A Scoping Review of Influences on HPV Vaccine Uptake in the Rural US †
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Source of Evidence Screening and Selection
2.3. Data Extraction
2.4. Data Analysis
3. Results
3.1. Study Descriptions
3.2. The Characteristics of HPV Vaccine Interventions
3.3. Theoretical Models
3.4. Multilevel Interventions and Change
3.5. HPV Vaccination Outcomes of Initiation, Completion, or Both
3.6. Predictors of HPV Vaccine Initiation
4. Discussion
4.1. Summary of the Results
4.2. Sociodemographic Influences
4.3. Healthcare Provider Influences
4.4. Multilevel Observations and Interventions
4.5. Theory as a Guide
4.6. Limitations
5. Conclusions
6. Next Steps
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Search Strategy
Appendix A.1.1. Medline
- exp Papillomavirus Infections/or exp Alphapapillomavirus/or (“papillomavirus infection*” or “Human Papillomavirus” or “Human papilloma virus” or HPV).tw,kw.
- Exp immunization/or exp Immunization programs/or exp Papillomavirus Vaccines/or Vaccines/or (vaccin* OR immuniz* OR immunis* OR inoculat* OR Nine-valent OR “nine valent” OR bivalent OR quadrivalent OR Gardasil OR cervarix).tw,kw.
- Exp patient acceptance of health care/OR exp Health Knowledge, Attitudes, Practice/OR vaccination refusal/OR anti-vaccination movement/OR exp decision making/OR trust/OR exp risk assessment OR exp religion/OR (accept* OR aware* OR attitude* OR knowledge OR belief* OR view* OR opinion* OR barrier* OR support* OR behave*OR decision OR decide OR intent* OR undecided OR hesita* OR doubt* OR refus* OR reject* OR omission* OR omit* OR object OR objection OR incomplet* OR delay* OR suboptimal* OR intent* OR know* OR perceive* OR percept* OR perspective* OR understand* OR prefer* OR risk* OR uptake* OR will* OR hesitan* OR reluctan* OR fear OR concern* OR trust OR uncertain* or distrust OR anti-vax* OR anti-vacc* OR antivax* or antivaccin* OR wary OR religion OR religious).tw,kw.
- Exp rural health services/OR rural population/OR rural health/OR “Hospitals, Rural”/OR Medically Underserved Area/OR exp population dynamics/OR exp residence characteristics/OR (remote OR rural OR Appalachia OR (regional adj3 disparit*) OR “small town*” OR (region* adj3 disparities) OR ((geographic* OR medical*) adj3 (underserv* OR underrepresent*)) OR (underserv* adj3 (population* OR communit* OR area*)) OR (shortage adj3 area)).tw,kw.
- (1 AND 2 AND 3 AND 4)
Appendix A.1.2. Embase
- ‘Papillomavirus Infection’/exp OR ‘Alphapapillomavirus’/exp OR (“Human Papillomavirus” OR “papillomavirus infection*” OR “Human papilloma virus” OR HPV): ti, ab, kw
- ‘immunization’/exp OR ‘wart virus Vaccine’/de OR ‘Vaccine’/de OR (vaccin* OR immuniz* OR immunis* OR inoculat* OR Nine-valent OR “nine valent” OR bivalent OR quadrivalent OR Gardasil OR cervarix): ti, ab, kw
- ‘Patient attitude’/exp OR ‘attitude to health’/de OR ‘anti-vaccination movement’/de OR ‘patient decision making’/de OR ‘trust’/de OR ‘risk assessment’/de OR ‘religion’/exp OR (accept* OR aware* OR attitude* OR knowledge OR belief* OR view* OR opinion* OR barrier* OR support* OR behave* OR decision OR decide OR intent* OR undecided OR hesita* OR doubt* OR refus* OR reject* OR omission* OR omit* OR object OR objection OR incomplet* OR delay* OR suboptimal* OR intent* OR know* OR perceive* OR percept* OR perspective* OR understand* OR prefer* OR risk* OR uptake* OR will* OR hesitan* OR reluctan* OR fear OR concern* OR trust OR uncertain* OR distrust OR anti-vax* OR anti-vacc* OR antivax* OR antivaccin* OR wary OR religion OR religious): ti, ab, kw
- ‘rural health care’/exp OR ‘rural population’/de OR ‘rural health’/de OR ‘rural hospital’/de OR ‘Medically Underserved’/de OR ‘population dynamics’/exp OR ‘demography”/de OR (remote OR rural OR Appalachia OR (regional NEAR/3 disparit*) OR “small town*” OR (region* NEAR/3 disparities) OR ((geographic* OR medical*) NEAR/3 (underserv* OR underrepresent*)) OR (underserv* NEAR/3 (population* OR communit* OR area*)) OR (shortage NEAR/3 area)): ti, ab, kw
- #5:
- [06-07-2023]/sd
- #6:
- [embase]/lim NOT ([embase]/lim AND [medline]/lim)
- #7.
- (#1 AND #2 AND #3 AND #4 AND #5 AND #6)
Appendix A.1.3. CINAHL
- (MH “Papillomavirus Infections+”) OR (TI “papillomavirus infection*” OR AB “papillomavirus infection*”) OR (TI “Human Papillomavirus” OR AB “Human Papillomavirus”) OR (TI “Human papilloma virus” OR AB “Human papilloma virus”) OR (TI HPV OR AB HPV)
- (MH “immunization”) OR (MH “Papillomavirus Vaccines”) OR (MH “Viral Vaccines”) OR ((TI vaccin* OR AB vaccin*) OR (TI immuniz* OR AB immuniz*) OR (TI immunis* OR AB immunis*) OR (TI inoculat* OR AB inoculat*) OR (TI Nine-valent OR AB Nine-valent) OR (TI “nine valent” OR AB “nine valent”) OR (TI bivalent OR AB bivalent) OR (TI quadrivalent OR AB quadrivalent) OR (TI Gardasil OR AB Gardasil) OR (TI cervarix OR AB cervarix)
- (MH “Patient Attitudes”) OR (MH “Attitude to Vaccines”) OR (MH “Health Knowledge”) OR (MH “anti-vaccination movement”) OR (MH “decision making, patient”) OR (MH “trust”) OR (MH “risk assessment”) OR (MH “religion and relgions+”) OR ((TI accept* OR AB accept*) OR (TI aware* OR AB aware*) OR (TI attitude* OR AB attitude*) OR (TI knowledge OR AB knowledge) OR (TI belief* OR AB belief*) OR (TI view* OR AB view*) OR (TI opinion* OR AB opinion*) OR (TI barrier* OR AB barrier*) OR (TI support* OR AB support*) OR (TI behave* OR AB behave*) OR (TI decision OR AB decision) OR (TI decide OR AB decide) OR (TI intent* OR AB intent*) OR (TI undecided OR AB undecided) OR (TI hesita* OR AB hesita*) OR (TI doubt* OR AB doubt*) OR (TI refus* OR AB refus*) OR (TI reject* OR AB reject*) OR (TI omission* OR AB omission*) OR (TI omit* OR AB omit*) OR (TI object OR AB object) OR (TI objection OR AB objection) OR (TI incomplet* OR AB incomplet*) OR (TI delay* OR AB delay*) OR (TI suboptimal* OR AB suboptimal*) OR (TI intent* OR AB intent*) OR (TI know* OR AB know*) OR (TI perceive* OR AB perceive*) OR (TI percept* OR AB percept*) OR (TI perspective* OR AB perspective*) OR (TI understand* OR AB understand*) OR (TI prefer* OR AB prefer*) OR (TI risk* OR AB risk*) OR (TI uptake* OR AB uptake*) OR (TI will* OR AB will*) OR (TI hesitan* OR AB hesitan*) OR (TI reluctan* OR AB reluctan*) OR (TI fear OR AB fear) OR (TI concern* OR AB concern*) OR (TI trust OR AB trust) OR (TI uncertain* OR AB uncertain*) OR (TI distrust OR AB distrust) OR (TI anti-vax* OR AB anti-vax*) OR (TI anti-vacc* OR AB anti-vacc*) OR (TI antivax* OR AB antivax*) OR (TI antivaccin* OR AB antivaccin*) OR (TI wary OR AB wary) OR (TI religion OR AB religion) OR (TI religious OR AB religious)
- (MH “rural health services”) OR (MH “rural population”) OR (MH “rural health”) OR (MH “Hospitals, Rural”) OR (MH “Medically Underserved Area”) OR (MH “population characteristics”) OR (MH “residence characteristics”) OR ((TI remote OR AB remote) OR (TI rural OR AB rural) OR (TI Appalachia OR AB Appalachia) OR ((TI regional OR AB regional) N3 (TI disparit* OR AB disparit*)) OR (TI “small town*” OR AB “small town*”) OR ((TI region* OR AB region*) N3 (TI disparities OR AB disparities)) OR (((TI geographic* OR AB geographic*) OR (TI medical* OR AB medical*)) N3 ((TI underserv* OR AB underserv*) OR (TI underrepresent* OR AB underrepresent*))) OR ((TI underserv* OR AB underserv*) N3 ((TI population* OR AB population*) OR (TI communit* OR AB communit*) OR (TI area* OR AB area*))) OR ((TI shortage OR AB shortage) N3 (TI area OR AB area)))
- #5:
- (1 AND 2 AND 3 AND 4 AND 5)
Appendix A.1.4. Scopus
- TITLE-ABS-KEY (“Papillomavirus Infection*”) OR TITLE-ABS-KEY (“papillomavirus infection*”) OR TITLE-ABS-KEY (“Alphapapillomavirus”) OR TITLE-ABS-KEY (“Human Papillomavirus”) OR TITLE-ABS-KEY (“Human papilloma virus”) OR TITLE-ABS-KEY (“HPV”)
- TITLE-ABS-KEY (“immunization”) OR TITLE-ABS-KEY (“Vaccin*”) OR TITLE-ABS-KEY (“immuniz*”) OR TITLE-ABS-KEY (“immunis*”) OR TITLE-ABS-KEY (“inoculat*”) OR TITLE-ABS-KEY (“Nine-valent”) OR TITLE-ABS-KEY (“nine valent”) OR TITLE-ABS-KEY (“bivalent”) OR TITLE-ABS-KEY (“quadrivalent”) OR TITLE-ABS-KEY (“Gardasil”) OR TITLE-ABS-KEY (“cervarix”)
- INDEXTERMS (“patient acceptance of health care”) OR INDEXTERMS (“Health Knowledge, Attitudes, Practice”) OR INDEXTERMS (“anti-vaccination movement”) OR INDEXTERMS (“decision making”) OR INDEXTERMS (“trust”) OR INDEXTERMS (“risk assessment”) OR TITLE-ABS-KEY (“accept*”) OR TITLE-ABS-KEY (“aware*”) OR TITLE-ABS-KEY (“attitude*”) OR TITLE-ABS-KEY (“knowledge”) OR TITLE-ABS-KEY (“belief*”) OR TITLE-ABS-KEY (“view*”) OR TITLE-ABS-KEY (“opinion*”) OR TITLE-ABS-KEY (“barrier*”) OR TITLE-ABS-KEY (“support*”) OR TITLE-ABS-KEY (“behave*”) OR TITLE-ABS-KEY (“decision”) OR TITLE-ABS-KEY (“decide”) OR TITLE-ABS-KEY (“intent*”) OR TITLE-ABS-KEY (“undecided”) OR TITLE-ABS-KEY (“hesita*”) OR TITLE-ABS-KEY (“doubt*”) OR TITLE-ABS-KEY (“refus*”) OR TITLE-ABS-KEY (“reject*”) OR TITLE-ABS-KEY (“omission*”) OR TITLE-ABS-KEY (“omit*”) OR TITLE-ABS-KEY (“object”) OR TITLE-ABS-KEY (“objection”) OR TITLE-ABS-KEY (“incomplet*”) OR TITLE-ABS-KEY (“delay*”) OR TITLE-ABS-KEY (“suboptimal*”) OR TITLE-ABS-KEY (“intent*”) OR TITLE-ABS-KEY (“know*”) OR TITLE-ABS-KEY (“perceive*”) OR TITLE-ABS-KEY (“percept*”) OR TITLE-ABS-KEY (“perspective*”) OR TITLE-ABS-KEY (“understand*”) OR TITLE-ABS-KEY (“prefer*”) OR TITLE-ABS-KEY (“risk*”) OR TITLE-ABS-KEY (“uptake*”) OR TITLE-ABS-KEY (“will*”) OR TITLE-ABS-KEY (“hesitan*”) OR TITLE-ABS-KEY (“reluctan*”) OR TITLE-ABS-KEY (“fear”) OR TITLE-ABS-KEY (“concern*”) OR TITLE-ABS-KEY (“trust”) OR TITLE-ABS-KEY (“uncertain*”) OR TITLE-ABS-KEY (“distrust”) OR TITLE-ABS-KEY (“anti-vax*”) OR TITLE-ABS-KEY (“anti-vacc*”) OR TITLE-ABS-KEY (“antivax*”) OR TITLE-ABS-KEY (“antivaccin*”) OR TITLE-ABS-KEY (“wary”) OR TITLE-ABS-KEY (“religion”) OR TITLE-ABS-KEY (“religious”)
- INDEXTERMS (“Medically Underserved Area”) OR INDEXTERMS (“population dynamics”) OR “exp residence characteristics” OR (TITLE-ABS-KEY (“remote”) OR TITLE-ABS-KEY (“rural”) OR TITLE-ABS-KEY (“Appalachia”) OR (TITLE-ABS-KEY (“regional”) W/3 TITLE-ABS-KEY (“disparit*”)) OR TITLE-ABS-KEY (“small town*”) OR (TITLE-ABS-KEY (“region*”) W/3 TITLE-ABS-KEY (“disparities”)) OR ( (TITLE-ABS-KEY (“geographic*”) OR TITLE-ABS-KEY (“medical*”)) W/3 (TITLE-ABS-KEY (“underserv*”) OR TITLE-ABS-KEY (“underrepresent*”))) OR (TITLE-ABS-KEY (“underserv*”) W/3 (TITLE-ABS-KEY (“population*”) OR TITLE-ABS-KEY (“communit*”) OR TITLE-ABS-KEY (“area*”))) OR (TITLE-ABS-KEY (“shortage”) W/3 TITLE-ABS-KEY (“area”)))
- #5:
- ORIG-LOAD-DATE AFT 20230706
- #5
- (#1 AND #2 AND #3 AND #4 AND #5)
Appendix A.1.5. PsycInfo
- (DE “Human Papillomavirus”) OR ((TI “Human Papillomavirus” OR AB “Human Papillomavirus”) OR (TI “papillomavirus infection*” OR AB “papillomavirus infection*”) OR (TI “Human papilloma virus” OR AB “Human papilloma virus”) OR (TI HPV OR AB HPV))
- (DE “immunization”) OR ((TI vaccin* OR AB vaccin*) OR (TI immuniz* OR AB immuniz*) OR (TI immunis* OR AB immunis*) OR (TI inoculat* OR AB inoculat*) OR (TI Nine-valent OR AB Nine-valent) OR (TI “nine valent” OR AB “nine valent”) OR (TI bivalent OR AB bivalent) OR (TI quadrivalent OR AB quadrivalent) OR (TI Gardasil OR AB Gardasil) OR (TI cervarix OR AB cervarix))
- (DE “Health Knowledge”) OR (DE “decision making”) OR (DE “trust”) OR (DE “risk assessment”) OR (DE “religion+”) OR ((TI accept* OR AB accept*) OR (TI aware* OR AB aware*) OR (TI attitude* OR AB attitude*) OR (TI knowledge OR AB knowledge) OR (TI belief* OR AB belief*) OR (TI view* OR AB view*) OR (TI opinion* OR AB opinion*) OR (TI barrier* OR AB barrier*) OR (TI support* OR AB support*) OR (TI behave* OR AB behave*) OR (TI decision OR AB decision) OR (TI decide OR AB decide) OR (TI intent* OR AB intent*) OR (TI undecided OR AB undecided) OR (TI hesita* OR AB hesita*) OR (TI doubt* OR AB doubt*) OR (TI refus* OR AB refus*) OR (TI reject* OR AB reject*) OR (TI omission* OR AB omission*) OR (TI omit* OR AB omit*) OR (TI object OR AB object) OR (TI objection OR AB objection) OR (TI incomplet* OR AB incomplet*) OR (TI delay* OR AB delay*) OR (TI suboptimal* OR AB suboptimal*) OR (TI intent* OR AB intent*) OR (TI know* OR AB know*) OR (TI perceive* OR AB perceive*) OR (TI percept* OR AB percept*) OR (TI perspective* OR AB perspective*) OR (TI understand* OR AB understand*) OR (TI prefer* OR AB prefer*) OR (TI risk* OR AB risk*) OR (TI uptake* OR AB uptake*) OR (TI will* OR AB will*) OR (TI hesitan* OR AB hesitan*) OR (TI reluctan* OR AB reluctan*) OR (TI fear OR AB fear) OR (TI concern* OR AB concern*) OR (TI trust OR AB trust) OR (TI uncertain* OR AB uncertain*) OR (TI distrust OR AB distrust) OR (TI anti-vax* OR AB anti-vax*) OR (TI anti-vacc* OR AB anti-vacc*) OR (TI antivax* OR AB antivax*) OR (TI antivaccin* OR AB antivaccin*) OR (TI wary OR AB wary) OR (TI religion OR AB religion) OR (TI religious OR AB religious))
- (DE “rural environments”) OR (DE “rural health”) OR ((TI remote OR AB remote) OR (TI rural OR AB rural) OR (TI Appalachia OR AB Appalachia) OR ((TI regional OR AB regional) N3 (TI disparit* OR AB disparit*)) OR (TI “small town*” OR AB “small town*”) OR ((TI region* OR AB region*) N3 (TI disparities OR AB disparities)) OR (((TI geographic* OR AB geographic*) OR (TI medical* OR AB medical*)) N3 ((TI underserv* OR AB underserv*) OR (TI underrepresent* OR AB underrepresent*))) OR ((TI underserv* OR AB underserv*) N3 ((TI population* OR AB population*) OR (TI communit* OR AB communit*) OR (TI area* OR AB area*))) OR ((TI shortage OR AB shortage) N3 (TI area OR AB area)))
- (#1 AND #2 AND #3 AND #4)
Appendix A.1.6. Cochrane
- [mh “Papillomavirus Infections”] OR [mh Alphapapillomavirus] OR (“papillomavirus infection*” OR “Human Papillomavirus” OR “Human papilloma virus” OR HPV): ti, ab, kw.
- [mh immunization] OR [mh “Immunization programs”] OR [mh “Papillomavirus Vaccines”] OR [mh Vaccines] OR (vaccin* OR immuniz* OR immunis* OR inoculat* OR Nine-valent OR “nine valent” OR bivalent OR quadrivalent OR Gardasil OR cervarix): ti, ab, kw.
- [mh “patient acceptance of health care”] OR [mh “Health Knowledge, Attitudes, Practice”] OR [mh “vaccination refusal”] OR [mh “anti-vaccination movement”] OR [mh “decision making”] OR [mh trust] OR [mh “risk assessment OR exp religion”] OR (accept* OR aware* OR attitude* OR knowledge OR belief* OR view* OR opinion* OR barrier* OR support* OR behave* OR decision OR decide OR intent* OR undecided OR hesita* OR doubt* OR refus* OR reject* OR omission* OR omit* OR object OR objection OR incomplet* OR delay* OR suboptimal* OR intent* OR know* OR perceive* OR percept* OR perspective* OR understand* OR prefer* OR risk* OR uptake* OR will* OR hesitan* OR reluctan* OR fear OR concern* OR trust OR uncertain* OR distrust OR anti-vax* OR anti-vacc* OR antivax* OR antivaccin* OR wary OR religion OR religious): ti, ab, kw.
- [mh “rural health services”] OR [mh “rural population”] OR [mh “rural health”] OR [mh “Hospitals, Rural”] OR [mh “Medically Underserved Area”] OR [mh “population dynamics”] OR [mh “residence characteristics”] OR (remote OR rural OR Appalachia): ti, ab, kw. OR (regional NEAR/3 disparit*): ti, ab, kw. OR (“small” NEAR/2 town*): ti, ab, kw. OR (region* NEAR/3 disparities): ti, ab, kw. OR ((geographic* OR medical*) NEAR/3 (underserv* OR underrepresent*)): ti, ab, kw. OR (underserv* NEAR/3 (population* OR communit* OR area*)): ti, ab, kw. OR (shortage NEAR/3 area): ti, ab, kw.
