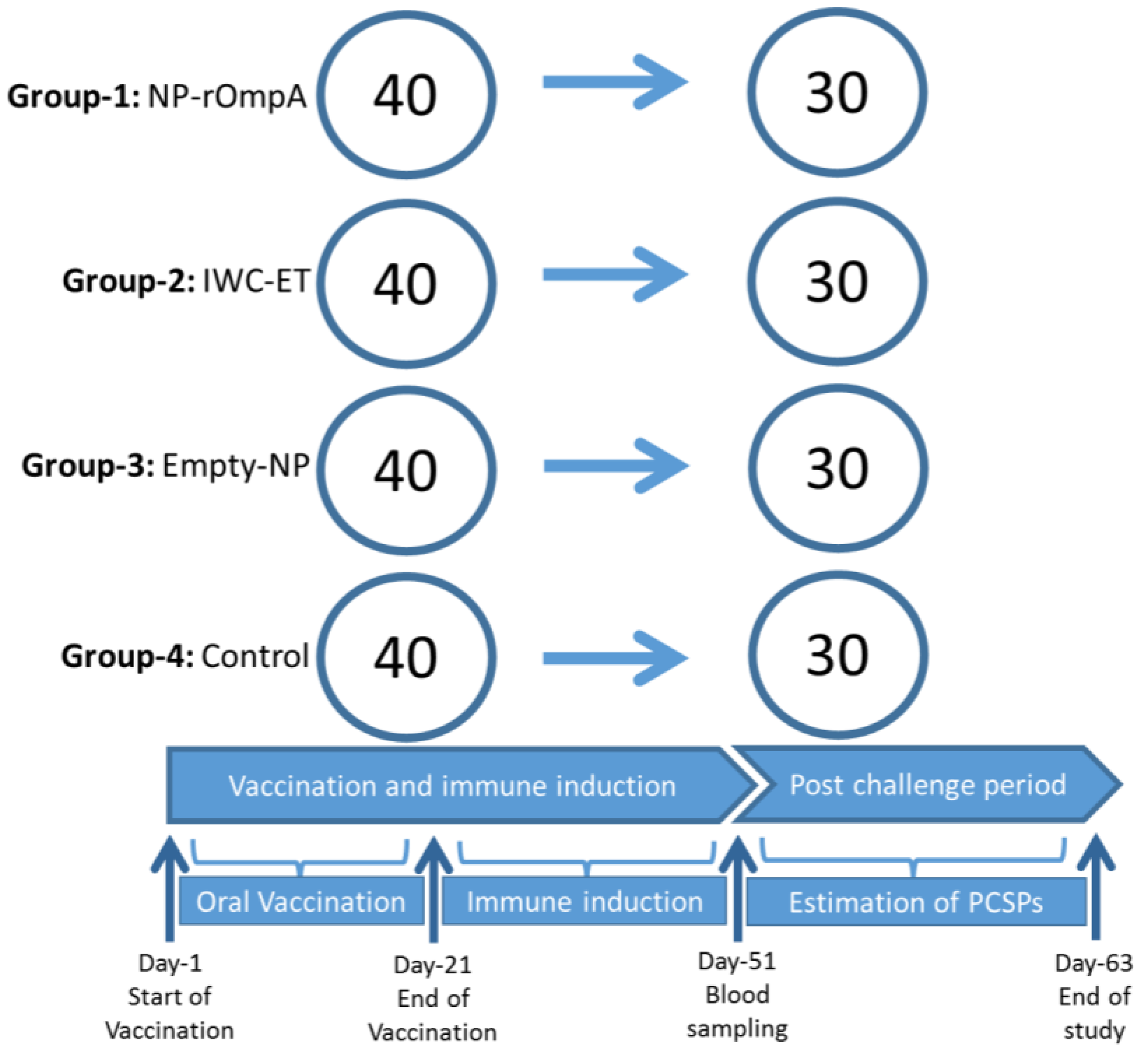
2.6. Vaccination and Challenge Study
2.7. Antibody Responses Detected by Enzyme Linked Immunosorbent Assay
2.8. Serum Inhibitory Assay
2.9. Statistical Analysis
3. Results
3.1. Expression, Purification and Concentration of Recombinant OmpA
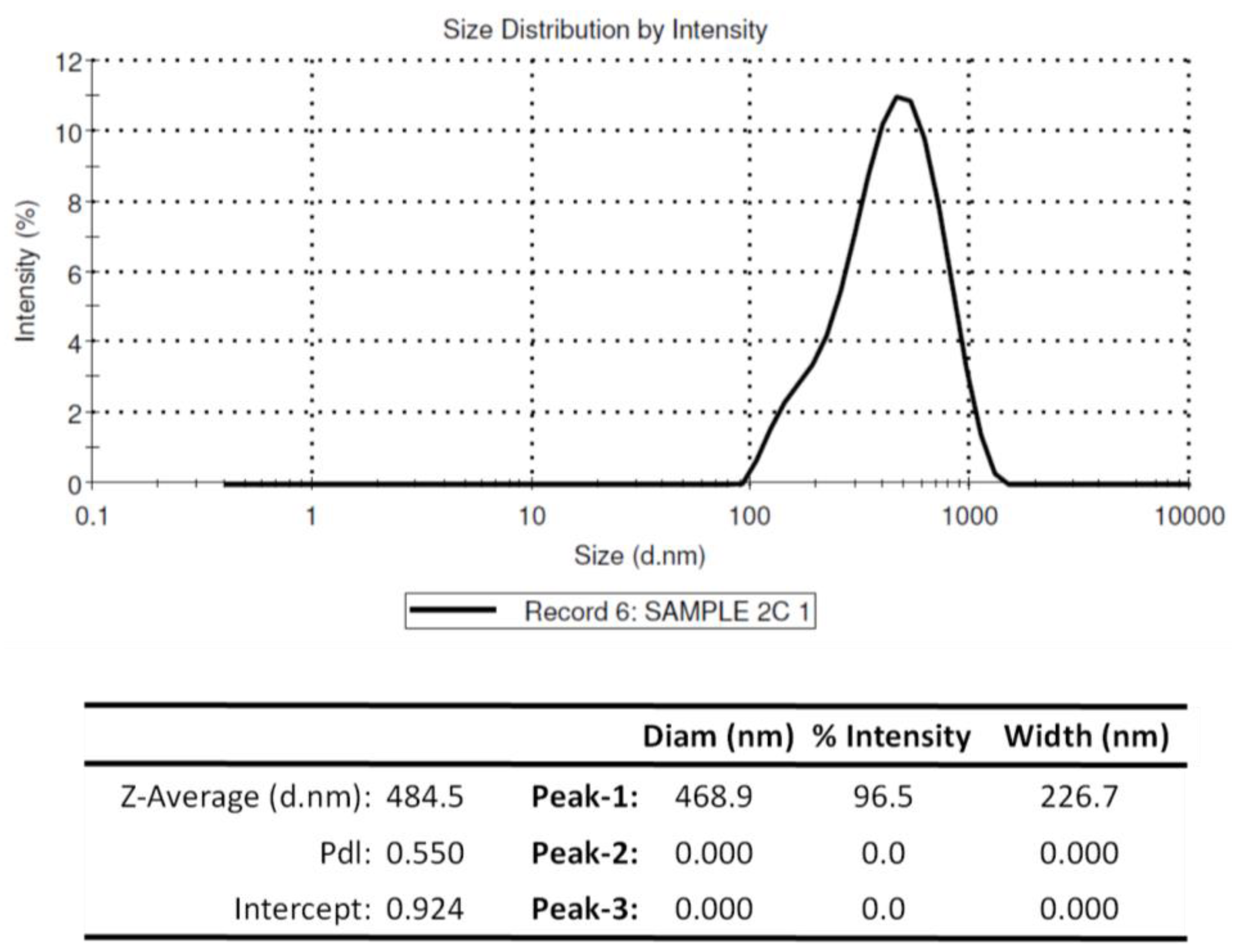
3.2. Physiochemical Properties of Chitosan Nanoparticles Encapsulating rOmpA
3.3. In Vitro Release Assay
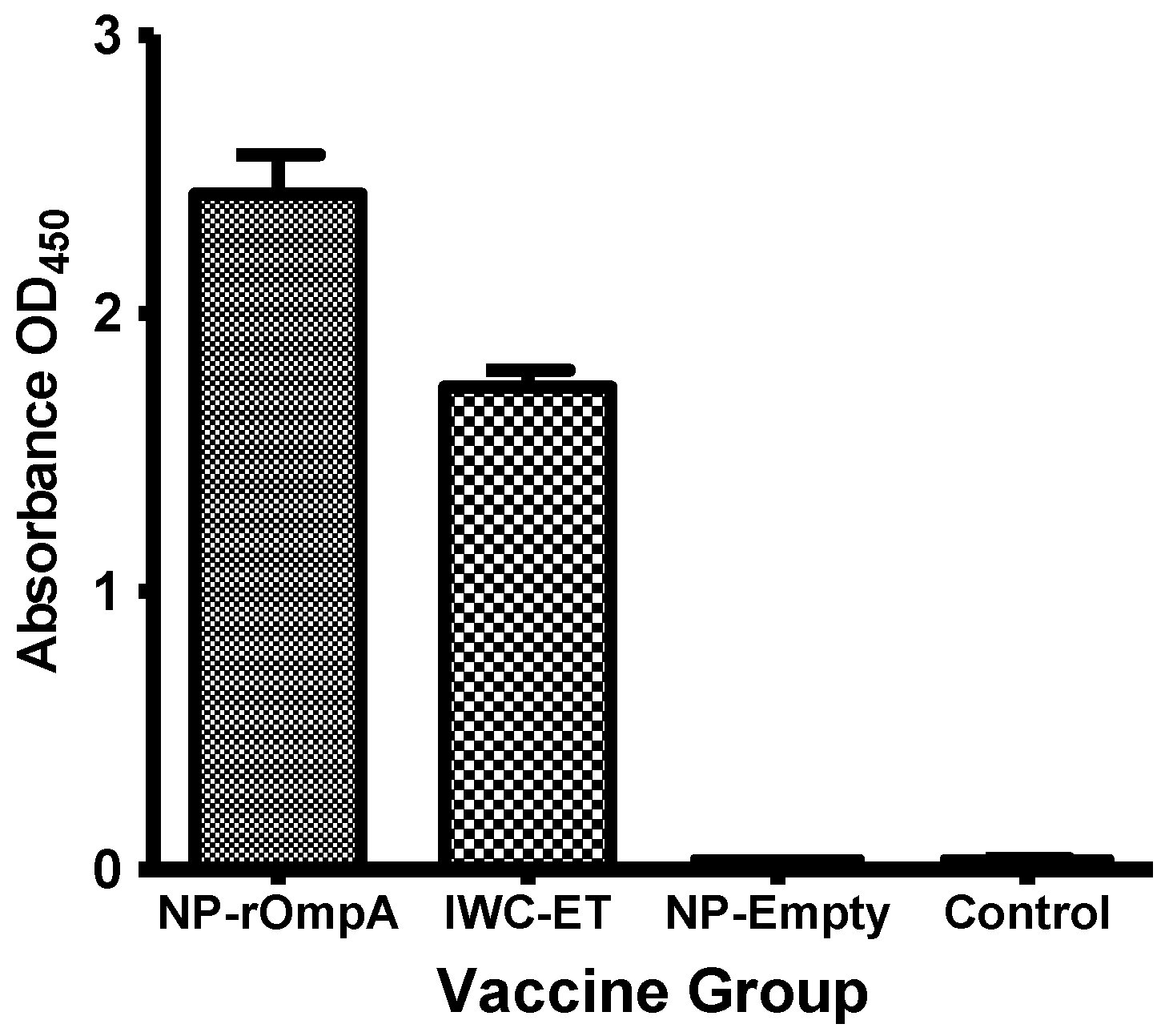
3.4. Antibody Responses
3.5. Serum Inhibition Test
3.6. Kaplan Meyer’s Survival Analysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mohanty, B.R.; Sahoo, P.K. Edwardsiellosis in fish: A brief review. J. Biosci. 2007, 32, 1331–1344. [Google Scholar] [CrossRef]
- Li, J.; Mo, Z.L.; Li, G.Y.; Xiao, P.; Huang, J. Generation and evaluation of virulence attenuated mutants of Edwardsiella tarda as vaccine candidates to combat edwardsiellosis in flounder (Paralichthys olivaceus). Fish Shellfish Immun. 2015, 43, 175–180. [Google Scholar] [CrossRef]
- Clarridge, J.E.; Musher, D.M.; Fainstein, V.; Wallace, R.J., Jr. Extraintestinal human infection caused by Edwardsiella tarda. J. Clin. Microbiol. 1980, 11, 511–514. [Google Scholar]
- Wilson, J.P.; Waterer, R.R.; Wofford, J.D., Jr.; Chapman, S.W. Serious infections with edwardsiella tarda: A case report and review of the literature. Arch. Int. Med. 1989, 149, 208–210. [Google Scholar] [CrossRef]
- Tamura, K.; Sakazaki, R.; McWhorter, A.C.; Kosako, Y. Edwardsiella tarda serotyping scheme for international use. J. Clin. Microbiol. 1988, 26, 2343–2346. [Google Scholar]
- Roger, O.E.; Michel, G.; Stewart, M.; Devanand, M.P.; Neil, W.R. Identification of the major outer membrane proteins of Aeromonas salmonicida. Dis. Aquat. Org. 2005, 68, 29–38. [Google Scholar]
- Vàzquez-Juárez, R.C.; Romero, M.J.; Ascencio, F. Adhesive properties of a LamB-like outer-membrane protein and its contribution to Aeromonas veronii adhesion. J. Appl. Microbiol. 2004, 96, 700–708. [Google Scholar] [CrossRef]
