An In-House ELISA for Anti-Porcine Circovirus Type 2d (PCV2d) IgG: Analytical Validation and Serological Correlation
Abstract
1. Introduction
2. Materials and Methods
2.1. Vaccine Preparation Process
2.2. Quantitative Real-Time Polymerase Chain Reaction of PCV2d DNA for Viral Titration
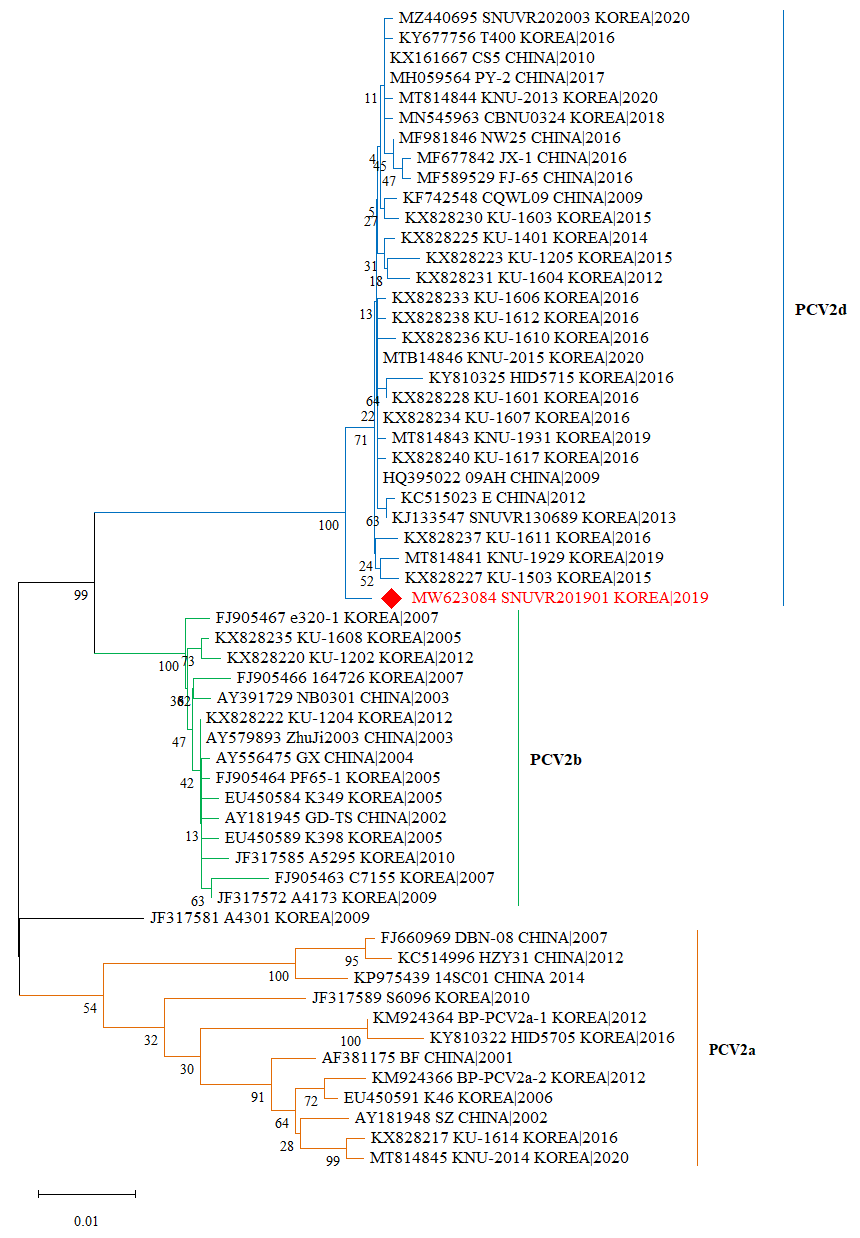
2.3. Phylogenetic Analysis
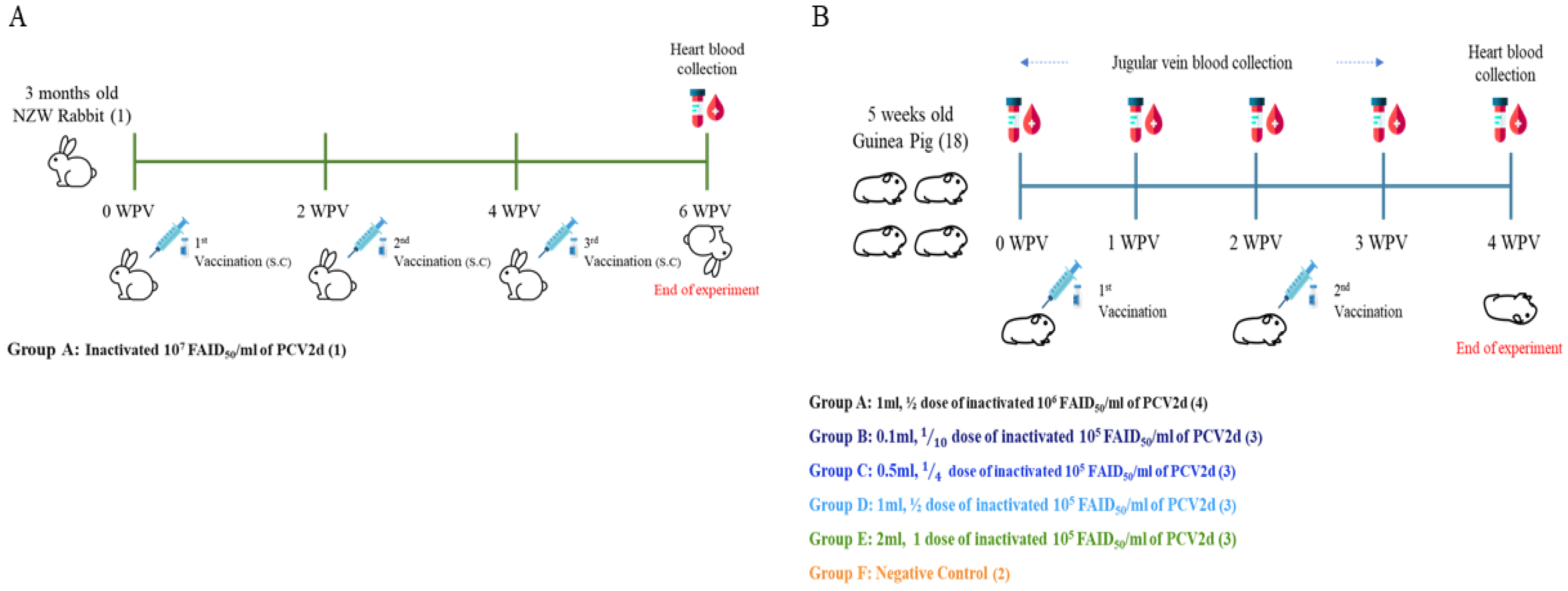
2.4. Animal Study
2.4.1. Production of In-House Polyclonal Antibodies (Rabbit Anti-PCV2d IgG)
2.4.2. Evaluation of PCV2d-Specific Antibodies by Guinea Pig Antiserum
