Twenty-Eight Years of Invasive Meningococcal Disease Surveillance in the Autonomous Province of Vojvodina, Serbia: Epidemiological Trends and Implications for Enhanced Surveillance and Vaccination Policy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Data Sources, and Variables
- Presence of a purpuric rash with N. meningitidis considered the most likely cause (linked to a confirmed case, with other causes of hemorrhagic rash excluded or deemed less likely);
- Detection of Gram-negative diplococci in a normally sterile site (blood, CSF) or in a purpuric skin lesion;
- Detection of N. meningitidis antigen (e.g., by latex agglutination) from a normally sterile site or a purpuric skin lesion.
2.2. Statistical Analysis
2.3. Ethical Considerations
3. Results
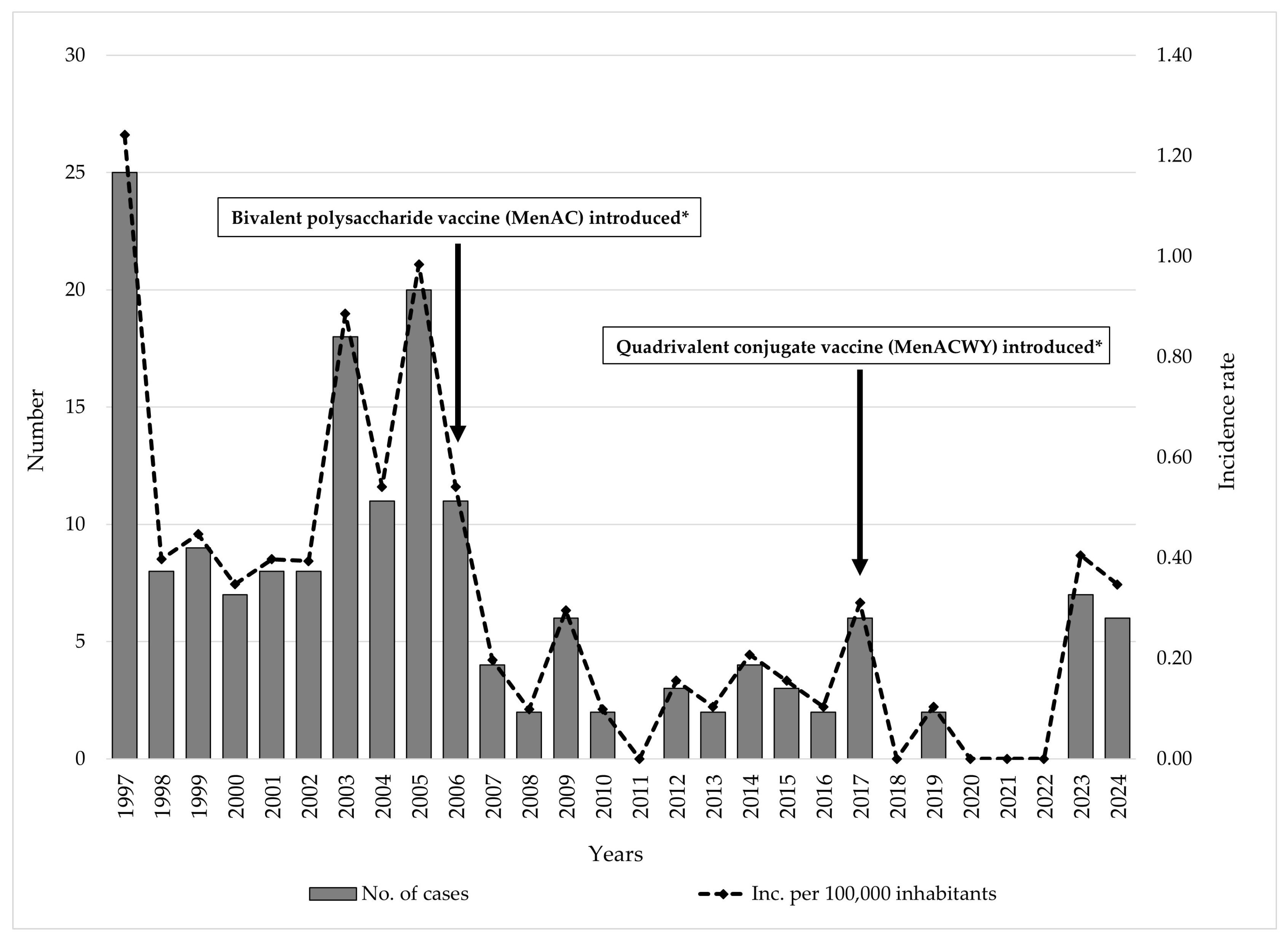
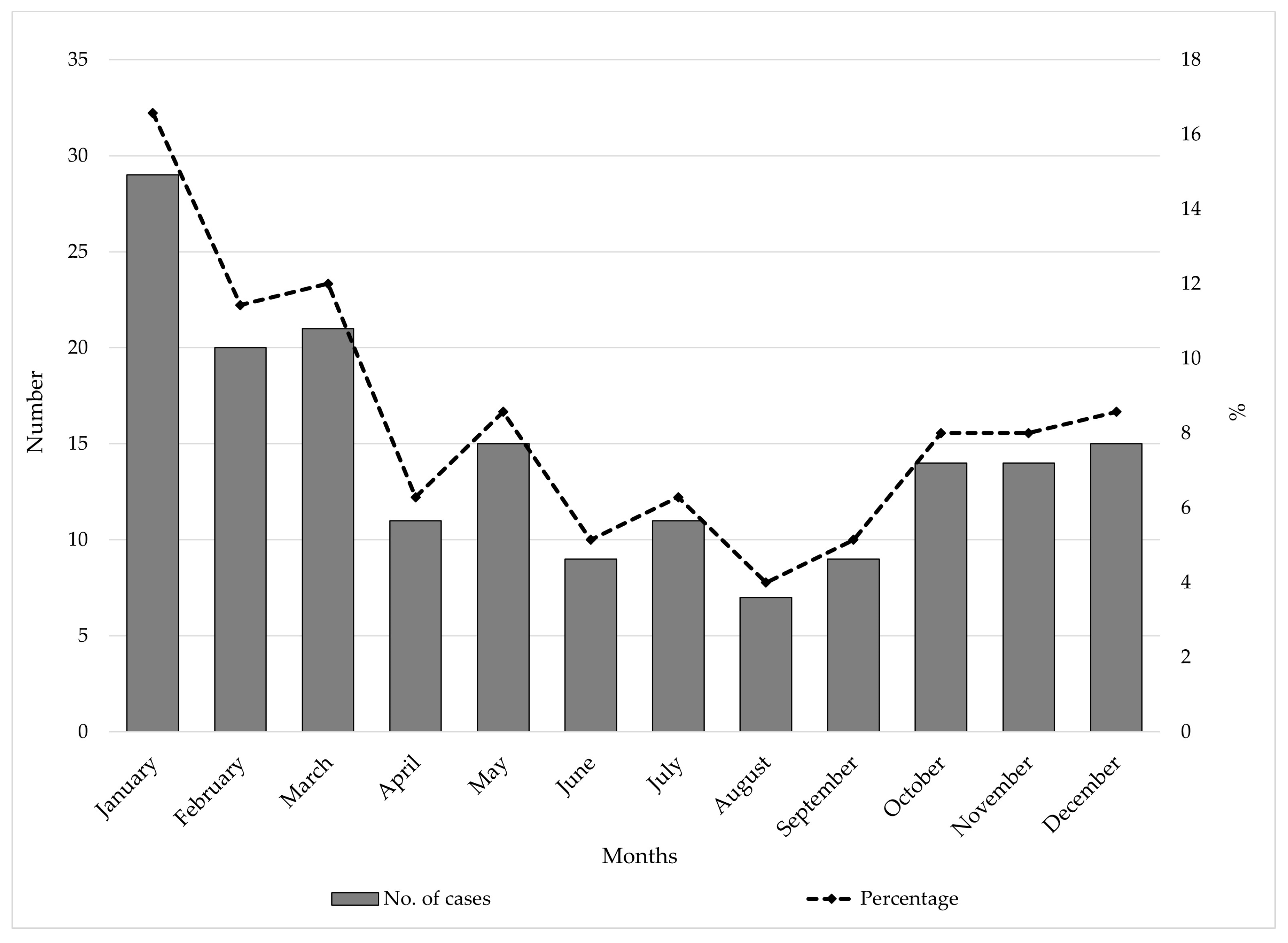
3.1. Trends and Seasonal Patterns
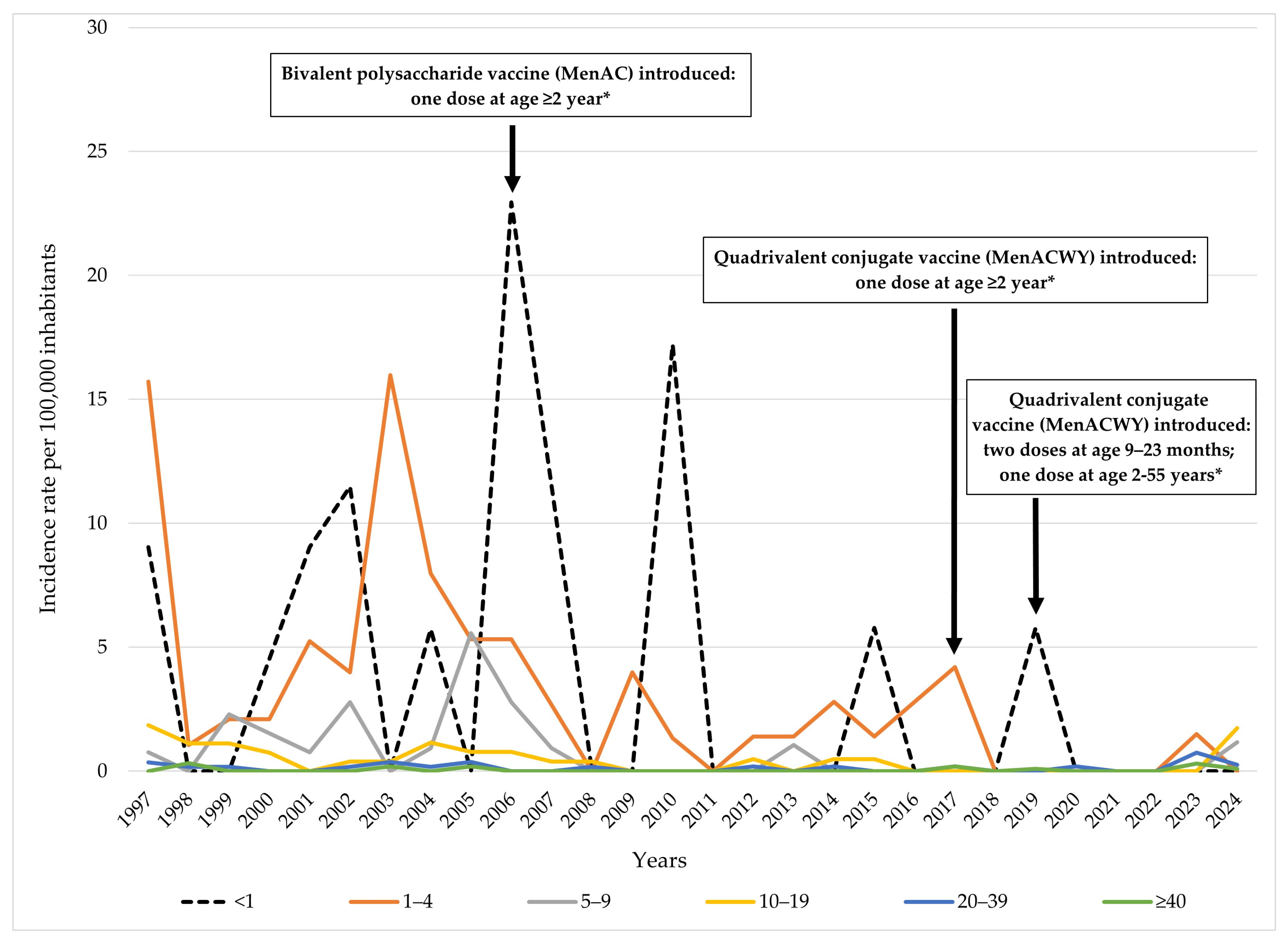
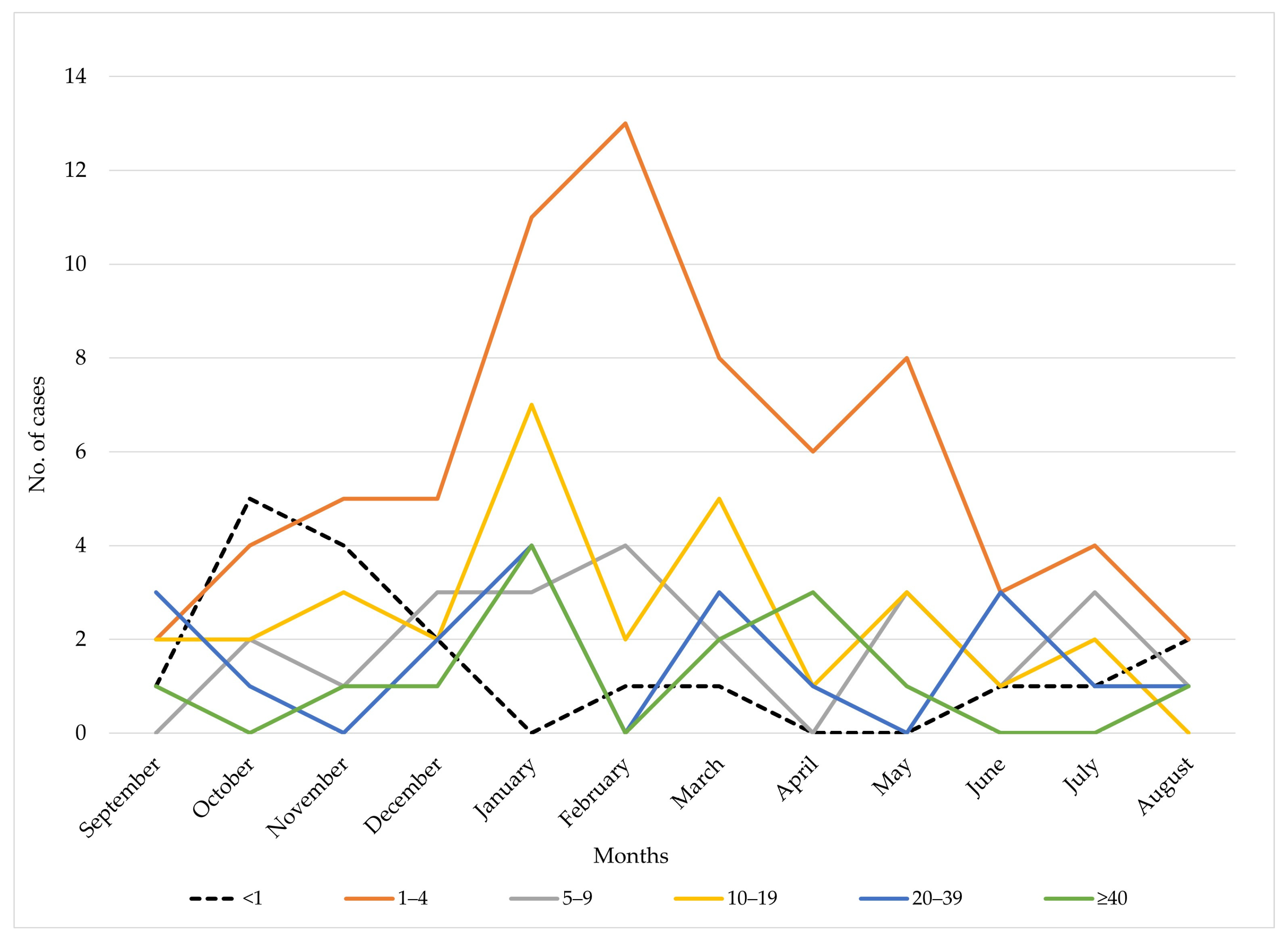
3.2. Age-Specific Distribution
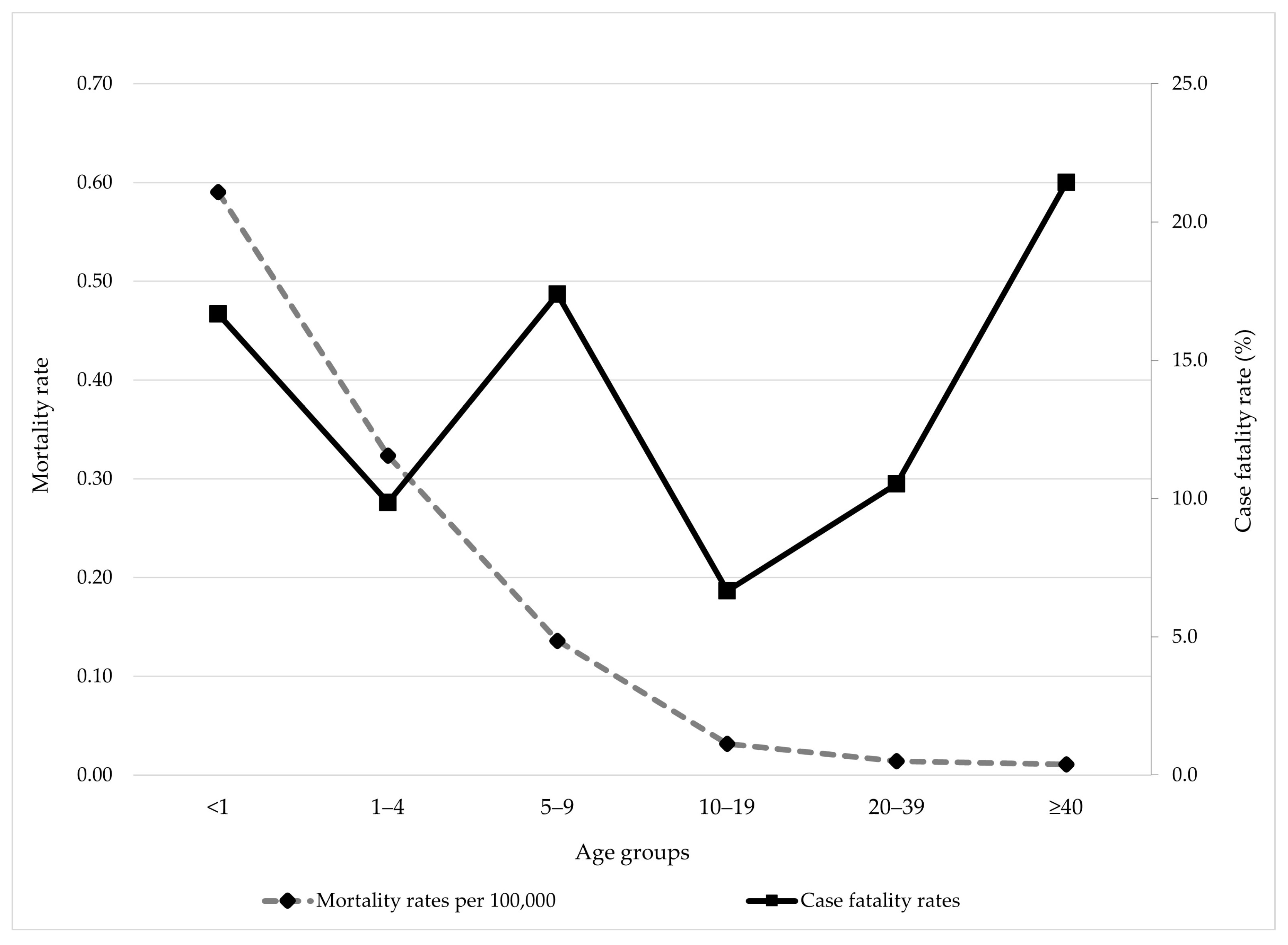
3.3. Mortality and Case Fatality Rates
3.4. Clinical Outcomes
4. Discussion
4.1. Temporal Trends
4.2. Age-Specific Patterns
4.3. Seasonal Variation
4.4. Case Fatality Rates
4.5. Global, Regional, and Local Serogroup Distribution and Vaccination Policies
4.6. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Meningococcal vaccines: WHO position paper, November 2011. Wkly. Epidemiol. Rec. 2011, 86, 521–539. [Google Scholar] [PubMed]
