High Effectiveness of the Changchun Baike Varicella Vaccine in a Real-World Outbreak Setting: An Observational Study from Yanji City, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Definition and Diagnostic Criteria
2.2. Data Sources
2.3. Data Quality and Completeness
2.4. Methods
2.5. Statistical Analysis
2.6. Ethical Reflections
3. Results
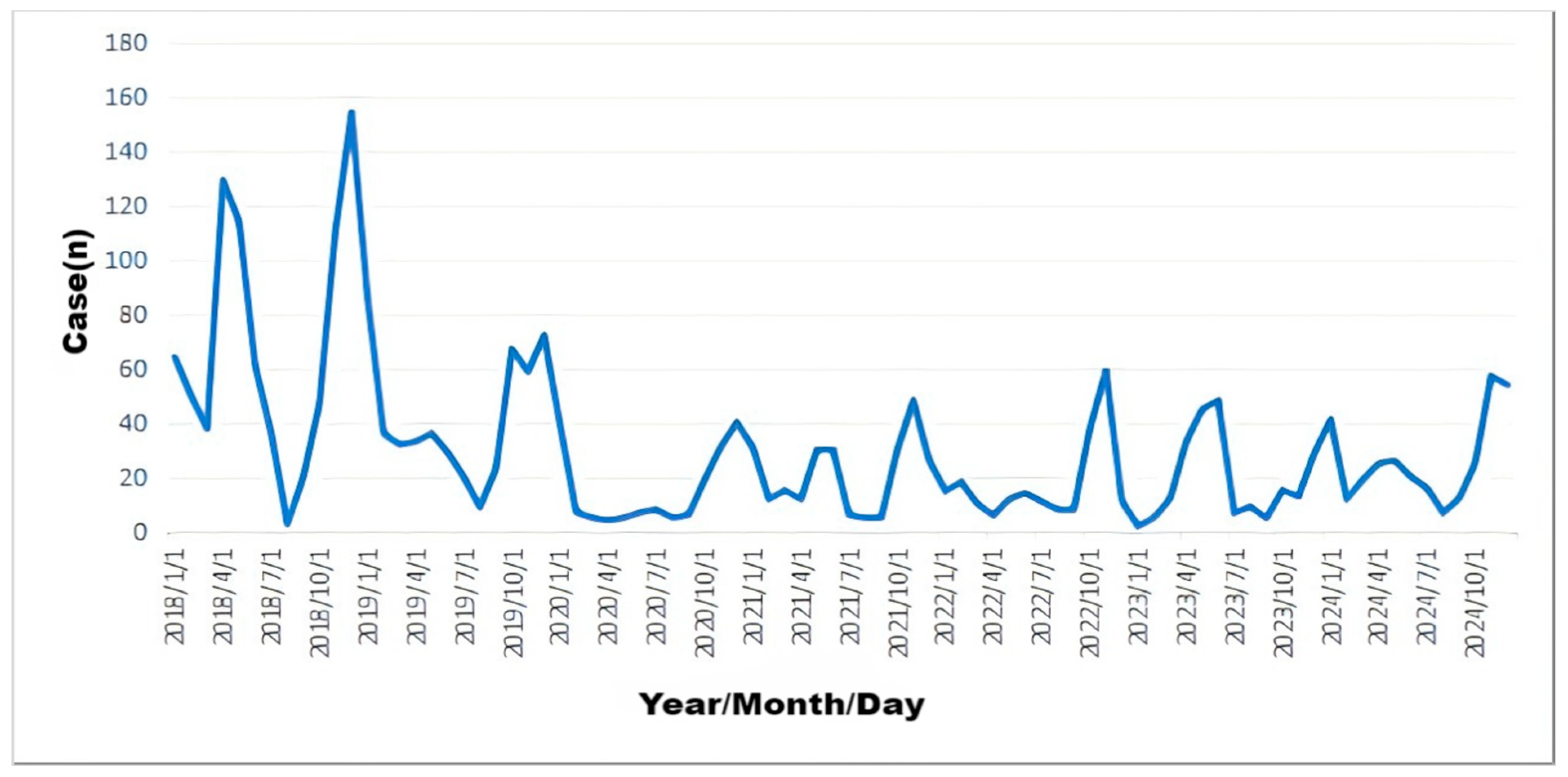
3.1. Temporal Distribution
3.2. Spatial Distribution
3.3. Population Distribution
3.3.1. Sex Distribution
3.3.2. Age Distribution
3.3.3. Occupational Distribution
3.3.4. Vaccination Status
3.4. Vaccine Protective Efficacy
4. Discussion
Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rasizadeh, R.; Shamekh, A.; Shiri Aghbash, P.; Bannazadeh Baghi, H. Comparison of human monkeypox, chickenpox and smallpox: A comprehensive review of pathology and dermatological manifestations. Curr. Med. Res. Opin. 2023, 39, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Bai, S. Epidemiological characteristics of varicella among primary and secondary school students in Shenyang City, 2006–2018. Chin. J. Sch. Health 2020, 41, 148–150. [Google Scholar]
- Ayoade, F.; Kumar, S. Varicella-Zoster Virus (Chickenpox). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Overview of National Notifiable Infectious Diseases in 2022 [EB/OL]; China Center for Disease Control and Prevention: Beijing, China, 2023.
- Dong, P.M.; Wang, M.; Liu, Y.M. Epidemiological characteristics of varicella in China, 2016–2019. Chin. J. Vaccines Immun. 2020, 26, 403–406. [Google Scholar]
- Wang, H.Y.; Liu, F. Progress in research on epidemiological characteristics and vaccine protective efficacy of varicella in China. Prev. Med. Forum 2021, 27, 800–802. [Google Scholar]
- Di Pietrantonj, C.; Rivetti, A.; Marchione, P.; Debalini, M.G.; Demicheli, V. Vaccines for measles, mumps, rubella, and varicella in children. Cochrane Database Syst. Rev. 2021, 11, CD004407. [Google Scholar]
- Pan, X.; Shu, M.; Ma, R.; Fang, T.; Dong, H.; Sun, Y.; Xu, G. Varicella breakthrough infection and effectiveness of 2-dose varicella vaccine in China. Vaccine 2021, 39, 729–736. [Google Scholar] [CrossRef]
- Lin, M.; Yang, T.; Deng, P.; Yang, L.; Xue, C. Analysis on the Epidemiological Characteristics of Breakthrough Varicella Cases and Incremental Effectiveness of 2-Dose Varicella Vaccine in China. Vaccines 2025, 13, 160. [Google Scholar] [CrossRef]