- Kawai, K.; Liu, Y.; Ohnishi, K.; Oshima, S. A conserved 37 kDa outer membrane protein of Edwardsiella tarda is an effective vaccine candidate. Vaccine 2004, 22, 3411–3418. [Google Scholar] [CrossRef]
- Kumar, G.; Rathore, G.; El-Matbouli, M. Outer membrane protein assembly factor YaeT (omp85) and GroEL proteins of Edwardsiella tarda are immunogenic antigens for Labeo rohita (Hamilton). J. Fish Dis. 2014, 37, 1055–1059. [Google Scholar] [CrossRef]
- Kumar, G.; Rathore, G.; Sengupta, U.; Singh, V.; Kapoor, D.; Lakra, W.S. Isolation and characterization of outer membrane proteins of Edwardsiella tarda and its application in immunoassays. Aquaculture 2007, 272, 98–104. [Google Scholar] [CrossRef]
- Maiti, B.; Shetty, M.; Shekar, M.; Karunasagar, I.; Karunasagar, I. Recombinant outer membrane protein A (OmpA) of Edwardsiella tarda, a potential vaccine candidate for fish, common carp. Microbiol. Res. 2011, 167, 1–7. [Google Scholar] [CrossRef]
- Tafalla, C.; Bøgwald, J.; Dalmo, R.A.; Munangandu, H.M.; Evensen, Ø. Adjuvants in Fish Vaccines. In Fish Vaccination; Wiley Blackwell: West Sussex, UK, 2014; pp. 68–84. [Google Scholar]
- Munang’andu, H.M.; Mutoloki, S.; Evensen, O. An Overview of Challenges Limiting the Design of Protective Mucosal Vaccines for Finfish. Front. Immunol. 2015. [Google Scholar] [CrossRef] [Green Version]
- Munang’andu, H.M.; Mutoloki, S.; Evensen, Ø. Non-replicating Vaccines. In Fish Vaccination; Wiley Blackwell: West Sussex, UK, 2014; pp. 68–84. [Google Scholar]
- Munang’andu, H.M.; Mutoloki, S.; Evensen, O. A Review of the Immunological Mechanisms Following Mucosal Vaccination of Finfish. Front. Immunol. 2015. [Google Scholar] [CrossRef] [Green Version]
- Salinas, I. The mucosal immune system of teleost fish. Biology 2015, 4, 525–539. [Google Scholar] [CrossRef]
- Salinas, I.; Zhang, Y.-A.; Sunyer, J.O. Mucosal immunoglobulins and B cells of teleost fish. Dev. Comp. Immunol. 2011, 35, 1346–1365. [Google Scholar] [CrossRef]