Appendix A.1.7. Sociological Abstracts
- TI (“papillomavirus infection*” OR “Human Papillomavirus” or “Human papilloma virus” or HPV) OR AB (“papillomavirus infection*” OR “Human Papillomavirus” or “Human papilloma virus” or HPV)
- SU (“immunization”) OR SU (“vaccination”) or TI (vaccin* OR immuniz* OR immunis* OR inoculat* OR Nine-valent OR “nine valent” OR bivalent OR quadrivalent OR Gardasil OR cervarix) OR AB (vaccin* OR immuniz* OR immunis* OR inoculat* OR Nine-valent OR “nine valent” OR bivalent OR quadrivalent OR Gardasil OR cervarix)
- SU (“Health attitudes”) OR MAINSUBJECT.EXACT.EXPLODE (“Decision Making”) OR SU (“trust”) OR SU (“risk”) OR SU (“religions”) OR (TI (accept* OR aware* OR attitude* OR knowledge OR belief* OR view* OR opinion* OR barrier* OR support* OR behave* OR decision OR decide OR intent* OR undecided OR hesita* OR doubt* OR refus* OR reject* OR omission* OR omit* OR object OR objection OR incomplet* OR delay* OR suboptimal* OR intent* OR know* OR perceive* OR percept* OR perspective* OR understand* OR prefer* OR risk* OR uptake* OR will* OR hesitan* OR reluctan* OR fear OR concern* OR trust OR uncertain* or distrust OR anti-vax* OR anti-vacc* OR antivax* or antivaccin* OR wary OR religion OR religious) OR AB (accept* OR aware* OR attitude* OR knowledge OR belief* OR view* OR opinion* OR barrier* OR support* OR behave* OR decision OR decide OR intent* OR undecided OR hesita* OR doubt* OR refus* OR reject* OR omission* OR omit* OR object OR objection OR incomplet* OR delay* OR suboptimal* OR intent* OR know* OR perceive* OR percept* OR perspective* OR understand* OR prefer* OR risk* OR uptake* OR will* OR hesitan* OR reluctan* OR fear OR concern* OR trust OR uncertain* or distrust OR anti-vax* OR anti-vacc* OR antivax* or antivaccin* OR wary OR religion OR religious))
- SU (“Rural Population”) OR SU (“Rural Areas”) OR SU (“Rurality”) OR SU (“Rural Communities”) OR SU (“Rural Urban Differences”) OR SU (“residence”) OR TI (remote OR rural OR Appalachia OR (regional adj3 disparit*) OR “small town*” OR (region* adj3 disparities) OR ((geographic* OR medical*) adj3 (underserv* OR underrepresent*)) OR (underserv* adj3 (population* OR communit* OR area*)) OR (shortage adj3 area)) OR AB (remote OR rural OR Appalachia OR (regional adj3 disparit*) OR “small town*” OR (region* adj3 disparities) OR ((geographic* OR medical*) adj3 (underserv* OR underrepresent*)) OR (underserv* adj3 (population* OR communit* OR area*)) OR (shortage adj3 area))
- (1 AND 2 AND 3 AND 4)
Appendix B. Full Study Characteristics (N = 101)
| Reference | Publication Year | Location | Study Years | Purpose/Aims | Study Type | Sample Size | Participant Type | Theory, Model, and Framework | Primary Outcomes Measured | Other Outcomes Measured | Primary Findings | Other Findings |
| Bhatta MP, Phillips L. Human papillomavirus vaccine awareness, uptake, and parental and healthcare provider communication among 11- to 18-year-old adolescents in a rural Appalachian Ohio county in the United States. J Rural Health Winter. 2015;31(1):67–75. doi:10.1111/jrh.12079 [26] | 2014 | Midwest | 2012 | Examine the levels of adolescent HPV vaccine awareness, uptake, and parental and healthcare provider communication; assess the relationship between the parental and healthcare provider communication regarding the HPV vaccine, and the vaccine uptake from the adolescent perspective | Cross-sectional survey | 1299 | Parents of adolescents | HPV vaccine initiation and awareness | Parental and provider communication about HPV vaccine | 49.2% of respondents reported that they have heard of the HPV vaccine. Overall, 19.4% of the adolescents indicated having a discussion with their parents about the HPV vaccine. Nearly a quarter (24.6%) of the adolescents indicated having a healthcare provider discuss the HPV vaccine with them. | Both parental and healthcare provider communication were significantly associated with HPV vaccine uptake in this population (p < 0.0001) | |
| Crosby RA, Casey BR, Vanderpool R, Collins T, Moore GR. Uptake of free HPV vaccination among young women: a comparison of rural versus urban rates. J Rural Health Winter. 2011;27(4):380–384. doi:10.1111/j.1748-0361.2010.00354.x [27] | 2011 | South | 2007–2009 | Compare rates of initial HPV vaccine uptake, offered at no cost, between a rural clinic, a rural community college, and an urban college clinic and to identify rural–urban differences in uptake of free booster doses | quasi-experimental study | 706 | Adults | HPV vaccine Initiation and Completion | The contrast in initial uptake between urban clinic women and rural college women was significant (p < 0.0001), but the difference in initial uptake between urban clinic women and rural clinic women was not significant (p = 0.42). Rural clinic women were about 7 times more likely than urban clinic women (p < 0.0001) to not return for at least 1 follow-up dose. The difference between urban clinic women and rural college women was significant for follow-up vaccine doses (p = 0.014). | |||
| * Osaghae I, Chido-Amajuoyi OG, Shete S. Healthcare Provider Recommendations and Observed Changes in HPV Vaccination Acceptance during the COVID-19 Pandemic. Vaccines. 2022;10(9):1515. doi:10.3390/vaccines10091515 [36] | 2022 | South | 2021 | Examine the association between HPV vaccination recommendation by HCPs and their observed changes in HPV Vaccination acceptance during the COVID-19 pandemic | Cross-sectional study | 1283 | Providers | HPV vaccine initiation | 554 (77.5%) reported no change, 99 (13.9%) reported a decrease, and 62 (8.7%) reported an increase in HPV vaccination acceptance during the COVID-19 pandemic. Providers who recommended the vaccine often/always had 46% (OR = 0.54; 95%CI: 0.30–0.96) lower odds of reporting a decrease in HPV vaccination acceptance during the COVID-19 pandemic | |||
| Ryan G, Gilbert PA, Ashida S, Charlton ME, Scherer A, Askelson NM. Challenges to Adolescent HPV Vaccination and Implementation of Evidence-Based Interventions to Promote Vaccine Uptake During the COVID-19 Pandemic: “HPV Is Probably Not at the Top of Our List.” Prev Chronic Dis. 2022;19. doi:10.5888/pcd19.210378 [38] | 2022 | Midwest | 2020 | Assess how the COVID-19 pandemic impacted opportunities for HPV vaccination delivery and EBI implementation | Qualitative | 18 | Clinic Managers and administrators | Consolidated Framework for Implementation Research | Clinic challenges to implementing HPV vaccines during COVID-19 pandemic | The pandemic led to an overall decrease in HPV vaccinations as well as routine care. Additionally, the pandemic disrupted EBI work (evidence-based interventions) | ||
| Adjei Boakye E, Fedorovich Y, White M, et al. Rural–Urban Disparities in HPV Vaccination Coverage Among Adolescents in the Central Part of the State of Illinois, USA. J Community Health. 2022;48(1):24–29. doi:10.1007/s10900-022-01136-x [52] | 2022 | Midwest | 2015–2020 | Quantify the rates of HPV vaccine initiation and completion in an academic medical center in central Illinois and identify factors associated with both outcomes | Retrospective Chart Review | 9351 | Adolescents | HPV vaccine initiation and completion | Vaccine initiation for HPV was 46.2% and completion was 24.7% among the participants. Older age and being female increased the odds of initiating and completing the HPV vaccination. Adolescents residing in rural areas were 38% and 24% less likely to initiate (aOR = 0.62; 95 CI: 0.54–0.72) and complete (aOR = 0.76, 95 CI: 0.65–0.88) the HPV vaccine compared to those in urban areas. Adolescents were less likely to initiate and complete the HPV vaccine if they were not up-to-date on the hepatitis A, meningococcal, and TDaP vaccines. | |||
| Adjei Boakye E, McKinney SL, Whittington KD, et al. Association between Sexual Activity and Human Papillomavirus (HPV) Vaccine Initiation and Completion among College Students. Vaccines. 2022;10(12):2079. doi:10.3390/vaccines10122079 [53] | 2022 | Midwest | 2021 | Examine if sexual activity was associated with HPV vaccination uptake among university students | Cross-sectional study | 951 | Adults | HPV vaccine initiation and completion | Students who had ever engaged in sexual activity were more likely to have initiated (aOR = 2.06, 95% CI: 1.34–3.17) the HPV vaccine; however, no difference was observed for HPV vaccine completion. | |||
| Askelson N, Ryan G, McRee AL, et al. Using concept mapping to identify opportunities for HPV vaccination efforts: Perspectives from the Midwest and West Coast. Eval Program Plann. 2021;89:102010. doi:10.1016/j.evalprogplan.2021.102010 [54] | 2021 | Midwest, West | 2018–2019 | Solicit perspectives on barriers and facilitators to HPV vaccination from state-level stakeholders | Observational | 134 | Other (stakeholders) | Barriers and facilitators to HPV vaccination from state-level stakeholders | Clusters rated most feasible included coordinated/consistent messaging and education. Clusters rated as most important for improving vaccination in rural areas were education (Mean [M] = 4.21), provider influence (M = 4.10), and evidence-based interventions (M = 4.07). All items except coordinated/consistent messaging were rated as more important than feasible. | |||
| Askelson NM, Campo S, Lowe JB, Smith S, Dennis LK, Andsager J. Using the Theory of Planned Behavior to Predict Mothers’ Intentions to Vaccinate Their Daughters Against HPV. J Sch Nurs. 2010;26(3):194–202. doi:10.1177/1059840510366022 [55] | 2010 | Midwest | 2007 | Investigate the influences of mothers’ intentions to vaccinate their daughters against HPV | Cross-sectional survey | 217 | Parents of adolescents | Theory of Planned Behavior | Intention to vaccinate | Attitudes were the strongest predictor of mothers’ intentions to vaccinate (β = 0.61, p < 0.001). Mothers with subjective norms that were in support of the vaccine were more likely to intend to vaccinate (β = 0.16, p < 0.05). | ||
| Askelson NM, Campo S, Smith S, Lowe JB, Dennis L, Andsager J. Assessing physicians’ intentions to talk about sex when they vaccinate nine-year-old to 15-year-old girls against HPV. Sex Educ. 2011;11(4):431–441. doi:10.1080/14681811.2011.595252 [56] | 2011 | Midwest | Not listed | Assess whether physicians would use HPV vaccination to communicate with young female patients about sex | Cross-sectional study | 207 | Provider | Theory of Planned Behavior | Intention to vaccinate | Most physicians intended to talk about sexually transmitted infections when they vaccinate against HPV (90.3%). Physicians’ intentions to talk about sex are influenced by attitudes (β = 0.18, p < 0.05), subjective norms (β = 0.53, p < 0.001), and perceived behavioral control (β = 0.15, p < 0.05). | ||
| Askelson NM, Ryan G, Seegmiller L, Pieper F, Kintigh B, Callaghan D. Implementation Challenges and Opportunities Related to HPV Vaccination Quality Improvement in Primary Care Clinics in a Rural State. J Community Health Aug. 2019;44(4):790–795. doi:10.1007/s10900-019-00676-z [57] | 2019 | Midwest | 2017 | Understand the decision-making process of intervention selection and implementation from the perspective of Vaccine for Children (VHC) liaisons | Cross-sectional study | 115 | Clinics | How HPV intervention selection decisions are made and the extent of implementation | Respondents (VFC liaisons) reported decisions about vaccine QI were made by multiple actors within their own clinics (45.1%), by a clinic manager in charge of multiple clinics (33.0%) and/or at a centralized administrative office (35.2%). Additionally, the majority of respondents considered external actors, like insurance companies (52.7%) or Medicaid/Medicare (50.5%), important to the decision-making process. | |||
| Ayres S, Gee A, Kim S. Human Papillomavirus Vaccination Knowledge, Barriers, and Recommendations Among Healthcare Provider Groups in the Western United States. J Cancer Educ Dec. 2022;37(6):1816–1823. doi:10.1007/s13187-021-02047-6 [58] | 2021 | West | 2019 | Compare differences in same-day HPV vaccination recommendation at clinics Mountain West (MW) in states between healthcare provider and staff groups, and compare different provider groups’ perceived challenges associated with HPV vaccination, HPV vaccination knowledge, HPV recommendation practices, and same-day HPV vaccination recommendation | Cross-sectional study | 99 | Providers | Provider challenges and knowledge, and recommendation practices, and same-day HPV vaccination | Clinicians had a higher knowledge of HPV vaccination, identified more challenges that limit HPV vaccination, and had better HPV recommendation practices. There was no difference between clinicians and OTMs on the tendency of the patients to receive vaccine on same day as recommended. No significant differences were found between rural and urban subgroups on demographics or survey responses. | |||
| Beck A, Bianchi A, Showalter D. Evidence-Based Practice Model to Increase Human Papillomavirus Vaccine Uptake: A Stepwise Approach. Nurs Womens Health. 2021;25(6):430–436. doi:10.1016/j.nwh.2021.09.006 [59] | 2021 | South | 2018–2019 | Increase uptake of HPV vaccination by implementing HPV education along with a strong provider recommendation to parents of youth and adolescents | Controlled Trial | 24 | Clinic | Evidence-based practice model | HPV vaccine initiation and completion | Of all the 24 vaccine-eligible patients, all 24 ended up receiving initiation of the vaccine or completed a previously started series. | ||
| Bednarczyk RA, Whitehead JL, Stephenson R. Moving beyond sex: Assessing the impact of gender identity on human papillomavirus vaccine recommendations and uptake among a national sample of rural-residing LGBT young adults. Papillomavirus Res. 2017;3:121–125. doi:10.1016/j.pvr.2017.04.002 [60] | 2017 | National | 2014 | Compare HPV vaccine recommendation and uptake by self-reported sex assigned at birth and current gender identity | Cross-sectional survey | 660 | Adults | Healthcare provider HPV vaccine recommendation and HPV vaccine Initiation | Receipt of HPV vaccination recommendation and at least one HPV vaccine dose was higher for female SAAB (47% and 44%, respectively) compared to male SAAB (17% and 14%, respectively), as well as female or transmale gender identity compared to male or transfemale gender identity. Approximately half of vaccinated respondents reported receiving HPV vaccine between 13 and 17 years of age. | |||
| Berenson AB, Hirth JM, Kuo YF, Rupp RE. Quantitative and qualitative assessment of an all-inclusive postpartum human papillomavirus vaccination program. Am J Obstet Gynecol. 2021;224(5):504.e1–504.e9. doi:10.1016/j.ajog.2020.11.033 [61] | 2021 | South | 2012–2019 | Examine the success and limitations of a program that promotes HPV vaccination to young adult women postpartum after expansion | Mixed methods | 6961 | Other (young postpartum women) | HPV vaccine completion | In the initial program, 76.9% completed the series, and in the expansion program, 73.5% completed the series. | |||
| Blake KD, Ottenbacher AJ, Finney Rutten LJ, et al. Predictors of Human Papillomavirus Awareness and Knowledge in 2013. Am J Prev Med. 2015;48(4):402–410. doi:10.1016/j.amepre.2014.10.024 [62] | 2015 | National | 2013 | Assess current population awareness of and knowledge about HPV and the HPV vaccine and the contribution of sociodemographic characteristics to disparities in HPV awareness and knowledge. | Cross-sectional Survey | 3103 | Adults | HPV and HPV vaccine awareness and knowledge | Sociodemographic characteristics associated with HPV knowledge/awareness | 68% had heard of HPV and the HPV vaccine, and 62% knew that HPV causes cervical cancer. | Age and sex impacted awareness and knowledge of HPV and the vaccine. Education, race, health insurance access, and internet access affected HPV and vaccine awareness, while rurality, education, and race affected some HPV knowledge questions. Those in rural areas were less likely than those in urban areas to know that HPV causes cervical cancer [aOR = 0.54 (0.30–0.98), p < 0.05]. | |
| Boitano TKL, Daniel C, Kim Y il, Straughn JM, Peral S, Scarinci I. Beyond words: Parental perceptions on human papillomavirus vaccination recommendations and its impact on uptake. Prev Med Rep. 2021;24:101596. doi:10.1016/j.pmedr.2021.101596 [63] | 2021 | South | 2019–2020 | Evaluate the impact of provider recommendations regarding HPV vaccination uptake in a rural setting | Cross-sectional survey | 368 | Parents | HPV vaccine Initiation and Intention to vaccinate | Approximately 40% indicated receiving a recommendation from a provider to vaccinate their child. Parental impression from the recommendation of HPV vaccination being “important” was significantly associated with the child being vaccinated that day (OR = 7.31, 95% CI: 2.20–24.3) as well as scheduling HPV vaccination (OR = 3.17, 95% CI: 1.01–9.92). Parents who got the impression that “there was no hurry” were less likely to vaccinate their child that day (OR = 0.23, 95% CI: 0.09–0.59). | |||
| Boyce TG, Christianson B, Hanson KE, et al. Factors associated with human papillomavirus and meningococcal vaccination among adolescents living in rural and urban areas. Vaccine X. 2022;11:100180. doi:10.1016/j.jvacx.2022.100180 [64] | 2022 | Midwest | 2019 | Assess factors and barriers associated with adolescent HPV and MenACWY vaccination to understand the determinants of rural–urban differences | Cross-sectional study | 536 | Parents | Parents' perception of importance placed on HPV vaccine by HCP and HPV vaccine initiation | 60% of teens received one or more doses of HPV vaccine. Among teens who received Tdap, HPV, and MenACWY, more rural teens received the three vaccines on the same day than urban teens (62% vs. 44%, p = 0.02). Fewer rural parents reported discussion with their provider and HPV vaccination as being “very important” for their teen according to their provider (45% vs. 54%, p = 0.08). The HPV vaccine harms factor had the lowest mean score (least favorable toward vaccination) among the factors assessed and differed by residency. Mean HPV vaccine harms score was significantly lower among rural parents than urban parents (5.49 (SD = 2.32) vs. 6.05 (SD = 2.35), p = 0.006). | |||
| Brennan LP, Rodriguez NM, Head KJ, Zimet GD, Kasting ML. Obstetrician/gynecologists’ HPV vaccination recommendations among women and girls 26 and younger. Prev Med Rep. 2022;27:101772. doi:10.1016/j.pmedr.2022.101772 [65] | 2022 | National | 2019 | Identify the factors that are most associated with an OB/GYN being a strong and frequent HPV vaccine recommender to girls and women 26 years of age or younger | Cross-sectional study | 205 | Providers | Competing Demands Model | Strength and frequency of provider recommendations | 56.3% (n = 116) were categorized as strong and frequent recommenders of the HPV vaccine. The clinic-level attributes were having the vaccine stocked (aOR = 2.66, 95%CI:1.02–6.93) and suburban (aOR = 3.31, 95%CI:1.07–10.19) or urban (aOR = 3.54, 95%CI:1.07–11.76) location versus rural for strong and frequent vaccine recommendations. Being a strong and frequent recommender was positively associated with believing other gynecologists frequently recommend the vaccine (aOR = 24.33, 95%CI:2.56–231.14) and believing that 50% or more of their patients are interested in receiving the vaccine (aOR = 2.77, 95%CI: 1.25–6.13). | ||
| Brewer NT, Hall ME, Malo TL, Gilkey MB, Quinn B, Lathren C. Announcements Versus Conversations to Improve HPV Vaccination Coverage: A Randomized Trial. Pediatrics. 2017;139(1). doi:10.1542/peds.2016-1764 [66] | 2017 | South | 2015 | Determine the effectiveness of training providers to improve their recommendations using either presumptive “announcements” or participatory “conversations” | randomized clinical trial | 17,173 | Providers | HPV vaccine initiation | Six-month difference in HPV vaccination coverage for 13–17-year-olds and 3-month difference in all measures | 5.4% (95% CI: 1.1–9.7%) increase in vaccination initiation for patients who received announcement training compared to control clinics for 11–12-year-olds after 6 months. Conversation training did not differ from control clinics | At 6 months, neither announcement or conversation training was effective for changing coverage for other vaccination outcomes or for adolescents aged 13 through 17. After 3 months, clinics' announcement training had higher HPV initiation rates compared to control clinics. | |
| Britt R, Britt BC. The need to develop health campaigns for obtaining the HPV vaccine in rural and medically underserved college campuses. Educ Health. 2016;34:74–78 [67] | 2016 | Midwest | Not listed | Examine behavioral factors and their association with HPV vaccination | Cross-sectional study | 327 | Adults | Theory of Planned Behavior | Intention to vaccinate | There was no significant relationship between gender, intent, normative beliefs, or attitudes towards receiving the HPV vaccine. Neither attitudes nor perceived behavioral control were identified as significant predictors of intent, but subjective norms did serve as a significant predictor of receiving the HPV vaccine. | ||
| Britt RK, Englebert AM. Behavioral determinants for vaccine acceptability among rurally located college students. Health Psychol Behav Med. 2018;6(1):262–276. doi:10.1080/21642850.2018.1505519 [68] | 2018 | South | Not listed | Investigate the demands of family, school, social, and work and the potential relationships and their potential impact on attitudes, subjective norms, and perceived behavioral control related to vaccination uptake | Cross-sectional study | 208 | Adults | Theory of Planned Behavior | Intention to vaccinate and HPV vaccine initiation | Attitudes towards vaccination uptake were positively related to increased work demands (r = 0.223, p < 0.001). Subjective norms were not significant with any variable. PBC and vaccination uptake were associated with work demands (r = 0.168, p < 0.001), school demands (r = 0.227, p < 0.01), and social demands (r = 0.056, p < 0.001). Intent to vaccinate was predicted by work demands (r = 0.143, p < 0.01), school demands (r = 0.130, p < 0.01), and social demands (r = 0.080, p < 0.01). | ||
| Brumbaugh JT, Sokoto KC, Wright CD, et al. Vaccination intention and uptake within the Black community in Appalachia. Health Psychol. 2023;42(8):557–566. doi:10.1037/hea000126 [69] | 2023 | South | 2020 | Identify and compare psychosocial predictors of COVID-19, flu, and HPV vaccination intention or behavior | Cross-sectional study | 336 | Adults | Andersen model | HPV vaccine initiation | Age was negatively associated (OR = 0.96, p = 0.023) and vaccine confidence was positively associated with HPV vaccination uptake (OR = 1.77, p < 0.001). Vaccine calculation remained significantly associated with HPV vaccination uptake in the final step of the overall model (OR = 1.32, p = 0.050) | ||
| Carman AL, McGladrey ML, Goodman Hoover A, Crosby RA. Organizational Variation in Implementation of an Evidence-Based Human Papillomavirus Intervention. Am J Prev Med. 2015;49(2):301–308. doi:10.1016/j.amepre.2015.03.011 [70] | 2015 | South | 2013–2014 | Implement the 1-2-3 Pap intervention in a public health setting and identify site-specific variations in its implementation | Quasi-experimental (pre- and post-implementation study) | 18 | Other (health departments) | HPV vaccine initiation and Implementation outcome: organizational readiness for change | The ORCA revealed variation in implementation strategies was widespread despite the “controls” provided by each site receiving the same instructions, incentives, and technical assistance. There was no statistical difference between ORCA scores and either channel selection or vaccine uptake. Among female patients, clinics using the waiting room channel had a mean total dose of 17.40. For clinics using the Internet distribution channel, the mean was 36.92. Interviews reinforced that there were wide implementation strategies among the LHD | |||
| Cataldi JR, Brewer SE, Perreira C, et al. Rural Adolescent Immunization: Delivery Practices and Barriers to Uptake. J Am Board Fam Med. 2021;34(5):937–949. doi:10.3122/jabfm.2021.05.210107 [71] | 2021 | West | 2019 | Assess whether there were rural–urban differences in perceived parental vaccine confidence and beliefs, and adolescent immunization delivery practices among Colorado vaccine providers | Cross-sectional study | 437 | Providers | Health belief model | Barriers to adolescent vaccination and perceived parental vaccine attitudes and immunization practices | Percentage of clinicians that think parents would agree with vaccine benefits | Rural respondents were less likely than urban respondents to agree that most patients have insurance that covers vaccination (86% vs. 97%; p = 0.02). Rural respondents were less likely than urban respondents to indicate most parents in their practice would agree with statements about vaccine benefits (p = 0.02) and trust in medical providers (p = 0.05). Fewer providers strongly recommended HPV vaccine (81% for females, 80% for males 11 to 12 years) than other adolescent immunizations (Tdap, MenACWY, influenza: 87–97%). There were no significant differences between rural and urban responses for perceived parental HPV vaccination beliefs. | |
| Cates JR, Ortiz RR, North S, Martin A, Smith R, Coyne-Beasley T. Partnering with middle school students to design text messages about HPV vaccination. Health Promot Pract. 2015 Mar;16(2):244–55. doi: 10.1177/1524839914551365. Epub 2014 Sep 25. PMID: 25258431; PMCID: PMC5319196. [72] | 2015 | South | 2011–2012 | Examine the acceptability of text messages about HPV vaccination and message preferences among adolescents | Cross-sectional survey | 43 | Adolescents | Health Belief Model | Preferences for proposed text messages about HPV and HPV vaccine | Acceptability of using text messages to convey HPV vaccine information | More than 70% used text messaging with a cell phone. The text message with the best composite score (M = 2.33, SD = 0.72) for likeability, trustworthiness, and motivation to seek more information was a gain frame emphasizing reduction in HPV infection if vaccinated against HPV. Text messages with lower scores emphasized threats of disease if not vaccinated. | Participants (68%) preferred doctors as their information source. |
| Cates JR, Shafer A, Diehl SJ, Deal AM. Evaluating a County-Sponsored Social Marketing Campaign to Increase Mothers’ Initiation of HPV Vaccine for Their Preteen Daughters in a Primarily Rural Area. Soc Mark Q. 2011;17(1):4–26. doi:10.1080/15245004.2010.546943 [73] | 2012 | South | 2009 | Evaluate a social marketing campaign initiated by 13 North Carolina counties to raise awareness among parents and reduce barriers to accessing the vaccine in a primarily rural area | Quasi-experimental | 294 | Parents of adolescents and healthcare providers | Health Belief Model | HPV vaccine initiation | Awareness of Media campaign | HPV vaccination rates within six months of campaign launch were 2% higher for 9–13-year-old girls in two of the four intervention counties compared to 96 non-intervention counties. | Most respondents (82%) were aware of HPV messages, logos, or both. Overall awareness did not differ by daughters’ age, mother’s race, income level, or rural/urban residence. Mothers in the target age group were less likely to see posters “frequently” or “occasionally” than mothers with older daughters (44% vs. 69%, p < 0.05). Of respondent providers (n = 35), 94% used campaign brochures regularly or occasionally in conversations with parents. |
| Chido-Amajuoyi OG, Jackson I, Yu R, Shete S. Declining awareness of HPV and HPV vaccine within the general US population. Hum Vaccines Immunother. 2020;17(2):420–427. doi:10.1080/21645515.2020.1783952 [74] | 2020 | National | 2008–2018 | Determine awareness of HPV and HPV vaccine in the US over the 10-year period | Cross-sectional survey | 21,325 | Adults | HPV and HPV vaccine awareness | Sociodemographic factors affecting awareness over time | The awareness of HPV decreased by 4.4%, and HPV vaccine awareness declined by 4.9% over time. | The lowest awareness was among racial minorities, rural residents, male respondents, those aged 65 years and older, as well as those with the lowest educational and socioeconomic standing | |
| Cunningham-Erves J, Koyama T, Huang Y, et al. Providers’ Perceptions of Parental Human Papillomavirus Vaccine Hesitancy: Cross-Sectional Study. JMIR Cancer. 2019;5(2):e13832. doi:10.2196/13832 [75] | 2019 | South | 2018 | Characterize the reasons for and level of parental HPV vaccine hesitancy as perceived by pediatric providers in Middle Tennessee and identify provider-level and clinic-level factors influencing perceived parental hesitancy | Cross-sectional survey | 187 | Providers | Perceived parental barriers to HPV vaccine hesitancy among pediatric providers | The most common parental barriers to HPV vaccination Perceived by providers were concerns about HPV vaccine safety (88%), child being too young (78%), low risk of HPV infection for child through sexual activity (70%), and mistrust in vaccines (59%). Perceived parental HPV vaccine hesitancy was significantly associated with several provider-level factors: self-efficacy (p = 0.001), outcome expectations (p < 0.001), and confidence in HPV vaccine safety (p = 0.009). | |||
