Impact of COVID-19 on Mucosal Immunity and Antibody Responses in COVID Vaccinees
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Anti-SARS-CoV-2 Spike and Nucleoprotein Antibodies
2.3. SARS-CoV-2 Spike-Specific IgG ELISA
2.4. SARS-CoV-2 Spike-Specific Total IgA ELISA
2.5. SARS-CoV-2 Spike-Specific Secretory IgA (sIgA) ELISA
2.6. Cytokine Assays
2.7. Statistical Analysis
3. Results
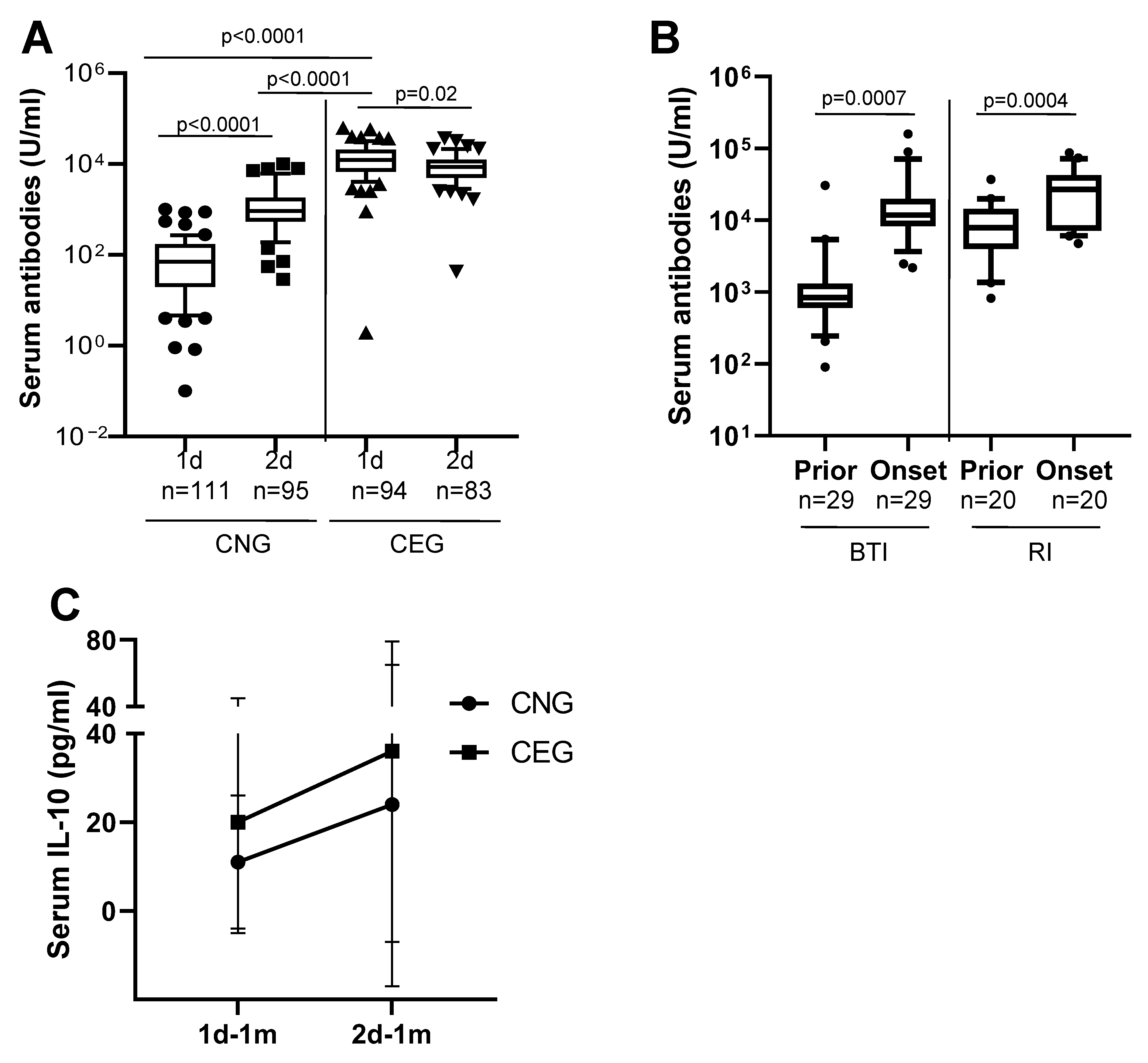
3.1. Antibody Anergy in Previously COVID-19 Exposed Vaccinees but Not in Previously Vaccinated COVID-19 Patients
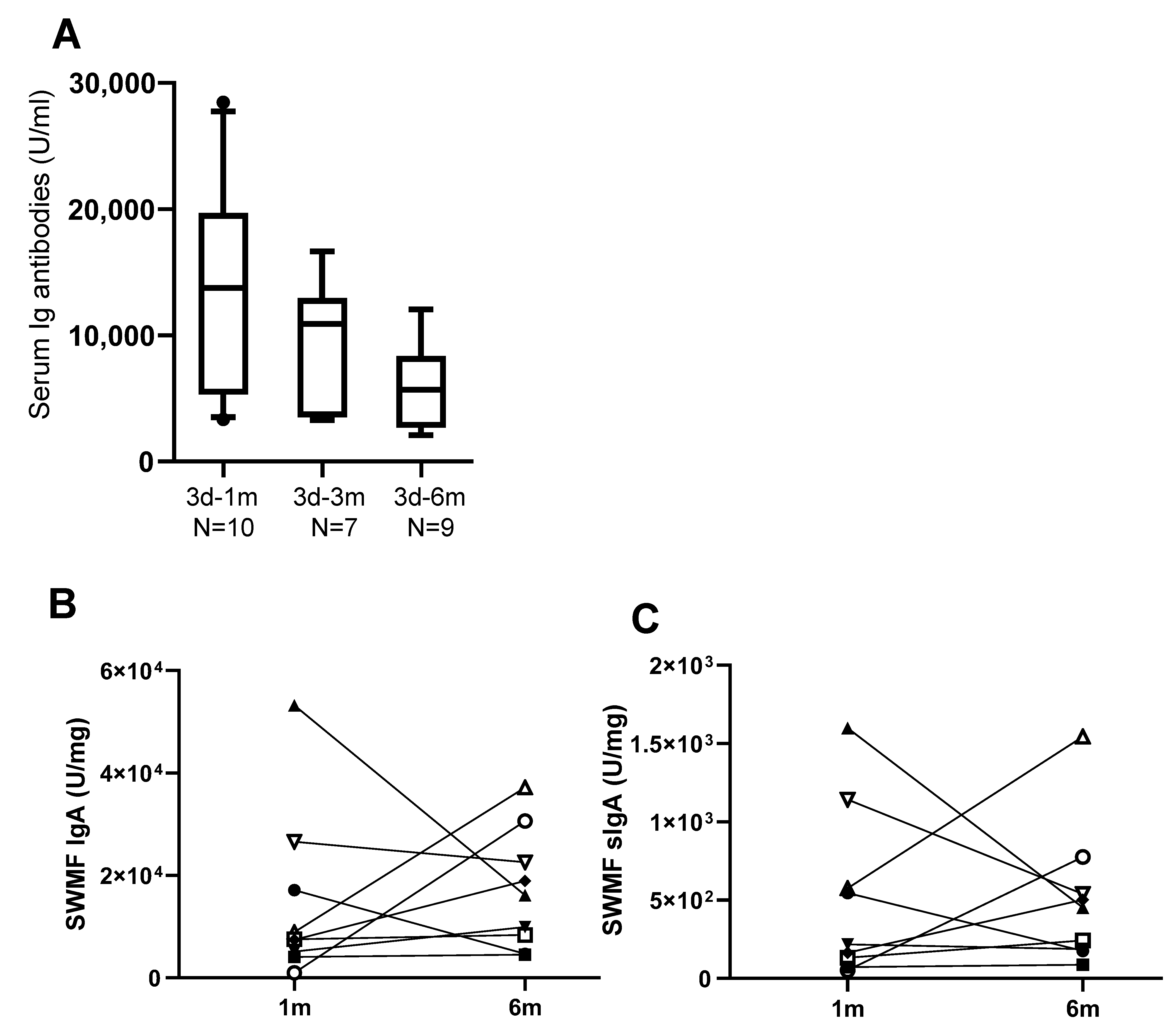
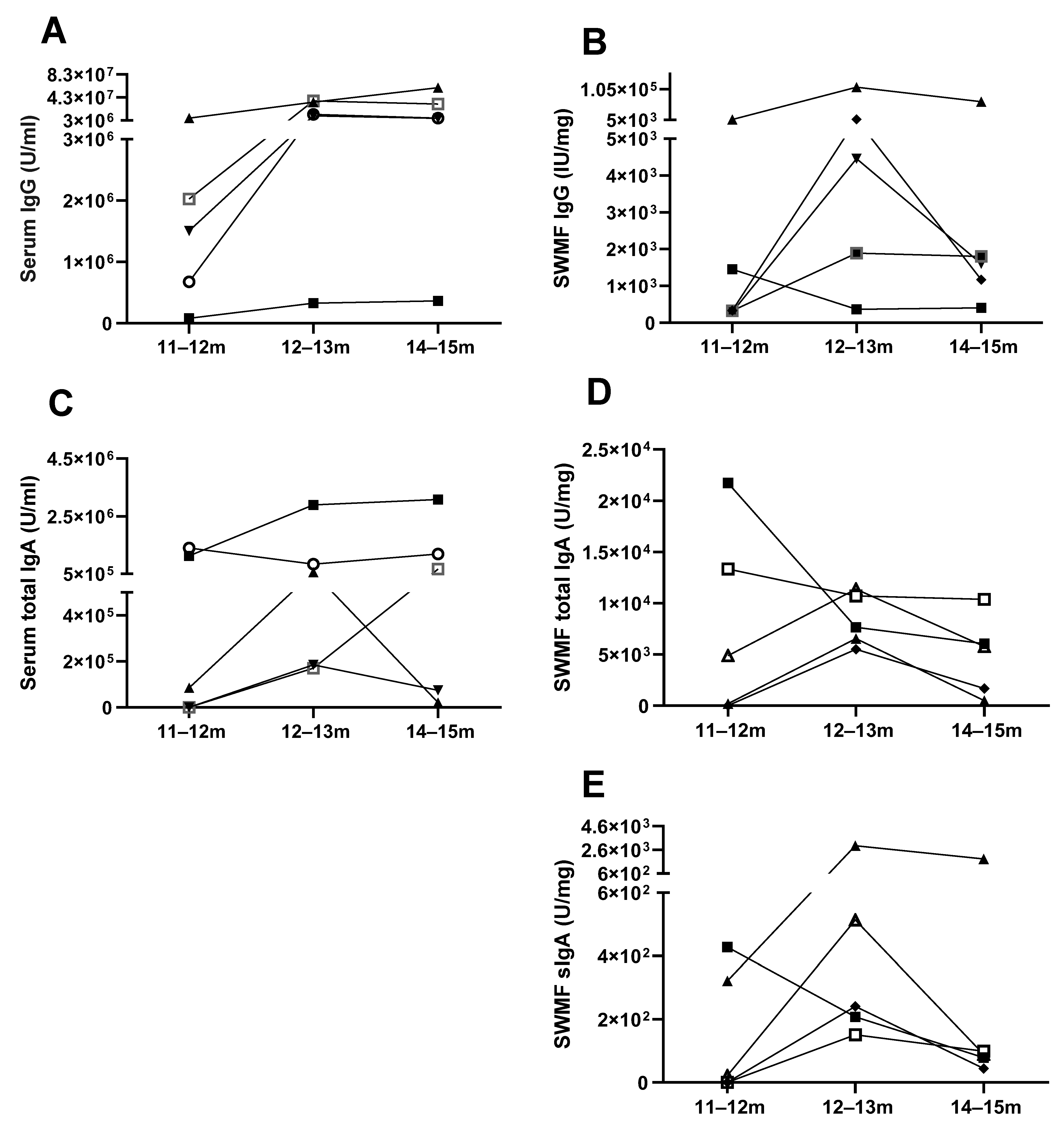