- Fredriksen, B.N.; Grip, J. PLGA/PLA micro- and nanoparticle formulations serve as antigen depots and induce elevated humoral responses after immunization of Atlantic salmon (Salmo salar L.). Vaccine 2012, 30, 656–667. [Google Scholar] [CrossRef]
- Munang’andu, H.M.; Fredriksen, B.N.; Mutoloki, S.; Brudeseth, B.; Kuo, T.Y.; Marjara, I.S.; Dalmo, R.A.; Evensen, O. Comparison of vaccine efficacy for different antigen delivery systems for infectious pancreatic necrosis virus vaccines in Atlantic salmon (Salmo salar L.) in a cohabitation challenge model. Vaccine 2012, 30, 4007–4016. [Google Scholar] [CrossRef]
- Bozkir, A.; Saka, O.M. Chitosan–DNA Nanoparticles: Effect on DNA Integrity, Bacterial Transformation and Transfection Efficiency. J. Drug Target. 2004, 12, 281–288. [Google Scholar] [CrossRef]
- Lee, M.; Nah, J.-W.; Kwon, Y.; Koh, J.J.; Ko, K.S.; Kim, S.W. Water-Soluble and Low Molecular Weight Chitosan-Based Plasmid DNA Delivery. Pharm. Res. 2011, 18, 427–431. [Google Scholar] [CrossRef]
- Pan, Y.; Li, Y.; Zhao, H.; Zheng, J.; Xu, H.; Wei, G.; Hao, J.; Cui, F. Bioadhesive polysaccharide in protein delivery system: Chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int. J. Pharm. 2002, 249, 139–147. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, T. Chitosan nanoparticle as protein delivery carrier—Systematic examination of fabrication conditions for efficient loading and release. Colloids Surf. B Biointerfaces 2007, 59, 24–34. [Google Scholar] [CrossRef]
- Mao, H.-Q.; Roy, K.; Troung-Le, V.L.; Janes, K.A.; Lin, K.Y.; Wang, Y.; August, J.T.; Leong, K.W. Chitosan-DNA nanoparticles as gene carriers: Synthesis, characterization and transfection efficiency. J. Control. Release 2001, 70, 399–421. [Google Scholar] [CrossRef]
- Mao, S.; Sun, W.; Kissel, T. Chitosan-based formulations for delivery of DNA and siRNA. Adv. Drug Deliv. Rev. 2010, 62, 12–27. [Google Scholar] [CrossRef]
- Dahanukar, N. Labeo fimbriatus; The IUCN Red List of Threatened Species, 2011. e.T166500A622. Available online: http://www.iucnredlist.org/details/166500/0 (accessed on 3 November 2016).