2.4.3. Application of Guinea Pig and Field Pig Sera for ELISA Validation
2.5. Purification and Optimization of an In-House Antibody (WG-PCV2d pAb)
2.6. Analytical Methods of In-House Antibody Characterization
2.7. Construction and Standardization of an In-House PCV2d Indirect ELISA
2.7.1. Standard Curve
2.7.2. LOD (Limited of Detection)
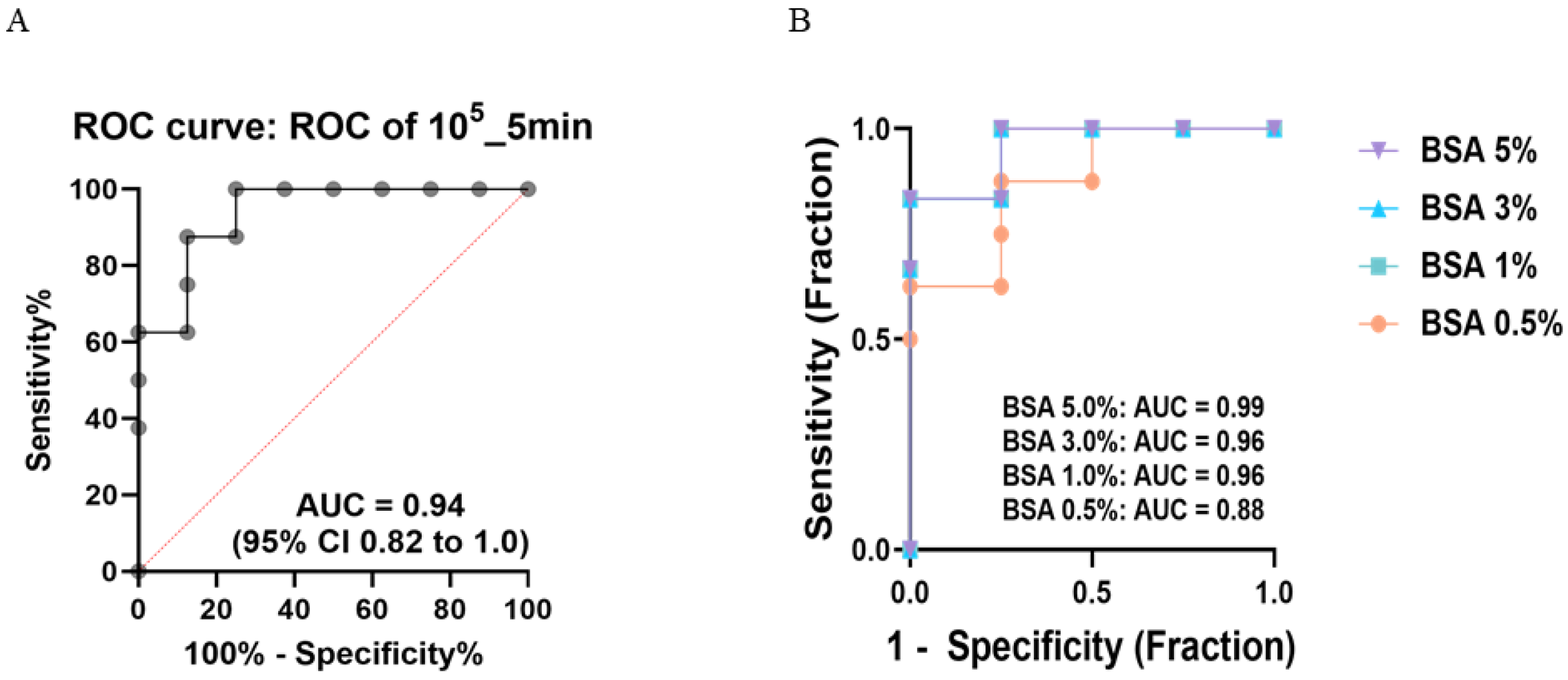
2.7.3. ROC (Receiver Operating Characterization)
2.7.4. SNR (Signal-to-Noise Ratio)
2.7.5. Establish the Cut-Off Value of an In-House ELISA
- *Bmean: The mean of the blank’s OD;
- #σB: The standard deviation of the blank.