3.2. Antibody Memory to SARS-CoV-2 and Protection
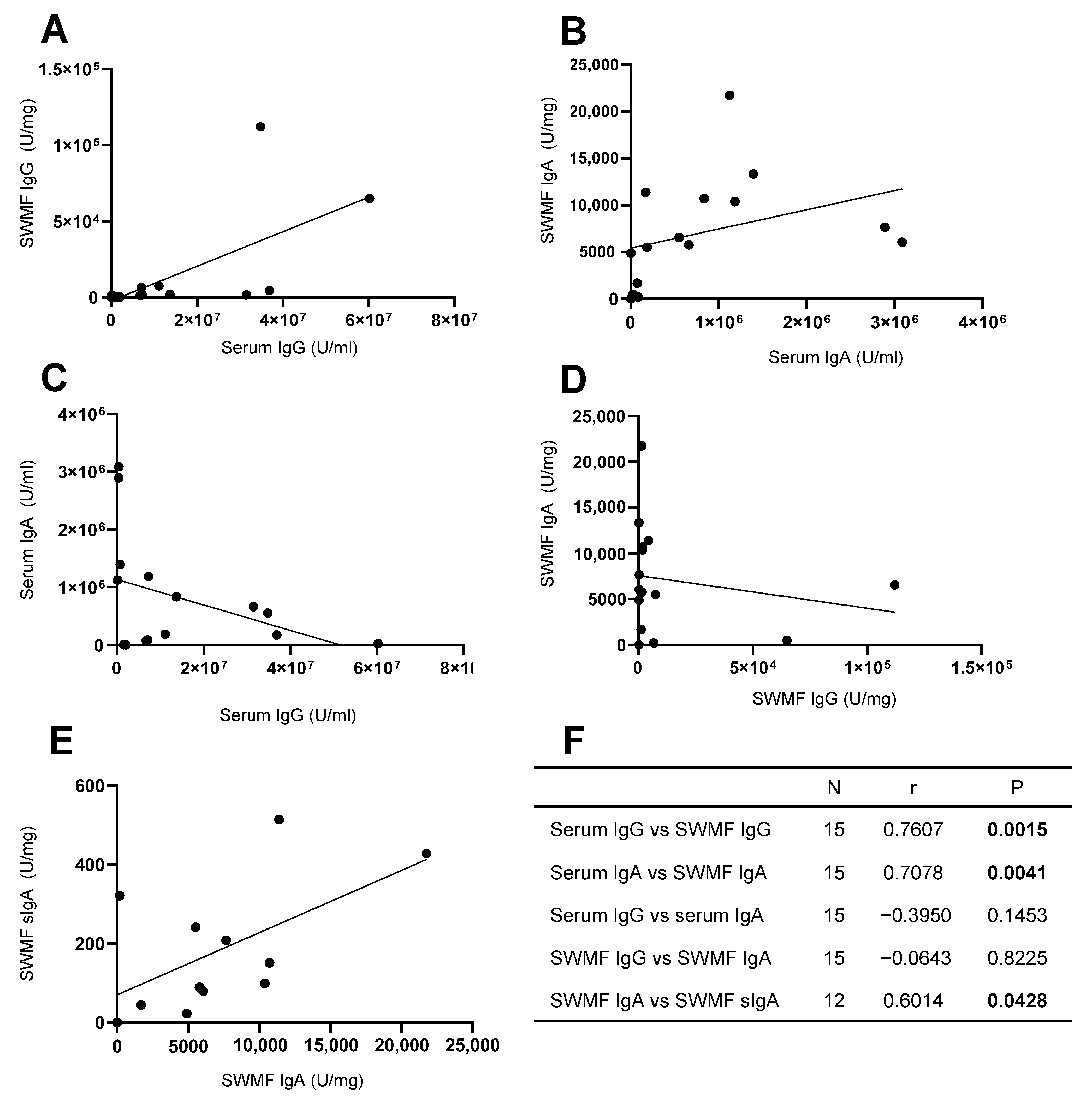
3.3. Mucosal Antibodies Elicited by Vaccination/Natural Infection
3.4. Cytokines Indicate Sustained Innate Mucosal Immunity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegrist, C.A. Vaccine Immunology. In Plotkin’s Vaccines; Elsevier: Amsterdam, The Netherlands, 2018; pp. 16–34.e7. [Google Scholar] [CrossRef]
- Ahmadivand, S.; Fux, R.; Palic, D. Role of T follicular helper cells in viral infections and vaccine design. Cells 2025, 14, 508. [Google Scholar] [CrossRef]