- Centers for Disease Prevention and Control (CDC). Chapter 8: Meningococcal Disease. In Epidemiology and Prevention of Vaccine-Preventable Diseases; US Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2024. Available online: https://www.cdc.gov/surv-manual/php/table-of-contents/chapter-8-meningococcal-disease.html?CDC_AAref_Val=https://www.cdc.gov/vaccines/pubs/surv-manual/chpt08-mening.html?utm_source=chatgpt.com (accessed on 13 July 2025).
- Cohn, A.; MacNeil, J. The Changing Epidemiology of Meningococcal Disease. Infect. Dis. Clin. N. Am. 2015, 29, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Pardo de Santayana, C.; Tin Tin Htar, M.; Findlow, J.; Balmer, P. Epidemiology of invasive meningococcal disease worldwide from 2010–2019: A literature review. Epidemiol. Infect. 2023, 151, e57. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khatami, A.; Pollard, A.J. The epidemiology of meningococcal disease and the impact of vaccines. Expert Rev. Vaccines 2010, 9, 285–298, Erratum in Expert Rev. Vaccines 2011, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- McNamara, L.A.; Topaz, N.; Wang, X.; Hariri, S.; Fox, L.; MacNeil, J.R. High Risk for Invasive Meningococcal Disease Among Patients Receiving Eculizumab (Soliris) Despite Receipt of Meningococcal Vaccine. MMWR. Morb. Mortal. Wkly. Rep. 2017, 66, 734–737. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ohm, M.; Hahné, S.J.M.; van der Ende, A.; Sanders, E.A.M.; Berbers, G.A.M.; Ruijs, W.L.M.; van Sorge, N.M.; de Melker, H.E.; Knol, M.J. Vaccine Impact and Effectiveness of Meningococcal Serogroup ACWY Conjugate Vaccine Implementation in the Netherlands: A Nationwide Surveillance Study. Clin. Infect. Dis. 2022, 74, 2173–2180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- European Centre for Disease Prevention and Control (ECDC). Invasive Meningococcal Disease-Annual Epidemiological Report for 2022; ECDC: Stockholm, Sweden, 2024. Available online: https://www.ecdc.europa.eu/en/publications-data/invasive-meningococcal-disease-annual-epidemiological-report-2022 (accessed on 13 July 2025).