| Dang JHT, McClure S, Gori ACT. Implementation and evaluation of a multilevel intervention to increase uptake of the human papillomavirus vaccine among rural adolescents. J Rural Health Jan. 2023;39(1):136–141. doi:10.1111/jrh.12690 [76] | 2023 | West | 2018–2020 | Evaluate the effectiveness of a multilevel evidence-based intervention aimed at increasing HPV vaccination coverage among rural adolescent patients in a rural health clinic | Controlled Trial | 498 | Adolescents | Initiation and completion | Adolescent patients ages 11–17 who had initiated the HPV vaccine series (82.7% vs. 52.4%, p < 0.0001) and completed the vaccine series (58.0% vs. 27.0%, p < 0.0001) were significantly greater at follow-up compared to baseline. | |||
| Daniel CL, Lawson F, Vickers M, et al. Enrolling a rural community pharmacy as a Vaccines for Children provider to increase HPV vaccination: a feasibility study. BMC Public Health. 2021;21(1). doi:10.1186/s12889-021-11304-8 [77] | 2021 | South | 2019–2020 | Examine the feasibility and potential effectiveness of enrolling a rural, community pharmacy as a VFC provider | pilot study | 1 | HPV-eligible community members | HPV vaccine initiation | Pharmacy VFC enrollment feasibility measures | 166 vaccines were administered to 89 adolescents, which included 55 HPV doses, 53 Tdap doses, 45 Meningococcal doses, and 13 Influenza doses. 64% (64) were VFC patients. The VFC intervention had positive feedback in the community and improved access to VFC-approved providers | The pharmacy increased overall prescription revenue by 34.1% (compared to a 6.9% increase for this time period in the previous year) and had a 17.8% increase in Medicaid prescriptions filled, thought to be heavily influenced by the added Medicaid/VFC services. Total revenue increased 24.4% after introduction of the intervention, compared to an 8.0% increase the previous year | |
| Fernandez-Pineda M, Cianelli R, Villegas N, et al. Preferred HPV and HPV Vaccine Learning Methods to Guide Future HPV Prevention Interventions Among Rural Hispanics. J Pediatr Nurs. 2021;60:139–145. doi:10.1016/j.pedn.2021.04.026 [78] | 2021 | South | Not listed | Determine rural Hispanic parents’ preferred HPV and HPVV learning methods | qualitative | 23 | Parents | Rural Hispanic parents’ preferred HPV and HPVV learning methods | For parents, small educational sessions (“charlas”) were the most preferred way to learn about HPV and HPVV. Other possible modes were healthcare providers, community-wide campaigns, mail, pharmacy, radio/tv, word of mouth, research studies, CDs/DVDs, email, pamphlets, social media videos, and webpage posts. For families/children to learn about HPV and HPVV, school-based events were most preferred. Other modes included healthcare providers/teachers, healthcare centers/clinic short video clips, health fairs, educational sessions, telephone(texts), and social media posts. | |||
| Fish LJ, Harrison SE, McDonald JA, et al. Key stakeholder perspectives on challenges and opportunities for rural HPV vaccination in North and South Carolina. Hum Vaccines Immunother. 2022;18(5). doi:10.1080/21645515.2022.2058264 [79] | 2022 | South | 2019–2020 | Learn about barriers and opportunities to scaling up adolescent vaccination, including HPV vaccination, in rural areas | qualitative | 14 | Other (stakeholders) | Social Ecological framework | Key stakeholder perspectives on challenges to HPV vaccination in rural areas | Individual: misinformation/vaccine beliefs and attitudes to preventive care; Provider: provider shortage, hard to participate in VFC programs, lack of strong provider HPV vaccine recommendations; System: no state mandate for HPV vaccine and school enrollment, school nurses could help address provider gaps, expand current programs for adolescents to include vaccines | ||
| Ford M, Cartmell K, Malek A, et al. Evaluation of the First-Year Data from an HPV Vaccination Van Program in South Carolina, U.S. J Clin Med. 2023;12(4):1362. doi:10.3390/jcm12041362 [80] | 2023 | South | 2021–2022 | Assess the program’s effectiveness by increasing the HPV vaccine uptake in SC | observational | 552 | Adolescents and Adults | HPV vaccine initiation | 552 participants received vaccinations from the HPV Van Program with 243 of them receiving the HPV vaccine | |||
| Gilbert PA, Lee AA, Pass L, et al. Queer in the Heartland: Cancer Risks, Screenings, and Diagnoses among Sexual and Gender Minorities in Iowa. J Homosex. Published online October 19, 2020:1–17. doi:10.1080/00918369.2020.1826832 [81] | 2020 | Midwest | 2017 | Develop detailed epidemiologic profiles of Iowa’s SGM for cancer prevention | Cross-sectional study | 567 | Adults | HPV vaccine initiation | Less than half (41.8%) of those plausibly eligible individuals reported HPV vaccine initiation. The majority (80.0%) reported receiving two or three doses. Compared to ciswomen, cismen had 78% lower odds of reporting HPV vaccination initiation (OR = 0.22, 95% CI: 0.11–0.45) but there was no difference for transgender/genderqueer individuals. Examining sexual orientation differences, bisexual/pansexual respondents had over four-times higher odds and queer/other individuals had 2 times higher odds of reporting HPV vaccination initiation compared to gay/lesbian respondents (OR = 4.34, 95% CI: 2.18–8.62 and OR = 2.10, 95% CI: 1.11–3.97, respectively). | |||
| Goessl CL, Christianson B, Hanson KE, et al. Human papillomavirus vaccine beliefs and practice characteristics in rural and urban adolescent care providers. BMC Public Health. 2022;22(1). doi:10.1186/s12889-022-13751-3 [82] | 2022 | Midwest | 2019 | Identify the HPV vaccine attitudes and practices that were most strongly associated with rural vs. urban providers | Cross-sectional survey | 437 | Providers | Provider HPV Vaccine Resources, Practices and Attitudes | Five vaccine factors were different between rural and urban providers, including evening/weekend appointments (aOR = 0.21, 95% CI: 0.12, 0.36), standing vaccination orders (aOR = 2.81, 95% CI: 1.61, 4.91), prior experience with vaccine quality improvement projects (aOR = 0.52, 95% CI: 0.28, 0.98), providing HPV vaccine information before it is due (aOR = 3.10, 95% CI: 1.68, 5.71), and recommending HPV vaccine during urgent care visit (aOR = 0.37, 95% CI: 0.18, 0.79). Other practices and attitudinal exposures were statistically similar between rural and urban providers. | |||
| Gunn R, Ferrara LK, Dickinson C, et al. Human Papillomavirus Immunization in Rural Primary Care. Am J Prev Med. 2020;59(3):377–385. doi:10.1016/j.amepre.2020.03.018 [83] | 2020 | West | 2018 | Identify the organizational structures and clinical workflows that enable rural, high-performing primary care clinics to support HPV vaccine delivery | mixed methods | 12 | Clinics | Positive Deviance framework | organizational structures and workflows | Four key themes were identified: (1) standardized workflows to identify patients due for the vaccine and had vaccine administration protocols, (2) have a vaccine champion, (3) clinical staff were comfortable providing immunizations regardless of visit type, and (4) clear, persuasive language to recommend or educate parents/patients about the vaccine’s importance | ||
| Harris KL, Tay D, Kaiser D. The perspectives, barriers, and willingness of Utah dentists to engage in human papillomavirus (HPV) vaccine practices. Hum Vaccin Immunother. 2020;16(2):436–444. doi:10.1080/21645515.2019.1649550 [84] | 2019 | West | 2017–2018 | Examine the relationship between dental providers’ perspectives about their scope of practice, barriers, and willingness to engage and collaborate in HPV vaccination practices in the dental setting | Cross-sectional Survey | 203 | Other (Dentists) | Barriers to HPV vaccine among dentists and dentists’ willingness to engage in HPV vaccination practices and collaborate with primary care providers. | Majority of Utah dentists surveyed perceived that discussing the link between HPV and OPC and recommending the HPV vaccine is within their scope of practice, but not administration of the HPV vaccine. A significantly higher proportion of urban Utah dentists disagreed that they were concerned about the safety of the HPV vaccine (n = 141, 73.43%, p = 0.011), or that they were concerned about the liability related to the HPV vaccine (n = 103, 53.34%, p = 0.004) were compared with rural dental providers (n = 13, 6.77%; n = 13, 6.77%). Discussing, recommending, and administering the HPV vaccine did not significantly differ by dentists’ age group, rurality, time spent on patient education, or length of dental experience. | |||
| Harry ML, Asche SE, Freitag LA, et al. Human Papillomavirus vaccination clinical decision support for young adults in an upper midwestern healthcare system: a clinic cluster-randomized control trial. Hum Vaccines Immunother. 2022;18(1). doi:10.1080/21645515.2022.2040933 [85] | 2022 | Midwest | 2018–2019 | Test Clinical Decision Support with or without shared decision-making tools (SDMTs) on HPV vaccination rates compared to usual care (UC) | Randomized controlled trial | 34 clinics | Adults | HPV vaccine completion | The HPV vaccination series was completed by 12 months in 2.3% (95% CI: 1.6–3.2%) of CDS, 1.6% (95% CI: 1.1–2.3%) of CDS + SDMT, and 2.2% (95% CI: 1.6–3.0%) of UC patients, and at least one HPV vaccine was received by 12 months in 13.1% (95% CI: 10.6–16.1%) of CDS, 9.2% (95% CI: 7.3–11.6%) of CDS + SDMT, and 11.2% (95% CI: 9.1–13.7%) of UC patients. | |||
| Hatch BA, Valenzuela S, Darden PM. Clinic-level differences in human papillomavirus vaccination rates among rural and urban Oregon primary care clinics. J Rural Health Mar. 2023;39(2):499–507. doi:10.1111/jrh.12724 [86] | 2023 | West | 2019 | Compare HPV vaccination between rural and urban primary care clinics and examine the association of rurality with HPV vaccination | Cross-sectional study | 537 | Clinics | HPV vaccine initiation and completion | The mean rate of HPV vaccine ≥ 1 dose was lower among rural clinics (46.9% vs. 51.1%, p = 0.039), as was vaccination UTD (40.5% vs. 49.9%, p < 0.001) when compared to urban clinics. The rural/urban disparity was not significant after adjusting for other individual- and clinic-level characteristics. | |||
| Henry KA, Swiecki-Sikora AL, Stroup AM, Warner EL, Kepka D. Area-based socioeconomic factors and Human Papillomavirus (HPV) vaccination among teen boys in the United States. BMC Public Health. 2017;18(1). doi:10.1186/s12889-017-4567-2 [87] | 2017 | National | 2012–2013 | Examine associations between both individual-level and area-based factors and HPV vaccine initiation and completion among boys | Secondary data analysis | 19,518 | Adolescents | HPV vaccine initiation and completion | Area-based poverty was not statistically significantly associated with HPV vaccination initiation, but it was associated with completion, with boys living in high-poverty areas having higher odds of completing the series than boys in low-poverty areas. Boys from urban or densely populated areas have higher odds of initiation and completion compared to boys living in non-urban, less densely populated areas. | |||
| Jafari SDG, Appel SJ, Shorter DG. Risk Reduction Interventions for Human Papillomavirus in Rural Maryland. J Dr Nurs Pract. 2020;13(2):134–141. doi:10.1891/jdnp-d-19-00047 [88] | 2020 | South | 2017–2018 | Address patient or parental perceptions Leading to vaccine hesitancy and identify the vaccine impact from provider to patient education | Mixed methods | 416 | Adolescents/ providers | HPV vaccine initiation | A documentary movie for women aged 12–26 was implemented to decrease HPV-related risks; the impact was not significant. Direct provider to patient recommendations resulted in a 15% increase in HPV immunizations. | |||
| Kepka D, Christini K, McGough E, et al. Successful Multi-Level HPV Vaccination Intervention at a Rural Healthcare Center in the Era of COVID-19. Front Digit Health. 2021;3. doi:10.3389/fdgth.2021.719138 [89] | 2021 | West | 2019–2021 | Test HPV vaccination intervention that includes healthcare team training activities and patient reminders to reduce missed opportunities and improve the rate of appointment scheduling for HPV vaccination in a rural medical clinic | Quasi-experimental study | 402 | Parents, adolescents and adults | Missed opportunities for HPV vaccination | Missed opportunities for HPV vaccination declined significantly between the pre-intervention and the post-intervention period (21.6 vs. 8.1%, respectively, p = 0.002). Participants who recalled receipt of a vaccination reminder had 7.0 (95% CI 2.4–22.8) times higher unadjusted odds of scheduling a visit compared with those who did not recall receiving a reminder. The unadjusted odds of confirming that they had scheduled or were intending to schedule a follow-up appointment to receive the HPV vaccine were 4.9 (95% CI 1.51–20.59) times greater among those who had not received the vaccine for themselves or for their child. | |||
| Kepka D, Coronado GD, Rodriguez HP, Thompson B. Evaluation of a Radionovela to Promote HPV Vaccine Awareness and Knowledge Among Hispanic Parents. J Community Health. 2011;36(6):957–965. doi:10.1007/s10900-011-9395-1 [90] | 2011 | West | 2008–2009 | Investigate the efficacy of messages delivered via a radionovela to improve HPV and HPV vaccine-related knowledge and attitudes | randomized controlled trial | 88 | Parents | HPV and HPV vaccine awareness and knowledge & attitudes/beliefs | Parents who listened to the HPV radionovela (intervention group) were more likely to confirm that HPV is a common infection (70% vs. 48%, p = 0.002), to deny that women are able to detect HPV (53% vs. 31%, p = 0.003), to know vaccine age recommendations (87% vs. 68%, p = 0.003), and to confirm multiple doses (48% vs. 26%, p = 0.03) than control group parents. | |||
| Kepka DL, Ulrich AK, Coronado GD. Low Knowledge of the Three-Dose HPV Vaccine Series among Mothers of Rural Hispanic Adolescents. J Health Care Poor Underserved. 2012;23(2):626–635. doi:10.1353/hpu.2012.0040 [91] | 2012 | West | 2009 | Investigate correlates of HPV vaccine uptake by adolescent daughters of rural Hispanic mothers | Cross-sectional survey | 78 | Parents | Social Ecological Framework | HPV vaccine initiation | Mothers who had heard of the HPV vaccine were more likely to have a vaccinated daughter (p < 0.01). Mothers who thought their daughter’s father would approve were more likely to have a vaccinated daughter (p = 0.004). Parents who believed that only one injection was necessary were more likely to have a vaccinated daughter (p = 0.009) | ||
| Kim S, Zhou K, Parker S, Kline KN, Montealegre JR, McGee LU. Perceived Barriers and Use of Evidence-Based Practices for Adolescent HPV Vaccination among East Texas Providers. Vaccines. 2023;11(4):728. doi:10.3390/vaccines11040728 [92] | 2023 | South | 2022 | Understand current clinical practices regarding HPV vaccination in rural East Texas primary health-care settings and assess health-care providers’ perceived barriers to HPV vaccination | Cross-sectional study | 27 | Clinics | Perceived barriers to HPV vaccination in clinics and strategies used by clinics to increase HPV vaccination rates | HPV vaccine-promoting clinical practices | The most prevalent perceived barrier was missed opportunities for vaccination (66.7%), and concern about vaccine hesitancy (44.4%) because of the pandemic. | Many clinics surveyed currently implement evidence-based practices to promote HPV vaccination, but using a “refusal to vaccinate” form (29.6%), having an identified HPV vaccine champion (29.6%), and recommending the HPV vaccine at age 9 (22.2%) were least implemented among these clinics. | |
| Koskan AM, Dominick LN, Helitzer DL. Rural Caregivers’ Willingness for Community Pharmacists to Administer the HPV Vaccine to Their Age-Eligible Children. J Cancer Educ Feb. 2021;36(1):189–198. doi:10.1007/s13187-019-01617-z [93] | 2021 | south | Not listed | Explore rural caregivers’ perceptions of receiving the HPV vaccine from their local pharmacist and determine preferences for education for both the vaccine and receiving vaccines from pharmacists | Qualitative | 26 | Providers | Caregivers’ perceptions of the HPV vaccine and their willingness for pharmacist- administered HPV vaccination | Awareness about the HPV vaccine, HPV vaccine barriers, and facilitators. | Most caregivers were unaware that pharmacists could offer adolescent vaccines, but most were willing to allow their children to receive the vaccine from this non-traditional source. The primary concern was pharmacist training for administering the HPV vaccine. | Caregivers preferred print fliers disseminated in various locations and Facebook for channels of health education about HPV vaccine availability in pharmacies. | |
| Kurani S, MacLaughlin KL, Jacobson RM, et al. Socioeconomic disadvantage and human papillomavirus (HPV) vaccination uptake. Vaccine. 2022;40(3):471–476. doi:10.1016/j.vaccine.2021.12.003 [94] | 2022 | Midwest | 2016–2018 | Examine HPV vaccine-related disparities by area deprivation using patient-level data from persons residing in a largely rural, Upper Midwest region | Retrospective cohort study | 54,573 | Adolescents | HPV vaccine initiation and completion | Individuals living in more deprived block groups were significantly less likely to initiate and complete HPV vaccinations compared to those living in the least deprived blocks. Individuals with rural residence had decreased probabilities of initiation compared to individuals living in urban areas. | |||
| Lee HY, Luo Y, Won CR, Daniel C, Coyne-Beasley T. HPV and HPV Vaccine Awareness Among African Americans in the Black Belt Region of Alabama. J Racial Ethn Health Disparities. 2023;11(2):808–814. doi:10.1007/s40615-023-01562-0 [95] | 2023 | South | Not listed | Examine HPV and HPV vaccine awareness and associated factors among rural, Southern African Americans | cross-sectional survey | 257 | Adults | HPV and HPV vaccine awareness | Slightly more than half of the participants were aware of HPV (62.5%) and HPV vaccine (62.1%). Being single, having a family cancer history, and good self-reported health status were positively associated with both HPV and HPV vaccine awareness. Employment was positively associated with HPV awareness, and participation in social groups was positively associated with HPV vaccine awareness. | |||
| Manganello JA, Chiang SC, Cowlin H, Kearney MD, Massey PM. HPV and COVID-19 vaccines: Social media use, confidence, and intentions among parents living in different community types in the United States. J Behav Med Apr. 2023;46(1–2):212–228. doi:10.1007/s10865-022-00316-3 [96] | 2022 | National | 2021 | Assess information seeking around children’s health and vaccines, and vaccine confidence and intention/uptake among parents living in different community types for HPV and COVID-19 | Cross-sectional study | 452 | Parents | Intention to vaccinate | Social media use | For both HPV and COVID-19 vaccines, political affiliation was the only common factor associated with both vaccine confidence and intention/uptake. Parents who identified as Democrats compared to Republicans had greater confidence in the vaccines and had higher odds of vaccine intention/uptake for their children. | Use of Facebook was not associated with vaccine confidence. | |
| McMann N, Trout KE. Assessing the Knowledge, Attitudes, and Practices Regarding Sexually Transmitted Infections Among College Students in a Rural Midwest Setting. J Community Health Feb. 2021;46(1):117–126. doi:10.1007/s10900-020-00855-3 [97] | 2020 | Midwest | 2019 | Assess the knowledge, attitudes, and practices regarding sexual health among rural college students in Nebraska | Cross-sectional survey | 125 | Adults | Knowledge, attitudes, and practices of sexual health (including percentage with HPV vaccination). | Prevalence of HPV vaccination was 51% (n = 63) and was different among females and males (60% vs. 18%, p < 0.001) | |||
| Mohammed KA, Subramaniam DS, Geneus CJ, et al. Rural–urban differences in human papillomavirus knowledge and awareness among US adults. Prev Med. 2018;109:39–43. doi:10.1016/j.ypmed.2018.01.016 [98] | 2018 | National | 2013–2017 | Determine the prevalence of knowledge and awareness of HPV, the HPV vaccine, and HPV-associated cancers among rural and urban residents, and examine the association of rural/urban status with knowledge and awareness | Cross-sectional survey | 10,147 | Adults | Awareness, knowledge of HPV, the HPV vaccine, and HPV-associated cancers | Knowledge about HPV causing cervical, oral, anal, and penile cancers, as well as the knowledge about HPV being transmitted through sexual contact. | In comparison to rural respondents, the prevalence of awareness of HPV (67.2%; 95% CI: 67.0–69.2) and the HPV vaccine (65.8%; 95% CI: 64.2–67.1) was higher among urban respondents. Compared to urban residents, rural residents were less likely to be aware of HPV (OR = 0.68, 95% CI = 0.53–0.86) and HPV vaccine (OR = 0.78, 95% CI = 0.63–0.97). | Additionally, the prevalence of knowing that HPV causes cervical (75.4%; 95% CI: 72.5–77.3) and oral cancer (30.9%; 95% CI: 28.4–32.1), and knowing HPV is transmitted through sexual contact (65.9%; 95% CI: 63.6–67.2) was higher among urban residents than rural residents. | |
| Morales-Campos DY, McDaniel MD, Amaro G, Flores BE, Parra-Medina D. Factors Associated with HPV Vaccine Adherence among Latino/a Adolescents in a Rural, Texas-Mexico Border County. Ethn Dis. 2022;32(4):275–284. doi:10.18865/ed.32.4.275 [99] | 2022 | South | 2015–2018 | Examine HPV vaccine initiation and completion among Hispanic adolescents in a rural, Texas-Mexico border county | Cross-sectional survey | 1832 | Parents | Ecological systems theory | HPV vaccine initiation and completion | Factors associated with HPV vaccine initiation and completion were female gender (p < 0.01), adolescent insurance status (p < 0.001), and receipt of required vaccines (p < 0.001). Adolescents who received mandatory vaccinations for school entry were five times more likely to initiate and complete the HPV vaccine series (OR = 5.39, p < 0.001) | ||
| Moss JL, Gilkey MB, Reiter PL, Brewer NT. Trends in HPV Vaccine Initiation among Adolescent Females in North Carolina, 2008–2010. Cancer Epidemiol Biomarkers Prev. 2012;21(11):1913–1922. doi:10.1158/1055-9965.epi-12-0509 [100] | 2012 | South | 2008–2010 | Assess trends and disparities in HPV vaccine initiation among female adolescents in North Carolina over 3 years | Secondary Data Analysis | 1427 | Parents | HPV vaccine initiation | HPV vaccine initiation increased over time (2008, 34%; 2009, 41%; 2010, 44%). This upward trend was present within 11 subpopulations of girls, including those who lived in rural areas, were of minority (non-black/non-white) race, or had not recently received a preventive check-up. | |||
| Moss JL, Gilkey MB, Rimer BK, Brewer NT. Disparities in collaborative patient–provider communication about human papillomavirus (HPV) vaccination. Hum Vaccines Immunother. 2016;12(6):1476–1483. doi:10.1080/21645515.2015.1128601 [101] | 2016 | National | 2010 | Understand how collaborative communication operates in vaccination decisions across demographic groups | Cross-sectional study | 4124 | Parents | Charles and Gafni framework (shared treatment decision-making model) | HPV vaccine initiation | Disparities in collaborative communication accounted for geographic variation in HPV vaccination, specifically, the higher rates of uptake in the urban/suburban vs. rural areas (p < 0.01). Half of parents (53%) in the survey reported collaborative communication. Poor, less educated, Spanish-speaking, Southern, and rural parents, and parents of non-privately insured and Hispanic adolescents, were least likely to report collaborative communication (all p < 0.05). | ||
| Newcomer SR, Caringi J, Jones B, Coyle E, Schehl T, Daley MF. A Mixed Methods Analysis of Barriers to and Facilitators of Human Papillomavirus Vaccination Among Adolescents in Montana. Public Health Reports®. 2020;135(6):842–850. doi:10.1177/0033354920954512 [102] | 2020 | West | 2013–3017 | Identify barriers to and facilitators of adolescent HPV vaccination in Montana | Mixed methods | 326 | Adolescents | Vaccine Perceptions, Accountability and Adherence Model | HPV vaccine initiation | In Montana, initiation of the HPV vaccine series among adolescents aged 13–17 increased from 34.4% in 2013 to 65.5% in 2017. In NIS-Teen 2017 data (n = 326 adolescents), receiving a medical provider recommendation was significantly associated with series initiation (aPR = 2.3; 95% CI, 1.5–3.6). Among parents who did not intend to initiate the vaccine series for their adolescent within 12 months (n = 71), vaccine safety was the top concern (aPR = 24.5%; 95% CI, 12.1–36.9%). The two most commonly referenced themes were medical providers’ recommendation style and parental vaccine hesitancy as factors for HPV vaccination. | ||
| Newcomer SR, Freeman RE, Albers AN, et al. Missed opportunities for human papillomavirus vaccine series initiation in a large, rural U.S. state. Hum Vaccines Immunother. 2022;18(1). doi:10.1080/21645515.2021.2016304 [103] | 2022 | West | 2020–2021 | Quantify the prevalence of missed opportunities to vaccinate adolescents against HPV when they presented for other vaccines, and to determine whether the risk of missed opportunities differed by vaccination clinics | Secondary data analysis | 47,622 | Adolescents | HPV vaccine initiation | Secondary: Immunization visits that were missed opportunities for initiating the human papillomavirus vaccine series for adolescents ages 11–17 years by clinic setting, Montana, 2014–2020. Tertiary: Associations between clinic setting, age, sex, and rurality with missed opportunities for initiating the human papillomavirus vaccine series for adolescents ages 11–17 years, Montana, 2014–2020 | Among 47,622 adolescents, 53.9% of 71,447 vaccination visits were missed opportunities. | Receiving vaccines in public health departments was significantly associated with higher risk of missed opportunities (aRR = 1.25, 95% confidence interval = 1.22–1.27, vs. private clinics). Receipt of vaccines in Indian Health Services and Tribal clinics was associated with fewer missed opportunities (aRR = 0.72, 95% CI: 0.69–0.75, vs. private clinics). | |
| Nguyen CG, Pogemiller MI, Cooper MT, Garbe MC, Darden PM. Characteristics of Oklahoma Pediatricians Who Dismiss Families for Refusing Vaccines. Clin Pediatr Phila Jan. 2023;62(1):24–32. doi:10.1177/00099228221108801 [104] | 2023 | South | 2019 | To assess the frequency of declining new patients or dismissing current patients who request to delay or refuse vaccines, the delay/refusal of specific vaccine(s) that prompt pediatricians to decline/dismiss patients, and the demographics of pediatricians who decline or dismiss patients | Cross-sectional study | 122 | Providers | Dismiss or decline for some (but not all) vaccines | Secondary: the specific vaccines causing the delay/refusal resulting in decline/dismissal. Tertiary: demographic information about physicians who decline or dismiss patients. | 35% (34/98) of pediatricians dismissed current patients for refusing/delaying vaccine. 47% (48/103) declined accepting new patients due to refusing/delaying vaccine. Of the 48 physicians who declined patients, 25 (52%) declined new patients for refusing some but not all vaccines, and 23 (19%) declined new patients for refusing” all vaccines. | Secondary: Over 90% of respondents would dismiss/decline patients who refuse Dtap, Hib, PCV13, IPV, MMR, Varicella, Hep A, or Tdap. For influenza and HPV vaccines, less than 20% would dismiss or decline a patient over refusal or delay. Tertiary: More than 10 years in practice and being rural are more likely to dismiss current patients or decline new patients due to refusal for one, some, but not all, vaccines. | |
| * Osaghae I, Darkoh C, Chido-Amajuoyi OG, et al. Healthcare Provider’s Perceived Self-Efficacy in HPV Vaccination Hesitancy Counseling and HPV Vaccination Acceptance. Vaccines. 2023;11(2):300. doi:10.3390/vaccines11020300 [105] | 2022 | South | 2021 | Examine the relationship between HPV vaccination training of HCPs and HPV vaccination status assessment and recommendation | Cross-sectional survey | 1283 | Providers | Provider HPV vaccination status assessment and recommendation | 482 (47%) reported that they often/always assess. 537 (53%) never/sometimes assess. 756 (59%) reported they often/always recommend. 527 (41%) reported that they never/sometimes recommend. | |||
