Heterologous DNA–Adenovirus Prime–Boost Strategy Expressing Bluetongue Virus VP2 and VP7 Proteins Protects Against Virulent Challenge
Abstract
1. Introduction
2. Material and Methods
2.1. Ethical Statement
2.2. Cell Lines and Viruses
2.3. Generation of pPAL-VP7 and VP2 Plasmids
2.4. Immunofluorescence Study of VP7 and VP2 Expression
2.5. Mouse Vaccination, Serum Preparation and BTV-8 Challenge
2.6. BTV Viremia Detection by RT-qPCR
2.7. Splenocyte Isolation
2.8. Mouse IFN-γ ELISpot Assay
2.9. Flow Cytometry: Intracellular Cytokine Staining (ICS) and T Cell Activation Marker Staining
2.10. Anti-BTV Total IgG, IgG1 and IgG2a/c ELISA
2.11. BTV Seroneutralization Assays
2.12. Statistical Analysis
3. Results
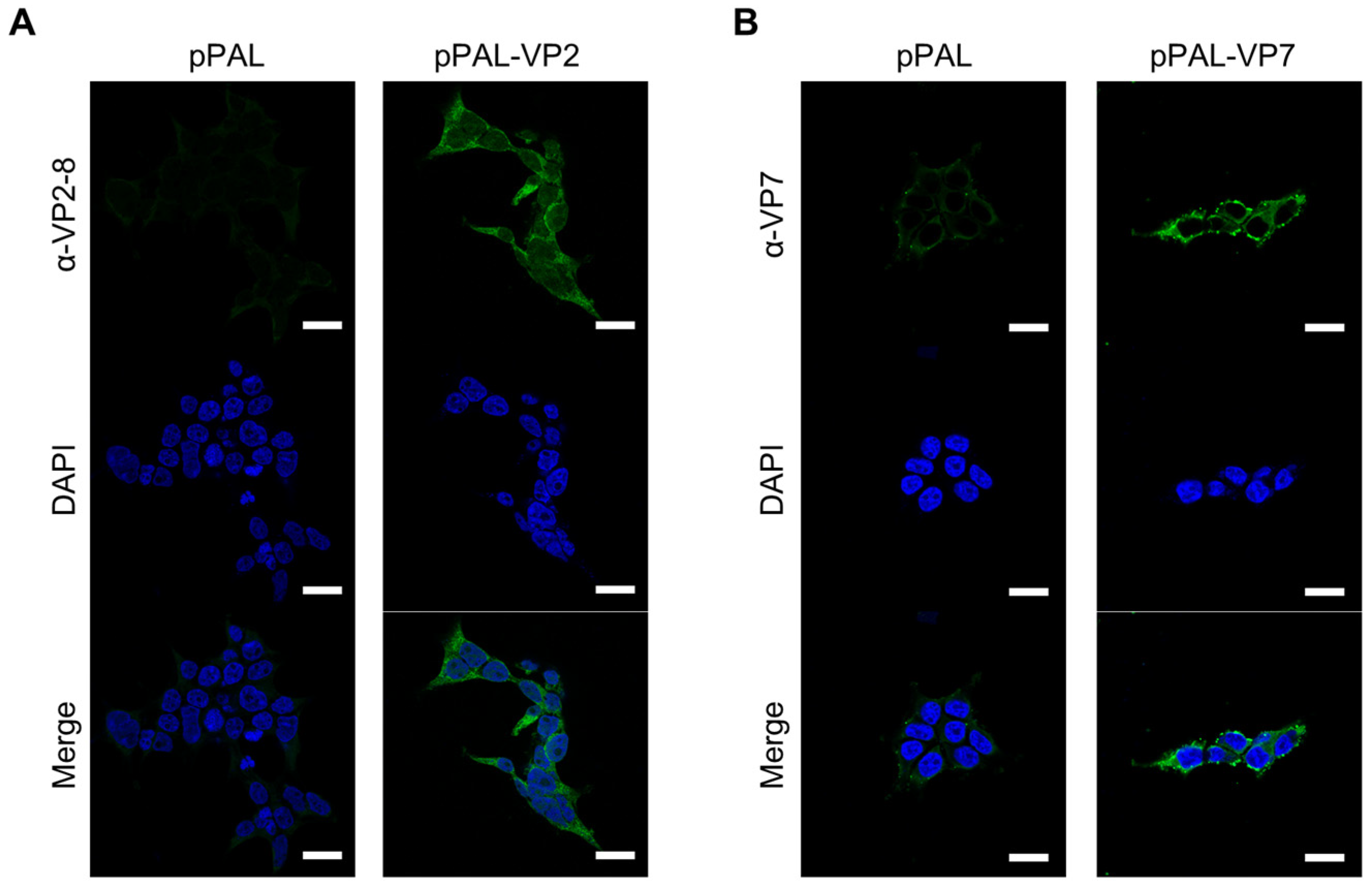
3.1. pPAL-Mediated Expression of BTV Proteins VP7 and VP2 in Transfected Cells
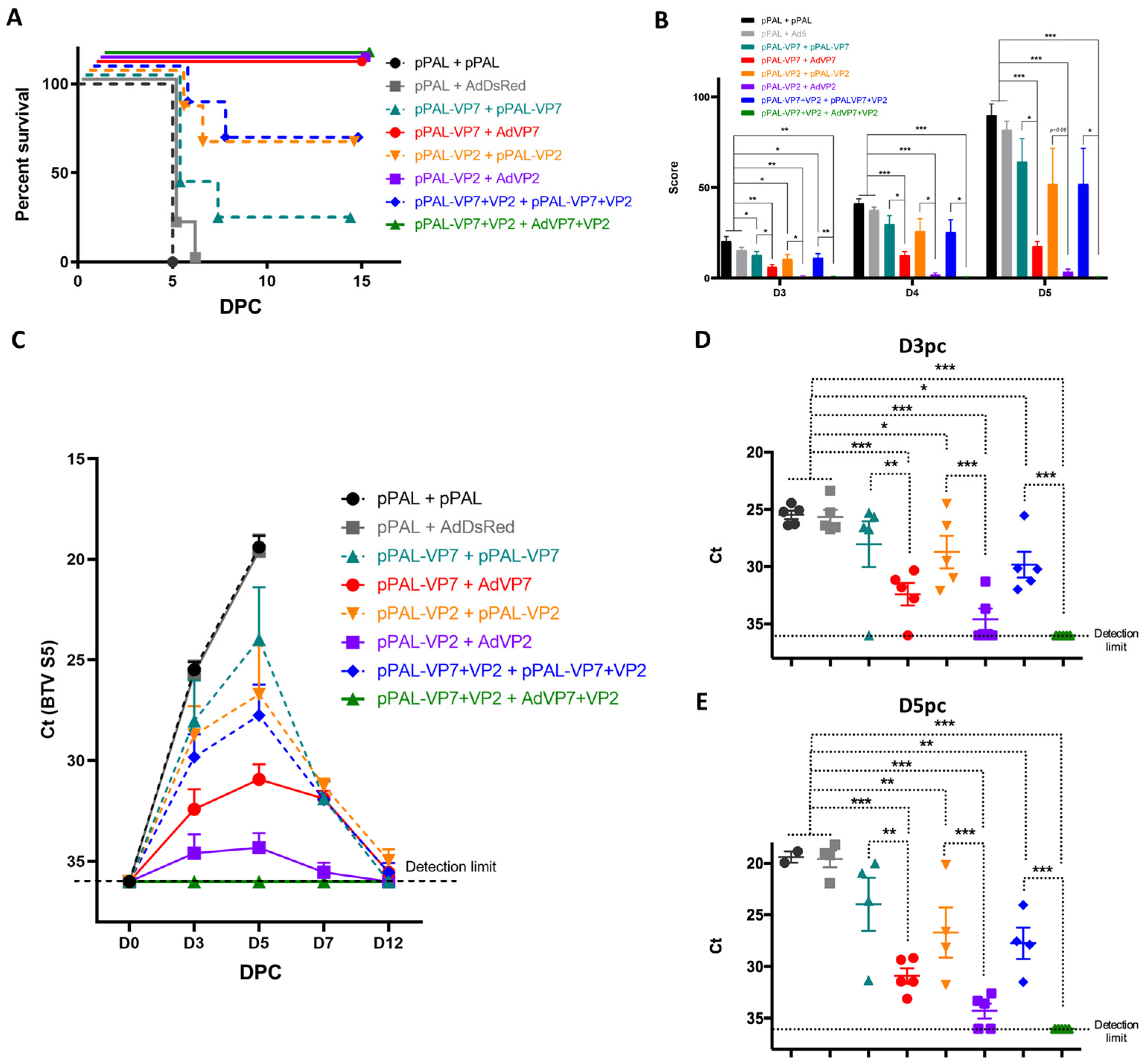
3.2. Heterologous pPAL Prime + Adenovirus Booster Vaccination Protects Against BTV Lethal Challenge
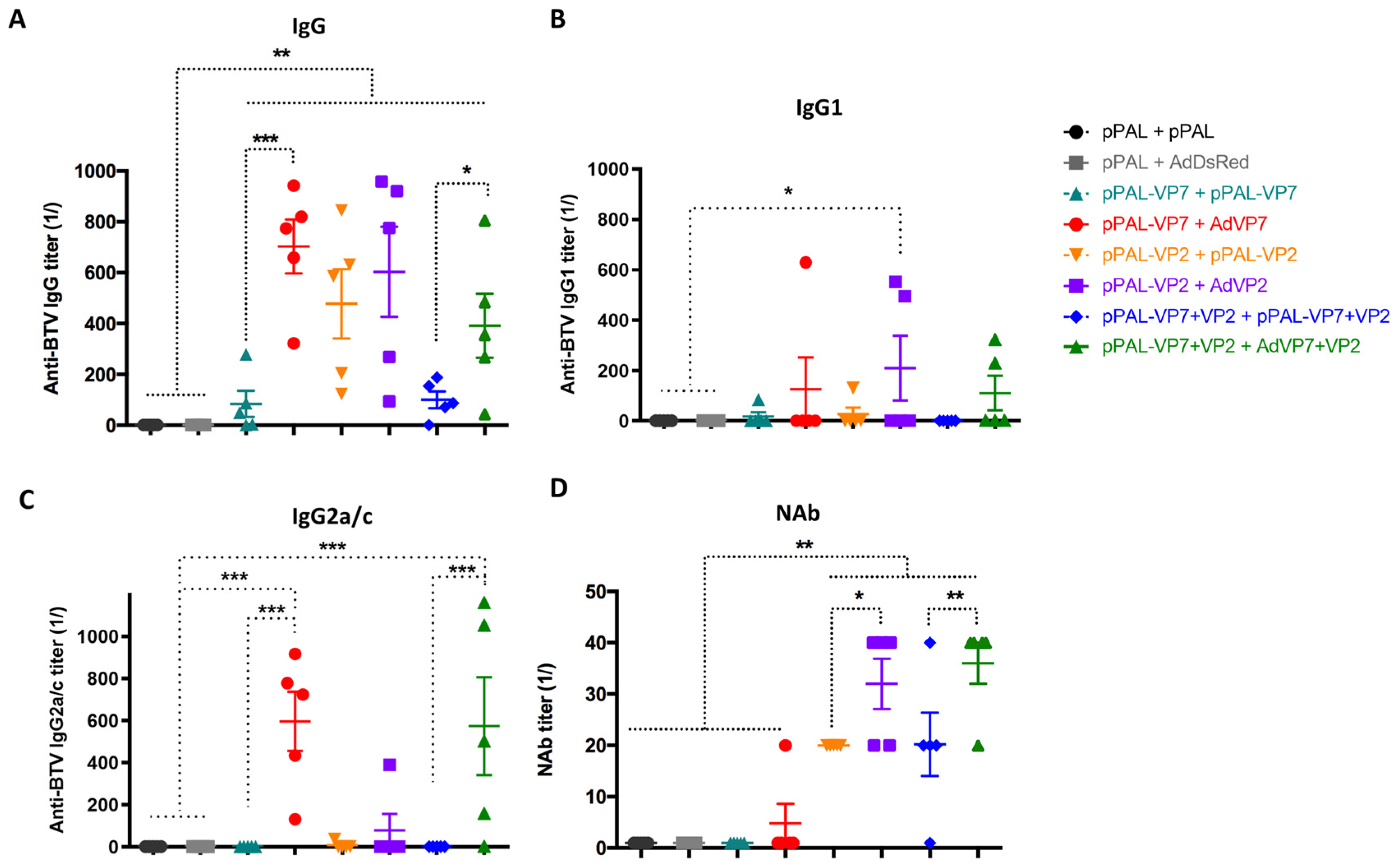
3.3. Heterologous pPAL Prime + Adenovirus Booster Vaccination Induces Potent Humoral Responses
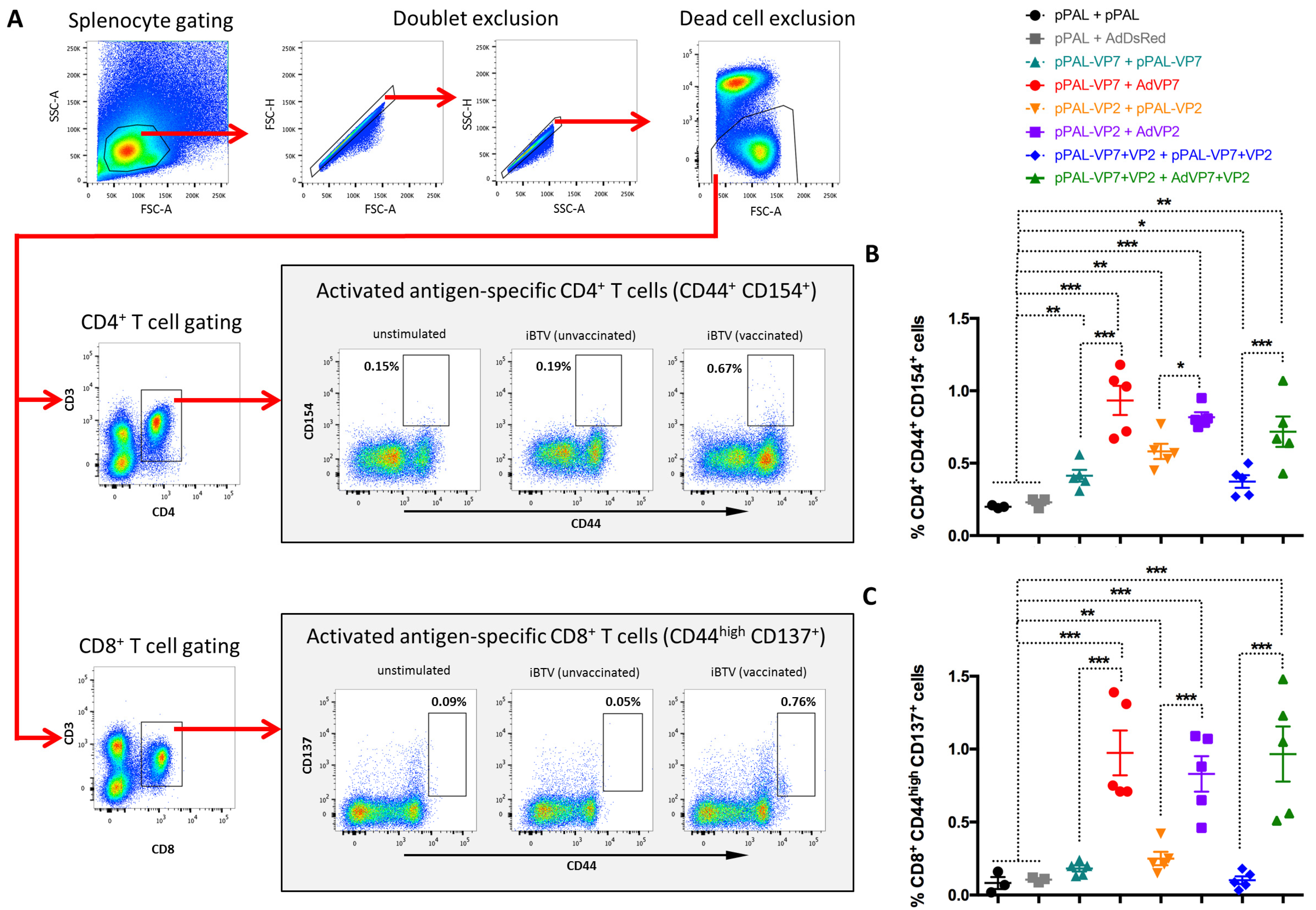
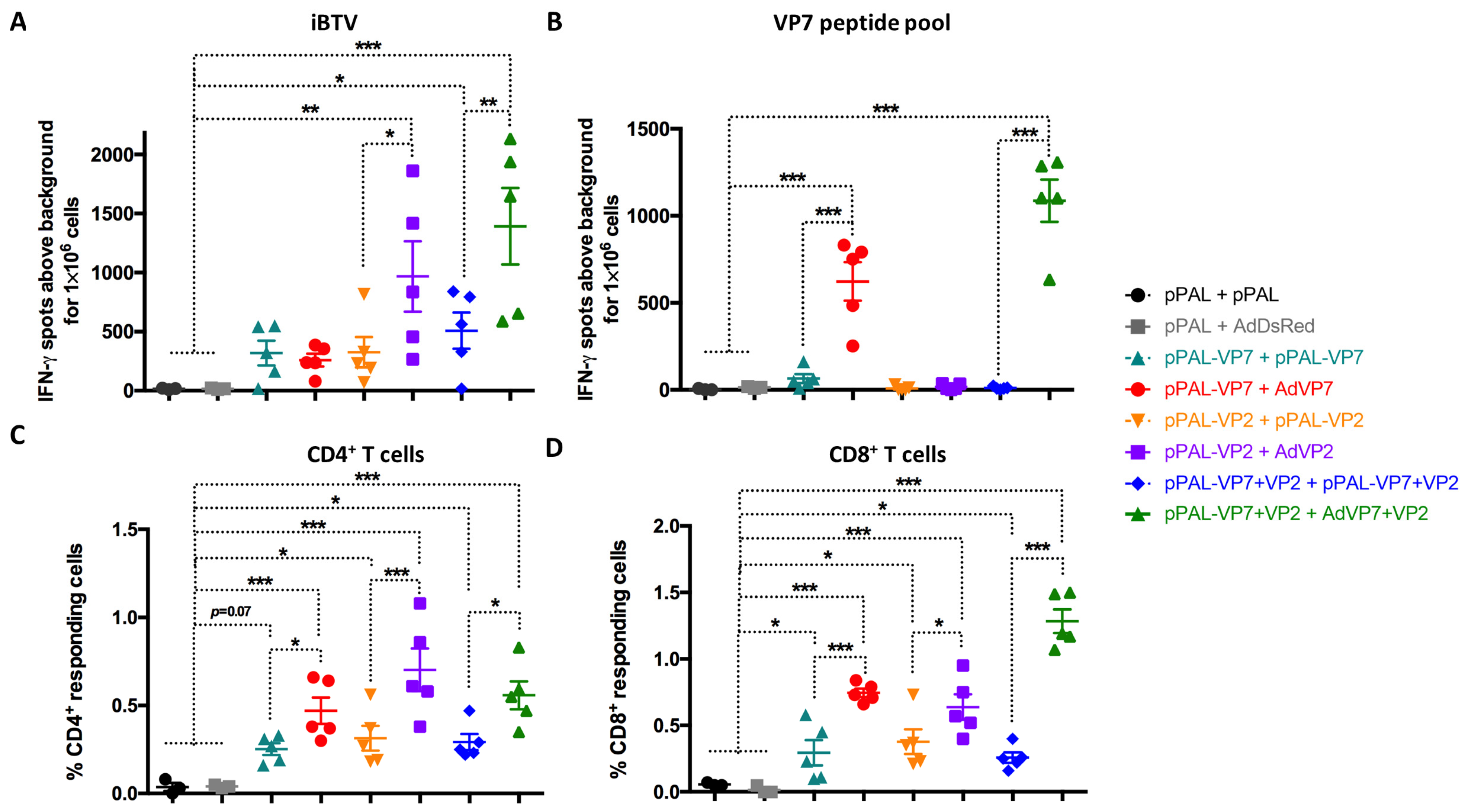
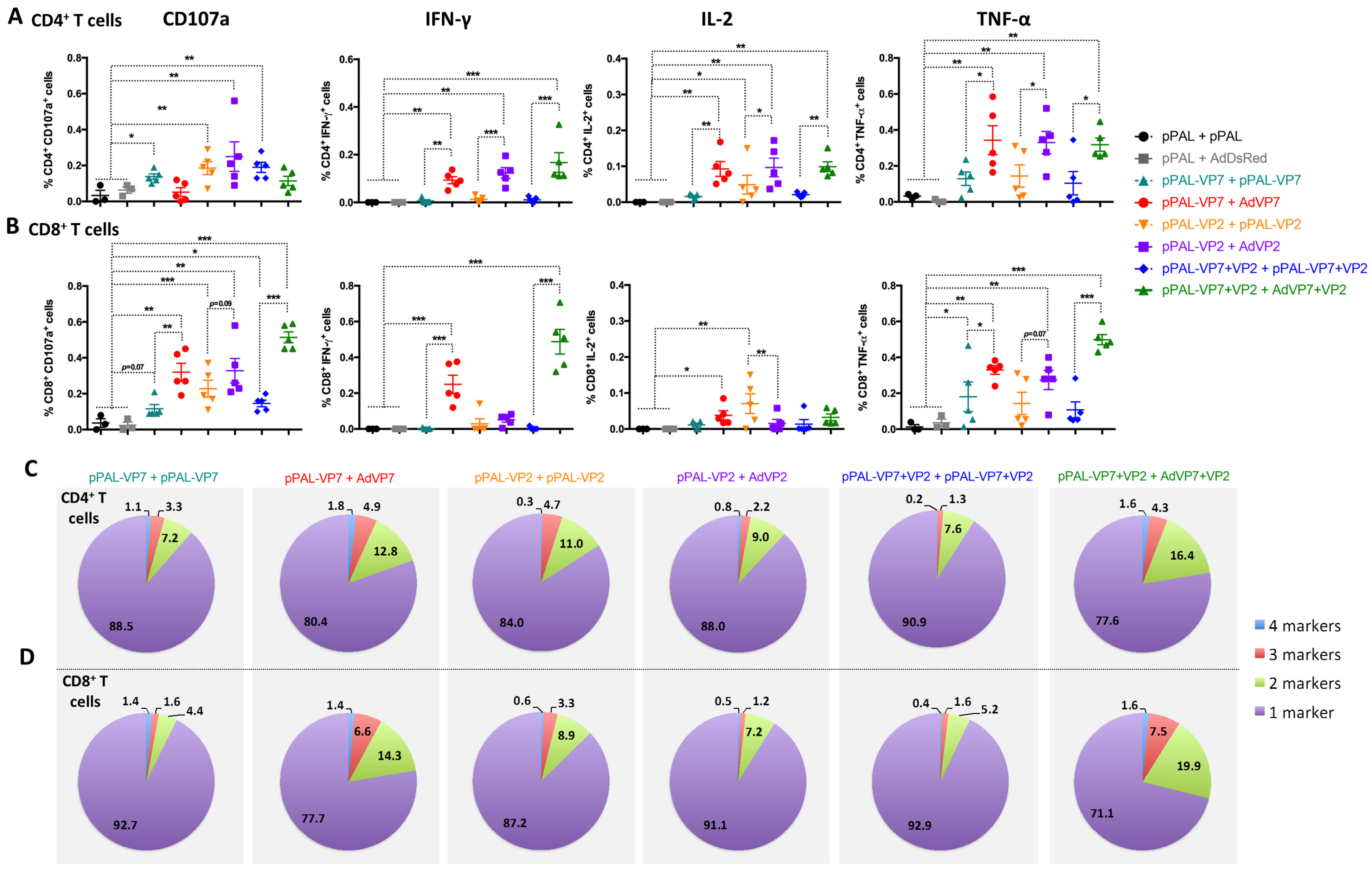
3.4. Heterologous pPAL Prime + Adenovirus Booster Vaccination Induces Potent Anti-BTV Cellular Responses
3.5. Vaccine-Induced Adaptive Immune Response Parameters Correlate with Protection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rojas, J.M.; Rodríguez-Martín, D.; Martín, V.; Sevilla, N. Diagnosing bluetongue virus in domestic ruminants: Current perspectives. Vet. Med. 2019, 10, 17–27. [Google Scholar] [CrossRef]
- Rojas, J.M.; Martín, V.; Sevilla, N. Vaccination as a Strategy to Prevent Bluetongue Virus Vertical Transmission. Pathogens 2021, 10, 1528. [Google Scholar] [CrossRef]
- Ben Salem, A.; Ben Aicha, E.; Kalthoum, S.; Dhaouadi, A.; Hajlaoui, H.; Bel Haj Mohamed, B.; Ben Slimen, I.; Khalfaoui, W.; Gharbi, R.; Guesmi, K.; et al. Estimation of the economic impact of a bluetongue serotype 4 outbreak in Tunisia. Front. Vet. Sci. 2024, 11, 1310202. [Google Scholar] [CrossRef]
- Gethmann, J.; Probst, C.; Conraths, F.J. Economic Impact of a Bluetongue Serotype 8 Epidemic in Germany. Front. Vet. Sci. 2020, 7, 65. [Google Scholar] [CrossRef]
- Rushton, J.; Lyons, N. Economic impact of Bluetongue: A review of the effects on production. Vet. Ital. 2015, 51, 401–406. [Google Scholar] [CrossRef]