2.7.6. Precision
- *σintra: The standard deviation of replicate OD values within a single plate;
- #μintra: The mean OD value of the replicates within the plate.
- *σintra: The standard deviation of mean OD values from multiple plates;
- #μintra: The overall mean OD value across plates.
2.7.7. Analytical Specificity via a Competitive Binding Assay
2.7.8. Robustness
2.7.9. Validation Through Comparison with a Commercialized ELISA Kit
2.7.10. Data Analysis
3. Results
3.1. Phylogenetic Analysis of IMMUNIS® DMVac
3.2. Characterization of the In-House Antibody (Rabbit Anti PCV2d IgG)
3.3. Optimization of Key ELISA Parameters: The Blocking Agent, Antigen Coating, and Substrate Reaction Time
3.4. Precision Evaluation of the In-House ELISA
3.5. Specificity and Signal Responsiveness of the In-House ELISA Under Optimized Conditions
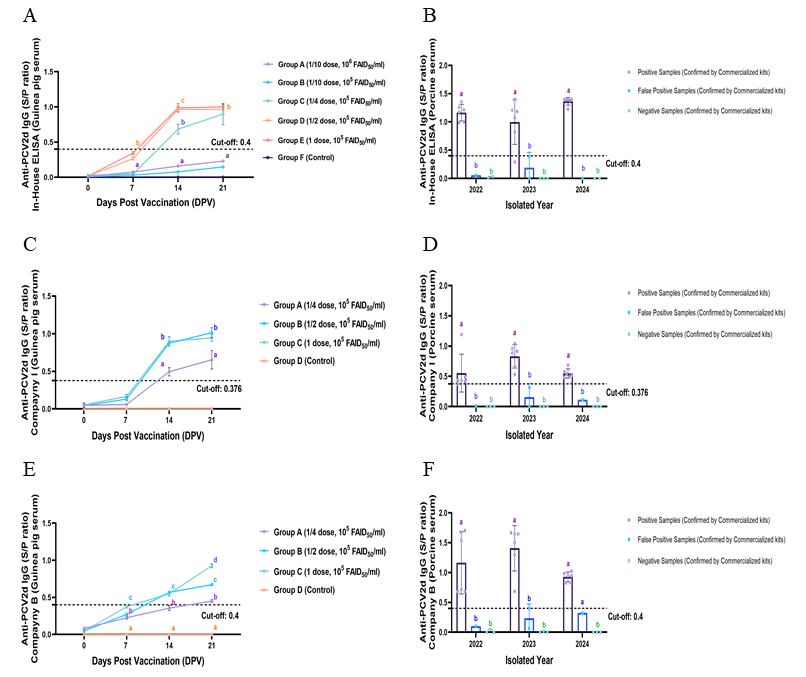
3.6. Comparative Diagnostic Performance of In-House and Commercial ELISA Kits
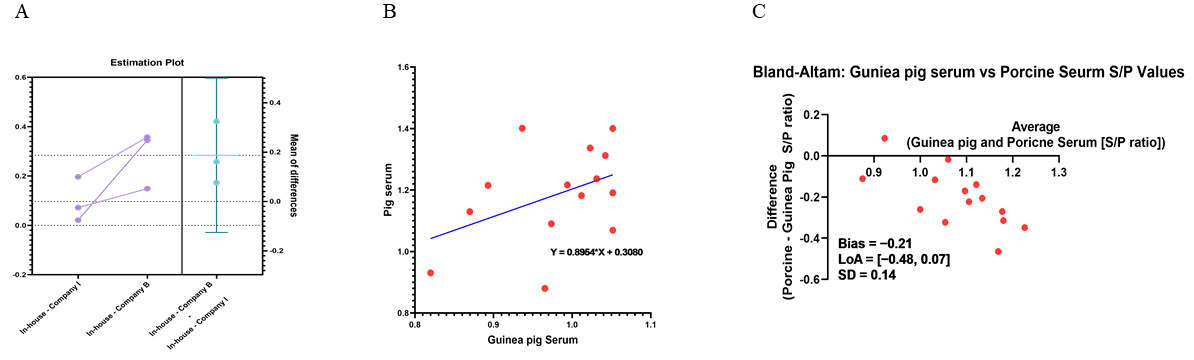
3.7. Consistency and Predictive Validity of the In-House ELISA Compared to Commercial Kits and Porcine Sera
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCV | Porcine Circovirus |
| PRRSV | Porcine Reproductive and Respiratory Syndrome Virus |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| BSA | Bovine Serum Albumin |
| APQA | Animal and Plant Quarantine Agency |
| FAID50 | Fluorescent Antibody Infectious Dose50 |
| OD | Optical Density |
| ORF | Open Reading Frame |
| DPV | Days Post Vaccination |
| WPV | Weeks Post Vaccination |
| AVMS | American Veterinary Medical Association |
| NRC | National Research Council |
| HPLC | High Performance Liquid Chromatography |
| SDS-PAGE | Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis |
| kDa | Kilodalton |
| LOD | Limited of Detection |
| ROC | Receiver Operating Characterization |
| SNR | Signal to Noise Ratio |
| CV | Coefficient of Variation |
| AUC | Area Under Curve |
| IgG | Immunoglobulin G |
References
- Allan, G.M.; Ellis, J.A. Porcine circoviruses: A review. J. Vet. Diagn. Investig. 2000, 12, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Finsterbusch, T.; Steinfeldt, T.; Caliskan, R.; Mankertz, A. Analysis of the subcellular localization of the proteins Rep, Rep′ and Cap of porcine circovirus type 1. Virology 2005, 343, 36–46. [Google Scholar] [CrossRef]
- Opriessnig, T.; Karuppannan, A.K.; Castro, A.M.; Xiao, C.-T. Porcine circoviruses: Current status, knowledge gaps and challenges. Virus Res. 2020, 286, 198044. [Google Scholar] [CrossRef]
- Palinski, R.; Piñeyro, P.; Shang, P.; Yuan, F.; Guo, R.; Fang, Y.; Byers, E.; Hause, B.M. A novel porcine circovirus distantly related to known circoviruses is associated with porcine dermatitis and nephropathy syndrome and reproductive failure. J. Virol. 2017, 91, e01879-16. [Google Scholar] [CrossRef]
- Tischer, I.; Rasch, R.; Tochtermann, G. Characterization of papovavirus-and picornavirus-like particles in permanent pig kidney cell lines. Zentrablatt Bakteriol. Parasitenkd. Infekt. Hyg. Erste Abt. Orig. 1974, 226A, 153–167. [Google Scholar]