- Filip, C.; Fletcher, G.; Wulff, J.L.; Earhart, C. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J. Bacteriol. 1973, 115, 717–722. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin pheno reagents. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Hori, M.; Onishi, H.; Machida, Y. Evaluation of Eudragit-coated chitosan microparticles as an oral immune delivery system. Int. J. Pharm. 2005, 297, 223–234. [Google Scholar] [CrossRef]
- Caipang, C.M.A.; Hynes, N.; Puangkaew, J.; Brinchmann, M.F.; Kiron, V. Intraperitoneal vaccination of Atlantic cod, Gadus morhua with heat-killed Listonella anguillarum enhances serum antibacterial activity and expression of immune response genes. Fish Shellfish Immun. 2008, 24, 314–322. [Google Scholar] [CrossRef]
- Rajesh Kumar, S.; Ishaq Ahmed, V.P.; Parameswaran, V.; Sudhakaran, R.; Sarath Babu, V.; Sahul Hameed, A.S. Potential use of chitosan nanoparticles for oral delivery of DNA vaccine in Asian sea bass (Lates calcarifer) to protect from Vibrio (Listonella) anguillarum. Fish Shellfish Immun. 2008, 25, 47–56. [Google Scholar] [CrossRef]
- Hamod, M.A.; Nithin, M.S.; Shukur, Y.N.; Karunasagar, I.; Karunasagar, I. Outer membrane protein K as a subunit vaccine against V. anguillarum. Aquaculture 2012, 354, 107–110. [Google Scholar] [CrossRef]
- Gudding, R.; Lillehaug, A.; Evensen, O. Recent developments in fish vaccinology. Vet. Immunol. Immunopathol. 1999, 72, 203–212. [Google Scholar] [CrossRef]
- Evensen, O.; Brudeseth, B.; Mutoloki, S. The vaccine formulation and its role in inflammatory processes in fish—Effects and adverse effects. Dev. Biol. 2005, 121, 117–125. [Google Scholar]
- Koppolu, B.; Zaharoff, D.A. The effect of antigen encapsulation in chitosan particles on uptake, activation and presentation by antigen presenting cells. Biomaterials 2013, 34, 2359–2369. [Google Scholar] [CrossRef]