- Santman-Berends, I.M.G.A.; Hage, J.J.; van Rijn, P.A.; Stegeman, J.A.; van Schaik, G. Bluetongue virus serotype 8 (BTV-8) infection reduces fertility of Dutch dairy cattle and is vertically transmitted to offspring. Theriogenology 2010, 74, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Attoui, H.; Nomikou, K.; Maan, S.; Belaganahalli, M.; Mertens, P.P.C. Orbiviruses. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Roy, P. Bluetongue virus assembly and exit pathways. Adv. Virus Res. 2020, 108, 249–273. [Google Scholar] [CrossRef]
- Stewart, M.; Hardy, A.; Barry, G.; Pinto, R.M.; Caporale, M.; Melzi, E.; Hughes, J.; Taggart, A.; Janowicz, A.; Varela, M.; et al. Characterization of a second open reading frame in genome segment 10 of bluetongue virus. J. Gen. Virol. 2015, 96, 3280–3293. [Google Scholar] [CrossRef]
- Baylis, M.; O’Connell, L.; Mellor, P.S. Rates of bluetongue virus transmission between Culicoides sonorensis and sheep. Med. Vet. Entomol. 2008, 22, 228–237. [Google Scholar] [CrossRef]
- Barratt-Boyes, S.M.; MacLachlan, N.J. Dynamics of viral spread in bluetongue virus infected calves. Vet. Microbiol. 1994, 40, 361–371. [Google Scholar] [CrossRef]
- Takamatsu, H.; Mellor, P.S.; Mertens, P.P.C.; Kirkham, P.A.; Burroughs, J.N.; Parkhouse, R.M.E. A possible overwintering mechanism for bluetongue virus in the absence of the insect vector. J. Gen. Virol. 2003, 84 Pt 1, 227–235. [Google Scholar] [CrossRef]
- Goffredo, M.; Catalani, M.; Federici, V.; Portanti, O.; Marini, V.; Mancini, G.; Quaglia, M.; Santilli, A.; Teodori, L.; Savini, G. Vector species of Culicoides midges implicated in the 2012–2014 Bluetongue epidemics in Italy. Vet. Ital. 2015, 51, 131–138. [Google Scholar] [CrossRef]
- van Rijn, P.A. Prospects of Next-Generation Vaccines for Bluetongue. Front. Vet. Sci. 2019, 6, 407. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.M.; Barba-Moreno, D.; Avia, M.; Sevilla, N.; Martín, V. Vaccination with Recombinant Adenoviruses Expressing the Bluetongue Virus Subunits VP7 and VP2 Provides Protection Against Heterologous Virus Challenge. Front. Vet. Sci. 2021, 8, 645561. [Google Scholar] [CrossRef]
- Calvo-Pinilla, E.; Navasa, N.; Anguita, J.; Ortego, J. Multiserotype protection elicited by a combinatorial prime-boost vaccination strategy against bluetongue virus. PLoS ONE 2012, 7, e34735. [Google Scholar] [CrossRef]