- Allan, G.; McNeilly, F.; Cassidy, J.; Reilly, G.; Adair, B.; Ellis, W.; McNulty, M. Pathogenesis of porcine circovirus; experimental infections of colostrum deprived piglets and examination of pig foetal material. Vet. Microbiol. 1995, 44, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Allan, G.; McNeilly, F.; McNair, I.; Curran, M.; Walker, I.; Ellis, J.; Konoby, C.; Kennedy, S.; Meehan, B. Absence of evidence for porcine circovirustype 2 in cattle and humans, and lack of seroconversion or lesions in experimentally infected sheep. Arch. Virol. 2000, 145, 853–857. [Google Scholar] [CrossRef]
- Matzinger, S.R.; Opriessnig, T.; Xiao, C.-T.; Catanzaro, N.; Beach, N.M.; Slade, D.E.; Nitzel, G.P.; Meng, X.-J. A chimeric virus created by DNA shuffling of the capsid genes of different subtypes of porcine circovirus type 2 (PCV2) in the backbone of the non-pathogenic PCV1 induces protective immunity against the predominant PCV2b and the emerging PCV2d in pigs. Virology 2016, 498, 82–93. [Google Scholar] [CrossRef]
- Segalés, J. Porcine circovirus type 2 (PCV2) infections: Clinical signs, pathology and laboratory diagnosis. Virus Res. 2012, 164, 10–19. [Google Scholar] [CrossRef]
- Kim, J.; Chung, H.-K.; Chae, C. Association of porcine circovirus 2 with porcine respiratory disease complex. Vet. J. 2003, 166, 251–256. [Google Scholar] [CrossRef]
- Kixmöller, M.; Ritzmann, M.; Eddicks, M.; Saalmüller, A.; Elbers, K.; Fachinger, V. Reduction of PMWS-associated clinical signs and co-infections by vaccination against PCV2. Vaccine 2008, 26, 3443–3451. [Google Scholar] [CrossRef]
- Harding, J.C. The clinical expression and emergence of porcine circovirus 2. Vet. Microbiol. 2004, 98, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.H.; Hu, W.Q.; Li, J.Y.; Liu, T.N.; Zhou, J.Y.; Opriessnig, T.; Xiao, C.T. Novel circovirus species identified in farmed pigs designated as Porcine circovirus 4, Hunan province, China. Transbound. Emerg. Dis. 2020, 67, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.G.; Do, H.Q.; Huynh, T.M.L.; Park, Y.H.; Park, B.K.; Chung, H.C. Molecular-based detection, genetic characterization and phylogenetic analysis of porcine circovirus 4 from Korean domestic swine farms. Transbound. Emerg. Dis. 2022, 69, 538–548. [Google Scholar] [CrossRef]
- Kim, H.-R.; Park, J.; Kim, W.-I.; Lyoo, Y.S.; Park, C.-K. Prevalence and co-infection status of three pathogenic porcine circoviruses (PCV2, PCV3, and PCV4) by a newly established triplex real-time polymerase chain reaction assay. Korean J. Vet. Serv. 2022, 45, 87–99. [Google Scholar] [CrossRef]
- Sirisereewan, C.; Nguyen, T.C.; Piewbang, C.; Jittimanee, S.; Kedkovid, R.; Thanawongnuwech, R. Molecular detection and genetic characterization of porcine circovirus 4 (PCV4) in Thailand during 2019–2020. Sci. Rep. 2023, 13, 5168. [Google Scholar] [CrossRef]
- Hou, C.Y.; Zhang, L.H.; Zhang, Y.H.; Cui, J.T.; Zhao, L.; Zheng, L.L.; Chen, H.Y. Phylogenetic analysis of porcine circovirus 4 in Henan Province of China: A retrospective study from 2011 to 2021. Transbound. Emerg. Dis. 2022, 69, 1890–1901. [Google Scholar] [CrossRef]
- Tian, R.B.; Zhao, Y.; Cui, J.T.; Zheng, H.H.; Xu, T.; Hou, C.Y.; Wang, Z.Y.; Li, X.S.; Zheng, L.L.; Chen, H.Y. Molecular detection and phylogenetic analysis of Porcine circovirus 4 in Henan and Shanxi Provinces of China. Transbound. Emerg. Dis. 2021, 68, 276–282. [Google Scholar] [CrossRef]