- Zaharoff, D.A.; Rogers, C.J.; Hance, K.W.; Schlom, J.; Greiner, J.W. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine 2007, 25, 2085–2094. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Preat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Tsuji, T.; Yoshitomi, H.; Usukura, J. Endocytic mechanism of transferrin-conjugated nanoparticles and the effects of their size and ligand number on the efficiency of drug delivery. Microscopy 2013, 62, 341–352. [Google Scholar] [CrossRef]
- Win, K.Y.; Feng, S.S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005, 26, 2713–2722. [Google Scholar] [CrossRef]
- Florence, A.T.; Hillery, A.M.; Hussain, N.; Jani, P.U. Factors affecting the oral uptake and translocation of polystyrene nanoparticles: Histological and analytical evidence. J. Drug Target. 1995, 3, 65–70. [Google Scholar] [CrossRef]
- Frohlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Pore, D.; Chowdhury, P.; Mahata, N.; Pal, A.; Yamasaki, S.; Mahalanabis, D.; Chakrabarti, M.K. Purification and characterization of an immunogenic outer membrane protein of Shigella flexneri 2a. Vaccine 2009, 27, 5855–5864. [Google Scholar] [CrossRef]
- Pore, D.; Mahata, N.; Chakrabarti, M.K. Outer membrane protein A (OmpA) of Shigella flexneri 2a links innate and adaptive immunity in a TLR2-dependent manner and involvement of IL-12 and nitric oxide. J. Biol. Chem. 2012, 287, 12589–12601. [Google Scholar] [CrossRef]
- Pore, D.; Mahata, N.; Pal, A.; Chakrabarti, M.K. Outer membrane protein A (OmpA) of Shigella flexneri 2a, induces protective immune response in a mouse model. PLoS ONE 2011, 6, e22663. [Google Scholar] [CrossRef]
- Pore, D.; Chakrabarti, M.K. Outer membrane protein A (OmpA) from Shigella flexneri 2a: A promising subunit vaccine candidate. Vaccine 2013, 31, 3644–3650. [Google Scholar] [CrossRef]
- Medina, A.; Mancera, J.M.; Martinez-Manzanares, E.; Morinigo, M.A.; Arijo, S. Identification of Vibrio harveyi proteins involved in the specific immune response of Senegalese sole (Solea senegalensis, Kaup). Fish Shellfish Immunol. 2015, 47, 377–380. [Google Scholar] [CrossRef]
- Tang, X.; Zhan, W.; Sheng, X.; Chi, H. Immune response of Japanese flounder Paralichthys olivaceus to outer membrane protein of Edwardsiella tarda. Fish Shellfish Immunol. 2010, 28, 333–343. [Google Scholar] [CrossRef]