- Utrilla-Trigo, S.; Jiménez-Cabello, L.; Calvo-Pinilla, E.; Marín-López, A.; Lorenzo, G.; Sánchez-Cordón, P.; Moreno, S.; Benavides, J.; Gilbert, S.; Nogales, A.; et al. The Combined Expression of the Nonstructural Protein NS1 and the N-Terminal Half of NS2 (NS2(1-180)) by ChAdOx1 and MVA Confers Protection against Clinical Disease in Sheep upon Bluetongue Virus Challenge. J. Virol. 2022, 96, e01614-21. [Google Scholar] [CrossRef]
- Jeggo, M.H.; Wardley, R.C.; Brownlie, J. A study of the role of cell-mediated immunity in bluetongue virus infection in sheep, using cellular adoptive transfer techniques. Immunology 1984, 52, 403–410. [Google Scholar]
- Jeggo, M.H.; Wardley, R.C.; Taylor, W.P. Role of neutralising antibody in passive immunity to bluetongue infection. Res. Vet. Sci. 1984, 36, 81–86. [Google Scholar] [CrossRef]
- Rojas, J.M.; Peña, L.; Martín, V.; Sevilla, N. Ovine and murine T cell epitopes from the non-structural protein 1 (NS1) of bluetongue virus serotype 8 (BTV-8) are shared among viral serotypes. Vet. Res. 2014, 45, 30. [Google Scholar] [CrossRef]
- Rojas, J.M.; Rodriguez-Calvo, T.; Pena, L.; Sevilla, N. T cell responses to bluetongue virus are directed against multiple and identical CD4+ and CD8+ T cell epitopes from the VP7 core protein in mouse and sheep. Vaccine 2011, 29, 6848–6857. [Google Scholar] [CrossRef]
- Martin, V.; Pascual, E.; Avia, M.; Pena, L.; Valcarcel, F.; Sevilla, N. Protective Efficacy in Sheep of Adenovirus-Vectored Vaccines against Bluetongue Virus Is Associated with Specific T Cell Responses. PLoS ONE 2015, 10, e0143273. [Google Scholar] [CrossRef]
- Marín-López, A.; Calvo-Pinilla, E.; Barriales, D.; Lorenzo, G.; Brun, A.; Anguita, J.; Ortego, J. CD8 T Cell Responses to an Immunodominant Epitope within the Nonstructural Protein NS1 Provide Wide Immunoprotection against Bluetongue Virus in IFNAR(−/−) Mice. J. Virol. 2018, 92, e00938-18. [Google Scholar] [CrossRef]
- Alonso, A.; Alcolea, P.J.; Larraga, J.; Peris, M.P.; Esteban, A.; Cortés, A.; Ruiz-García, S.; Castillo, J.A.; Larraga, V. A non-replicative antibiotic resistance-free DNA vaccine delivered by the intranasal route protects against canine leishmaniasis. Front. Immunol. 2023, 14, 1213193. [Google Scholar] [CrossRef]
- Alcolea, P.J.; Larraga, J.; Rodriguez-Martin, D.; Alonso, A.; Loayza, F.J.; Rojas, J.M.; Ruiz-Garcia, S.; Louloudes-Lazaro, A.; Carlon, A.B.; Sanchez-Cordon, P.J.; et al. Non-replicative antibiotic resistance-free DNA vaccine encoding S and N proteins induces full protection in mice against SARS-CoV-2. Front. Immunol. 2022, 13, 1023255. [Google Scholar] [CrossRef]