| * Osaghae I, Darkoh C, Chido-Amajuoyi OG, et al. Association of provider HPV vaccination training with provider assessment of HPV vaccination status and recommendation of HPV vaccination. Hum Vaccines Immunother. 2022;18(6). doi:10.1080/21645515.2022.2132755 [106] | 2023 | South | 2021 | Determine the association between healthcare providers’ self-efficacy in HPV vaccination hesitancy counseling and HPV vaccination acceptance after initial and follow-up counseling sessions | Cross-sectional survey | 1283 | Providers | HPV vaccine initiation | HCPs who believed that they were very/completely confident in counseling HPV vaccine-hesitant parents had higher odds of observing HPV vaccination acceptance very often/always after an initial counseling session (aOR= 3.50; 95% CI: 2.25–5.44) and after follow-up counseling sessions (aOR = 2.58; 95% CI: 1.66–4.00) compared to HCPs that perceived they were not at all/somewhat/moderately confident. | |||
| Osegueda ER, Chi X, Hall JM, Vadaparampil ST, Christy SM, Staras SAS. County-Level Factors Associated With HPV Vaccine Coverage Among 11-Year-Olds to 12-Year-Olds Living in Florida in 2019. J Adolesc Health. 2023;72(1):130–137. doi:10.1016/j.jadohealth.2022.09.005 [107] | 2023 | South | 2019 | Understand county-level characteristics associated with HPV vaccination rates | Cross-sectional study | 481,846 | Adolescents | HPV vaccine initiation and Completion | On average, the HPV vaccine initiation rate among the most urban counties at 65% (95%CI = 58.1–72.2) was higher than the 43% (95%CI = 36.4–50.5) HPV vaccine initiation rate among the most rural counties. HPV vaccine UTD prevalence is 21% in more urban counties and 10% for those living in rural counties. | |||
| Panagides R, Voges N, Oliver J, Bridwell D, Mitchell E. Determining the Impact of a Community-Based Intervention on Knowledge Gained and Attitudes Towards the HPV Vaccine in Virginia. J Cancer Educ Apr. 2023;38(2):646–651. doi:10.1007/s13187-022-02169-5 [108] | 2023 | South | 2016–2019 | Compare the impact of an educational film intervention on HPV intention to vaccinate and knowledge gained in urban and rural areas; To increase knowledge and intent to receive the HPV vaccine | quasi-experimental | 149 | Community | Health Belief Model | Attitudes, beliefs (intention), and knowledge | Changes in knowledge about HPV were statistically significant in two out of seven questions (p < 0.05). Changes in attitude were statistically significant in every attitude-based question about HPV (p < 0.05). There were significant differences in knowledge gained and attitudes towards the HPV vaccine when comparing urban and rural locations. | ||
| Paskett ED, Krok-Schoen JL, Pennell ML, et al. Results of a Multilevel Intervention Trial to Increase Human Papillomavirus (HPV) Vaccine Uptake among Adolescent Girls. Cancer Epidemiol Biomarkers Prev. 2016;25(4):593–602. doi:10.1158/1055-9965.epi-15-1243 [109] | 2016 | Midwest | 2010–2015 | Test the efficacy of a multilevel intervention to improve the uptake of the HPV vaccine among Appalachian girls aged 9 to 17 years old in 12 counties in Appalachian Ohio | Randomized controlled trial | 456 | Multilevel | Intervention guided by the Health Belief Model, Theory of Reasoned Action, Extended Parallel Process Model, and Organizational Developmental Theory | HPV vaccine initiation | HPV vaccine uptake at 6 months and uptake of the second and third HPV vaccine shots. | 10 (7.7%) daughters of intervention participants received the first shot of the HPV vaccine within 3 months of being sent the intervention materials compared with 4 (3.2%) daughters of comparison group participants (p = 0.061). Provider knowledge about HPV increased (p < 0.001, from baseline to after education). | By 6 months, 17 (13.1%) daughters of intervention participants received the first HPV vaccine shot compared with eight (6.5%) daughters of comparison group participants (p = 0.002). |
| Pham D, Shukla A, Welch K, Villa A. Assessing knowledge of human papillomavirus among men who have sex with men (MSM) using targeted dating applications. Vaccine. 2022;40(36):5376–5383. doi:10.1016/j.vaccine.2022.07.048 [110] | 2022 | National | Not listed | Investigate knowledge regarding HPV, HPV-related cancers, and HPV vaccination rates among men who have sex with men (MSM) who had active accounts on two LGBTQ+ online dating applications | Cross-sectional survey | 3342 | Adults | HPV-related knowledge and HPV vaccine initiation and completion | What source has recommended the vaccine to the participant and comfort level receiving a vaccine from a dentist. | Half of the HPV vaccine-eligible respondents reported having received at least one dose of the HPV vaccine, while only 37.9% of the individuals aged 9–26 reported being vaccinated against HPV. Among the unvaccinated, 63.3% reported being interested in future vaccination, or learning more about it. There were no significant differences in vaccination status or HPV knowledge (except for cancers associated with HPV) between respondents from rural vs. urban locations. | Doctors/physicians/ nurses were reported to be the largest source of HPV vaccine recommendation. 42.2% of participants are comfortable receiving the HPV vaccine from a dentist. | |
| Pourebrahim N, Shah P, VoPham T, et al. Time and geographic variations in human papillomavirus vaccine uptake in Washington state. Prev Med. 2021;153:106753. doi:10.1016/j.ypmed.2021.106753 [111] | 2021 | West | 2008–2018 | Identify priority areas in Washington State that have had persistently low HPV vaccine rates over time, and the contributing sociodemographic factors associated with low HPV vaccine rates | Longitudinal study | 564,493 | Adolescents | Initiation and completion at the census tract level | Moran’s I for HPV initiation was 0.44 and 0.47 for completion. Average vaccine initiation and completion for urban areas were higher compared to rural tracts. In urban areas, initiation rose from 11% to 34% and completion rose from 4% to 19% from 2010 to 2018. In rural areas, the rate rose from 9% to 22% initiation and 3–11% completion. Percentage of White population was positively associated with being in low vaccine areas. | |||
| Pruitt SL, Tiro JA, Kepka D, Henry K. Missed Vaccination Opportunities Among U.S. Adolescents by Area Characteristics. Am J Prev Med. 2022;62(4):538–547. doi:10.1016/j.amepre.2021.10.014 [112] | 2022 | National | 2015–2017 | Identify adolescent-level, area-level, and household-level characteristics for coverage patterns in Tdap, HPV, and MenACWY | Secondary data analysis | 63,299 | Adolescents | Vaccination coverage of 1 or 2 vaccines in combination, and missed opportunity for HPV vaccine | Missed HPV vaccination opportunities were common in those in rural areas, living in the Midwest or South (not West), and not having private insurance | |||
| Rabarison KM, Bish CL, Massoudi MS, Giles WH. Economic Evaluation Enhances Public Health Decision Making. Front Public Health. 2015;3. doi:10.3389/fpubh.2015.00164 [113] | 2015 | South | 2010–2012 | Test the cost effectiveness of 1-2-3 Pap intervention per number of completed HPV vaccine series would decrease when offered to more women in the target population | Cost Analysis | 344 | Adults | Implementation: cost of completion of HPV vaccine series. | Assuming the same success rate as the efficacy study, the 1-2-3 Pap adaptation scenario would cover 1000 additional women aged 18 through 26 years (344 in efficacy study; 1346 in adaptation scenario), and almost three times as many completed series (130 in efficacy study; 412 in adaptation scenario) as in the original 1-2-3 Pap efficacy study. | |||
| Ramsay JM, Kaddas HK, Ou JY, Kepka D, Kirchhoff AC. Missed opportunities for concomitant HPV vaccination among childhood cancer survivors. Cancer Med. 2022;11(4):1181–1191. doi:10.1002/cam4.4492 [114] | 2022 | West | 2013–2016 | Assess if there are higher rates of missed opportunities to vaccinate for HPV among adolescent cancer survivors | Cohort study | 2238 | Adolescents | Missed opportunities for HPV vaccination | Childhood cancer survivors had more missed opportunities than the sample population (70% healthcare encounters had MOs, vs. 59%). 48.2% of the sample population and 39.8% of survivors received 1 or more doses of the HPV vaccine throughout the study. 10.2% of sample population completed the 3-dose series compared to 7.3% of the survivor group. | |||
| Robison SG. The Impact of the Number of Injections per Visit on the Likelihood of Human Papillomavirus Immunization. J Pediatr X. 2020;3:100024. [115] | 2020 | West | 2015–2019 | Assess whether single injection visits among teens correlated with lower rates of HPV series completion, and examine whether childhood patterns of injection-limited were correlated with decreased HPV vaccine completion | Cohort study | 241,453 | Adolescents | Limited number of injections per visit (tdap, MenACWY, HPV), HPV initiation and completion | For adolescents who received more than 1 vaccine per visit, HPV vaccine completion rates were 62.2%, while rates were 7.7% among those considered injection-limited. 16.3% of adolescents were considered injection-limited. Of those that were not up-to-date for HPV vaccination, 76.1% were either injection-limited or did not receive their HPV vaccine at their Tdap visit. Children who had not received more than 1 injection per visit since age 4 had HPV vaccine completion rates of 3.9%. | |||
| Rodriguez AM, Do TQN, Chen L, Schmeler KM, Montealegre JR, Kuo YF. Human papillomavirus vaccinations at recommended ages: How a middle school-based educational and vaccination program increased uptake in the Rio Grande Valley. Hum Vaccines Immunother. 2022;18(6). doi:10.1080/21645515.2022.2133315 [116] | 2022 | South | 2016–2022 | Evaluate how a community-based education and school-based HPV vaccination program increased HPV vaccination rates among medically underserved students in rural middle school districts in Texas by age of initiation | Quasi-experimental study | 1766 | Adolescents | HPV vaccine initiation and completion | The majority of students initiated the HPV vaccine at 11 (39.5%) and 12 (30.5%). 72.4% of students who received HPV vaccines through the program received them bundled with other vaccines. Those who initiated the HPV vaccine before age 11 had higher UTD percentages | |||
| Rodriguez AM, Do TQN, Eyada MF, Chen L, Schmeler KM, Montealegre JR. Human Papillomavirus Vaccination Uptake in the Rio Grande Valley: Results from a Pilot Community-Based Educational and School-Based Vaccination Program and Its Expansion. Vaccines. 2023;11(2):329. doi:10.3390/vaccines11020329 [117] | 2023 | South | 2016–2022 | Assess the effectiveness of a physician-run HPV education campaign and middle school-based HPV vaccination program in rural Texas | Cross-sectional | 19,951 | Adolescents | HPV vaccine initiation and completion | The overall HPV-UTD was 58.8%. A total of 19,951 students received HPV vaccines either directly or indirectly from the program in the 6 years of intervention. 1549 HPV vaccine initiations, 1042 vaccine completions were delivered at schools throughout the program (total of 2145 students vaccinated directly in the school-based program). 18,172 HPV vaccine initiations and 17,075 HPV vaccine completions were completed through collaborating healthcare practices. Male students and students older at initiation were less likely to be HPV-UTD | |||
| Rosen BL, DiClemente R, Shepard AL, Wilson KL, Fehr SK. Factors associated with school nurses’ HPV vaccine attitudes for school-aged youth. Psychol Health Med Jun. 2017;22(5):535–545. doi:10.1080/13548506.2016.1173710 [118] | 2016 | National | Not listed | Describe school nurses’ knowledge, perception of role as opinion leader, perceived school district support and attitudes regarding the HPV vaccine for school-aged youth, and determine which factors are associated with positive HPV vaccine attitudes in school nurses | Cross-sectional study | 413 | (Other) School Nurses | Theory of Planned Behavior | School nurse attitudes towards HPV vaccine initiation | Positive attitudes regarding the HPV vaccine were predicted by higher HPV and vaccine knowledge (β = 0.096, p < 0.001) and school nurses’ stronger perceptions of role as opinion leaders for the vaccine (β = 0.665, p < 0.001). | ||
| Ryan G, Ashida S, Gilbert PaulA, et al. The Use of Medical Claims Data for Identifying Missed Opportunities for HPV Immunization Among Privately Insured Adolescents in the State of Iowa. J Community Health. 2022;47(5):783–789. doi:10.1007/s10900-022-01110-7 [119] | 2022 | Midwest | 2012–2017 | Quantify the number of MOs for HPV vaccination that adolescents experienced between the ages of 11 and 13 using medical claims data and conduct subgroup comparisons by gender and rurality | Cohort study | 14,505 | Adolescents | Missed opportunities for HPV vaccination | Average number of missed opportunities by subgroup: gender and rurality | 16.8% of females and 11.4% of males had completed the vaccine series (3 doses by age 13). Adolescents experienced 5–6 MOs between ages 11 and 13. There were more MOs experienced by non-initiators of the vaccine compared to initiators (7 MOs vs. fewer than 2 MOs). | Urban adolescents experienced more MOs than rural counterparts. Female patients had fewer MOs than males. | |
| Ryan G, Daly E, Askelson N, Pieper F, Seegmiller L, Allred T. Exploring Opportunities to Leverage Pharmacists in Rural Areas to Promote Administration of Human Papillomavirus Vaccine. Prev Chronic Dis. 2020;17. doi:10.5888/pcd17.190351 [120] | 2020 | Midwest | 2018 | Assess rural pharmacists’ role in administering and promoting the HPV vaccine in counties in Iowa with low rates of HPV vaccine uptake | Qualitative | 11 | Other (Pharmacists) | HPV vaccination barriers among rural pharmacists | Pharmacists were willing to administer HPV vaccinations and saw it within their role to do so. Barriers to offering the vaccine included sensitivity of the subject, lack of information, concerns about safety and misinformation | |||
| Ryan GW, Perry SS, Scherer A, et al. Factors contributing to missed opportunities for human papillomavirus vaccination among adolescents, ages 11 to 13, in Iowa. Vaccine X. 2022;11:100192. doi:10.1016/j.jvacx.2022.100192 [121] | 2022 | Midwest | 2012–2020 | Explore associations between adolescent and provider characteristics and the number of MOs adolescents experience between ages 11 and 13 | Retrospective Cohort study | 14,104 | Adolescents | Missed opportunities for HPV vaccination | Non-initiators of HPV vaccination had more MOs, fewer well-child visits and fewer other adolescent vaccinations compared to HPV vaccination initiators. MOs decreased with age. Among those who had initiated the vaccine, MOs were higher among those whose PCP was not a pediatrician and those who saw rural providers. Most MOs occurred at acute care visits rather than well-child visits. | |||
| Schrote K, Hersh A, Bruegl A, Rodriguez MI. Women’s perspectives on receiving and expanding access to essential health services in pharmacies in the United States. J Am Pharm Assoc. 2022;62(3):711–716.e3. doi:10.1016/j.japh.2021.11.034 [122] | 2022 | National | 2020 | Determine whether there were differences by rurality in women’s perspectives/willingness to receive essential preventative/diagnostic reproductive health services in community pharmacies | Cross-sectional | 544 | Adults | Women’s perspectives; willingness to receive preventative services in community pharmacies | Women in rural settings were less likely to have reported receiving the HPV vaccine (56.2% vs. 41.7%), and 13.9% of rural respondents reported that they were unsure if they had received the HPV vaccine (p= 0.02). Both rural and urban women want to receive preventative reproductive health services in community pharmacies. | |||
| Shah SFA, Ginossar T, Bentley JM, Zimet G, McGrail JP. Using the Theory of Planned behavior to identify correlates of HPV vaccination uptake among college students attending a rural university in Alabama. Vaccine. 2021;39(51):7421–7428. doi:10.1016/j.vaccine.2021.10.082 [123] | 2021 | South | 2019 | Examine college students’ intentions to receive the HPV vaccine and examine the relationship between religious beliefs and HPV vaccination uptake status among college students | Cross-sectional survey | 257 | Adults | Theory of Planned Behavior | Intention to vaccinate | Attitudes and subjective norms were significant predictors of intention to receive vaccinated. Three knowledge statements about HPV and its vaccine were associated with higher attitude scores. The odds of receiving at least one HPV shot were higher for females than for males, for non-Caucasians than for Caucasians. Students who were not vaccinated were more likely to report that religion influenced their health beliefs. | ||
| Shato T, Humble S, Anandarajah A, et al. Influences of sociodemographic characteristics and parental HPV vaccination hesitancy on HPV vaccination coverage in five US states. Vaccine. 2023;41(25):3772–3781. doi:10.1016/j.vaccine.2023.04.082 [124] | 2023 | Midwest, South | 2021 | Examine the association of sociodemographic characteristics and HPV vaccination hesitancy with HPV vaccination coverage in five US states with disproportionately low adolescent coverage rates compared to the national average | Cross-sectional survey | 926 | Adults | HPV vaccination initiation | Children of vaccine hesitant parents were less likely to have received any doses of the HPV vaccine than children of non-vaccine hesitant parents (AOR: 0.17, 95% CI:0.11–0.27). Male children were less likely to have initiated the HPV vaccine series than female children (AOR: 0.70, 95% CI:0.50– 0.97). Older children (13–17 vs. 9–12 years), receiving the meningococcal conjugate or most recent seasonal influenza vaccine were all associated with higher likelihoods of receiving any doses of the HPV vaccine (aOR = 6.01, 95% CI:3.98–9.08; aOR = 2.24, 95% CI:1.27–3.95; aOR = 2.41, 95% CI:1.73–3.36, respectively) | |||
| Song S, White A, Kucik JE. Use of Selected Recommended Clinical Preventive Services—Behavioral Risk Factor Surveillance System, United States, 2018. MMWR Morb Mortal Wkly Rep Apr. 2021;70(13):461–466. doi:10.15585/mmwr.mm7013a1 [125] | 2021 | National | 2018 | Ascertain prevalence of the use of selected recommended clinical preventive services among persons aged ≥18 years | Cross-sectional survey | 437,436 | Adults | HPV vaccine initiation and other preventative services | The overall prevalence of HPV vaccination was 16.5%. There was no statistical difference between rural and urban prevalence for the HPV vaccination (PRR = 1.29, 95% CI: 0.77–2.16, reference = rural). Income was not significant with HPV vaccination prevalence, but having insurance was associated with higher HPV vaccination (PRR = 1.95, 95% CI: 1.17–3.25) compared to uninsured. | |||
| Stewart T, Lee YA, Damiano EA. Do Transgender and Gender Diverse Individuals Receive Adequate Gynecologic Care? An Analysis of a Rural Academic Center. Transgender Health. 2020;5(1):50–58. doi:10.1089/trgh.2019.0037 [126] | 2020 | Northeast | 2015–2018 | Compare utilization rates of gynecologic screening services between transgender individuals in a rural setting and cisgender individuals nationally, and determine if utilization rates differed by insurance type or gender identity | retrospective chart review | 255 | Adults | HPV vaccine initiation | 84% (N = 218) of the sample were eligible to receive the HPV vaccination, with 47% (N = 102) receiving the vaccination using the 2018 HPV guidelines. There was a statistically significant difference, with 20% of transgender men, 60% of transgender women, and 60% of GNB/GNC/Genderqueer/gender diverse individuals receiving the vaccination (p < 0.001). | |||
| Swiecki-Sikora AL, Henry KA, Kepka D. HPV Vaccination Coverage Among US Teens Across the Rural–Urban Continuum. J Rural Health Sep. 2019;35(4):506–517. doi:10.1111/jrh.12353 [127] | 2019 | National | 2012–2013 | Examine associations between HPV vaccination uptake and rural and urban residence, and examine whether vaccine uptake in rural and urban places was modified by area-based poverty | Secondary data analysis | 37,115 | Adolescents | HPV vaccine initiation and completion | Lower HPV vaccination initiation and completion among teens from isolated small rural towns and small rural towns than among urban teens. Girls from small rural towns had lower odds of completion (OR = 0.74, 95% CI: 0.60–0.91) than girls from urban areas. Boys from isolated small rural towns had statistically significant lower odds of initiation (OR = 0.68, 95% CI: 0.52–0.88) and completion (OR = 0.63, 95% CI: 0.41–0.97) than boys from urban areas. | |||
| Teferra AA, Keller-Hamilton B, Roberts ME, Reiter PL. HPV Vaccine Coverage Among Adolescent Males in Ohio: Results of a Longitudinal Study. Ohio J Public Health. 2019;2(2):15–23. doi:10.18061/ojph.v2i2.9030 [128] | 2019 | Midwest | 2015–2018 | Examine HPV vaccine coverage among adolescent males in Ohio and identify predictors of vaccination | Secondary Data Analysis | 1126 | Adolescents | HPV vaccine initiation | HPV vaccination initiation predictors | 42.4% had initiated the HPV vaccine series at the time of the baseline survey. Among sons who were unvaccinated at baseline and whose parents completed a follow-up survey, 36.3% had initiated the HPV vaccine series at follow-up | Initiation was lower among sons of parents with an associate’s degree, or some college education, compared to parents with a high school degree or less (RR = 0.28, 95% CI = 0.46–0.99). Sons whose parents indicated they had received influenza vaccine were more likely to initiate the HPV vaccine series (RR = 1.54, 95% CI = 1.08–2.18), and whose parents indicated a lack of a recent visit to the doctor as a reason for not vaccinating at baseline (RR = 1.41, 95% CI = 1.02–1.95). | |
| Thaker J, Albers AN, Newcomer SR. Nurses’ perceptions, experiences, and practices regarding human papillomavirus vaccination: results from a cross-sectional survey in Montana. BMC Nurs. 2023;22(1). doi:10.1186/s12912-023-01379-6 [129] | 2023 | Midwest | 2020–2021 | Determine nurses’ perceptions, experiences, and practices regarding human papillomavirus vaccination in a Rural and medically underserved region of the United States. | Cross-sectional survey | 227 | Providers | Nurses’ perceptions of clinic vaccination practices & barriers to vaccine uptake & potential strategies to improve HPV vaccination rates | Secondary: Nurses’ report of the estimated percentage of parents who defer HPV vaccination, by age group and sex of adolescent. Tertiary: Nurses’ support of strategies to improve HPV vaccination rates | 91.8% (n = 179) of nurses agreed or strongly agreed that it was important that older children and adolescents be vaccinated against HPV, and 89.8% (n = 177) expressed confidence in the safety of the HPV vaccine. Only 34.5% (n = 68) of respondents reported anticipating an uncomfortable conversation while discussing the HPV vaccine with parents of 9 to 12-year-old children. | The highest perceived barriers to recommending and administering the HPV vaccine are parents not thinking that the vaccine is necessary for their sons (n = 146, 74.5%), misinformation that parents receive from the internet or social media (n = 139,71.6%), parental concerns about the safety of the HPV vaccine (n = 132, 67.7%), and irregular well-child visits (n = 130, 66.7%). | |
| Thomaier L, Aase DA, Vogel RI, Parsons HM, Sadak KT, Teoh D. HPV vaccination coverage for pediatric, adolescent and young adult patients receiving care in a childhood cancer survivor program. Prev Med Rep. 2022;29:101972. doi:10.1016/j.pmedr.2022.101972 [130] | 2022 | Midwest | 2014–2019 | Determine HPV vaccination coverage among individuals participating in a childhood cancer survivor program (CCSP) | Retrospective cohort study | 592 | Adolescents and Adults | HPV vaccine initiation | Vaccination initiation among CCSP patients was not statistically significantly different from controls [60.0% vs. 66.3%, OR = 0.82, 95% CI: (0.55, 1.23), p = 0.35], and neither was completion (28.5% vs. 30.1%, p = 0.09). | |||
| Thomas TL, Caldera M, Maurer J. A short report: parents HPV vaccine knowledge in rural South Florida. Hum Vaccines Immunother. 2019;15(7–8):1666–1671. doi:10.1080/21645515.2019.1600986 [131] | 2019 | South | 2016 | Explore parental knowledge and hesitancy of HPV vaccination | Pilot study | 123 | Parents | HPV vaccine initiation and parental knowledge regarding HPV and the HPV vaccines | Less than 45% of parents/caregivers had vaccinated their child with the HPV vaccine, and 80% of the participants had low or no knowledge of HPV vaccination. Participants with a high school education or less (64%) and conservative religious affiliation, e.g., Baptist and Catholic (74%), did not decline HPV vaccination. | |||
| Thomas TL, Strickland O, Diclemente R, Higgins M. An Opportunity for Cancer Prevention During Preadolescence and Adolescence: Stopping Human Papillomavirus (HPV)-Related Cancer Through HPV Vaccination. J Adolesc Health. 2013;52(5):S60-S68. doi:10.1016/j.jadohealth.2012.08.011 [132] | 2013 | South | 2009–2011 | Determine correlates of refusal and acceptance of HPV vaccination by rural parents of preadolescent and adolescent children | Cross-sectional study | 519 | Parents | Health Belief Model | Intention to vaccinate and HPV vaccine initiation | Being African American and being Baptist lowers the likelihood of parents who choose to vaccinate or intend to vaccinate their children. Parents who had vaccinated or intended to vaccinate had significantly higher scores on perceived barriers (1.02 times more likely to vaccinate) and lower scores on perceived benefits (1.01 times more likely to vaccinate) (model p < 0.001). | ||
| Thomas TL, Strickland OL, DiClemente R, Higgins M, Haber M. Rural African American Parents’ Knowledge and Decisions About Human Papillomavirus Vaccination. J Nurs Scholarsh. 2012;44(4):358–367. doi:10.1111/j.1547-5069.2012.01479.x [133] | 2014 | South | 2010–2011 | Identify predictors of HPV vaccination among rural African American families, and find culturally specific points of intervention that would increase HPV vaccination rates among children in these communities | Cross-sectional study | 400 | Parents | Health Belief Model | Intention to vaccinate and HPV vaccine initiation | Intention to vaccinate was significantly different across the three counties (p < 0.01). Non-Baptists were 3.6 (95% CI: 2.0–6.6, p < 0.001) times more likely to vaccinate compared to Baptists after adjusting for perceived vulnerability and perceived barriers. | ||
| Vamos CA, Kline N, Vázquez-Otero C. Stakeholders’ perspectives on system-level barriers to and facilitators of HPV vaccination among Hispanic migrant farmworkers. Ethn Health Aug. 2022;27(6):1442–1464. doi:10.1080/13557858.2021.1887820 [134] | 2021 | South | 2020 | Inform intervention development targeting vaccination uptake and completion, ultimately decreasing HPV-related cancer disparities | Qualitative | 13 | Other (Stakeholders) | Social Ecological Model, Precede-Proceed Model, CBPR, Intervention Mapping | Stakeholder perceptions of barriers and facilitators to HPV vaccination among Latinx migrant farmworkers | Barriers included lack of healthcare access, language barriers, limited knowledge about HPV and the vaccine, financial constraints, and concerns about immigration status. Facilitators included the presence of outreach programs, culturally tailored interventions, supportive healthcare providers, and social networks within the community that promote vaccination awareness and acceptance. | ||
| Vanderpool RC, Cohen E, Crosby RA, Jones MG, Bates W, Casey BR, Collins T. “1-2-3 Pap” Intervention Improves HPV Vaccine Series Completion among Appalachian Women. J Commun. 2013 Feb;63(1):95–115. doi: 10.1111/jcom.12001. Epub 2013 Jan 10. PMID: 26560123; PMCID: PMC4639462. [135] | 2015 | South | 2010–2011 | This study identified correlates of intent to complete the vaccine series and actual series completion. The study tested the efficacy of a DVD intervention to promote series completion. | Randomized controlled trial | 344 | Adults | Theory of Planned Behavior | HPV vaccine completion | Women’s beliefs that all three doses reduced cancer risk predicted intent and completion. Intention predicted completion, as did the belief that having a friend accompany the woman would promote completion. Beyond these effects, women assigned to the intervention were 2.44 times more likely than women in the control group to complete the series. | ||
| Vielot NA, Lane RM, Loefstedt K, et al. Acceptability and readiness to promote human papillomavirus vaccination at ages 9–10 years: a feasibility study among North Carolina clinics. Pilot Feasibility Stud. 2023;9(1). doi:10.1186/s40814-023-01379-y [136] | 2022 | South | 2022 | Assess the feasibility of the age-9 recommendation of HPV vaccination in rural clinics | Pilot study | 10 | Providers | Attitudes towards recommending HPV vaccination to 9-and 10-year-olds | There are four predominant themes from the interviews: (1) clinics have created opportunities to recommend HPV vaccination during well-child visits; (2) providers educate caregivers who are hesitant about HPV vaccination; (3) providers often consider the benefits of HPV vaccination in the context of adolescent social and physical development; and (4) providers are generally willing and able to promote age-9 HPV vaccination in the clinic. | |||