- Li, W.; Liu, L.L.; Tan, H.L.; Zhuang, C.Y.; Zeng, Y.; Zhu, Y.F.; Ye, B.L.; Wei, H.M.; Li, G. Protective efficacy evaluation of trivalent inactivated influenza vaccine among primary and secondary school students in Longgang District, Shenzhen, during the 2023–2024 influenza season. Chin. J. Vaccines Immun. 2025, 31, 132–137. [Google Scholar]
- Zhang, R.Y.; Jiang, Z.; Gao, L. Epidemiological characteristics of varicella in Guiyang City, 2010–2022. Mod. Prev. Med. 2023, 50, 4386–4391. [Google Scholar]
- Luo, R.J.; Wen, Y.; Cheng, Y.P.; Chen, N.X.; Huang, F.; Chen, Z.G.; Zhang, Z.; Lü, Q.Y. Epidemiological trends of major respiratory infectious diseases among people aged 6–19 years in Shenzhen, 2013–2022. Chin. J. Trop. Med. 2024, 24, 184–189. [Google Scholar]
- Zhang, X.Q.; Lü, Y.; Wang, Y.; Pan, F.; Chen, Y.F.; Zhang, H.; Yang, H.; Shao, M.H.; Cheng, K.; Qin, W. Epidemiological characteristics and vaccine protective efficacy of varicella in Liu’an City, 2010–2022. Chin. J. Vaccines Immun. 2023, 29, 285–289. [Google Scholar]
- Lin, M.Z.; Ding, M.H.; Deng, P.F.; Wang, Q.Z.; Fei, Y.; Xue, C.Y. Epidemiological characteristics of varicella in Pudong New Area, Shanghai, 2011–2020. Chin. J. Biol. 2022, 35, 695–699+705. [Google Scholar]
- Ding, X.; Ren, D.F.; Gao, Q.R.; Ning, L.T.; Xiao, Y.F. Epidemiological characteristics of varicella in Tongren City, 2010–2020. Mod. Prev. Med. 2021, 48, 3411–3414. [Google Scholar]
- Chen, Q.; Wang, M.C.; Zeng, X.P. Epidemiological characteristics of infectious diseases in schools and childcare facilities in Haikou City, 2015–2019. Mod. Prev. Med. 2021, 48, 2214–2217+2225. [Google Scholar]
- Wu, J.J.; Zou, L.P.; He, Y.J.; Guo, L.; Xie, Y.Z.; Han, Y.; Wang, Q.F. Epidemiological characteristics and vaccine protective efficacy of varicella in Jinan City, 2006–2022. Mod. Prev. Med. 2024, 51, 3422–3427+3441. [Google Scholar]
- Wang, Z.; Chen, L.; Lu, F.; Peng, J.; Huang, F.; Xie, X.; Kong, D. Analysis of the implementation effect and evaluation of the vaccine protection effect of the live attenuated varicella vaccine program for school-age children in Bao’an district of Shenzhen, China. Hum. Vaccin. Immunother. 2024, 20, 2364485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Y.; Yu, J.; Dong, C.; Zhang, J.; Liu, N.; Qian, C.; Luan, L. Seroprevalence rates in children aged 3–6 years after implementing a two-dose varicella vaccination: A observational study. Hum. Vaccin. Immunother. 2023, 19, 2211465. [Google Scholar] [CrossRef]