- Avia, M.; Rojas, J.M.; Miorin, L.; Pascual, E.; Van Rijn, P.A.; Martín, V.; García-Sastre, A.; Sevilla, N. Virus-induced autophagic degradation of STAT2 as a mechanism for interferon signaling blockade. EMBO Rep. 2019, 20, e48766. [Google Scholar] [CrossRef]
- Rodriguez-Calvo, T.; Rojas, J.M.; Martin, V.; Sevilla, N. Type I interferon limits the capacity of bluetongue virus to infect hematopoietic precursors and dendritic cells in vitro and in vivo. J. Virol. 2014, 88, 859–867. [Google Scholar] [CrossRef]
- Calvo-Pinilla, E.; Rodriguez-Calvo, T.; Anguita, J.; Sevilla, N.; Ortego, J. Establishment of a bluetongue virus infection model in mice that are deficient in the alpha/beta interferon receptor. PLoS ONE 2009, 4, e5171. [Google Scholar] [CrossRef]
- Puigbò, P.; Guzmán, E.; Romeu, A.; Garcia-Vallvé, S. OPTIMIZER: A web server for optimizing the codon usage of DNA sequences. Nucleic Acids Res. 2007, 35, W126–W131. [Google Scholar] [CrossRef]
- Louloudes-Lazaro, A.; Rojas, J.M.; Garcia-Garcia, I.; Rodriguez-Martin, D.; Morel, E.; Martin, V.; Sevilla, N. Comprehensive immune profiling reveals that Orbivirus infection activates immune checkpoints during acute T cell immunosuppression. Front. Immunol. 2023, 14, 1255803. [Google Scholar] [CrossRef]
- Stevens, T.L.; Bossie, A.; Sanders, V.M.; Fernandez-Botran, R.; Coffman, R.L.; Mosmann, T.R.; Vitetta, E.S. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature 1988, 334, 255–258. [Google Scholar] [CrossRef]
- Frentsch, M.; Arbach, O.; Kirchhoff, D.; Moewes, B.; Worm, M.; Rothe, M.; Scheffold, A.; Thiel, A. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat. Med. 2005, 11, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Wölfl, M.; Kuball, J.; Eyrich, M.; Schlegel, P.G.; Greenberg, P.D. Use of CD137 to study the full repertoire of CD8+ T cells without the need to know epitope specificities. Cytom. Part A J. Int. Soc. Anal. Cytol. 2008, 73, 1043–1049. [Google Scholar] [CrossRef]
- Proctor, J.; Wolf, I.; Brodsky, D.; Cortes, L.M.; Frias-De-Diego, A.; Almond, G.W.; Crisci, E.; Negrão Watanabe, T.T.; Hammer, J.M.; Käser, T. Heterologous vaccine immunogenicity, efficacy, and immune correlates of protection of a modified-live virus porcine reproductive and respiratory syndrome virus vaccine. Front. Microbiol. 2022, 13, 977796. [Google Scholar] [CrossRef]