- Kumar, S.R.; Parameswaran, V.; Ahmed, V.P.; Musthaq, S.S.; Hameed, A.S. Protective efficiency of DNA vaccination in Asian seabass (Lates calcarifer) against Vibrio anguillarum. Fish Shellfish Immunol. 2007, 23, 316–326. [Google Scholar] [CrossRef]
- Meenakshi, M.; Bakshi, C.S.; Butchaiah, G.; Bansal, M.P.; Siddiqui, M.Z.; Singh, V.P. Adjuvanted outer membrane protein vaccine protects poultry against infection with Salmonella enteritidis. Vet. Res. Commun. 1999, 23, 81–90. [Google Scholar] [CrossRef]
- Gomez-Casado, E.; Estepa, A.; Coll, J.M. A comparative review on European-farmed finfish RNA viruses and their vaccines. Vaccine 2011, 29, 2657–2671. [Google Scholar] [CrossRef]
- Ji, J.; Torrealba, D.; Ruyra, A.; Roher, N. Nanodelivery Systems as New Tools for Immunostimulant or Vaccine Administration: Targeting the Fish Immune System. Biology 2015, 4, 664–696. [Google Scholar] [CrossRef]
- Munang’andu, H.M.; Evensen, O. A Review of Intra- and Extracellular Antigen Delivery Systems for Virus Vaccines of Finfish. J. Immunol. Res. 2015. [Google Scholar] [CrossRef]
- Shaalan, M.; Saleh, M.; El-Mahdy, M.; El-Matbouli, M. Recent progress in applications of nanoparticles in fish medicine: A review. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 701–710. [Google Scholar] [CrossRef]
- Rivas-Aravena, A.; Fuentes, Y.; Cartagena, J.; Brito, T.; Poggio, V.; La Torre, J.; Mendoza, H.; Gonzalez-Nilo, F.; Sandino, A.M.; Spencer, E. Development of a nanoparticle-based oral vaccine for Atlantic salmon against ISAV using an alphavirus replicon as adjuvant. Fish Shellfish Immunol. 2015, 45, 157–166. [Google Scholar] [CrossRef]
- Rauta, P.R.; Nayak, B. Parenteral immunization of PLA/PLGA nanoparticle encapsulating outer membrane protein (Omp) from Aeromonas hydrophila: Evaluation of immunostimulatory action in Labeo rohita (rohu). Fish Shellfish Immunol. 2015, 44, 287–294. [Google Scholar] [CrossRef]