- Li, Y.; Xu, F.; Liu, M.; Teng, S.; Liang, F.; Wang, F. Effectiveness of two-dose vs. one-dose varicella vaccine in children in Shanghai, China: A prospective cohort study. Front. Public. Health 2024, 12, 1320407. [Google Scholar] [CrossRef]
- Wang, L.; Wang, M.M.; Xu, C.D.; Wang, P.H.; You, M.Y.; Li, Z.H.; Chen, X.M.; Liu, X.Y.; Li, X.D.; Wang, Y.Y.; et al. Spatial Dynamics of Chickenpox Outbreaks in Rapidly Developing Regions: Implications for Global Public Health. Biomed. Environ. Sci. 2024, 37, 687–697. [Google Scholar] [PubMed]
- Williame, I.; George, M.; Shah, H.A.; Homer, N.; Alderson, D.; Jamet, N. Healthcare resource use and costs of varicella and its complications: A systematic literature review. Hum. Vaccin. Immunother. 2023, 19, 2266225. [Google Scholar] [CrossRef]
- Zeng, T.; Lian, C.-X.; Zhang, X.-Y.; Liu, P.-Q.; Ao, J.; Zhou, G.-F.; Chen, X.-D.; Huang, D.-D.; Hu, D.-G.; Chen, X. Clinical symptoms and molecular epidemiologic characteristics of varicella patients among children and adults in Ganzhou, China. Virol. J. 2025, 22, 44. [Google Scholar] [CrossRef]
- Luan, G.; Yao, H.; Yin, D.; Liu, J. Trends and Age-Period-Cohort Effect on Incidence of Varicella Under Age 35—China, 2005–2021. China CDC Wkly. 2024, 6, 390–395. [Google Scholar] [CrossRef]
- Zhang, M.; Gui, G.-P.; Guo, F.; Fan, X.-F.; Zha, R.-S. A Centralized Outbreak of Varicella among Children Attending Preschool in Suzhou, China. Biomed. Res. Int. 2020, 2020, 6183936. [Google Scholar] [CrossRef]
- Ribeiro, M.Z.; Kupek, E.; Ribeiro, P.V.Z.; Pinheiro, C.E.A. Impact of the tetra viral vaccine introduction on varicella morbidity and mortality in the Brazilian macro regions. J. Pediatr. 2020, 96, 702–709. [Google Scholar] [CrossRef]
- Hu, Y.H.; Luo, X.F.; Lyu, M.; Yin, D.P. A Meta-analysis on varicella-zoster virus antibody levels in healthy population in China. Zhonghua Liu Xing Bing Xue Za Zhi 2021, 42, 1650–1661. (In Chinese) [Google Scholar]
- Liu, X.Y.; Wang, M.M.; You, M.Y.; Wang, P.H.; Wang, T.Q.; Chen, X.M.; Xu, C.D.; Li, X.D.; Wang, L.; Hu, Y.H.; et al. Epidemiological characteristics and influencing factors of public health emergency events of varicella in the Beijing-Tianjin-Hebei region, 2006–2021. Zhonghua Yu Fang Yi Xue Za Zhi 2024, 58, 1999–2004. [Google Scholar]