- Calvo-Pinilla, E.; Rodríguez-Calvo, T.; Sevilla, N.; Ortego, J. Heterologous prime boost vaccination with DNA and recombinant modified vaccinia virus Ankara protects IFNAR(-/-) mice against lethal bluetongue infection. Vaccine 2009, 28, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Marín-López, A.; Calvo-Pinilla, E.; Barriales, D.; Lorenzo, G.; Benavente, J.; Brun, A.; Martínez-Costas, J.M.; Ortego, J. Microspheres-prime/rMVA-boost vaccination enhances humoral and cellular immune response in IFNAR(−/−) mice conferring protection against serotypes 1 and 4 of bluetongue virus. Antivir. Res. 2017, 142, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Utrilla-Trigo, S.; Jiménez-Cabello, L.; Alonso-Ravelo, R.; Calvo-Pinilla, E.; Marín-López, A.; Moreno, S.; Lorenzo, G.; Benavides, J.; Gilbert, S.; Nogales, A.; et al. Heterologous Combination of ChAdOx1 and MVA Vectors Expressing Protein NS1 as Vaccination Strategy to Induce Durable and Cross-Protective CD8+ T Cell Immunity to Bluetongue Virus. Vaccines 2020, 8, 346. [Google Scholar] [CrossRef] [PubMed]
- Caddy, S.L.; Vaysburd, M.; Wing, M.; Foss, S.; Andersen, J.T.; O’Connell, K.; Mayes, K.; Higginson, K.; Iturriza-Gómara, M.; Desselberger, U.; et al. Intracellular neutralisation of rotavirus by VP6-specific IgG. PLoS Pathog. 2020, 16, e1008732. [Google Scholar] [CrossRef]
- Corthésy, B.; Benureau, Y.; Perrier, C.; Fourgeux, C.; Parez, N.; Greenberg, H.; Schwartz-Cornil, I. Rotavirus anti-VP6 secretory immunoglobulin A contributes to protection via intracellular neutralization but not via immune exclusion. J. Virol. 2006, 80, 10692–10699. [Google Scholar] [CrossRef]
- Li, X.; Yang, L.; Chen, S.; Zheng, J.; Zhang, H.; Ren, L. Multiple Roles of TRIM21 in Virus Infection. Int. J. Mol. Sci. 2023, 24, 1683. [Google Scholar] [CrossRef]
- White, J.R.; Eaton, B.T. Conformation of the VP2 protein of bluetongue virus (BTV) determines the involvement in virus neutralization of highly conserved epitopes within the BTV serogroup. J. Gen. Virol. 1990, 71 Pt 6, 1325–1332. [Google Scholar] [CrossRef]
- Jiménez-Cabello, L.; Utrilla-Trigo, S.; Calvo-Pinilla, E.; Lorenzo, G.; Illescas-Amo, M.; Benavides, J.; Moreno, S.; Marín-López, A.; Nogales, A.; Ortego, J. Co-expression of VP2, NS1 and NS2-Nt proteins by an MVA viral vector induces complete protection against bluetongue virus. Front. Immunol. 2024, 15, 1440407. [Google Scholar] [CrossRef]