- Paireau, J.; Chen, A.; Broutin, H.; Grenfell, B.; Basta, N.E. Seasonal dynamics of bacterial meningitis: A time-series analysis. Lancet Glob. Health 2016, 4, e370–e377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zografaki, I.; Detsis, M.; Del Amo, M.; Iantomasi, R.; Maia, A.; Montuori, E.A.; Mendez, C. Invasive meningococcal disease epidemiology and vaccination strategies in four Southern European countries: A review of the available data. Expert Rev. Vaccines 2023, 22, 545–562. [Google Scholar] [CrossRef] [PubMed]
- Pravilnik o Imunizaciji i Načinu Zastite Lekovima. Službeni Glasnik Republike Srbije, broj 11/2006, 25/2013, 63/2013, 99/2013, 118/2013, 65/2014, 32/2015. Available online: https://pravno-informacioni-sistem.rs/SlGlasnikPortal/reg/viewAct/7648144d-9226-46bc-90f5-16fe5dc2201d (accessed on 15 July 2025). (In Serbian).
- Pravilnik o Imunizaciji i Načinu Zastite Lekovima. Službeni Glasnik Republike Srbije, broj 88/2017, 11/2018, 14/2018, 45/2018, 48/2018, 58/2018, 104/2018, 6/2021, 52/2021 i 66/2022. Available online: https://pravno-informacioni-sistem.rs/eli/rep/sgrs/ministarstva/pravilnik/2018/14/1 (accessed on 15 July 2025). (In Serbian).
- Pravilnik o Programu Obavezne i Preporučene Imunizacije Stanovništva Protiv Određenih Zaraznih Bolesti. Službeni Glasnik Republike Srbije, broj 65/2020. Available online: https://www.paragraf.rs/propisi/pravilnik-o-programu-obavezne-i-preporucene-imunizacije-stanovnistva-protiv-odredjenih-zaraznih-bolesti.html (accessed on 15 July 2025). (In Serbian).
- Institut za Javno Zdravlje Srbije “Dr Milan Jovanović Batut”. Stručno-Metodološκo Uputstvo Za Sprovođenje Obavezne I Preporučene Imunizacije Stanovništva Za 2025. Godinu; Institut za javno zdravlje Srbije: Belgrade, Serbia, 2025; Available online: https://www.batut.org.rs/index.php?category_id=186 (accessed on 8 August 2025). (In Serbian)
- Statistical Office of the Republic of Serbia. Census of Population, Households and Dwellings in the Republic of Serbia (1991, 2002, 2011, and 2022). Available online: https://www.stat.gov.rs/en-us/oblasti/popis/ (accessed on 15 July 2025). (In Serbian)
- World Health Organization. Meningococcus. Vaccine Preventable Diseases. Surveillance Standards. 2018. Available online: https://www.who.int/publications/m/item/vaccine-preventable-diseases-surveillance-standards-meningococcus (accessed on 15 July 2025).
- Findlow, J.; Htar, M.T.T.; Villena, R.; Balmer, P. Invasive Meningococcal Disease in the Post-COVID World: Patterns of Disease Rebound. Vaccines 2025, 13, 165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tzanakaki, G.; Cabrnochová, H.; Delić, S.; Draganescu, A.; Hilfanova, A.; Onozó, B.; Pokorn, M.; Skoczyńska, A.; Tešović, G. Invasive meningococcal disease in South-Eastern European countries: Do we need to revise vaccination strategies? Hum. Vaccines Immunother. 2024, 20, 2301186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harrison, L.H.; Trotter, C.L.; Ramsay, M.E. Global epidemiology of meningococcal disease. Vaccine 2009, 27 (Suppl. S2), B51–B63. [Google Scholar] [CrossRef] [PubMed]
- Institute of Public Health of Vojvodina. Communicable Diseases in Vojvodina, 2018; Annual Report; Institute of Public Health of Vojvodina: Novi Sad, Serbia, 2019. (In Serbian) [Google Scholar]
- Brundage, J.F. Interactions between influenza and bacterial respiratory pathogens: Implications for pandemic preparedness. Lancet Infect. Dis. 2006, 6, 303–312. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, B.; Santoreneos, R.; Giles, L.; Haji Ali Afzali, H.; Marshall, H. Case fatality rates of invasive meningococcal disease by serogroup and age: A systematic review and meta-analysis. Vaccine 2019, 37, 2768–2782. [Google Scholar] [CrossRef] [PubMed]