- Segalés, J.; Olvera, A.; Grau-Roma, L.; Charreyre, C.; Nauwynck, H.; Larsen, L.; Dupont, K.; McCullough, K.; Ellis, J.; Krakowka, S. PCV-2 genotype definition and nomenclature. Vet. Rec. 2008, 162, 867. [Google Scholar] [CrossRef]
- Chae, C. An emerging porcine circovirus type 2b mutant (mPCV2b) originally known as PCV2d. Vet. J. (Lond. Engl. 1997) 2014, 203, 6–9. [Google Scholar] [CrossRef]
- Franzo, G.; Segalés, J. Porcine circovirus 2 (PCV-2) genotype update and proposal of a new genotyping methodology. PLoS ONE 2018, 13, e0208585. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Park, G.-N.; Choe, S.; Cha, R.M.; Kim, S.-Y.; Hyun, B.-H.; Park, B.-K.; An, D.-J. Genetic diversity of porcine circovirus isolated from Korean wild boars. Pathogens 2020, 9, 457. [Google Scholar] [CrossRef]
- Kim, S.-C.; Nazki, S.; Kwon, S.; Juhng, J.-H.; Mun, K.-H.; Jeon, D.-Y.; Jeong, C.-G.; Khatun, A.; Kang, S.-J.; Kim, W.-I. The prevalence and genetic characteristics of porcine circovirus type 2 and 3 in Korea. BMC Vet. Res. 2018, 14, 294. [Google Scholar] [CrossRef]
- Wongchanapai, P.; Yamsakul, P.; Arunorat, J.; Guntawang, T.; Sittisak, T.; Srivorakul, S.; Photichai, K.; Thanawongnuwech, R.; Sukmak, M.; Pringproa, K. Comparative efficacy of chimeric porcine circovirus (PCV) vaccines against experimental heterologous PCV2d challenges. Vet. Sci. 2023, 10, 80. [Google Scholar] [CrossRef]
- Xiao, C.-T.; Halbur, P.G.; Opriessnig, T. Global molecular genetic analysis of porcine circovirus type 2 (PCV2) sequences confirms the presence of four main PCV2 genotypes and reveals a rapid increase of PCV2d. J. Gen. Virol. 2015, 96, 1830–1841. [Google Scholar] [CrossRef]
- Chae, C. Porcine circovirus type 2 and its associated diseases in Korea. Virus Res. 2012, 164, 107–113. [Google Scholar] [CrossRef]
- Moon, B.-M.; Chu, K.-S.; Kim, S.-C.; Kim, H.-J.; Kim, D.-J.; Kim, W.-I. Genotypic diversity and prevalence of Porcine circoviruses for slaughter pigs in Korea. Korean J. Vet. Serv. 2023, 46, 315–324. [Google Scholar] [CrossRef]
- Kang, S.-J.; Kang, H.; You, S.-H.; Lee, H.J.; Lee, N.; Hyun, B.-H.; Cha, S.-H. Genetic diversity and different cross-neutralization capability of porcine circovirus type 2 isolates recently circulating in South Korea. BMC Vet. Res. 2020, 16, 334. [Google Scholar] [CrossRef] [PubMed]
- Eddicks, M.; Fux, R.; Szikora, F.; Eddicks, L.; Majzoub-Altweck, M.; Hermanns, W.; Sutter, G.; Palzer, A.; Banholzer, E.; Ritzmann, M. Detection of a new cluster of porcine circovirus type 2b strains in domestic pigs in Germany. Vet. Microbiol. 2015, 176, 337–343. [Google Scholar] [CrossRef]
- Salgado, R.L.; Vidigal, P.M.; de Souza, L.F.; Onofre, T.S.; Gonzaga, N.F.; Eller, M.R.; Bressan, G.C.; Fietto, J.L.; Almeida, M.R.; Silva Júnior, A. Identification of an emergent porcine circovirus-2 in vaccinated pigs from a Brazilian farm during a postweaning multisystemic wasting syndrome outbreak. Genome Announc. 2014, 2, e00163-14. [Google Scholar] [CrossRef]