- Kozak, M.; Hu, J. DNA Vaccines: Their Formulations, Engineering and Delivery. Vaccines 2024, 12, 71. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, B.; Pan, J.; Feng, Y.; Ye, W.; Xu, J.; Lan, M.; Sun, H.; Zhang, X.; Sun, Y.; et al. Construction and evaluation of DNA vaccine encoding Ebola virus glycoprotein fused with lysosome-associated membrane protein. Antivir. Res. 2021, 193, 105141. [Google Scholar] [CrossRef]
- Cenerenti, M.; Saillard, M.; Romero, P.; Jandus, C. The Era of Cytotoxic CD4 T Cells. Front. Immunol. 2022, 13, 867189. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, D.; Bangs, D.J.; Sidney, J.; Kolla, R.V.; De Silva, A.D.; de Silva, A.M.; Crotty, S.; Peters, B.; Sette, A. Dengue virus infection elicits highly polarized CX3CR1+ cytotoxic CD4+ T cells associated with protective immunity. Proc. Natl. Acad. Sci. USA 2015, 112, E4256–E4263. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, T.M.; Li, C.K.; Chui, C.S.; Huang, A.K.; Perkins, M.; Liebner, J.C.; Lambkin-Williams, R.; Gilbert, A.; Oxford, J.; Nicholas, B.; et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 2012, 18, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Kuo, H.H.; Boucau, J.; Farmer, J.R.; Allard-Chamard, H.; Mahajan, V.S.; Piechocka-Trocha, A.; Lefteri, K.; Osborn, M.; Bals, J.; et al. Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19. Cell 2020, 183, 143–157.e13. [Google Scholar] [CrossRef]
- Newbrook, K.; Khan, N.; Fisher, A.; Chong, K.; Gubbins, S.; Davies, W.C.; Sanders, C.; Busquets, M.G.; Cooke, L.; Corla, A.; et al. Specific T-cell subsets have a role in anti-viral immunity and pathogenesis but not viral dynamics or onwards vector transmission of an important livestock arbovirus. Front. Immunol. 2024, 15, 1328820. [Google Scholar] [CrossRef]
- Rodriguez-Martin, D.; Louloudes-Lazaro, A.; Avia, M.; Martin, V.; Rojas, J.M.; Sevilla, N. The Interplay between Bluetongue Virus Infections and Adaptive Immunity. Viruses 2021, 13, 1511. [Google Scholar] [CrossRef]
- Rojas, J.M.; Rodriguez-Calvo, T.; Sevilla, N. Recall T cell responses to bluetongue virus produce a narrowing of the T cell repertoire. Vet. Res. 2017, 48, 38. [Google Scholar] [CrossRef]
- Wong, G.; Qiu, X.G. Type I interferon receptor knockout mice as models for infection of highly pathogenic viruses with outbreak potential. Zool. Res. 2018, 39, 3–14. [Google Scholar] [CrossRef]