- Rosenstein, N.E.; Perkins, B.A.; Stephens, D.S.; Popovic, T.; Hughes, J.M. Meningococcal disease. N. Engl. J. Med. 2001, 344, 1378–1388. [Google Scholar] [CrossRef] [PubMed]
- Poulikakos, P.; Kapnisis, D.; Xirogianni, A.; Liakou, I.; Tsolia, M.; Michos, A.; Mantadakis, E.; Papaevangelou, V.; Iliadis, A.; Gkentzi, D.; et al. Invasive Meningococcal Disease in Children: Outcomes and Risk Factors for Sequelae and Fatal Cases in Greece. Microorganisms 2025, 13, 705. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pace, D.; Pollard, A.J. Meningococcal disease: Clinical presentation and sequelae. Vaccine 2012, 30 (Suppl. S2), B3–B9. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.H.; Pelton, S.I.; Wilder-Smith, A.; Holst, J.; Safadi, M.A.; Vazquez, J.A.; Taha, M.K.; LaForce, F.M.; von Gottberg, A.; Borrow, R.; et al. The Global Meningococcal Initiative: Recommendations for reducing the global burden of meningococcal disease. Vaccine 2011, 29, 3363–3371. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, Y.L.; Stephens, D.S. Epidemiology and pathogenesis of Neisseria meningitidis. Microbes Infect. 2000, 2, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Delic, S.; Mijac, V.; Gajic, I.; Kekic, D.; Ranin, L.; Jegorovic, B.; Culic, D.; Cirkovic, V.; Siljic, M.; Stanojevic, M.; et al. A Laboratory-Based Surveillance Study of Invasive Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae Diseases in a Serbian Pediatric Population-Implications for Vaccination. Diagnostics 2021, 11, 1059. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- European Centre for Disease Prevention and Control (ECDC). Vaccine Scheduler. Available online: https://vaccine-schedule.ecdc.europa.eu/ (accessed on 31 July 2025).
- Falguières, M.; Hong, E.; Denizon, M.; Terrade, A.; Taha, M.K.; Deghmane, A.E. Fluctuations in serogroup B meningococcal vaccine antigens prior to routine MenB vaccination in France. Commun. Med. 2025, 5, 87. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taha, S.; Terrade, A.; Doucoure, O.; Deghmane, A.E.; Taha, M.K. Troubled Times, Changing Tides: A Seroprevalence Study on Meningococcal Immunity in France Between 2016 and 2024. Vaccines 2025, 13, 647. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martinón-Torres, F.; Taha, M.K.; Knuf, M.; Abbing-Karahagopian, V.; Pellegrini, M.; Bekkat-Berkani, R.; Abitbol, V. Evolving strategies for meningococcal vaccination in Europe: Overview and key determinants for current and future considerations. Pathog. Glob. Health 2022, 116, 85–98. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ladhani, S.N.; Campbell, H.; Andrews, N.; Parikh, S.R.; White, J.; Edelstein, M.; Clark, S.A.; Lucidarme, J.; Borrow, R.; Ramsay, M.E. First Real-world Evidence of Meningococcal Group B Vaccine, 4CMenB, Protection Against Meningococcal Group W Disease: Prospective Enhanced National Surveillance, England. Clin. Infect. Dis. 2021, 73, e1661–e1668. [Google Scholar] [CrossRef] [PubMed]
- Schillie, S.; Loehr, J.; Chen, W.H.; Moser, C.A.; Cooper, G.; Isenhour, C.; McNamara, L.A. New Dosing Interval and Schedule for the Bexsero MenB-4C Vaccine: Updated Recommendations of the Advisory Committee on Immunization Practices—United States, October 2024. MMWR. Morb. Mortal. Wkly. Rep. 2024, 73, 1124–1128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Health Organization. Defeating Meningitis by 2030: A Global Road Map; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240026407 (accessed on 8 August 2025).