| Vielot NA, Butler AM, Brookhart MA, Becker-Dreps S, Smith JS. Patterns of Use of Human Papillomavirus and Other Adolescent Vaccines in the United States. J Adolesc Health. 2017;61(3):281–287. doi:10.1016/j.jadohealth.2017.05.016 [137] | 2017 | National | 2009–2014 | Describe the patterns of use of universally recommended adolescent vaccines in the United States | Observational Study | 1,691,223 | Adolescents | HPV vaccine initiation | Only 18.4% of residents received the HPV vaccine compared to Tdap (52.1%) and MenACWY (45.8%). Rural adolescents were less likely than urban adolescents to receive each vaccination except in the Northeast, where they were more likely to receive HPV vaccination (IRR: 1.09, 95% Cl: 1.20–1.13). Timely HPV vaccination was associated with female sex, urbanicity, Western residence, and later birth cohort. | |||
| Walker TY, Elam-Evans LD, Williams CL, et al. Trends in human papillomavirus (HPV) vaccination initiation among adolescents aged 13–17 by metropolitan statistical area (MSA) status, National Immunization Survey—Teen, 2013–2017. Hum Vaccines Immunother. 2019;16(3):554–561. doi:10.1080/21645515.2019.1671765 [138] | 2020 | National | 2013–2017 | Examine trends in HPV vaccination initiation coverage by MSA, and examine trends in disparities in HPV vaccination initiation coverage by MSA status over time | Secondary analysis | 103,047 | Adolescents | HPV vaccine initiation | The five-year average annual percentage point increases in HPV vaccination initiation coverage were similar between MSA designations (4.9–5.2). Coverage was significantly lower among teens living in mostly rural areas, regardless of poverty status, sex, and race/ethnicity, except among black, non-Hispanic adolescents. There was no significant change in the magnitude of the disparity between mostly urban areas and mostly rural areas over time (p = 0.98). | |||
| Walker TY, Elam-Evans LD, Yankey D, et al. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years—United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(33):718–723. doi:10.15585/mmwr.mm6833a2 [139] | 2019 | National | 2017- 2018 | Examine trends in HPV vaccination initiation coverage by MSA status, and examine trends in disparities in HPV vaccination initiation coverage by MSA status during 2013–2017 | Cross-sectional study | 103,074 | Adolescents | HPV vaccine initiation and completion | In 2018, 51.1% of adolescents aged 13–17 years were up-to-date with the HPV vaccine series, and 68.1% had received ≥1 dose of HPV vaccine. During 2017–2018, the increase in HPV vaccination coverage was attributable to increases among males only. | Small | ||
| Warner EL, Fowler B, Martel L, Kepka D. Improving HPV Vaccination Through a Diverse Multi-state Coalition. J Community Health. 2017;42(5):911–920. doi: 10.1007/s10900-017-0334-7 [140] | 2017 | West | 2015–2016 | Assess coalition members’ perceptions of barriers and facilitators to HPV vaccination in their communities and evaluate the efficacy, strengths, and future directions of the IWHVC | Mixed methods | 122 | Adults | HPV vaccination facilitators and barriers | Perceived barriers to vaccination were a lack of education/low knowledge about the HPV vaccine (55.8%), concerns about sexuality/promiscuity (44.2%), and not knowing the vaccine is recommended for boys (38.4%). Top facilitators to HPV vaccination included a strong provider recommendation (53.5%), improved messaging/education (51.2%), and increasing parental buy-in (32.6%). | |||
| Warren BR, Gillette-Walch H, Adler J, et al. Assessment of human papillomavirus vaccination rates of adolescents in California, 2018–2019. Prev Med Rep. 2023;32:102144. doi:10.1016/j.pmedr.2023.102144 [141] | 2023 | West | 2018–2019 | Evaluate the vaccine registries (National Immunization Survey (NIS)-Teen, commercial HMOs in California, Medi-Cal, and California Immunization Registry) data for HPV vaccine series completion, and compare their completeness | Secondary data analysis | 664,795 | Adolescents | HPV vaccination series completion | Secondary: HPV vaccine series completion differences among adolescent females and males. Tertiary: HPV vaccine series initiation versus completion among 13-year-olds by county | HPV series completion among 13-year-olds in 2018 for commercial HMOs was 50%, Medi-Cal was 45%, and the California Immunization Registry was 28%, with NIS-Teen rates for 13 to 17-year-olds at 50% in 2018 and 54% in 2019 | Series completion increased for females from 50.1% in 2018 to 61.5% in 2019, but dropped for males from 55.1% to 51.4% in the same time period | |
| Wheeler DC, Miller CA, Do EK, et al. Identifying Area-Level Disparities in Human Papillomavirus Vaccination Coverage Using Geospatial Analysis. Cancer Epidemiol Biomarkers Prev. 2021;30(9):1689–1696. doi:10.1158/1055-9965.epi-21-0331 [142] | 2021 | South | 2010–2018 | Determine whether neighborhood sociodemographic variables explain variation in HPV vaccination, and identify areas with significantly depressed vaccination coverage | Secondary data analysis | 294,948 | Adolescents | HPV vaccine completion | 42,145 (28.9%) of girls and 34,760 (23.8%) of boys had completed HPV vaccination; girls had overall higher completion probabilities. Predominantly rural areas had significantly lower vaccination completion rates compared to others. | |||
| Wick JA, Elswick BM. Impact of Pharmacist-Delivered Education on Early Parent Awareness and Perceptions Regarding Human Papillomavirus (HPV) Vaccination in the Community Pharmacy Setting in West Virginia. Innov Pharm. 2018;9(3):8. doi:10.24926/iip.v9i3.1396 [143] | 2018 | South | 2018 | Determine parental perceptions of the Human Papillomavirus Vaccine and awareness of vaccine administration at community pharmacies; and describe parental intentions to have children vaccinated against HPV, and assess the impact of pharmacist-led education on these perceptions and intentions | Prospective pretest, post-test study (quasi-experimental) | 34 | Parents | Intention to vaccinate and awareness of HPV vaccine availability at community pharmacies | Prior to the educational session, 35% of participants planned to vaccinate their child. Following completion of the intervention, 44% of the population intended to vaccinate at the ACIP-recommended age. Participants demonstrated increased awareness of HPV vaccine availability at community pharmacies from 32% (n = 11) to 100% (n = 34). | |||
| Williams CL, Walker TY, Elam-Evans LD. Factors associated with not receiving HPV vaccine among adolescents by metropolitan statistical area status, United States, National Immunization Survey-Teen, 2016–2017. Hum Vaccin Immunother Mar. 2020;16(3):562–572. doi:10.1080/21645515.2019.1670036 [144] | 2020 | National | 2016–2017 | Identify sociodemographic factors associated with not initiating the HPV vaccine series, and determine whether these factors differed by MSA status | Secondary data analysis | 41,424 | Adolescents | Non-initiation of the HPV vaccine series | A significantly higher percentage of suburban (39.2%) and mostly rural (45.4%) teens had not received any doses of the HPV vaccine compared to mostly urban teens (32.0%). Regardless of MSA designation, factors for not receiving HPV included living in the South, having a mother with some college education, not having an 11–12-year-old well-child visit, and not receiving a provider recommendation for vaccination. There was no difference in the percentage of mostly rural teens (78.9%) with missed opportunities for HPV vaccination when compared to mostly urban teens (79.3%). | |||
| Yoost JL, Starcher RW, King-Mallory RA, Hussain N, Hensley CA, Gress TW. The Use of Telehealth to Teach Reproductive Health to Female Rural High School Students. J Pediatr Adolesc Gynecol. 2017;30(2):193–198. doi:10.1016/j.jpag.2016.10.002 [145] | 2017 | South | 2015 | Evaluate the use of telehealth to teach reproductive health in rural areas with high rates of teen pregnancy | Prospective cohort study | 55 | Adolescents | HPV vaccine initiation | Those reporting vaccine initiation or completion was 38% (10/26) at the time of the educational session post-test. This report increased to 71.4%, 15/21 (p = 0.03) at 6 months among those who attended that session and increased to 70%, 26/37 (p = 0.001) among all subjects who completed the 6-month survey (n = 37). | |||
| Zahnd WE, Harrison SE, Stephens HC, et al. Expanding access to HPV vaccination in South Carolina through community pharmacies: A geospatial analysis. J Am Pharm Assoc. 2020;60(6):e153-e157. doi:10.1016/j.japh.2020.05.005 [146] | 2020 | South | 2019 | Determine whether spatial access to pharmacies among adolescents and young adults in South Carolina varied by rurality and geographic access to primary care providers | Secondary data analysis | 1010 | Pharmacies (other) | Spatial access to pharmacies | There were statistically significantly higher spatial accessibility scores in non-HPSA–designated CTs across South Carolina as a whole, as well as in both metropolitan and rural and small-town areas. However, among CTs in micropolitan areas, no difference in spatial accessibility scores was found between HPSA-designated and non-HPSA–designated CTs. | |||
| Zhang J, Xue H, Calabrese C, Chen H, Dang JHT. Understanding Human Papillomavirus Vaccine Promotions and Hesitancy in Northern California Through Examining Public Facebook Pages and Groups. Front Digit Health. 2021;3. doi:10.3389/fdgth.2021.683090 [147] | 2021 | West | 2010–2021 | Understand HPV vaccine promotions and hesitancy in Northern California by examining public Facebook pages and groups | Observational study | 212 | Adults | Sentiments, Negative emotions, and Thematic Topics in Facebook posts and comments | There was significantly more positive sentiment in comments than in posts, more negative sentiment in comments than in posts, and more anger in comments than in posts. Post themes included awareness and screening of HPV and cervical cancer, STI testing services, information sources, and calls to action for health services. Comment themes were related to vaccine hesitancy, discussing vaccine risks, safety concerns, and distrust in vaccine science, citing misinformation. When comparing high-coverage counties, there were no significant differences across all dimensions of sentiment and emotions for posts. For comments, there was a significantly higher level of anger in high-coverage counties than in low-coverage counties. | |||
| Zoellner JM, Porter KJ, Brock DJP, et al. Advancing engagement and capacity for rural cancer control: a mixed methods case study of a Community-Academic Advisory Board in the Appalachia region of Southwest Virginia. Res Involv Engagem. 2021;7(1). doi:10.1186/s40900-021-00285-y [148] | 2021 | South | 2017–2020 | Describe engagement processes used to prioritize and address regional comprehensive cancer control needs among a Community-Academic Advisory Board (CAB) in the medically underserved, rural Appalachian region | Convergent parallel mixed methods | 69 | Other | Community-Based Participatory Research, Comprehensive Participatory Planning and Evaluation | Habits of community advisory boards, challenges, and strengths across those habits | Across habits and at both Time 2 and Time 3 interviews, strengths reported by CAB members outweighed the challenges in both quantity and frequency. An exception was for diversified funding, where reported strengths and challenges were relatively more even. Related to challenges, limited time was consistently mentioned across most habits and was viewed as a limiting factor at both time points. Also, implications of COVID-19, especially as it related to effective communication and diversified funding, emerged as a major challenge at Time 3. | ||
| * These papers (as well as Chido-Amajuoyi et al., 2022, reference [37]) rely on one statewide cross-sectional survey of healthcare professionals in Texas (N = 1283). | ||||||||||||
Description of Process Measures in Implementation and Intervention Studies (N = 20)
| Reference | General Description of Intervention | Study Type | Intervention Type | Free Vaccination Offered | Intervention Level | Intended Audience for Intervention | Intervention Duration | Duration of Training, Educational Sessions | Number of Sessions; Number of media Outlets, Flyers | Mode of Intervention | Who Intervened (Directly Performed Intervention) |
| Crosby RA, Casey BR, Vanderpool R, Collins T, Moore GR. Uptake of free HPV vaccination among young women: a comparison of rural versus urban rates. J Rural Health Winter. 2011;27(4):380–384. doi:10.1111/j.1748-0361.2010.00354.x [27] | Young rural women attending rural clinics (n = 246), young women attending a rural community college (n = 251) and young women attending an urban university health clinic (n = 209) were recruited in Kentucky. After completing a brief questionnaire, women received a free voucher for HPV vaccination. | Interventional | Vaccine voucher | Yes | Individual-level | College women | 23 months | N/A | N/A | In-person | Research assistant |
| Beck A, Bianchi A, Showalter D. Evidence-Based Practice Model to Increase Human Papillomavirus Vaccine Uptake: A Stepwise Approach. Nurs Womens Health. 2021;25(6):430–436. doi:10.1016/j.nwh.2021.09.006 [59] | Education targeting parental HPV vaccine hesitancy and strong recommendations for immunization was administered by healthcare providers to parents of youth and adolescents who are vaccine-eligible. | Interventional | Parental Education | No | Clinic | Parents of unvaccinated children from 11 to 17 | 6 weeks (additional 6 weeks for control period) | N/A | N/A | In-person | Clinic staff and vaccine providers |
| Berenson AB, Hirth JM, Kuo YF, Rupp RE. Quantitative and qualitative assessment of an all-inclusive postpartum human papillomavirus vaccination program. Am J Obstet Gynecol. 2021;224(5):504.e1–504.e9. doi:10.1016/j.ajog.2020.11.033 [61] | Postpartum women eligible for HPV vaccine were offered education on the HPV vaccination and the CDC facts sheet. Patients who gave consent were administered a dose prior to discharge, or were scheduled for outpatient vaccination due to time purposes with follow-up doses along with the postpartum doctor visits. | Interventional | Patient Education | Yes | Individual-level | Postpartum women | 3 years (evaluation portion of intervention) | N/A | N/A | In-person | Patient Navigators |
| Brewer NT, Hall ME, Malo TL, Gilkey MB, Quinn B, Lathren C. Announcements Versus Conversations to Improve HPV Vaccination Coverage: A Randomized Trial. Pediatrics. 2017;139(1). doi:10.1542/peds.2016-1764 [66] | Randomized clinics to receive no training (control), announcement training, or conversation training. | Interventional | Vaccine provider education | No | Providers | Vaccine providers | 4 months | 1 h | 1 training session | In-person | physician educator |
| Carman AL, McGladrey ML, Goodman Hoover A, Crosby RA. Organizational Variation in Implementation of an Evidence-Based Human Papillomavirus Intervention. Am J Prev Med. 2015;49(2):301–308. doi:10.1016/j.amepre.2015.03.011 [70] | Pragmatic implementation study focused on LHDs the option of showing the HPV vaccine informational video after the first dose of the vaccine but also pilot tested the feasibility and acceptability of other delivery options | Implementation | Patient Education (Implementation at LHD clinics) | No | Clinic-level * (local public health department clinics) | Local health department clinics | 6 months | N/A | N/A | Unspecified (mainly remote and indirect) | Mainly self-directed (Research team gave general guidelines) |
| Cates JR, Shafer A, Diehl SJ, Deal AM. Evaluating a County-Sponsored Social Marketing Campaign to Increase Mothers’ Initiation of HPV Vaccine for Their Preteen Daughters in a Primarily Rural Area. Soc Mark Q. 2011;17(1):4–26. doi:10.1080/15245004.2010.546943 [73] | They placed posters and brochures in English and/or Spanish with a goal of one location for every ten mothers of 11–12-year-old girls in each city in the four counties, according to census data. On launch date and six weeks later, media releases about the campaign were sent to 10 newspapers and PSAs were sent to 15 radio or television stations. | Interventional | HPV Awareness/Media Campaign | No | Community social marketing campaign | Mothers of 11–12 y/o girls | 3 months | N/A | 10 newspapers and 15 radio/TV stations were used in the campaign (sent media on launch date and then 6 weeks later) | Indirect | Research team |
| Dang JHT, McClure S, Gori ACT. Implementation and evaluation of a multilevel intervention to increase uptake of the human papillomavirus vaccine among rural adolescents. J Rural Health Jan. 2023;39(1):136–141. doi:10.1111/jrh.12690 [76] | There was intervention strategies applied on three levels. On the parent level there was tailored HPV vaccination reminder postcards sent out. On the primary care team level there were 3 clinic-wide HPV vaccination trainings and a quarterly review of HPV vaccination data. On the clinic level there was a physician champion (the clinic’s Medical Director) and clinic visual cues (examination room posters, educational handouts, lanyards, and pins). | Interventional | Other (Parental-education/awareness, Clinic-awareness; Provider Education) | No | Multilevel | clinics, primary care providers, and parents/guardians | 1.5 years | 1 h | 3 sessions for PCPs | In-person (PCPs), indirect (parental postcards) | Research team |
| Daniel CL, Lawson F, Vickers M, et al. Enrolling a rural community pharmacy as a Vaccines for Children provider to increase HPV vaccination: a feasibility study. BMC Public Health. 2021;21(1). doi:10.1186/s12889-021-11304-8 [77] | Enrolled a rural community pharmacy as a Vaccines for Children (VFC) provider to provide free vaccines to eligible adolescents. Development and execution of a health communication campaign for the community | Feasibility-Interventional | Other (Pharmacy VFC enrollment) | VFC-eligible only | Community | Pharmacy and surrounding community | 12 months | N/A | N/A | Both | Research team |
| Ford M, Cartmell K, Malek A, et al. Evaluation of the First-Year Data from an HPV Vaccination Van Program in South Carolina, U.S. J Clin Med. 2023;12(4):1362. doi:10.3390/jcm12041362 [80] | HPV Vaccination Van Program; conducted a town hall meeting prior to the HPV vaccine clinic in their district with several speakers who spoke about their experience (a teacher who had HPV, her son telling why he received it, a physician telling facts about the vaccine). Putting vaccination clinics at school and sharing the video of the town hall meeting. | Interventional | Community education/awareness | VFC-eligible only | Community-level | Adolescents 13–17 | 1 year | 1 h | 1 town hall | virtual | town hall speakers: science teacher, her son, 2 physicians from nearby medical school |
| Harry ML, Asche SE, Freitag LA, et al. Human Papillomavirus vaccination clinical decision support for young adults in an upper midwestern healthcare system: a clinic cluster-randomized control trial. Hum Vaccines Immunother. 2022;18(1). doi:10.1080/21645515.2022.2040933 [85] | Randomized control trial to test clinical decision support among 34 clinics with three treatment arms: Clinical Decision Support only, Clinical Decision Support with Shared Decision-Making Tools, and Usual Care over 12 months from first visit by eligible patients | Interventional | Vaccine provider education | No | Clinic-level | Clinic staff | 20 months | unspecified | 4 sessions (2 in-peron, 2 virtual webinars) | Both | research team (for staff education) |
| Jafari SDG, Appel SJ, Shorter DG. Risk Reduction Interventions for Human Papillomavirus in Rural Maryland. J Dr Nurs Pract. 2020;13(2):134–141. doi:10.1891/jdnp-d-19-00047 [88] | Advertisement of a film screening event was undertaken via flyers on the college campus, in the office, and through social media posts. Women’s Health clinic office staff were instructed to review HPV immunization records at the time of the annual Well Woman Visit for females aged 12–26 years. The CDC HPV education sheet was distributed to parents of those aged 12–17 and to patients aged 18–26 years. For the public awareness campaign, screenings of the documentary Someone You Love; The HPV Epidemic © by Lumiere Media Inc. (2015), were undertaken with permission. The screening served as the focal point for part one of this initiative. The screenings were heavily advertised at the local community college and on social media. The second component of the project measured the impact of patient education. | Interventional | Community education/awareness | No | Individual-level | Parents of eligible children and adult patients | Unspecified | N/A | unspecified | Both | Research team |
| Kepka D, Christini K, McGough E, et al. Successful Multi-Level HPV Vaccination Intervention at a Rural Healthcare Center in the Era of COVID-19. Front Digit Health. 2021;3. doi:10.3389/fdgth.2021.719138 [89] | Human papillomavirus vaccination training for the healthcare team included two 1 h early morning video calls that focused on training providers and support staff at TMC on evidence-based HPV vaccination systems, vaccine recommendations, and patient education materials relevant to their patient population. Healthcare team members were given evidence-based patient center HPV vaccination education materials. An HPV vaccination reminder campaign was performed for patients/caregivers with age-eligible children for the HPV vaccine (children ages 11–17) and young adults (ages 18–26) who are also age-eligible for the HPV vaccine. | Interventional | Vaccine provider education | No | Multilevel | Vaccine providers | 2019–2021 | 1 h | 2 main training sessions (1 optional/refresher) | virtual | Research team |
| Kepka D, Coronado GD, Rodriguez HP, Thompson B. Evaluation of a Radionovela to Promote HPV Vaccine Awareness and Knowledge Among Hispanic Parents. J Community Health. 2011;36(6):957–965. doi:10.1007/s10900-011-9395-1 [90] | Intervention Arm: The radionovela addresses facts about cervical cancer, HPV, and the HPV vaccine, concerns about the HPV vaccine, and decision-making activities related to vaccine uptake. Control Arm: the same 5 min of Spanish radio programming prior to the control message, but included public service announcement related to prostate cancer prevention | Interventional | HPV Awareness/Media Campaign | No | Individual-level | Parents of female children (9–17 y/o) | 3 months | N/A | 1 listening activity | In-person | Research team and local health educators |
| Panagides R, Voges N, Oliver J, Bridwell D, Mitchell E. Determining the Impact of a Community-Based Intervention on Knowledge Gained and Attitudes Towards the HPV Vaccine in Virginia. J Cancer Educ Apr. 2023;38(2):646–651. doi:10.1007/s13187-022-02169-5 [108] | Showed documentary “Someone You Love: The HPV Epidemic” and evaluated intention to vaccinate and HPV knowledge through surveys before and after participants watched the film. | Interventional | Community education/awareness | No | Individual-level | 18+ adults in community | 2016–2019 | unspecified | >1 movie showing | In-person | Research team |
| Paskett ED, Krok-Schoen JL, Pennell ML, et al. Results of a Multilevel Intervention Trial to Increase Human Papillomavirus (HPV) Vaccine Uptake among Adolescent Girls. Cancer Epidemiol Biomarkers Prev. 2016;25(4):593–602. doi:10.1158/1055-9965.epi-15-1243 [109] | Clinical level: posters, brochures, and tabletop tent cards for the HPV vaccine intervention. Provider level: For the HPV vaccine education session, we modified an evidence-based tobacco cessation program (38) focused on the “5 A’s” and “5 R’s.” The session was on current evidence-based HPV vaccine information and strategies designed to assist physicians in discussing HPV vaccination with parents Parent level: an educational brochure and DVD video about HPV and HPV vaccination, a magnet reminder to receive the 2nd and 3rd HPV vaccine shots, and a Centers for Disease Control and Prevention (CDC) HPV vaccine information statement. (Provider control: Providers were given information on the flu and flu vaccine. Parent level Control: The comparison group was mailed a packet that included similar items, a flu vaccine information statement from the CDC and flu information sheets from Ohio Department of Health.) | Interventional | Other (Clinic/Provider/Parental education) | No | Multilevel | Vaccine providers and parents | 12 months (provider), 6 months (measuring secondary outcomes in patients) | 1 h (provider education) | 1 educational session for providers | indirect (parent/clinic) | Research team |
| Rodriguez AM, Do TQN, Chen L, Schmeler KM, Montealegre JR, Kuo YF. Human papillomavirus vaccinations at recommended ages: How a middle school-based educational and vaccination program increased uptake in the Rio Grande Valley. Hum Vaccines Immunother. 2022;18(6). doi:10.1080/21645515.2022.2133315 [116] | A comprehensive school-based intervention was conducted to encourage middle school students to become vaccinated for HPV. Several school districts participated in the intervention that included physician-led educational events about HPV and its vaccine, five school-based vaccination events at participating schools (prior to COVID-19), and remote/outdoor events (during COVID-19). | Interventional | Community education/awareness | Yes | Individual-level/Community-level | middle schoolers | 2016–2022 | unspecified | 5 school vaccination events; unspecified number of physician-led educational events | In-person (some adaptations during the pandemic) | Physician-led educational events |
| Rodriguez AM, Do TQN, Eyada MF, Chen L, Schmeler KM, Montealegre JR. Human Papillomavirus Vaccination Uptake in the Rio Grande Valley: Results from a Pilot Community-Based Educational and School-Based Vaccination Program and Its Expansion. Vaccines. 2023;11(2):329. doi:10.3390/vaccines11020329 [117] | A comprehensive school-based intervention was conducted to encourage middle school students to become vaccinated for HPV. Several school districts participated in the intervention that included physician-led educational events about HPV and its vaccine, five school-based vaccination events at participating schools (prior to COVID-19), and remote/outdoor events (during COVID-19). | Interventional | Community education/awareness | Yes | Individual-level/Community-level | middle schoolers | 2016–2022 | unspecified | 5 school vaccination events; unspecified number of physician-led educational events | In-person (some adaptations during the pandemic) | Physician-led educational events |
| Vanderpool RC, Cohen E, Crosby RA, Jones MG, Bates W, Casey BR, Collins T. “1-2-3 Pap” Intervention Improves HPV Vaccine Series Completion among Appalachian Women. J Commun. 2013 Feb;63(1):95–115. doi: 10.1111/jcom.12001. Epub 2013 Jan 10. PMID: 26560123; PMCID: PMC4639462. [135] | Women in the intervention group viewed a 13 min educational DVD, called “1–2–3 Pap.” The DVD focused on the importance of HPV vaccination and guideline-concordant Pap testing for Appalachian Kentucky women. | Interventional | HPV Awareness/Media Campaign | Yes * (only first dose was free, the rest of series was the participants’ financial obligation) | Individual-level | Women (18–26) | 1 year | 13 min video (for intervention arm) | 1 video or pamphlet session (13 min for intervention video) | Unspecified | Self-directed |
| Wick JA, Elswick BM. Impact of Pharmacist-Delivered Education on Early Parent Awareness and Perceptions Regarding Human Papillomavirus (HPV) Vaccination in the Community Pharmacy Setting in West Virginia. Innov Pharm. 2018;9(3):8. doi:10.24926/iip.v9i3.1396 [143] | An educational session regarding HPV vaccination | Interventional | Parental Education | No | Individual-level | Parents of children under 9 | 5 months | 30 min | 1 session per parent (4 sessions held) | In-person | Pharmacist |
| Yoost JL, Starcher RW, King-Mallory RA, Hussain N, Hensley CA, Gress TW. The Use of Telehealth to Teach Reproductive Health to Female Rural High School Students. J Pediatr Adolesc Gynecol. 2017;30(2):193–198. doi:10.1016/j.jpag.2016.10.002 [145] | Teleconferencing equipment connected rural high schools to a distant academic institution. Telehealth sessions included reproductive health and life skills topics. Demographic information, session pre- and post-tests, and 6-month assessment were obtained. | Interventional | Patient Education | No | Individual-level | Female high school students | 4 weeks | 1 h | 8 sessions | Virtual | Faculty/residents/medical students 3 h from study locations |
| * These papers (as well as Chido-Amajuoyi et al., 2022, reference [37]) rely on one statewide cross-sectional survey of healthcare professionals in Texas (N = 1283). | |||||||||||
References
- HPV Vaccine Safety and Effectiveness. Available online: https://www.cdc.gov/vaccines/vpd/hpv/hcp/safety-effectiveness.html#hpvvxeff (accessed on 15 November 2025).