- Marín-Lopez, A.; Calvo-Pinilla, E.; Moreno, S.; Utrilla-Trigo, S.; Nogales, A.; Brun, A.; Fikrig, E.; Ortego, J. Modeling Arboviral Infection in Mice Lacking the Interferon Alpha/Beta Receptor. Viruses 2019, 11, 35. [Google Scholar] [CrossRef]
- Rojas, J.M.; Alejo, A.; Martín, V.; Sevilla, N. Viral pathogen-induced mechanisms to antagonize mammalian interferon (IFN) signaling pathway. Cell. Mol. Life Sci. 2021, 78, 1423–1444. [Google Scholar] [CrossRef]
- Rojas, J.M.; Avia, M.; Pascual, E.; Sevilla, N.; Martin, V. Vaccination with recombinant adenovirus expressing peste des petits ruminants virus-F or -H proteins elicits T cell responses to epitopes that arises during PPRV infection. Vet. Res. 2017, 48, 79. [Google Scholar] [CrossRef]
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): A single-blind, randomised, non-inferiority trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef]
- Sakurai, F.; Tachibana, M.; Mizuguchi, H. Adenovirus vector-based vaccine for infectious diseases. Drug Metab. Pharmacokinet. 2022, 42, 100432. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Schönbrunn, A.; Fischer, D.; Liu, Y.; Hocher, J.-G.; Weinerth, J.; Klemm, K.; von Baehr, V.; Krämer, B.K.; Elitok, S.; et al. Immune response of heterologous versus homologous prime-boost regimens with adenoviral vectored and mRNA COVID-19 vaccines in immunocompromised patients. Front. Immunol. 2023, 14, 1187880. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Bagheri, N.; Bradshaw, E.M.; Hafler, D.A.; Lauffenburger, D.A.; Love, J.C. Polyfunctional responses by human T cells result from sequential release of cytokines. Proc. Natl. Acad. Sci. USA 2012, 109, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Ratto-Kim, S.; Currier, J.R.; Cox, J.H.; Excler, J.L.; Valencia-Micolta, A.; Thelian, D.; Lo, V.; Sayeed, E.; Polonis, V.R.; Earl, P.L.; et al. Heterologous prime-boost regimens using rAd35 and rMVA vectors elicit stronger cellular immune responses to HIV proteins than homologous regimens. PLoS ONE 2012, 7, e45840. [Google Scholar] [CrossRef]
- Siddiqui, A.; Adnan, A.; Abbas, M.; Taseen, S.; Ochani, S.; Essar, M.Y. Revival of the heterologous prime-boost technique in COVID-19: An outlook from the history of outbreaks. Health Sci. Rep. 2022, 5, e531. [Google Scholar] [CrossRef]