| Variable | Survived (n = 154) | Fatal Cases (n = 21) | p-Value | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Gender | Male | 88 | 57.14 | 14 | 66.67 | 0.5524 |
| Female | 66 | 42.86 | 7 | 33.33 | ||
| Age group (years) | <1 | 15 | 9.74 | 3 | 14.29 | 0.6480 |
| 1–4 | 64 | 41.56 | 7 | 33.33 | ||
| 5–9 | 19 | 12.34 | 4 | 19.05 | ||
| 10–19 | 28 | 18.18 | 2 | 9.52 | ||
| 20–39 | 17 | 11.04 | 2 | 9.52 | ||
| ≥40 | 11 | 7.14 | 3 | 14.29 | ||
| Place of residence | Urban | 93 | 60.39 | 10 | 47.62 | 0.3793 |
| Rural | 61 | 39.61 | 11 | 52.38 | ||
| District of the AP Vojvodina | North Bačka | 12 | 7.79 | 4 | 19.05 | 0.6513 |
| West Bačka | 29 | 18.83 | 4 | 19.05 | ||
| South Bačka | 75 | 48.70 | 8 | 38.10 | ||
| North Banat | 7 | 4.55 | 0 | 0.00 | ||
| Central Banat | 8 | 5.19 | 1 | 4.76 | ||
| South Banat | 5 | 3.25 | 1 | 4.76 | ||
| Srem | 18 | 11.69 | 3 | 14.29 | ||
| Quarter of the year | January–March | 58 | 37.66 | 12 | 57.14 | 0.3368 |
| April–June | 33 | 21.43 | 2 | 9.52 | ||
| July–September | 24 | 15.58 | 3 | 14.29 | ||
| October–December | 39 | 25.32 | 4 | 19.05 | ||
| Diagnosis | Meningococcal meningitis | 105 | 68.18 | 5 | 23.81 | 0.0002 |
| Meningococcal sepsis | 49 | 31.82 | 16 | 76.19 | ||
| Patient | Year of Death | Gender | Age (in Years) | Area of Residence | Diagnosis | Days Between Disease Notification and Exitus Letalis | Classification | Month of Death |
|---|---|---|---|---|---|---|---|---|
| 1 | 1997 | Female | 1 | Urban | MM | 2 | CC | February |
| 2 | 1997 | Female | 4 | Urban | MM | 1 | LCC | March |
| 3 | 1997 | Female | 35 | Rural | MM | 11 | LCC | April |
| 4 | 1997 | Male | 1 | Urban | MS | 4 | CC | May |
| 5 | 1998 | Male | 61 | Rural | MM | 10 | LCC | September |
| 6 | 1999 | Female | 7 | Rural | MS | 1 | CC | March |
| 7 | 2001 | Male | 2 | Rural | MS | 1 | LCC | February |
| 8 | 2001 | Male | 7 | Urban | MS | 2 | LCC | October |
| 9 | 2002 | Female | 24 | Urban | MS | 6 | LCC | February |
| 10 | 2002 | Male | 9 | Urban | MS | 1 | CC | July |
| 11 | 2003 | Male | 1 | Rural | MS | 1 | LCC | March |
| 12 | 2003 | Male | 49 | Rural | MS | 18 | LCC | June |
| 13 | 2003 | Male | 1 | Rural | MS | 2 | LCC | November |
| 14 | 2005 | Male | 1 | Rural | MS | 1 | CC | February |
| 15 | 2005 | Female | 7 | Urban | MS | 1 | LCC | July |
| 16 | 2005 | Male | 61 | Urban | MM | 10 | LCC | November |
| 17 | 2005 | Male | <1 | Rural | MS | 2 | CC | February |
| 18 | 2006 | Male | <1 | Rural | MS | 1 | LCC | October |
| 19 | 2007 | Male | 17 | Rural | MS | 1 | LCC | March |
| 20 | 2009 | Female | <1 | Urban | MS | 2 | LCC | March |
| 21 | 2024 | Male | 14 | Urban | MS | 2 | LCC | January |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ristić, M.; Vuković, V.; Pustahija, T.; Medić, S.; Dragovac, G.; Petrović, V. Twenty-Eight Years of Invasive Meningococcal Disease Surveillance in the Autonomous Province of Vojvodina, Serbia: Epidemiological Trends and Implications for Enhanced Surveillance and Vaccination Policy. Vaccines 2025, 13, 945. https://doi.org/10.3390/vaccines13090945
Ristić M, Vuković V, Pustahija T, Medić S, Dragovac G, Petrović V. Twenty-Eight Years of Invasive Meningococcal Disease Surveillance in the Autonomous Province of Vojvodina, Serbia: Epidemiological Trends and Implications for Enhanced Surveillance and Vaccination Policy. Vaccines. 2025; 13(9):945. https://doi.org/10.3390/vaccines13090945
Chicago/Turabian StyleRistić, Mioljub, Vladimir Vuković, Tatjana Pustahija, Snežana Medić, Gorana Dragovac, and Vladimir Petrović. 2025. "Twenty-Eight Years of Invasive Meningococcal Disease Surveillance in the Autonomous Province of Vojvodina, Serbia: Epidemiological Trends and Implications for Enhanced Surveillance and Vaccination Policy" Vaccines 13, no. 9: 945. https://doi.org/10.3390/vaccines13090945
APA StyleRistić, M., Vuković, V., Pustahija, T., Medić, S., Dragovac, G., & Petrović, V. (2025). Twenty-Eight Years of Invasive Meningococcal Disease Surveillance in the Autonomous Province of Vojvodina, Serbia: Epidemiological Trends and Implications for Enhanced Surveillance and Vaccination Policy. Vaccines, 13(9), 945. https://doi.org/10.3390/vaccines13090945