- Kim, K.; Choi, K.; Shin, M.; Hahn, T.-W. A porcine circovirus type 2d-based virus-like particle vaccine induces humoral and cellular immune responses and effectively protects pigs against PCV2d challenge. Front. Microbiol. 2024, 14, 1334968. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Hou, L.; Zhou, J.; Wang, D.; Cui, Y.; Feng, X.; Liu, J. Porcine circovirus type 2 vaccines: Commercial application and research advances. Viruses 2022, 14, 2005. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.; Suh, J.; Kim, C.; Seo, B.J.; Park, G.S.; Chae, C. A field evaluation of a new porcine circovirus type 2d and Mycoplasma hyopneumoniae bivalent vaccine in herds suffering from subclinical PCV2d infection and enzootic pneumonia. Vet. Med. Sci. 2024, 10, e70001. [Google Scholar] [CrossRef]
- Suh, J.; Ham, S.; Oh, T.; Chae, C. Cross-protection of a porcine circovirus types 2a/b (PCV-2a/b) and Mycoplasma hyopneumoniae trivalent vaccine against a dual PCV-2e and Mycoplasma hyopneumoniae challenge. Can. J. Vet. Res. 2023, 87, 297–302. [Google Scholar]
- Kim, K.; Hahn, T.-W. Evaluation of novel recombinant porcine circovirus type 2d (PCV2d) vaccine in pigs naturally infected with PCV2d. Vaccine 2021, 39, 529–535. [Google Scholar] [CrossRef]
- Ju, L.; Jayaramaiah, U.; Lee, M.-A.; Jeong, Y.-J.; You, S.-H.; Lee, H.-S.; Hyun, B.-H.; Lee, N.; Kang, S.-J. A Field Efficacy Trial of Recombinant Porcine Circovirus Type 2d Vaccine in Three Herds. Vaccines 2023, 11, 1497. [Google Scholar] [CrossRef]
- Ju, L.; You, S.-H.; Lee, M.-A.; Jayaramaiah, U.; Jeong, Y.-J.; Lee, H.-S.; Hyun, B.-H.; Lee, N.; Kang, S.-J. Selection and Evaluation of Porcine circovirus (PCV) 2d Vaccine Strains to Protect against Currently Prevalent PCV2. Vaccines 2023, 11, 1447. [Google Scholar] [CrossRef]
- Park, S.-C.; Park, J.Y.; Choi, J.Y.; Lee, S.-G.; Eo, S.K.; Oem, J.-K.; Tark, D.-S.; You, M.; Yu, D.-H.; Chae, J.-S. Pathogenicity of severe fever with thrombocytopenia syndrome virus in mice regulated in type I interferon signaling: Severe fever with thrombocytopenia and type I interferon. Lab. Anim. Res. 2020, 36, 38. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.; Suh, J.; Oh, T.; Kim, C.; Seo, B.-J.; Chae, C. Efficacy of a novel bivalent vaccine containing porcine circovirus type 2d and Mycoplasma hyopneumoniae against a dual PCV2d and Mycoplasma hyopneumoniae challenge. Front. Vet. Sci. 2023, 10, 1176091. [Google Scholar] [CrossRef]
- Kang, S.-J.; Bae, S.-M.; Lee, H.-J.; Jeong, Y.-J.; Lee, M.-A.; You, S.-H.; Lee, H.-S.; Hyun, B.-H.; Lee, N.; Cha, S.-H. Porcine circovirus (PCV) genotype 2d-based virus-like particles (VLPs) induced broad cross-neutralizing antibodies against diverse genotypes and provided protection in dual-challenge infection of a PCV2d virus and a type 1 porcine reproductive and respiratory syndrome virus (PRRSV). Pathogens 2021, 10, 1145. [Google Scholar]
- Noh, Y.-H.; Kim, S.-C.; Jeong, C.-G.; Lee, S.-C.; Lee, D.-U.; Yoon, I.-J.; Kim, W.-I. Pathological evaluation of porcine circovirus 2d (PCV2d) strain and comparative evaluation of PCV2d and PCV2b inactivated vaccines against PCV2d infection in a specific pathogen-free (SPF) Yucatan miniature pig model. Vaccines 2022, 10, 1469. [Google Scholar] [CrossRef] [PubMed]
- Leenaars, M.; Hendriksen, C.F. Critical steps in the production of polyclonal and monoclonal antibodies: Evaluation and recommendations. ILAR J. 2005, 46, 269–279. [Google Scholar] [CrossRef]