- Dubey, S.; Avadhani, K.; Mutalik, S.; Sivadasan, S.M.; Maiti, B.; Paul, J.; Girisha, S.K.; Venugopal, M.N.; Mutoloki, S.; Evensen, O.; et al. Aeromonas hydrophila OmpW PLGA Nanoparticle Oral Vaccine Shows a Dose-Dependent Protective Immunity in Rohu (Labeo rohita). Vaccines 2016. [Google Scholar] [CrossRef]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubey, S.; Avadhani, K.; Mutalik, S.; Sivadasan, S.M.; Maiti, B.; Girisha, S.K.; Venugopal, M.N.; Mutoloki, S.; Evensen, Ø.; Karunasagar, I.; et al. Edwardsiella tarda OmpA Encapsulated in Chitosan Nanoparticles Shows Superior Protection over Inactivated Whole Cell Vaccine in Orally Vaccinated Fringed-Lipped Peninsula Carp (Labeo fimbriatus). Vaccines 2016, 4, 40. https://doi.org/10.3390/vaccines4040040
Dubey S, Avadhani K, Mutalik S, Sivadasan SM, Maiti B, Girisha SK, Venugopal MN, Mutoloki S, Evensen Ø, Karunasagar I, et al. Edwardsiella tarda OmpA Encapsulated in Chitosan Nanoparticles Shows Superior Protection over Inactivated Whole Cell Vaccine in Orally Vaccinated Fringed-Lipped Peninsula Carp (Labeo fimbriatus). Vaccines. 2016; 4(4):40. https://doi.org/10.3390/vaccines4040040
Chicago/Turabian StyleDubey, Saurabh, Kiran Avadhani, Srinivas Mutalik, Sangeetha Madambithara Sivadasan, Biswajit Maiti, Shivani Kallappa Girisha, Moleyur Nagarajappa Venugopal, Stephen Mutoloki, Øystein Evensen, Indrani Karunasagar, and et al. 2016. "Edwardsiella tarda OmpA Encapsulated in Chitosan Nanoparticles Shows Superior Protection over Inactivated Whole Cell Vaccine in Orally Vaccinated Fringed-Lipped Peninsula Carp (Labeo fimbriatus)" Vaccines 4, no. 4: 40. https://doi.org/10.3390/vaccines4040040
APA StyleDubey, S., Avadhani, K., Mutalik, S., Sivadasan, S. M., Maiti, B., Girisha, S. K., Venugopal, M. N., Mutoloki, S., Evensen, Ø., Karunasagar, I., & Munang′andu, H. M. (2016). Edwardsiella tarda OmpA Encapsulated in Chitosan Nanoparticles Shows Superior Protection over Inactivated Whole Cell Vaccine in Orally Vaccinated Fringed-Lipped Peninsula Carp (Labeo fimbriatus). Vaccines, 4(4), 40. https://doi.org/10.3390/vaccines4040040