- Muñoz, N.; Bosch, F.X.; de Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K.V.; Snijders, P.J.F.; Meijer, C.J.L.M. Epidemiologic Classification of Human Papillomavirus Types Associated with Cervical Cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Facts & Figures 2020. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2020.html (accessed on 11 November 2025).
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Estimating the world cancer burden: Globocan 2000. Int. J. Cancer 2001, 94, 153–156. [Google Scholar] [CrossRef] [PubMed]
- DeVita, V.T., Jr.; Lawrence, T.S.; Rosenberg, S.A. DeVita, Hellman, and Rosenberg’s Cancer: Principles & Practice of Oncology; Lippincott Williams & Wilkins: London, UK, 2015; Available online: https://oncology.lwwhealthlibrary.com/book.aspx?bookid=1172§ionid=0 (accessed on 19 January 2026).
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R. (Eds.) SEER Cancer Statistics Review, 1975–2017; National Cancer Institute: Bethesda, MD, USA, 2020. Available online: https://seer.cancer.gov/csr/1975_2017 (accessed on 3 April 2025).
- U.S. Department of Health and Human Services Centers for Disease Control Prevention and National Cancer Institute. U.S. Available online: https://gis.cdc.gov/cancer/USCS/#/AtAGlance/value,1,2,1,1,1,1 (accessed on 3 December 2025).
- Meites, E.; Szilagyi, P.G.; Chesson, H.W.; Unger, E.R.; Romero, J.R.; Markowitz, L.E. Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Office of Disease Prevention and Health Promotion. Increase the proportion of adolescents who get recommended doses of the HPV vaccine―IID-08. Healthy People. 2023. Available online: https://health.gov/healthypeople/objectives-and-data/browse-objectives/vaccination/increase-proportion-adolescents-who-get-recommended-doses-hpv-vaccine-iid-08 (accessed on 13 October 2025).
- Peterson, C.E.; Silva, A.; Holt, H.K.; Balanean, A.; Goben, A.H.; Dykens, J.A. Barriers and facilitators to HPV vaccine uptake among US rural populations: A scoping review. Cancer Causes Control 2020, 31, 801–814. [Google Scholar] [CrossRef]
- Pingali, C.; Yankey, D.; Elam-Evans, L.D.; Markowitz, L.E.; Valier, M.R.; Fredua, B.; Crowe, S.J.; DeSisto, C.L.; Stokley, S.; Singleton, J.A. Vaccination Coverage Among Adolescents Aged 13–17 Years—National Immunization Survey–Teen, United States, 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Zahnd, W.E.; Rodriguez, C.; Jenkins, W.D. Rural-Urban Differences in Human Papillomavirus-associated Cancer Trends and Rates. J. Rural Health 2019, 35, 208–215. [Google Scholar] [CrossRef]
- Pingali, C. Vaccination Coverage Among Adolescents Aged 13–17 Years—National Immunization Survey-Teen, United States, 2024. MMWR Morb. Mortal. Wkly. Rep. 2025, 74, 466–472. [Google Scholar] [CrossRef]
- Ratcliffe, M.; Burd, C.; Holder, K.; Fields, A. Defining Rural at the U.S. Census Bur. Am. Community Surv. Geogr. Brief 2016, 1, 1–8. [Google Scholar]
- Childs, E.M.; Boyas, J.F.; Blackburn, J.R. Off the beaten path: A scoping review of how ‘rural’ is defined by the U.S. government for rural health promotion. Health Promot. Perspect. 2022, 12, 10–21. [Google Scholar] [CrossRef]
- MacKinney, A.; Coburn, A.; Lundblad, J.; McBride, T.; Mueller, K.; Watson, S. Access to Rural Health Care—A Literature Review and New Synthesis; Prepared by the RUPRI Health Panel; Rural Policy Research Institute: Iowa City, IA, USA, 2014. [Google Scholar]
- U.S. Department of Health and Human Services (HHS); Health Resources and Services Administration. A Guide for Rural Health Care Collaboration; HHS: Washington, DC, USA, 2019.
- Citizens Research Council of Michigan. Where are the Primary Care Doctors? A Look at Michigan’s Primary Care Physician Shortage. Report 390. 2015. Available online: https://crcmich.org/wp-content/uploads/primary_care_physician_shortage-2015.pdf (accessed on 11 December 2025).
- Hirko, K.A.; Lennon, S.A.; Lucas, T.; Miller, D.C.; Jimbo, M.; Leibfritz, S.J.; Knoff, S.J.; Janney, C.A.; Berg, P.D. Improving Colorectal Cancer Screening in a Rural Setting: A Randomized Study. Am. J. Prev. Med. 2020, 59, 404–411. [Google Scholar] [CrossRef]
- Abdullahi, L.H.; Kagina, B.M.; Ndze, V.N.; Hussey, G.D.; Wiysonge, C.S. Improving vaccination uptake among adolescents. Cochrane Database Syst. Rev. 2020, 2020, CD011895. [Google Scholar] [CrossRef]
- Dempsey, A.F.; O’Leary, S.T. Human Papillomavirus Vaccination: Narrative Review of Studies on How Providers’ Vaccine Communication Affects Attitudes and Uptake. Acad. Pediatr. 2018, 18, S23–S27. [Google Scholar] [CrossRef]
- Krieger, J.L.; Katz, M.L.; Kam, J.A.; Roberto, A. Appalachian and Non-Appalachian Pediatricians’ Encouragement of the Human Papillomavirus Vaccine: Implications for Health Disparities. Women’s Health Issues 2012, 22, e19–e26. [Google Scholar] [CrossRef]
- Katz, M.L.; Reiter, P.L.; Heaner, S.; Ruffin, M.T.; Post, D.M.; Paskett, E.D. Acceptance of the HPV vaccine among women, parents, community leaders, and healthcare providers in Ohio Appalachia. Vaccine 2009, 27, 3945–3952. [Google Scholar] [CrossRef]
- Mills, L.A.; Vanderpool, R.C.; Crosby, R.A. Sexually Related Behaviors as Predictors of HPV Vaccination Among Young Rural Women. J. Women’s Health 2011, 20, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Boyd, E.D.; Phillips, J.M.; Schoenberger, Y.-M.M.; Simpson, T. Barriers and facilitators to HPV vaccination among rural Alabama adolescents and their caregivers. Vaccine 2018, 36, 4126–4133. [Google Scholar] [CrossRef]
- Bhatta, M.P.; Phillips, L. Human papillomavirus vaccine awareness, uptake, and parental and health care provider communication among 11- to 18-year-old adolescents in a rural Appalachian Ohio county in the United States. J. Rural Health 2015, 31, 67–75. [Google Scholar] [CrossRef]
- Crosby, R.A.; Casey, B.R.; Vanderpool, R.; Collins, T.; Moore, G.R. Uptake of free HPV vaccination among young women: A comparison of rural versus urban rates. J. Rural Health 2011, 27, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Vanderpool, R.C.; Casey, B.R.; Crosby, R.A. HPV-related risk perceptions and HPV vaccine uptake among a sample of young rural women. J. Community Health 2011, 36, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; Coronado, G.D.; Williams, R.S.; Glenn, B.; Escoffery, C.; Fernandez, M.; Tuff, R.A.; Wilson, K.M.; Mullen, P.D. A systematic review of measures used in studies of human papillomavirus (HPV) vaccine acceptability. Vaccine 2010, 28, 4027–4037. [Google Scholar] [CrossRef]
- Escoffery, C.; Petagna, C.; Agnone, C. A systematic review of interventions to promote HPV vaccination globally. BMC Public Health 2023, 23, 1262. [Google Scholar] [CrossRef]
- Company NOVAR. Administrative Supplements for NCI-Designated Cancer Centers to Investigate HPV Vaccine Uptake. A Summary Report Sciences DoCCaP. 2019. Available online: https://healthcaredelivery.cancer.gov/hpvuptake/CancCent_HPVsupp_finalRound2report.pdf (accessed on 11 December 2025).
- Paskett, E.D.; Young, G.S.; Bernardo, B.M.; Washington, C.; DeGraffinreid, C.R.; Fisher, J.L.; Huerta, T.R. The CITIES Project: Understanding the Health of Underrepresented Populations in Ohio. Cancer Epidemiol. Biomark. Prev. 2019, 28, 442–454. [Google Scholar] [CrossRef]
- Castañeda, S.F.; Rosenbaum, R.P.; Gonzalez, P.; Holscher, J.T. Breast and Cervical Cancer Screening Among Rural Midwestern Latina Migrant and Seasonal Farmworkers. J. Prim. Care Community Health 2012, 3, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Blake, K.D.; Croyle, R.T. Rurality, Rural Identity, and Cancer Control: Evidence from NCI’s Population Health Assessment in Cancer Center Catchment Areas Initiative. J. Rural Health 2019, 35, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Blake, K.D.; Ciolino, H.P.; Croyle, R.T. Population Health Assessment in NCI-Designated Cancer Center Catchment Areas. Cancer Epidemiol. Biomark. Prev. 2019, 28, 428–430. [Google Scholar] [CrossRef]
- Osaghae, I.; Chido-Amajuoyi, O.G.; Shete, S. Healthcare Provider Recommendations and Observed Changes in HPV Vaccination Acceptance during the COVID-19 Pandemic. Vaccines 2022, 10, 1515. [Google Scholar] [CrossRef]
- Chido-Amajuoyi, O.G.; Pande, M.; Agbajogu, C.; Yu, R.K.; Cunningham, S.; Shete, S. HPV Vaccination Uptake, Hesitancy, and Refusal: Observations of Health-Care Professionals During the COVID-19 Pandemic. JNCI Cancer Spectr. 2022, 6, pkac053. [Google Scholar] [CrossRef]
- Ryan, G.; Gilbert, P.A.; Ashida, S.; Charlton, M.E.; Scherer, A.; Askelson, N.M. Challenges to Adolescent HPV Vaccination and Implementation of Evidence-Based Interventions to Promote Vaccine Uptake During the COVID-19 Pandemic: “HPV Is Probably Not at the Top of Our List”. Prev. Chronic Dis. 2022, 19, 210378. [Google Scholar] [CrossRef] [PubMed]
- Brandt, H.M.; Vanderpool, R.C.; Pilar, M.; Zubizarreta, M.; Stradtman, L.R. A narrative review of HPV vaccination interventions in rural U.S. communities. Prev. Med. 2021, 145, 106407. [Google Scholar] [CrossRef]
- Bakare, D.; Gobbo, E.; Akinsola, K.O.; Bakare, A.A.; Salako, J.; Hanson, C.; Herzig van Wees, S.; Falade, A.; King, C. Healthcare worker practices for HPV vaccine recommendation: A systematic review and meta-analysis. Hum. Vaccines Immunother. 2024, 20, 2402122. [Google Scholar] [CrossRef]
- Camero, E. Broadband Connection in Rural Communities. ChangeLab Solutions Blog. 2023. Available online: https://www.changelabsolutions.org/blog/broadband-connection-rural-communities (accessed on 18 November 2025).
- Ejezie, C.L.; Choi, J.; Ayieko, S.; Burgoa, S.; Zerrouki, Y.; Lobaina, D.; Okwaraji, G.; Defeu, S.; Sacca, L. Digital Health Interventions for Cancer Prevention Among Racial and Ethnic Minority Groups in the United States: A Scoping Review. J. Racial Ethn. Health Disparities 2024, 12, 1251–1267. [Google Scholar] [CrossRef]
- Essa-Hadad, J.; Gorelik, Y.; Vervoort, J.; Jansen, D.; Edelstein, M. Understanding the health system barriers and enablers to childhood MMR and HPV vaccination among disadvantaged, minority or underserved populations in middle- and high-income countries: A systematic review. Eur. J. Public Health 2024, 34, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Zhetpisbayeva, I.; Kassymbekova, F.; Sarmuldayeva, S.; Semenova, Y.; Glushkova, N. Cervical Cancer Prevention in Rural Areas. Ann. Glob. Health 2023, 89, 75. [Google Scholar] [CrossRef]
- Rodriguez, S.A.; Mullen, P.D.; Lopez, D.M.; Savas, L.S.; Fernández, M.E. Factors associated with adolescent HPV vaccination in the U.S.: A systematic review of reviews and multilevel framework to inform intervention development. Prev. Med. 2020, 131, 105968. [Google Scholar] [CrossRef] [PubMed]
- Belbasis, L.; Bellou, V.; Ioannidis, J.P.A. Conducting umbrella reviews. BMJ Med. 2022, 1, e000071. [Google Scholar] [CrossRef]
- Pollock, D.; Tricco, A.C.; Peters, M.D.J.; Mclnerney, P.A.; Khalil, H.; Godfrey, C.M.; Alexander, L.A.; Munn, Z. Methodological quality, guidance, and tools in scoping reviews: A scoping review protocol. JBI Evid. Synth. 2022, 20, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- DistillerSR Inc. 2023. Available online: https://www.distillersr.com/ (accessed on 3 June 2023).
- Software Csr. Veritas Health Innovation. 2023. Available online: www.covidence.org (accessed on 15 June 2023).
- Van Der Mierden, S. Software tools for literature screening in systematic reviews in biomedical research. ALTEX 2019, 36, 508–517. [Google Scholar] [CrossRef]
- WHO. Ten Threats to Global Health in 2019. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 11 January 2026).
- Adjei Boakye, E.; Fedorovich, Y.; White, M.; Vohra, S.; Volle, M.; Osazuwa-Peters, N.; Gerend, M.A. Rural-Urban Disparities in HPV Vaccination Coverage Among Adolescents in the Central Part of the State of Illinois, USA. J. Community Health 2022, 48, 24–29. [Google Scholar] [CrossRef]
- Adjei Boakye, E.; McKinney, S.L.; Whittington, K.D.; Boyer, V.E.; Franca, M.C.; Lee, M.; McKinnies, R.C.; Collins, S.K.; Gerend, M.A. Association between Sexual Activity and Human Papillomavirus (HPV) Vaccine Initiation and Completion among College Students. Vaccines 2022, 10, 2079. [Google Scholar] [CrossRef]
- Askelson, N.; Ryan, G.; McRee, A.-L.; Farris, P.E.; Shannon, J.; Hanson, J.; Kenyon, D.B.; Daly, E.; Avdic, L. Using concept mapping to identify opportunities for HPV vaccination efforts: Perspectives from the Midwest and West Coast. Eval. Program Plan. 2021, 89, 102010. [Google Scholar] [CrossRef]
- Askelson, N.M.; Campo, S.; Lowe, J.B.; Smith, S.; Dennis, L.K.; Andsager, J. Using the Theory of Planned Behavior to Predict Mothers’ Intentions to Vaccinate Their Daughters Against HPV. J. Sch. Nurs. 2010, 26, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Askelson, N.M.; Campo, S.; Smith, S.; Lowe, J.B.; Dennis, L.; Andsager, J. Assessing physicians’ intentions to talk about sex when they vaccinate nine-year-old to 15-year-old girls against HPV. Sex Educ. 2011, 11, 431–441. [Google Scholar] [CrossRef]
- Askelson, N.M.; Ryan, G.; Seegmiller, L.; Pieper, F.; Kintigh, B.; Callaghan, D. Implementation Challenges and Opportunities Related to HPV Vaccination Quality Improvement in Primary Care Clinics in a Rural State. J. Community Health 2019, 44, 790–795. [Google Scholar] [CrossRef]
- Ayres, S.; Gee, A.; Kim, S. Human Papillomavirus Vaccination Knowledge, Barriers, and Recommendations Among Healthcare Provider Groups in the Western United States. J. Cancer Educ. 2022, 37, 1816–1823. [Google Scholar] [CrossRef]
- Beck, A.; Bianchi, A.; Showalter, D. Evidence-Based Practice Model to Increase Human Papillomavirus Vaccine Uptake: A Stepwise Approach. Nurs. Women’s Health 2021, 25, 430–436. [Google Scholar] [CrossRef]
- Bednarczyk, R.A.; Whitehead, J.L.; Stephenson, R. Moving beyond sex: Assessing the impact of gender identity on human papillomavirus vaccine recommendations and uptake among a national sample of rural-residing LGBT young adults. Papillomavirus Res. 2017, 3, 121–125. [Google Scholar] [CrossRef]
- Berenson, A.B.; Hirth, J.M.; Kuo, Y.-F.; Rupp, R.E. Quantitative and qualitative assessment of an all-inclusive postpartum human papillomavirus vaccination program. Am. J. Obstet. Gynecol. 2021, 224, 504.e1–504.e9. [Google Scholar] [CrossRef]
- Blake, K.D.; Ottenbacher, A.J.; Finney Rutten, L.J.; Grady, M.A.; Kobrin, S.C.; Jacobson, R.M.; Hesse, B.W. Predictors of Human Papillomavirus Awareness and Knowledge in 2013. Am. J. Prev. Med. 2015, 48, 402–410. [Google Scholar] [CrossRef]
- Boitano, T.K.L.; Daniel, C.; Kim, Y.; Straughn, J.M.; Peral, S.; Scarinci, I. Beyond words: Parental perceptions on human papilloma virus vaccination recommendations and its impact on uptake. Prev. Med. Rep. 2021, 24, 101596. [Google Scholar] [CrossRef] [PubMed]
- Boyce, T.G.; Christianson, B.; Hanson, K.E.; Dunn, D.; Polter, E.; VanWormer, J.J.; Williams, C.L.; Belongia, E.A.; McLean, H.Q. Factors associated with human papillomavirus and meningococcal vaccination among adolescents living in rural and urban areas. Vaccine X 2022, 11, 100180. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.P.; Rodriguez, N.M.; Head, K.J.; Zimet, G.D.; Kasting, M.L. Obstetrician/gynecologists’ HPV vaccination recommendations among women and girls 26 and younger. Prev. Med. Rep. 2022, 27, 101772. [Google Scholar] [CrossRef]
- Brewer, N.T.; Hall, M.E.; Malo, T.L.; Gilkey, M.B.; Quinn, B.; Lathren, C. Announcements Versus Conversations to Improve HPV Vaccination Coverage: A Randomized Trial. Pediatrics 2017, 139, e20161764. [Google Scholar] [CrossRef]
- Britt, R.; Britt, B.C. The need to develop health campaigns for obtaining the HPV vaccine in rural and medically-underserved college campuses. Educ. Health 2016, 34, 74–78. [Google Scholar]
- Britt, R.K.; Englebert, A.M. Behavioral determinants for vaccine acceptability among rurally located college students. Health Psychol. Behav. Med. 2018, 6, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Brumbaugh, J.T.; Sokoto, K.C.; Wright, C.D.; Francis, S.E.; Hubbard, J.; Alexander, L.; McNeil, D.W. Vaccination intention and uptake within the Black community in Appalachia. Health Psychol. 2023, 42, 557–566. [Google Scholar] [CrossRef]
- Carman, A.L.; McGladrey, M.L.; Goodman Hoover, A.; Crosby, R.A. Organizational Variation in Implementation of an Evidence-Based Human Papillomavirus Intervention. Am. J. Prev. Med. 2015, 49, 301–308. [Google Scholar] [CrossRef]
- Cataldi, J.R.; Brewer, S.E.; Perreira, C.; Furniss, A.; Nederveld, A.; Suresh, K.; Williams, C.; O’Leary, S.T.; Dempsey, A.F. Rural Adolescent Immunization: Delivery Practices and Barriers to Uptake. J. Am. Board Fam. Med. 2021, 34, 937–949. [Google Scholar] [CrossRef]
- Cates, J.R.; Ortiz, R.R.; North, S.; Martin, A.; Smith, R.; Coyne-Beasley, T. Partnering with middle school students to design text messages about HPV vaccination. Health Promot. Pract. 2015, 16, 244–255. [Google Scholar] [CrossRef]
- Cates, J.R.; Shafer, A.; Diehl, S.J.; Deal, A.M. Evaluating a County-Sponsored Social Marketing Campaign to Increase Mothers’ Initiation of HPV Vaccine for Their Preteen Daughters in a Primarily Rural Area. Soc. Mark. Q. 2011, 17, 4–26. [Google Scholar] [CrossRef]
- Chido-Amajuoyi, O.G.; Jackson, I.; Yu, R.; Shete, S. Declining awareness of HPV and HPV vaccine within the general US population. Hum. Vaccines Immunother. 2020, 17, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Cunningham-Erves, J.; Koyama, T.; Huang, Y.; Jones, J.; Wilkins, C.H.; Harnack, L.; McAfee, C.; Hull, P.C. Providers’ Perceptions of Parental Human Papillomavirus Vaccine Hesitancy: Cross-Sectional Study. JMIR Cancer 2019, 5, e13832. [Google Scholar] [CrossRef]
- Dang, J.H.T.; McClure, S.; Gori, A.C.T. Implementation and evaluation of a multilevel intervention to increase uptake of the human papillomavirus vaccine among rural adolescents. J. Rural Health 2023, 39, 136–141. [Google Scholar] [CrossRef]
- Daniel, C.L.; Lawson, F.; Vickers, M.; Green, C.; Wright, A.; Coyne-Beasley, T.; Lee, H.Y.; Turberville, S. Enrolling a rural community pharmacy as a Vaccines for Children provider to increase HPV vaccination: A feasibility study. BMC Public Health 2021, 21, 1266. [Google Scholar] [CrossRef]
- Fernandez-Pineda, M.; Cianelli, R.; Villegas, N.; Matsuda, Y.; Iriarte, E.; Fernandez, M.; Montano, N.P. Preferred HPV and HPV Vaccine Learning Methods to Guide Future HPV Prevention Interventions Among Rural Hispanics. J. Pediatr. Nurs. 2021, 60, 139–145. [Google Scholar] [CrossRef]
- Fish, L.J.; Harrison, S.E.; McDonald, J.-A.; Yelverton, V.; Williams, C.; Walter, E.B.; Vasudevan, L. Key stakeholder perspectives on challenges and opportunities for rural HPV vaccination in North and South Carolina. Hum. Vaccines Immunother. 2022, 18, 2058264. [Google Scholar] [CrossRef]
- Ford, M.; Cartmell, K.; Malek, A.; Le, P.; Keeve, C.; Sanders, I.; Ross, J.; Slan, M.; McLauren, J.; Platt, M.; et al. Evaluation of the First-Year Data from an HPV Vaccination Van Program in South Carolina. U.S. J. Clin. Med. 2023, 12, 1362. [Google Scholar] [CrossRef]
- Gilbert, P.A.; Lee, A.A.; Pass, L.; Lappin, L.; Thompson, L.; Sittig, K.W.; Baker, E.; Hoffman-Zinnel, D. Queer in the Heartland: Cancer Risks, Screenings, and Diagnoses among Sexual and Gender Minorities in Iowa. J. Homosex. 2020, 69, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Goessl, C.L.; Christianson, B.; Hanson, K.E.; Polter, E.J.; Olson, S.C.; Boyce, T.G.; Dunn, D.; Williams, C.L.; Belongia, E.A.; McLean, H.Q.; et al. Human papillomavirus vaccine beliefs and practice characteristics in rural and urban adolescent care providers. BMC Public Health 2022, 22, 1322. [Google Scholar] [CrossRef] [PubMed]
- Gunn, R.; Ferrara, L.K.; Dickinson, C.; Stock, I.; Griffith-Weprin, J.; Wiser, A.; Hatch, B.; Fagnan, L.J.; Carney, P.A.; Davis, M.M. Human Papillomavirus Immunization in Rural Primary Care. Am. J. Prev. Med. 2020, 59, 377–385. [Google Scholar] [CrossRef]
- Harris, K.L.; Tay, D.; Kaiser, D. The perspectives, barriers, and willingness of Utah dentists to engage in human papillomavirus (HPV) vaccine practices. Hum. Vaccines Immunother. 2020, 16, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Harry, M.L.; Asche, S.E.; Freitag, L.A.; Sperl-Hillen, J.M.; Saman, D.M.; Ekstrom, H.L.; Chrenka, E.A.; Truitt, A.R.; Allen, C.I.; O’Connor, P.J.; et al. Human Papillomavirus vaccination clinical decision support for young adults in an upper midwestern healthcare system: A clinic cluster-randomized control trial. Hum. Vaccines Immunother. 2022, 18, 2040933. [Google Scholar] [CrossRef] [PubMed]
- Hatch, B.A.; Valenzuela, S.; Darden, P.M. Clinic-level differences in human papillomavirus vaccination rates among rural and urban Oregon primary care clinics. J. Rural Health 2023, 39, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Henry, K.A.; Swiecki-Sikora, A.L.; Stroup, A.M.; Warner, E.L.; Kepka, D. Area-based socioeconomic factors and Human Papillomavirus (HPV) vaccination among teen boys in the United States. BMC Public Health 2017, 18, 19. [Google Scholar] [CrossRef]
- Jafari, S.D.G.; Appel, S.J.; Shorter, D.G. Risk Reduction Interventions for Human Papillomavirus in Rural Maryland. J. Dr. Nurs. Pract. 2020, 13, 134–141. [Google Scholar] [CrossRef]
- Kepka, D.; Christini, K.; McGough, E.; Wagner, A.; Del Fiol, G.; Gibson, B.; Ayres, S.; Brandt, H.M.; Mann, S.; Petrik, A.F.; et al. Successful Multi-Level HPV Vaccination Intervention at a Rural Healthcare Center in the Era of COVID-19. Front. Digit. Health 2021, 3, 719138. [Google Scholar] [CrossRef]
- Kepka, D.; Coronado, G.D.; Rodriguez, H.P.; Thompson, B. Evaluation of a Radionovela to Promote HPV Vaccine Awareness and Knowledge Among Hispanic Parents. J. Community Health 2011, 36, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Kepka, D.L.; Ulrich, A.K.; Coronado, G.D. Low Knowledge of the Three-Dose HPV Vaccine Series among Mothers of Rural Hispanic Adolescents. J. Health Care Poor Underserved 2012, 23, 626–635. [Google Scholar] [CrossRef]