- Tam, W.W.; Chan, J.; Lo, K.K.; Lee, A.; Chan, P.K.; Chan, D.; Nelson, E.A.S. Parental Attitudes and Factors Associated With Varicella Vaccination in Preschool and Schoolchildren in Hong Kong: A Cross-Sectional Study. Medicine 2015, 94, e1519. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, H.; Ou, R.; Zeng, Q.; Hu, L.; Qiu, H.; Sharma, M.; Ye, M. Spatiotemporal Epidemiology of Varicella in Chongqing, China, 2014–2018. Int. J. Environ. Res. Public Health 2020, 17, 662. [Google Scholar] [CrossRef]
- Liang, H.; Qi, X.; Chen, Y.; Pan, X. Surveillance of Adverse Events Following Varicella Vaccine Immunization in Zhejiang Province, China, from 2020 to 2022. Vaccines 2025, 13, 57. [Google Scholar] [CrossRef]
- Sabale, U.; Jarmale, L.; Murtagh, J.; Pawaskar, M.; Bencina, G. Impact assessment of immunization and the COVID-19 pandemic on varicella across Europe using digital epidemiology methods: A descriptive study. PLoS ONE 2023, 18, e0283465. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, Y.; Xia, J.; Wu, T.; Wang, D.; Lu, J. The impact of COVID-19 control measures on the morbidity of varicella, herpes zoster, rubella and measles in Guangzhou, China. Immun. Inflamm. Dis. 2020, 8, 844–846. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Zhou, Q.; Liu, J.-Y.; Gong, R.-J.; Mao, S.-Q.; Ye, Z.-J.; Wu, Q.-S. Characteristics of within-household varicella transmission events associated with school outbreaks in Shanghai, China, 2009–2018. Epidemiol. Infect 2020, 148, e127. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Wu, Q. Effectiveness of varicella vaccine as post-exposure prophylaxis: A meta-analysis. Hum. Vaccin. Immunother. 2021, 17, 5316–5324. [Google Scholar] [CrossRef] [PubMed]
- Varela, F.H.; Pinto, L.A.; Scotta, M.C. Global impact of varicella vaccination programs. Hum. Vaccines Immunother. 2019, 15, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Chemaitelly, H.; AlMukdad, S.; Ayoub, H.H.; Altarawneh, H.N.; Coyle, P.; Tang, P.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Hasan, M.R.; et al. COVID-19 Vaccine Protection among Children and Adolescents in Qatar. N. Engl. J. Med. 2022, 387, 1865–1876. [Google Scholar] [CrossRef] [PubMed]






| Year | Region | Total Varicella Vaccinations | Changchun Baike Vaccinations | Proportion (%) |
|---|---|---|---|---|
| 2018 | Yanji city | 12,342 | 3491 | 28.3 |
| Henan street | 1780 | 636 | 35.7 | |
| Sandaowan town | 0 | 0 | 0 | |
| 2019 | Yanji city | 8735 | 641 | 7.34 |
| Jin Xue street | 1203 | 137 | 11.3 | |
| Sandaowan town | 0 | 0 | 0 | |
| 2020 | Yanji city | 10,011 | 959 | 9.58 |
| Chaoyangchuan town | 210 | 160 | 76.1 | |