- Onder, S.; Van Grol, M.; Fidder, A.; Xiao, G.; Noort, D.; Yerramalla, U.; Tacal, O.; Schopfer, L.M.; Lockridge, O. Rabbit antidiethoxyphosphotyrosine antibody, made by single B cell cloning, detects chlorpyrifos oxon-modified proteins in cultured cells and immunopurifies modified peptides for mass spectrometry. J. Proteome Res. 2021, 20, 4728–4745. [Google Scholar] [CrossRef]
- Ghosh, P.; Bhaskar, K.R.; Hossain, F.; Khan, M.A.A.; Vallur, A.C.; Duthie, M.S.; Hamano, S.; Salam, M.A.; Huda, M.M.; Khan, M.G.M. Evaluation of diagnostic performance of rK28 ELISA using urine for diagnosis of visceral leishmaniasis. Parasites Vectors 2016, 9, 383. [Google Scholar] [CrossRef]
- Terato, K.; Do, C.; Chang, J.; Waritani, T. Preventing further misuse of the ELISA technique and misinterpretation of serological antibody assay data. Vaccine 2016, 34, 4643–4644. [Google Scholar] [CrossRef] [PubMed]
- Chabaud-Riou, M.; Moreno, N.; Guinchard, F.; Nicolai, M.C.; Niogret-Siohan, E.; Sève, N.; Manin, C.; Guinet-Morlot, F.; Riou, P. G-protein based ELISA as a potency test for rabies vaccines. Biologicals 2017, 46, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Gao, S.; Li, J.; Yang, J.; Zhu, Y.; Zhu, J.; Zhou, Y.; Shan, T.; Tong, W.; Zheng, H. Development and application of a blocking ELISA method based on Cap protein for detecting antibodies against porcine circovirus 2. Microbiol. Spectr. 2025, 13, e03040-24. [Google Scholar] [CrossRef]
- Thomsson, O.; Ström-Holst, B.; Sjunnesson, Y.; Bergqvist, A.-S. Validation of an enzyme-linked immunosorbent assay developed for measuring cortisol concentration in human saliva and serum for its applicability to analyze cortisol in pig saliva. Acta Vet. Scand. 2014, 56, 55. [Google Scholar] [CrossRef]
- Aydin, S.; Emre, E.; Ugur, K.; Aydin, M.A.; Sahin, İ.; Cinar, V.; Akbulut, T. An overview of ELISA: A review and update on best laboratory practices for quantifying peptides and proteins in biological fluids. J. Int. Med. Res. 2025, 53, 03000605251315913. [Google Scholar] [CrossRef]
- Gobena, D.; Gudina, E.K.; Degfie, T.T.; Girma, T.; Gebre, G.; Abdissa, A.; Tafesse, F.G.; Gelanew, T.; Mekonnen, Z. Comparative evaluation of in-house ELISA and two commercial serological assays for the detection of antibodies against SARS-CoV-2. Sci. Rep. 2025, 15, 13853. [Google Scholar] [CrossRef]
- Giavarina, D. Understanding bland Altman analysis. Biochem. Medica 2015, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bastit, L.; Marfil, S.; Pradenas, E.; Blanco, J.; Vergara-Alert, J.; Segalés, J. Comparison of Three Commercial ELISA Kits for Detection of Antibodies Against SARS-CoV-2 in Serum Samples from Different Animal Species. Viruses 2025, 17, 716. [Google Scholar] [CrossRef] [PubMed]
- Camargo, L.; Franklin, Y.V.; da Silva, G.F.R.; Santos, J.F.; Parreño, V.G.; Wigdorovitz, A.; Gomes, V. Serological Responses of Guinea Pigs and Heifers to Eight Different BoAHV-1 Vaccine Formulations. Vaccines 2024, 12, 615. [Google Scholar] [CrossRef]
- Keenan, J.I.; Rijpkema, S.G.; Durrani, Z.; Roake, J.A. Differences in immunogenicity and protection in mice and guinea pigs following intranasal immunization with Helicobacter pylori outer membrane antigens. FEMS Immunol. Med. Microbiol. 2003, 36, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Parreño, V. The Guinea Pig Model: An Alternative Method for Vaccine Potency Testing; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]