- Kim, S.; Zhou, K.; Parker, S.; Kline, K.N.; Montealegre, J.R.; McGee, L.U. Perceived Barriers and Use of Evidence-Based Practices for Adolescent HPV Vaccination among East Texas Providers. Vaccines 2023, 11, 728. [Google Scholar] [CrossRef]
- Koskan, A.M.; Dominick, L.N.; Helitzer, D.L. Rural Caregivers’ Willingness for Community Pharmacists to Administer the HPV Vaccine to Their Age-Eligible Children. J. Cancer Educ. 2021, 36, 189–198. [Google Scholar] [CrossRef]
- Kurani, S.; MacLaughlin, K.L.; Jacobson, R.M.; Sauver, J.L.; Jenkins, G.D.; Fan, C.; Jacobson, D.J.; Inselman, J.; Zhu, X.; Griffin, J.M.; et al. Socioeconomic disadvantage and human papillomavirus (HPV) vaccination uptake. Vaccine 2022, 40, 471–476. [Google Scholar] [CrossRef]
- Lee, H.Y.; Luo, Y.; Won, C.R.; Daniel, C.; Coyne-Beasley, T. HPV and HPV Vaccine Awareness Among African Americans in the Black Belt Region of Alabama. J. Racial Ethn. Health Disparities 2023, 11, 808–814. [Google Scholar] [CrossRef]
- Manganello, J.A.; Chiang, S.C.; Cowlin, H.; Kearney, M.D.; Massey, P.M. HPV and COVID-19 vaccines: Social media use, confidence, and intentions among parents living in different community types in the United States. J. Behav. Med. 2023, 46, 212–228. [Google Scholar] [CrossRef]
- McMann, N.; Trout, K.E. Assessing the Knowledge, Attitudes, and Practices Regarding Sexually Transmitted Infections Among College Students in a Rural Midwest Setting. J. Community Health 2021, 46, 117–126. [Google Scholar] [CrossRef]
- Mohammed, K.A.; Subramaniam, D.S.; Geneus, C.J.; Henderson, E.R.; Dean, C.A.; Subramaniam, D.P.; Burroughs, T.E. Rural-urban differences in human papillomavirus knowledge and awareness among US adults. Prev. Med. 2018, 109, 39–43. [Google Scholar] [CrossRef]
- Morales-Campos, D.Y.; McDaniel, M.D.; Amaro, G.; Flores, B.E.; Parra-Medina, D. Factors Associated with HPV Vaccine Adherence among Latino/a Adolescents in a Rural, Texas-Mexico Border County. Ethn. Dis. 2022, 32, 275–284. [Google Scholar] [CrossRef]
- Moss, J.L.; Gilkey, M.B.; Reiter, P.L.; Brewer, N.T. Trends in HPV Vaccine Initiation among Adolescent Females in North Carolina, 2008–2010. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.L.; Gilkey, M.B.; Rimer, B.K.; Brewer, N.T. Disparities in collaborative patient-provider communication about human papillomavirus (HPV) vaccination. Hum. Vaccines Immunother. 2016, 12, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- Newcomer, S.R.; Caringi, J.; Jones, B.; Coyle, E.; Schehl, T.; Daley, M.F. A Mixed-Methods Analysis of Barriers to and Facilitators of Human Papillomavirus Vaccination Among Adolescents in Montana. Public Health Rep. 2020, 135, 842–850. [Google Scholar] [CrossRef]
- Newcomer, S.R.; Freeman, R.E.; Albers, A.N.; Murgel, S.; Thaker, J.; Rechlin, A.; Wehner, B.K. Missed opportunities for human papillomavirus vaccine series initiation in a large, rural U.S. state. Hum. Vaccines Immunother. 2022, 18, 2016304. [Google Scholar] [CrossRef]
- Nguyen, C.G.; Pogemiller, M.I.; Cooper, M.T.; Garbe, M.C.; Darden, P.M. Characteristics of Oklahoma Pediatricians Who Dismiss Families for Refusing Vaccines. Clin. Pediatr. 2023, 62, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Osaghae, I.; Darkoh, C.; Chido-Amajuoyi, O.G.; Chan, W.; Padgett Wermuth, P.; Pande, M.; Cunningham, S.A.; Shete, S. Healthcare Provider’s Perceived Self-Efficacy in HPV Vaccination Hesitancy Counseling and HPV Vaccination Acceptance. Vaccines 2023, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Osaghae, I.; Darkoh, C.; Chido-Amajuoyi, O.G.; Chan, W.; Wermuth, P.P.; Pande, M.; Cunningham, S.A.; Shete, S. Association of provider HPV vaccination training with provider assessment of HPV vaccination status and recommendation of HPV vaccination. Hum. Vaccines Immunother. 2022, 18, 2132755. [Google Scholar] [CrossRef] [PubMed]
- Osegueda, E.R.; Chi, X.; Hall, J.M.; Vadaparampil, S.T.; Christy, S.M.; Staras, S.A.S. County-Level Factors Associated With HPV Vaccine Coverage Among 11-Year-Olds to 12-Year-Olds Living in Florida in 2019. J. Adolesc. Health 2023, 72, 130–137. [Google Scholar] [CrossRef]
- Panagides, R.; Voges, N.; Oliver, J.; Bridwell, D.; Mitchell, E. Determining the Impact of a Community-Based Intervention on Knowledge Gained and Attitudes Towards the HPV Vaccine in Virginia. J. Cancer Educ. 2023, 38, 646–651. [Google Scholar] [CrossRef]
- Paskett, E.D.; Krok-Schoen, J.L.; Pennell, M.L.; Tatum, C.M.; Reiter, P.L.; Peng, J.; Bernardo, B.M.; Weier, R.C.; Richardson, M.S.; Katz, M.L. Results of a Multilevel Intervention Trial to Increase Human Papillomavirus (HPV) Vaccine Uptake among Adolescent Girls. Cancer Epidemiol. Biomark. Prev. 2016, 25, 593–602. [Google Scholar] [CrossRef]
- Pham, D.; Shukla, A.; Welch, K.; Villa, A. Assessing knowledge of human papillomavirus among men who have sex with men (MSM) using targeted dating applications. Vaccine 2022, 40, 5376–5383. [Google Scholar] [CrossRef]
- Pourebrahim, N.; Shah, P.; VoPham, T.; Doody, D.R.; Bell, T.R.; deHart, M.P.; Madeleine, M.M. Time and geographic variations in human papillomavirus vaccine uptake in Washington state. Prev. Med. 2021, 153, 106753. [Google Scholar] [CrossRef]
- Pruitt, S.L.; Tiro, J.A.; Kepka, D.; Henry, K. Missed Vaccination Opportunities Among U.S. Adolescents by Area Characteristics. Am. J. Prev. Med. 2022, 62, 538–547. [Google Scholar] [CrossRef]
- Rabarison, K.M.; Bish, C.L.; Massoudi, M.S.; Giles, W.H. Economic Evaluation Enhances Public Health Decision Making. Front. Public Health 2015, 3, 164. [Google Scholar] [CrossRef]
- Ramsay, J.M.; Kaddas, H.K.; Ou, J.Y.; Kepka, D.; Kirchhoff, A.C. Missed opportunities for concomitant HPV vaccination among childhood cancer survivors. Cancer Med. 2022, 11, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Robison, S.G. The Impact of the Number of Injections per Visit on the Likelihood of Human Papillomavirus Immunization. J. Pediatr. X 2020, 3, 100024. [Google Scholar] [CrossRef]
- Rodriguez, A.M.; Do, T.Q.N.; Chen, L.; Schmeler, K.M.; Montealegre, J.R.; Kuo, Y.-F. Human papillomavirus vaccinations at recommended ages: How a middle school-based educational and vaccination program increased uptake in the Rio Grande Valley. Hum. Vaccines Immunother. 2022, 18, 2133315. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.M.; Do, T.Q.N.; Eyada, M.F.; Chen, L.; Schmeler, K.M.; Montealegre, J.R. Human Papillomavirus Vaccination Uptake in the Rio Grande Valley: Results from a Pilot Community-Based Educational and School-Based Vaccination Program and Its Expansion. Vaccines 2023, 11, 329. [Google Scholar] [CrossRef]
- Rosen, B.L.; DiClemente, R.; Shepard, A.L.; Wilson, K.L.; Fehr, S.K. Factors associated with school nurses’ HPV vaccine attitudes for school-aged youth. Psychol. Health Med. 2017, 22, 535–545. [Google Scholar] [CrossRef]
- Ryan, G.; Ashida, S.; Gilbert, P.A.; Scherer, A.; Charlton, M.E.; Kahl, A.; Askelson, N. The Use of Medical Claims Data for Identifying Missed Opportunities for HPV Immunization Among Privately Insured Adolescents in the State of Iowa. J. Community Health 2022, 47, 783–789. [Google Scholar] [CrossRef]
- Ryan, G.; Daly, E.; Askelson, N.; Pieper, F.; Seegmiller, L.; Allred, T. Exploring Opportunities to Leverage Pharmacists in Rural Areas to Promote Administration of Human Papillomavirus Vaccine. Prev. Chronic Dis. 2020, 17, E23. [Google Scholar] [CrossRef]
- Ryan, G.W.; Perry, S.S.; Scherer, A.; Charlton, M.E.; Ashida, S.; Gilbert, P.A.; Askelson, N. Factors contributing to missed opportunities for human papillomavirus vaccination among adolescents, ages 11 to 13, in Iowa. Vaccine X 2022, 11, 100192. [Google Scholar] [CrossRef] [PubMed]
- Schrote, K.; Hersh, A.; Bruegl, A.; Rodriguez, M.I. Women’s perspectives on receiving and expanding access to essential health services in pharmacies in the United States. J. Am. Pharm. Assoc. 2022, 62, 711–716.e3. [Google Scholar] [CrossRef]
- Shah, S.F.A.; Ginossar, T.; Bentley, J.M.; Zimet, G.; McGrail, J.P. Using the Theory of Planned behavior to identify correlates of HPV vaccination uptake among college students attending a rural university in Alabama. Vaccine 2021, 39, 7421–7428. [Google Scholar] [CrossRef] [PubMed]
- Shato, T.; Humble, S.; Anandarajah, A.; Barnette, A.; Brandt, H.M.; Garbutt, J.; Klesges, L.; Thompson, V.S.; Silver, M.I. Influences of sociodemographic characteristics and parental HPV vaccination hesitancy on HPV vaccination coverage in five US states. Vaccine 2023, 41, 3772–3781. [Google Scholar] [CrossRef]
- Song, S.; White, A.; Kucik, J.E. Use of Selected Recommended Clinical Preventive Services—Behavioral Risk Factor Surveillance System, United States, 2018. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 461–466. [Google Scholar] [CrossRef]
- Stewart, T.; Lee, Y.A.; Damiano, E.A. Do Transgender and Gender Diverse Individuals Receive Adequate Gynecologic Care? An Analysis of a Rural Academic Center. Transgender Health 2020, 5, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Swiecki-Sikora, A.L.; Henry, K.A.; Kepka, D. HPV Vaccination Coverage Among US Teens Across the Rural-Urban Continuum. J. Rural Health 2019, 35, 506–517. [Google Scholar] [CrossRef]
- Teferra, A.A.; Keller-Hamilton, B.; Roberts, M.E.; Reiter, P.L. HPV Vaccine Coverage Among Adolescent Males in Ohio: Results of a Longitudinal Study. Ohio J. Public Health 2019, 2, 15–23. [Google Scholar] [CrossRef]
- Thaker, J.; Albers, A.N.; Newcomer, S.R. Nurses’ perceptions, experiences, and practices regarding human papillomavirus vaccination: Results from a cross-sectional survey in Montana. BMC Nurs. 2023, 22, 211. [Google Scholar] [CrossRef]
- Thomaier, L.; Aase, D.A.; Vogel, R.I.; Parsons, H.M.; Sadak, K.T.; Teoh, D. HPV vaccination coverage for pediatric, adolescent and young adult patients receiving care in a childhood cancer survivor program. Prev. Med. Rep. 2022, 29, 101972. [Google Scholar] [CrossRef]
- Thomas, T.L.; Caldera, M.; Maurer, J. A short report: Parents HPV vaccine knowledge in rural South Florida. Hum. Vaccines Immunother. 2019, 15, 1666–1671. [Google Scholar] [CrossRef]
- Thomas, T.L.; Strickland, O.; Diclemente, R.; Higgins, M. An Opportunity for Cancer Prevention During Preadolescence and Adolescence: Stopping Human Papillomavirus (HPV)-Related Cancer Through HPV Vaccination. J. Adolesc. Health 2013, 52, S60–S68. [Google Scholar] [CrossRef]
- Thomas, T.L.; Strickland, O.L.; DiClemente, R.; Higgins, M.; Haber, M. Rural African American Parents’ Knowledge and Decisions About Human Papillomavirus Vaccination. J. Nurs. Scholarsh. 2012, 44, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Vamos, C.A.; Kline, N.; Vázquez-Otero, C. Stakeholders’ perspectives on system-level barriers to and facilitators of HPV vaccination among Hispanic migrant farmworkers. Ethn. Health 2022, 27, 1442–1464. [Google Scholar] [CrossRef] [PubMed]
- Vanderpool, R.C.; Cohen, E.; Crosby, R.A.; Jones, M.G.; Bates, W.; Casey, B.R.; Collins, T. “1-2-3 Pap” Intervention Improves HPV Vaccine Series Completion among Appalachian Women. J. Commun. 2013, 63, 95–115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vielot, N.A.; Lane, R.M.; Loefstedt, K.; Cunningham, J.L.; Everson, J.; Tiller, E.; Johnson Patel, S.E.; Smith, J.S. Acceptability and readiness to promote human papillomavirus vaccination at ages 9–10 years: A feasibility study among North Carolina clinics. Pilot Feasibility Stud. 2023, 9, 153. [Google Scholar] [CrossRef]
- Vielot, N.A.; Butler, A.M.; Brookhart, M.A.; Becker-Dreps, S.; Smith, J.S. Patterns of Use of Human Papillomavirus and Other Adolescent Vaccines in the United States. J. Adolesc. Health 2017, 61, 281–287. [Google Scholar] [CrossRef]
- Walker, T.Y.; Elam-Evans, L.D.; Williams, C.L.; Fredua, B.; Yankey, D.; Markowitz, L.E.; Stokley, S. Trends in human papillomavirus (HPV) vaccination initiation among adolescents aged 13–17 by metropolitan statistical area (MSA) status, National Immunization Survey—Teen, 2013–2017. Hum. Vaccines Immunother. 2019, 16, 554–561. [Google Scholar] [CrossRef]
- Walker, T.Y.; Elam-Evans, L.D.; Yankey, D.; Markowitz, L.E.; Williams, C.L.; Fredua, B.; Singleton, J.A.; Stokley, S. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years—United States, 2018. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 718–723. [Google Scholar] [CrossRef]
- Warner, E.L.; Fowler, B.; Martel, L.; Kepka, D. Improving HPV Vaccination Through a Diverse Multi-state Coalition. J. Community Health 2017, 42, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Warren, B.R.; Gillette-Walch, H.; Adler, J.; Arias, R.; Klausner, J.D.; Ashing, K.T.; Villa, A. Assessment of human papillomavirus vaccination rates of adolescents in California, 2018–2019. Prev. Med. Rep. 2023, 32, 102144. [Google Scholar] [CrossRef]
- Wheeler, D.C.; Miller, C.A.; Do, E.K.; Ksinan, A.J.; Trogdon, J.G.; Chukmaitov, A.; Fuemmeler, B.F. Identifying Area-Level Disparities in Human Papillomavirus Vaccination Coverage Using Geospatial Analysis. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Wick, J.A.; Elswick, B.M. Impact of Pharmacist Delivered Education on Early Parent Awareness and Perceptions Regarding Human Papillomavirus (HPV) Vaccination in the Community Pharmacy Setting in West Virginia. Innov. Pharm. 2018, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.L.; Walker, T.Y.; Elam-Evans, L.D. Factors associated with not receiving HPV vaccine among adolescents by metropolitan statistical area status, United States, National Immunization Survey-Teen, 2016–2017. Hum. Vaccines Immunother. 2020, 16, 562–572. [Google Scholar] [CrossRef]
- Yoost, J.L.; Starcher, R.W.; King-Mallory, R.A.; Hussain, N.; Hensley, C.A.; Gress, T.W. The Use of Telehealth to Teach Reproductive Health to Female Rural High School Students. J. Pediatr. Adolesc. Gynecol. 2017, 30, 193–198. [Google Scholar] [CrossRef]
- Zahnd, W.E.; Harrison, S.E.; Stephens, H.C.; Messersmith, A.R.; Brandt, H.M.; Hastings, T.J.; Eberth, J.M. Expanding access to HPV vaccination in South Carolina through community pharmacies: A geospatial analysis. J. Am. Pharm. Assoc. 2020, 60, e153–e157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xue, H.; Calabrese, C.; Chen, H.; Dang, J.H.T. Understanding Human Papillomavirus Vaccine Promotions and Hesitancy in Northern California Through Examining Public Facebook Pages and Groups. Front. Digit. Health 2021, 3, 683090. [Google Scholar] [CrossRef] [PubMed]
- Zoellner, J.M.; Porter, K.J.; Brock, D.-J.P.; Mitchell, E.M.K.; Chapman, H.; Clarkston, D.; Cohn, W.; Hauser, L.; Morris, D.W.; Ramey, S.Y.; et al. Advancing engagement and capacity for rural cancer control: A mixed-methods case study of a Community-Academic Advisory Board in the Appalachia region of Southwest Virginia. Res. Involv. Engagem. 2021, 7, 44. [Google Scholar] [CrossRef]
- United States Census Bureau. American Community Survey 5-Year Data. 2009. Available online: https://www.census.gov/data/developers/data-sets/acs-5year.2015.html (accessed on 12 January 2023).
- Prevention CfDCa. About the Vaccines for Children (VFC) Program. Available online: https://www.cdc.gov/vaccines-for-children/about/index.html (accessed on 11 December 2025).
- Ajzen, I. From Intentions to Actions: A Theory of Planned Behavior. In Action Control; Springer Nature: Berlin/Heidelberg, Germany, 1985; pp. 11–39. [Google Scholar] [CrossRef]
- Rosenstock, I.M. The health belief model: Explaining health behavior through expectancies. In Health Behavior and Health Education: Theory, Research, and Practice; Jossey-Bass/Wiley: San Francisco, CA, USA, 1990; pp. 39–62. [Google Scholar]
- Stokols, D. Establishing and maintaining healthy environments: Toward a social ecology of health promotion. Am. Psychol. 1992, 47, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.E.; Clifasefi, S.L.; Stanton, J.; The Leap Advisory Board; Straits, K.J.E.; Gil-Kashiwabara, E.; Rodriguez Espinosa, P.; Nicasio, A.V.; Andrasik, M.P.; Hawes, S.M.; et al. Community-based participatory research (CBPR): Towards equitable involvement of community in psychology research. Am. Psychol. 2018, 73, 884–898. [Google Scholar] [CrossRef]
- Aday, L.A.; Andersen, R. A framework for the study of access to medical care. Health Serv. Res. 1974, 9, 208–220. [Google Scholar]
- Charles, C.; Gafni, A.; Whelan, T. Decision-making in the physician–patient encounter: Revisiting the shared treatment decision-making model. Soc. Sci. Med. 1999, 49, 651–661. [Google Scholar] [CrossRef]
- Jaén, C.R.; Stange, K.C.; Nutting, P.A. Competing demands of primary care: A model for the delivery of clinical preventive services. J. Fam. Pract. 1994, 38, 166–171. [Google Scholar]
- Lefevre, P.; Kolsteren, P.; Wael, M.; Byekwaso, F.; Beghin, I. Comprehensive Participatory Planning and Evaluation Antwerp; Belgian Survival Fund for the Third World Joint Programme: Brussels, Belgium, 2000. [Google Scholar]
- Damschroder, L.J.; Aron, D.C.; Keith, R.E.; Kirsh, S.R.; Alexander, J.A.; Lowery, J.C. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implement. Sci. 2009, 4, 50. [Google Scholar] [CrossRef]
- Bronfenbrenner, U. Toward an experimental ecology of human development. Am. Psychol. 1977, 32, 513–531. [Google Scholar] [CrossRef]
- Kane, M.; Trochim, W.M. Applied Social Research Methods: Concept Mapping for Planning and Evaluation; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2007. [Google Scholar] [CrossRef]
- Bradley, E.H.; Curry, L.A.; Ramanadhan, S.; Rowe, L.; Nembhard, I.M.; Krumholz, H.M. Research in action: Using positive deviance to improve quality of health care. Implement. Sci. 2009, 4, 25. [Google Scholar] [CrossRef]
- McLean, H.Q.; VanWormer, J.J.; Chow, B.D.W.; Birchmeier, B.; Vickers, E.; DeVries, E.; Meyer, J.; Moore, J.; McNeil, M.M.; Stokley, S.; et al. Improving Human Papillomavirus Vaccine Use in an Integrated Health System: Impact of a Provider and Staff Intervention. J. Adolesc. Health 2017, 61, 252–258. [Google Scholar] [CrossRef]
- Brandt, H.M.; Footman, A.; Adsul, P.; Ramanadhan, S.; Kepka, D. Implementing interventions to start HPV vaccination at age 9: Using the evidence we have. Hum. Vaccines Immunother. 2023, 19, 2180250. [Google Scholar] [CrossRef]
- Biancarelli, D.L.; Drainoni, M.-L.; Perkins, R.B. Provider Experience Recommending HPV Vaccination Before Age 11 Years. J. Pediatr. 2020, 217, 92–97. [Google Scholar] [CrossRef]
- Perkins, R.B.; Humiston, S.; Oliver, K. Evidence supporting the initiation of HPV vaccination starting at age 9: Collection overview. Hum. Vaccines Immunother. 2023, 19, 2269026. [Google Scholar] [CrossRef]
- Velan, B.; Yadgar, Y. On the implications of desexualizing vaccines against sexually transmitted diseases: Health policy challenges in a multicultural society. Isr. J. Health Policy Res. 2017, 6, 30. [Google Scholar] [CrossRef]
- Harper, D.M.; Rego, R.; Tariq, M.; Patel, M.R.; Resnicow, K.; Sheinfeld Gorin, S. HPV vaccination initiation among white, black and Middle East North African (MENA) males. Prev. Med. Rep. 2022, 30, 102029. [Google Scholar] [CrossRef] [PubMed]
- Pingali, C.; Yankey, D.; Elam-Evans, L.D.; Markowitz, L.E.; Williams, C.L.; Fredua, B.; McNamara, L.A.; Stokley, S.; Singleton, J.A. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years—United States, 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1183–1190. [Google Scholar] [CrossRef]
- Boersma, P.; Black, L.I. Human Papillomavirus Vaccination Among Adults Aged 18–26, 2013–2018. NCHS Data Brief 2020, 354, 1–8. [Google Scholar]
- Adjei Boakye, E.; Lew, D.; Muthukrishnan, M.; Tobo, B.B.; Rohde, R.L.; Varvares, M.A.; Osazuwa-Peters, N. Correlates of human papillomavirus (HPV) vaccination initiation and completion among 18–26 year olds in the United States. Hum. Vaccines Immunother. 2018, 14, 2016–2024. [Google Scholar] [CrossRef] [PubMed]
- Ramphul, R.; Zamorano, A.S.; Upadhyay, S.; Desai, M.; Bauer, C. Spatiotemporal analysis of HPV vaccination and associated neighborhood-level disparities in Texas—An ecological study. Front. Public Health 2024, 12, 1418526. [Google Scholar] [CrossRef]
- Gilkey, M.B.; Grabert, B.K.; Heisler-MacKinnon, J.; Bjork, A.; Boynton, M.H.; Kim, K.; Alton Dailey, S.; Liu, A.; Todd, K.G.; Schauer, S.L.; et al. Coaching and Communication Training for HPV Vaccination: A Cluster Randomized Trial. Pediatrics 2022, 150, e2021052351. [Google Scholar] [CrossRef]
- Odebunmi, O.O.; Spees, L.P.; Biddell, C.B.; Yemeke, T.; Yanguela, J.; Higgins, C.; Gilkey, M.B.; Ozawa, S.; Wheeler, S.B. Benefits, challenges, and strategies related to using presumptive recommendations for HPV vaccination: A qualitative study with rural and non-rural-serving primary care professionals. Hum. Vaccines Immunother. 2024, 20, 2347018. [Google Scholar] [CrossRef]
- Brewer, N.T.; Chapman, G.B.; Rothman, A.J.; Leask, J.; Kempe, A. Increasing Vaccination: Putting Psychological Science Into Action. Psychol. Sci. Public Interest 2017, 18, 149–207. [Google Scholar] [CrossRef]
- Gorin, S.S.; Hirko, K. Primary Prevention of Cancer: A Multilevel Approach to Behavioral Risk Factor Reduction in Racially and Ethnically Minoritized Groups. Cancer J. 2023, 29, 354–361. [Google Scholar] [CrossRef]
- Gorin, S.S.; Badr, H.; Krebs, P.; Das, I.P. Multilevel Interventions and Racial/Ethnic Health Disparities. JNCI Monogr. 2012, 2012, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Newmann, S.J.; Garner, E.O. Social inequities along the cervical cancer continuum: A structured review. Cancer Causes Control 2005, 16, 63–70. [Google Scholar] [CrossRef]
- Dempsey, A.F. On the implications of desexualizing vaccines against sexually transmitted diseases: Reflections from a practicing pediatrician. Isr. J. Health Policy Res. 2017, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Martínez, D.; Díaz, L.; Maldonado, S. Nudging Parents with Tailored Informational SMS Can Increase HPV Vaccinations. 2021. Available online: https://behavioral.iadb.org/en/our-projects/nudging-parents-tailored-informational-sms-can-increase-hpv-vaccinations (accessed on 11 December 2025).