| Sandaowan town | 0 | 0 | 0 | |
| 2018–2020 | Yanji city | 31,088 | 5091 | 16.38 |
| Year | Variable | β | S.E | Z | p | HR (95% CI) | aVE (%) (95% CI) |
|---|---|---|---|---|---|---|---|
| 2018–2022 | Doses | ||||||
| 0 | - | - | - | - | 1.00 (Reference) | - | |
| 1 | −4.68 | 0.29 | −16.14 | <0.001 | 0.01 (0.01–0.02) | 99.0 (98.0–99.0) | |
| 2 | −4.70 | 0.30 | −15.51 | <0.001 | 0.01 (0.01–0.02) | 99.0 (98.0–99.0) | |
| All Doses | −4.22 | 0.21 | −20.09 | <0.001 | 0.01 (0.01–0.02) | 99.0 (98.0–99.0) | |
| 2019–2023 | Doses | ||||||
| 0 | - | - | - | - | 1.00 (Reference) | - | |
| 1 | −4.99 | 0.71 | −7.04 | <0.001 | 0.01 (0.00–0.03) | 99.0 (97.0–100.0) | |
| 2 | −4.99 | 0.71 | −7.04 | <0.001 | 0.01 (0.00–0.03) | 99.0 (97.0–100.0) | |
| All Doses | −4.59 | 0.58 | −7.91 | <0.001 | 0.01 (0.01–0.02) | 99.0 (97.0–100.0) | |
| 2020–2024 | Doses | ||||||
| 0 | - | - | - | - | 1.00 (Reference) | - | |
| 1 | −3.82 | 0.58 | −6.54 | <0.001 | 0.02 (0.00–0.07) | 98.0 (97.0–99.0) | |
| 2 | −5.23 | 0.58 | −9.04 | <0.001 | 0.01 (0.00–0.02) | 99.0 (98.0–100.0) | |
| All Doses | −4.63 | 0.41 | −10.65 | <0.001 | 0.01 (0.01–0.03) | 99.0 (98.0–100.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Wang, Z.; Shang, S.; Guo, X.; Song, S.; Guo, F.; Xu, N.; Ren, F.; Chen, Z.; Li, Y.; Gu, H. High Effectiveness of the Changchun Baike Varicella Vaccine in a Real-World Outbreak Setting: An Observational Study from Yanji City, China. Vaccines 2026, 14, 42. https://doi.org/10.3390/vaccines14010042
Wang Z, Shang S, Guo X, Song S, Guo F, Xu N, Ren F, Chen Z, Li Y, Gu H. High Effectiveness of the Changchun Baike Varicella Vaccine in a Real-World Outbreak Setting: An Observational Study from Yanji City, China. Vaccines. 2026; 14(1):42. https://doi.org/10.3390/vaccines14010042
Chicago/Turabian StyleWang, Zheng, Shuhan Shang, Xiaoguang Guo, Shiyuan Song, Feng Guo, Na Xu, Feifan Ren, Zijian Chen, Yihua Li, and Hanxue Gu. 2026. "High Effectiveness of the Changchun Baike Varicella Vaccine in a Real-World Outbreak Setting: An Observational Study from Yanji City, China" Vaccines 14, no. 1: 42. https://doi.org/10.3390/vaccines14010042
APA StyleWang, Z., Shang, S., Guo, X., Song, S., Guo, F., Xu, N., Ren, F., Chen, Z., Li, Y., & Gu, H. (2026). High Effectiveness of the Changchun Baike Varicella Vaccine in a Real-World Outbreak Setting: An Observational Study from Yanji City, China. Vaccines, 14(1), 42. https://doi.org/10.3390/vaccines14010042