| Blocked with 5% BSA | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Antigen Coating (Copies/mL) | 1st Antibody Dilution (From 100 ng/mL) | Plate 1 Mean O.D. | Plate 2 Mean O.D. | Plate 1 SD | Plate 2 SD | Plate 1 Intra-Assay CV (%) | Plate 2 Intra-Assay CV (%) | Inter-Assay CV (%) | Precision Met (Intra/Inter) |
| 104 | 1:2 | 3.2175 | 2.972 | 0.0771 | 0.2744 | 2.395 | 2.094 | 2.245 | O/O |
| 1:4 | 1.812 | 2.0145 | 0.1768 | 0.2305 | 9.756 | 2.913 | 6.335 | O/O | |
| 1:8 | 1.011 | 1.148 | 0.0191 | 0.0368 | 1.914 | 3.203 | 2.558 | O/O | |
| 1:16 | 0.623 | 0.603 | 0.0354 | 0.0057 | 5.675 | 0.938 | 3.307 | O/O | |
| 105 | 1:16 | 3.3625 | 3.433 | 0.0700 | 0.0094 | 2.082 | 2.760 | 2.421 | O/O |
| 1:32 | 2.4395 | 2.639 | 0.0007 | 0.0381 | 0.029 | 1.447 | 0.738 | O/O | |
| 1:64 | 1.7225 | 1.7485 | 0.0544 | 0.1110 | 3.161 | 6.349 | 4.755 | O/O | |
| 1:128 | 0.9075 | 0.9895 | 0.0657 | 0.0869 | 7.246 | 8.790 | 8.018 | O/O | |
| 1:256 | 0.518 | 0.5275 | 0.0026 | 0.0077 | 5.187 | 1.475 | 3.331 | O/O | |
| Blocked with 5% Skim milk | |||||||||
| Antigen Coating (Copies/mL) | 1st Antibody dilution (From 100 ng/mL) | Plate 1 Mean O.D. | Plate 2 Mean O.D. | Plate 1 SD | Plate 2 SD | Plate 1 Intra-assay CV (%) | Plate 2 Intra-assay CV (%) | Inter-assay CV (%) | Precision Met (Intra/Inter) |
| 104 | 1:2 | 2.675 | 2.9485 | 0.1032 | 0.3345 | 3.3859 | 11.343 | 7.601 | X/O |
| 1:4 | 1.6585 | 1.613 | 0.0530 | 0.1103 | 3.198 | 6.839 | 5.018 | O/O | |
| 1:8 | 0.7885 | 0.854 | 0.0049 | 0.0410 | 0.628 | 4.802 | 2.715 | O/O | |
| 1:16 | 0.4545 | 0.4045 | 0.0629 | 0.0474 | 13.847 | 11.712 | 12.779 | X/O | |
| 105 | 1:16 | 3.2915 | 3.3715 | 0.0728 | 0.0530 | 2.213 | 1.573 | 1.893 | O/O |
| 1:32 | 2.266 | 2.109 | 0.2065 | 0.3154 | 9.112 | 14.954 | 12.033 | X/O | |
| 1:64 | 1.2525 | 1.267 | 0.1209 | 0.0014 | 9.654 | 0.112 | 4.833 | O/O | |
| 1:128 | 0.5385 | 0.634 | 0.0728 | 0.0141 | 13.525 | 2.231 | 7.878 | O/O | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, G.-S.; Seo, B.J.; Kwon, W.J.; Seok, Y.L.; Lee, H.J.; Lee, S.H.; Kim, M.; Lee, M.; Chae, C.; Kim, C. An In-House ELISA for Anti-Porcine Circovirus Type 2d (PCV2d) IgG: Analytical Validation and Serological Correlation. Vaccines 2025, 13, 657. https://doi.org/10.3390/vaccines13060657
Park G-S, Seo BJ, Kwon WJ, Seok YL, Lee HJ, Lee SH, Kim M, Lee M, Chae C, Kim C. An In-House ELISA for Anti-Porcine Circovirus Type 2d (PCV2d) IgG: Analytical Validation and Serological Correlation. Vaccines. 2025; 13(6):657. https://doi.org/10.3390/vaccines13060657
Chicago/Turabian StylePark, Gyeong-Seo, Byoung Joo Seo, Woo Ju Kwon, Yeong Lee Seok, Hyo Jeong Lee, Sung Ho Lee, Minju Kim, MinChul Lee, Chanhee Chae, and Chonghan Kim. 2025. "An In-House ELISA for Anti-Porcine Circovirus Type 2d (PCV2d) IgG: Analytical Validation and Serological Correlation" Vaccines 13, no. 6: 657. https://doi.org/10.3390/vaccines13060657
APA StylePark, G.-S., Seo, B. J., Kwon, W. J., Seok, Y. L., Lee, H. J., Lee, S. H., Kim, M., Lee, M., Chae, C., & Kim, C. (2025). An In-House ELISA for Anti-Porcine Circovirus Type 2d (PCV2d) IgG: Analytical Validation and Serological Correlation. Vaccines, 13(6), 657. https://doi.org/10.3390/vaccines13060657