- McGlone, M.S.; Stephens, K.K.; Rodriguez, S.A.; Fernandez, M.E. Persuasive texts for prompting action: Agency assignment in HPV vaccination reminders. Vaccine 2017, 35, 4295–4297. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid. Synth. 2020, 18, 2119–2126. [Google Scholar] [CrossRef]
- Haider, A.; Roque, L. New Poverty and Food Insecurity Data Illustrate Persistent Racial Inequities. Available online: https://www.americanprogress.org/article/new-poverty-food-insecurity-data-illustrate-persistent-racial-inequities/ (accessed on 13 December 2025).
- New York State Department of Health. HPV Provider Education Project September 2018–August 2019. Available online: https://www.health.ny.gov/statistics/cancer/docs/hpv_provider_edu_report-2019.pdf (accessed on 9 November 2025).
- Knox, L.; Brach, C. The Practice Facilitation Handbook: Training Modules for New Facilitators and Their Trainers; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2013.
- Leeman, J.; Petermann, V.; Heisler-MacKinnon, J.; Bjork, A.; Brewer, N.T.; Grabert, B.K.; Gilkey, M.B. Quality Improvement Coaching for Human Papillomavirus Vaccination Coverage: A Process Evaluation in 3 States, 2018–2019. Prev. Chronic Dis. 2020, 17, E120. [Google Scholar] [CrossRef]
- Sheinfeld Gorin, S.N.; Glenn, B.A.; Perkins, R.B. The human papillomavirus (HPV) vaccine and cervical cancer: Uptake and next steps. Adv. Ther. 2011, 28, 615–639. [Google Scholar] [CrossRef]
- Boone-Heinonen, J.; Evenson, K.R.; Song, Y.; Gordon-Larsen, P. Built and socioeconomic environments: Patterning and associations with physical activity in U.S. adolescents. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Larsen, P.; Nelson, M.C.; Page, P.; Popkin, B.M. Inequality in the Built Environment Underlies Key Health Disparities in Physical Activity and Obesity. Pediatrics 2006, 117, 417–424. [Google Scholar] [CrossRef]
- Hawes, A.M.; Smith, G.S.; McGinty, E.; Bell, C.; Bower, K.; LaVeist, T.A.; Gaskin, D.J.; Thorpe, R.J. Disentangling Race, Poverty, and Place in Disparities in Physical Activity. Int. J. Environ. Res. Public Health 2019, 16, 1193. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.; Wong, C.A.; Perrin, E.; Page, S.; Sibley, L.; Skinner, A. Association of Physical Activity With Income, Race/Ethnicity, and Sex Among Adolescents and Young Adults in the United States. JAMA Pediatr. 2018, 172, 732. [Google Scholar] [CrossRef]
- CDC. Vaccines for Children (VFC) Program. Available online: https://www.cdc.gov/vaccines/programs/vfc/index.html (accessed on 13 November 2025).
- Sallis, J.F.; Floyd, M.F.; Rodríguez, D.A.; Saelens, B.E. Role of Built Environments in Physical Activity, Obesity, and Cardiovascular Disease. Circulation 2012, 125, 729–737. [Google Scholar] [CrossRef]
- Islami, F.; Goding Sauer, A.; Gapstur, S.M.; Jemal, A. Proportion of Cancer Cases Attributable to Excess Body Weight by US State, 2011–2015. JAMA Oncol. 2019, 5, 384. [Google Scholar] [CrossRef] [PubMed]
- Bramer, W.M.; Giustini, D.; de Jonge, G.B.; Holland, L.; Bekhuis, T. De-duplication of database search results for systematic reviews in EndNote. J. Med. Libr. Assoc. 2016, 104, 240–243. [Google Scholar] [CrossRef]
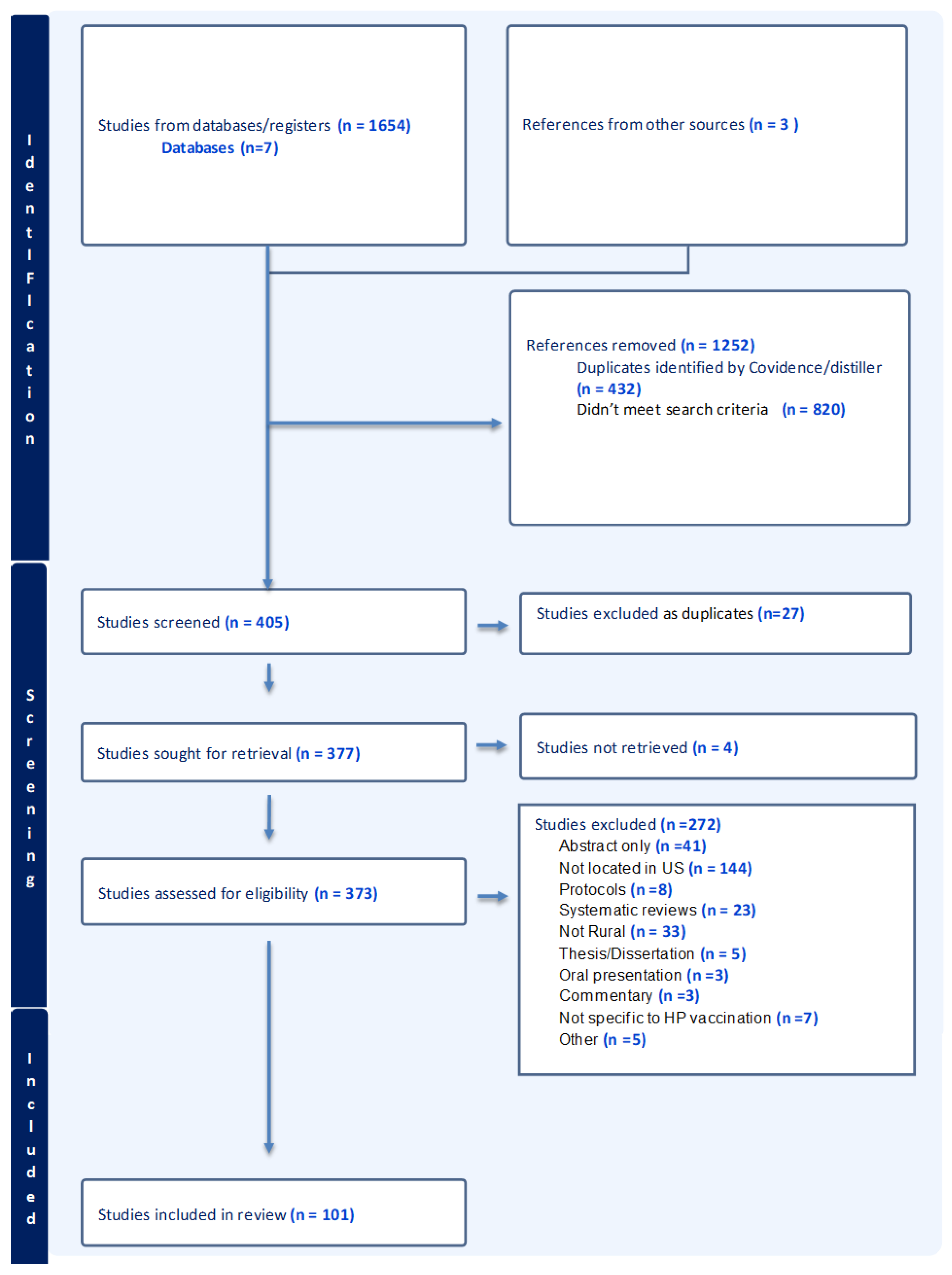
| Percentage/(Number) of Studies (N = 101) | References | |
|---|---|---|
| Study Type | ||
| Cross-sectional Survey | 45.5 (46) | [26,36,53,55,56,57,58,60,62,63,64,65,67,68,69,71,72,74,75,81,84,86,91,92,95,96,97,98,99,101,104,105,106,107,110,117,118,122,123,124,125,129,132,133,139] |
| Secondary analysis | 10.9 (11) | [87,100,103,112,127,128,138,141,142,144,146] |
| Cohort study | 6.9 (7) | [94,114,115,119,121,130,145] |
| Controlled trial | 6.9 (7) | [59,66,76,85,90,109,135] |
| Mixed methods | 6.9 (7) | [61,83,88,102,140,148] |
| Quasi-experimental | 6.9 (7) | [27,70,73,89,108,116,143] |
| Qualitative study | 5.9 (6) | [38,78,79,93,120,134] |
| Observational | 4 (4) | [54,80,137,147] |
| Pilot/Feasibility study | 3 (3) | [77,131,136] |
| Retrospective chart review | 2 (2) | [52,126] |
| Other | 2 (2) | [111,113] |
| Participant Types * | ||
| Healthcare providers | 14.9 (15) | [36,56,58,65,71,73,75,82,93,104,105,106,109,129,136] |
| Children and adolescents (17 and under) | 28.7 (29) | [52,72,76,80,87,88,89,94,102,103,107,111,112,114,115,116,117,119,121,127,128,130,137,138,139,141,142,144,145] |
| Adults (18–26) | 25.7 (26) | [27,53,60,62,67,68,69,74,80,85,89,95,97,98,108,110,113,122,123,124,125,126,130,135,140,147] |
| Parents of age-eligible children and youth | 18.8 (19) | [26,55,59,63,64,73,78,89,90,91,96,99,100,101,109,131,132,133,143] |
| Clinics | 5 (5) | [57,66,83,86,92] |
| Other | 11.9 (12) | [38,54,61,70,77,79,84,118,120,134,146,148] |
| Year of publication | ||
| Median year of publication 2021 | ||
| Modal publication year 2022 | ||
| Sample size | ||
| <50 | 14.9 (15) | [38,59,70,72,77,78,79,83,85,92,93,120,134,136,143] |
| 50–100 | 5.9 (6) | [58,63,90,91,145,148] |
| 101–500 | 32.7 (33) | [54,55,56,57,65,67,68,69,71,73,75,76,82,84,88,89,95,96,97,102,104,108,109,113,118,123,126,129,131,133,135,140,147] |
| 501–1000 | 10.9 (11) | [27,53,60,64,80,81,84,122,124,130,132] |
| 1001–5000 | 12.9 (13) | [26,36,62,99,100,101,105,106,110,114,116,128,146] |
| 5001–10,000 | 2 (2) | [52,61] |
| >10,000 | 20.8 (21) | [66,74,87,94,98,103,107,111,112,115,117,119,121,125,127,137,138,139,140,142,144] |
| Geographic region | ||
| Northeast | 1 (1) | [126] |
| Midwest | 20.8 (21) | [26,38,52,53,55,56,57,64,67,81,82,85,94,97,109,119,120,121,128,129,130] |
| South | 41.6 (42) | [27,36,59,61,63,66,68,69,70,72,73,75,77,78,79,80,88,92,93,95,99,100,104,105,106,107,108,113,116,117,123,131,132,133,134,135,136,142,143,145,146,148] |
| West | 16.8 (17) | [58,71,76,83,84,86,89,90,91,102,103,111,114,115,140,141,147] |
| More than one region | 2 (2) | [54,124] |
| National | 17.8 (18) | [60,62,65,74,87,96,98,101,110,112,118,122,125,127,137,138,139,144] |
| Characteristic | Percentage of Studies | Study References |
|---|---|---|
| Study Type | ||
| Feasibility | 5 | [77] |
| Intervention | 90 | [27,59,61,66,73,76,80,85,89,90,108,109,116,117,135,143,145] |
| Implementation | 5 | [70] |
| Study Purpose * | ||
| Parental Education | 20 | [59,76,109,143] |
| Patient Education | 20 | [61,70,135,145] |
| Vaccine Provider Education | 25 | [66,76,85,109] |
| Community education/awareness | 25 | [80,88,108,116,117] |
| Vaccine Voucher | 5 | [27] |
| HPV Awareness/Media Campaign | 10 | [73,90] |
| Other | 5 | [77] |
| Included Free Vaccination | ||
| Yes | 25 | [27,61,116,117,135] |
| VFC-eligible only | 10 | [77,80] |
| No | 65 | [59,66,70,73,76,85,88,89,90,108,109,141,145] |
| Intervention Duration ** | ||
| <0.5 year | 30 | [59,66,73,90,143,145] |
| 0.5 year–1 year | 20 | [70,77,80,135] |
| >1 year | 45 | [30,61,76,85,89,108,109,116,117] |
| Unspecified | 5 | [88] |
| Duration of Training and Education Sessions (N = 12) | ||
| Under 1 h | 17 | [135,143] |
| 1 h | 50 | [66,76,80,89,109,145] |
| Unspecified | 33 | [85,108,116,117] |
| Number of Sessions/Activities (N = 14) | ||
| One | 36 | [66,80,90,109,135] |
| Two to four | 29 | [76,85,89,143] |
| Five or more | 21 | [116,117,145] |
| Unspecified | 14 | [90,110] |
| Mode of Interaction * | ||
| Indirect | 20 | [73,76,89,109] |
| In-person | 65 | [27,59,61,66,76,77,85,88,90,108,116,117,143] |
| Virtual or remote | 25 | [77,80,85,88,145] |
| Unspecified | 10 | [70,135] |
| Intervener * | ||
| Research team | 50 | [27,73,76,77,85,88,89,109] |
| Self-directed | 10 | [70,135] |
| Community medical professionals | 25 | [59,80,116,117,143] |
| Other | 25 | [61,66,80,90,145] |
| Theory/Model/Framework | N | Study References |
|---|---|---|
| Anderson Model | 1 | [69] |
| Shared Treatment Decision-Making Model | 1 | [101] |
| Community-Based Participatory Research (CBPR) | 1 | [148] |
| Competing Demands Model | 1 | [65] |
| Comprehensive Participatory Planning | 1 | [148] |
| Consolidated Framework for Implementation Research (CFIR) | 1 | [38] |
| Ecological Systems Theory | 1 | [99] |
| Evidence-Based Practice Model | 1 | [59] |
| Extended Parallel Process Model | 1 | [109] |
| Health Belief Model | 7 | [71,72,73,108,109,132,133] |
| Concept Mapping | 1 | [54] |
| Organizational Developmental Theory | 1 | [109] |
| Positive Deviance Framework | 1 | [83] |
| Precede-Proceed Model | 1 | [134] |
| Social Ecological Framework | 3 | [79,91,134] |
| Theory of Planned Behavior | 7 | [55,56,67,68,118,123,135] |
| Theory of Reasoned Action | 1 | [109] |
| Vaccine Perceptions, Accountability and Adherence Model | 1 | [102] |
| Intervention Level | Outcome Trend * |
|---|---|
| Multilevel | |
| Increase | |
| Initiation and Completion [76] | |
| No Change | |
| Initiation Only [109] | |
| Decrease | |
| Missed Opportunities [89] | |
| Community-level | |
| Increase | |
| Completion and Initiation [80,116,117] | |
| Initiation [73,77] Attitudes toward, knowledge of HPV vaccination [108] | |
| Clinic-level | |
| No Change | |
| Completion Only [85] Initiation or Completion [59,70] | |
| Provider level/Team level | |
| Increase | |
| Initiation Only [66,88] | |
| Parent/Adult-level | |
| Increase | |
| Completion and Initiation [61] | |
| Initiation Only [59] | |
| Intention to Vaccinate [143] Awareness and knowledge [90] | |
| Individual Young Adult | No difference (rural women only) |
| Initiation [27,88] Increase Completion [135] | |
| Individual Child/Youth | Increase |
| Initiation [145] | |
| Completion and Initiation [116,117] |
| Initiation only: Increase | |
| Multilevel Intervention Significantly more multilevel intervention participants received the vaccine at 3 months than the comparison participants [109]. Vouchers for Free Vaccines, Social Marketing Campaigns Less than 50% of eligible individuals redeemed the voucher to receive dose one of the HPV vaccine for free in rural Appalachia [28]. | |
| A social marketing campaign initiated by county health departments in a primarily rural and a racially diverse part of North Carolina increased HPV vaccine uptake among preteen girls for whom the vaccine is routinely recommended [73]. Provider Recommendation Six-month increases in HPV vaccination coverage were larger for patients in clinics that received provider-based announcement training versus those in control clinics (5.4% difference, 95% confidence interval: 1.1–9.7%) [66]. In the NIS-Teen 2017 data, receiving a medical provider recommendation was significantly associated with series initiation [102] Provider recommendation that the HPV vaccine was significantly associated with the child being vaccinated that day, as well as scheduling vaccination in Alabama rural clinics. Parents who got the impression that “there was no hurry” were less likely to vaccinate their child that day [63]. Pharmacist-delivered educational presentation increased intention to vaccinate according to guidelines from 35% (N = 12) to 44% (N = 15) [143]. Individuals Engaging in Protective Behaviors Among Appalachian women, those engaging in behaviors that increase their risk for HPV infection were more likely to refuse the vaccine. Those women engaging in protective health behaviors were more likely to accept the vaccine [24]. | |
| Initiation: No change | |
| Provider conversation training did not differ from control clinics [66] | |
| Completion only: Increase | |
| Health campaigns to increase the HPV vaccine in rural and medically underserved college campuses need to target both genders to complete the vaccination process [67]. In rural Iowa VFC clinics, commonly implemented interventions focused on provider knowledge and patient education. Least commonly implemented interventions required systematic changes, such as reminder/recall and follow-up after missed appointments [57]. | |
| Initiation and Completion: Increase | |
| Rural clinics with higher HPV vaccine up-to-date rates differed from those with lower rates as they implemented standardized workflows to identify patients due for the vaccine and had vaccine administration protocols; they had vaccine champions. They provided immunizations regardless of visit type; clear, persuasive language to recommend or educate parents and patients [83]. | |
| Initiation and Completion: No Change | |
| HPV vaccination coverage was not statistically significantly different among CCSP patients (60.0%) compared to controls (66.3%). The proportions receiving 2 doses (CCSP patients 21.5% vs. controls 20.7%) and 3 doses (28.5% vs. 30.1%) were comparable between CCSP patients and controls [130]. | |
| Initiation and Completion: Decrease | |
| The COVID-19 pandemic had a negative impact on HPV vaccination [37]. | |
| Awareness of HPV | |
| In 2013, 68% of all Americans had heard of HPV and the HPV vaccine. Those in rural areas were less likely than those in urban areas to know that HPV causes cervical cancer [62]. Slightly more than half of the black participants in Alabama were aware of HPV (62.5%) and the HPV vaccine (62.1%). Marriage or partnership lowered awareness; family cancer history, self-reported health status, employment, and participation in social groups increased awareness [95]. | |
| Attitudes/Beliefs, Intentions to vaccinate, subjective norms | |
| Significant initial uptake difference between urban and rural college women; rural clinic women are less likely to follow up [26]. Attitudes were the strongest predictor of mothers’ intentions to vaccinate [95], but intentions were not high [55]. Subjective norms also influence intention [55]. | |
| Political Affiliation and Religion | |
| Increase in initiation associated with political affiliation (Democratic affiliation). Political affiliation explained most of the variation in vaccine confidence and intention/uptake between rural and other respondents [96]. Non-Baptists were 3.6 times more likely to vaccinate than Baptists [133]. | |
| Up-to-date with other vaccinations | |
| Adolescents were less likely to initiate and complete the HPV vaccine if they were not up-to-date on the hepatitis A, meningococcal, or Tdap vaccinations [52]. | |
| Gender | |
| Men aged 40 and younger were less likely to have any human papillomavirus vaccination than women [81]. | |
| Text messaging and DVD educational programs | |
| Providing messages through texting on cell phones could promote the HPV vaccine in rural middle school students [72]. DVD community education film, “Someone You Love: The HPV Epidemic,” increased HPV knowledge gained and attitudes towards the HPV vaccine in rural Virginia locations [108]. | |
| Provider access, influence, collaborative communication, and HPV education | |
| Relative to urban midwestern providers, significantly fewer rural providers had evening/weekend adolescent vaccination appointments available, had prior experience with adolescent vaccine quality improvement projects, and routinely recommended the HPV vaccine during urgent/acute care visits. Significantly more rural providers had standing orders to administer all recommended adolescent vaccines, and reported giving the HPV vaccine information to their patients/families before it was due [82]. Pediatricians in the western US reported a higher number of challenges limiting HPV vaccination, higher HPV vaccination knowledge, and more favorable HPV vaccination recommendation practices compared to other healthcare team members (OTM, including nurses, medical assistants clinic staff, administrators, and stakeholders (like community health workers) [58]. Collaborative communication affected urban–rural uptake disparity; poorer, less educated, and rural parents reported less communication [56]. Collaborative communication between providers and patients is less common among rural residents, and may account for differences—and lack of differences—in HPV vaccination among some subgroups of adolescent girls [101]. Clusters rated as most important by rural stakeholders included: education and provider influence; those rated as most feasible were education and coordinated/consistent messaging [54]. | |
| School-based programs | |
| Stakeholders in the Carolinas strongly supported school-based programs and approaches to strengthen confidence and demand for HPV vaccination [79]. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Sheinfeld Gorin, S.; Hyman, R.; Olson, C.; Fournier, E.A.; Yang, K.; Hanko, D.; HPV Review Working Group. A Scoping Review of Influences on HPV Vaccine Uptake in the Rural US. Vaccines 2026, 14, 156. https://doi.org/10.3390/vaccines14020156
Sheinfeld Gorin S, Hyman R, Olson C, Fournier EA, Yang K, Hanko D, HPV Review Working Group. A Scoping Review of Influences on HPV Vaccine Uptake in the Rural US. Vaccines. 2026; 14(2):156. https://doi.org/10.3390/vaccines14020156
Chicago/Turabian StyleSheinfeld Gorin, Sherri, Rebecca Hyman, Courtney Olson, Elizabeth Amber Fournier, Kaitlyn Yang, Diana Hanko, and HPV Review Working Group. 2026. "A Scoping Review of Influences on HPV Vaccine Uptake in the Rural US" Vaccines 14, no. 2: 156. https://doi.org/10.3390/vaccines14020156
APA StyleSheinfeld Gorin, S., Hyman, R., Olson, C., Fournier, E. A., Yang, K., Hanko, D., & HPV Review Working Group. (2026). A Scoping Review of Influences on HPV Vaccine Uptake in the Rural US. Vaccines, 14(2), 156. https://doi.org/10.3390/vaccines14020156