- Heinen, N.; Marheinecke, C.S.; Bessen, C.; Blazquez-Navarro, A.; Roch, T.; Stervbo, U.; Anft, M.; Plaza-Sirvent, C.; Busse, S.; Klöhn, M.; et al. In-depth analysis of T cell immunity and antibody responses in heterologous prime-boost-boost vaccine regimens against SARS-CoV-2 and Omicron variant. Front. Immunol. 2022, 13, 1062210. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.-C.; Li, Y.-H.; Guan, X.-H.; Hou, L.-H.; Wang, W.-J.; Li, J.-X.; Wu, S.-P.; Wang, B.-S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
| Immune Parameter | Ct(BTV S5) D3pi | Ct(BTV S5) D5pi | p-Value | |
|---|---|---|---|---|
| IgG titer | 0.541 | 0.611 | p < 10−3 | |
| NAb titer | 0.671 | 0.703 | p < 10−4 | |
| IFN-γ counts (ELISpot) | 0.626 | 0.682 | p < 10−5 | |
| CD4+CD44+CD154+ cells | 0.600 | 0.653 | p < 10−6 | |
| CD8+CD44highCD137+ cells | 0.696 | 0.674 | p < 10−7 | |
| CD4+(CD107a+/IFN-γ+/TNF-α+/IL-2+) cells | 0.694 | 0.768 | ||
| CD8+ (CD107a+/IFN-γ+/TNF-α+/IL-2+) cells | 0.791 | 0.814 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogales-Altozano, P.; Gómez-Marcos, L.; Carlón, A.B.; Louloudes-Lázaro, A.; Rivera-Rodríguez, A.; Larraga, J.; Alcolea, P.J.; Alonso, A.; Larraga, V.; Martín, V.; et al. Heterologous DNA–Adenovirus Prime–Boost Strategy Expressing Bluetongue Virus VP2 and VP7 Proteins Protects Against Virulent Challenge. Vaccines 2025, 13, 991. https://doi.org/10.3390/vaccines13090991
Nogales-Altozano P, Gómez-Marcos L, Carlón AB, Louloudes-Lázaro A, Rivera-Rodríguez A, Larraga J, Alcolea PJ, Alonso A, Larraga V, Martín V, et al. Heterologous DNA–Adenovirus Prime–Boost Strategy Expressing Bluetongue Virus VP2 and VP7 Proteins Protects Against Virulent Challenge. Vaccines. 2025; 13(9):991. https://doi.org/10.3390/vaccines13090991
Chicago/Turabian StyleNogales-Altozano, Pablo, Laro Gómez-Marcos, Ana Belén Carlón, Andrés Louloudes-Lázaro, Alicia Rivera-Rodríguez, Jaime Larraga, Pedro J. Alcolea, Ana Alonso, Vicente Larraga, Verónica Martín, and et al. 2025. "Heterologous DNA–Adenovirus Prime–Boost Strategy Expressing Bluetongue Virus VP2 and VP7 Proteins Protects Against Virulent Challenge" Vaccines 13, no. 9: 991. https://doi.org/10.3390/vaccines13090991
APA StyleNogales-Altozano, P., Gómez-Marcos, L., Carlón, A. B., Louloudes-Lázaro, A., Rivera-Rodríguez, A., Larraga, J., Alcolea, P. J., Alonso, A., Larraga, V., Martín, V., Rojas, J. M., & Sevilla, N. (2025). Heterologous DNA–Adenovirus Prime–Boost Strategy Expressing Bluetongue Virus VP2 and VP7 Proteins Protects Against Virulent Challenge. Vaccines, 13(9), 991. https://doi.org/10.3390/vaccines13090991