- Cobey, S. Vaccination against rapidly evolving pathogens and entanglements of memory. Nat. Immunol. 2024, 25, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Suthar, M.S. Durability of immune responses to SARS-CoV-2 infection and vaccination. Semin. Immunol. 2024, 73, 101884. [Google Scholar] [CrossRef] [PubMed]
- Sakre, M.; Agrawal, S.; Ravi, R.; Singh, D.; Bharadwaj, P. COVID-19 vaccination: Saviour or unfounded reliance? A cross sectional study among the air warriors. Med. J. Armed Forces India 2021, 77, S502–S504. [Google Scholar] [CrossRef]
- Bobdey, S.; Kaushik, S.K.; Sahu, R.; Naithani, N.; Vaidya, R.; Sharma, M.; Vashishtha, K.; Yadav, A.; Sen, S.; Karade, S. Effectiveness of ChAdOx1 nCoV-19 vaccine: Experience of a tertiary care institute. Med. J. Armed Forces India 2021, 77, S271–S277. [Google Scholar] [CrossRef]
- Ghosh, S.; Shankar, S.; Chatterjee, K.; Yadav, A.K.; Pandya, K.; Suryam, V.; Agrawal, S.; Ray, S.; Phutane, V.; Datta, R. Covishield (AZD1222) vaccine effectiveness among healthcare and frontline workers of Indian Armed Forces: Interim results of VIN-WIN cohort study. Med. J. Armed Forces India 2021, 77, S264–S270. [Google Scholar] [CrossRef]
- Muthukrishnan, J.; Vardhan, V.; Mangalesh, S.; Koley, M.; Shankar, S.; Yadav, A.K.; Khera, A. Vaccination status and COVID-19 related mortality: A hospital based cross sectional study. Med. J. Armed Forces 2021, 77, S278–S282. [Google Scholar] [CrossRef]
- Lozano-Ojalvo, D.; Camara, C.; Lopez-Granados, E.; Nozal, P.; del Pino-Molina, L.; Bravo-Gallego, L.Y.; Paz-Artal, E.; Pion, M.; Correa-Rocha, R.; Ortiz, A.; et al. Differential effects of the second SARS-CoV-2 mRNA vaccination on T cell immunity in naïve and COVID-19 recovered individuals. Cell Rep. 2021, 36, 109570. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, K.E.; Le Gars, M.; Sadoff, J.; de Groot, A.M.; Heerwegh, D.; Truyers, C.; Atyeo, C.; Loos, C.; Chandrashekar, A.; McMahan, K.; et al. Immunogenicity of the Ad26.COV2.S vaccine for COVID-19. JAMA 2021, 325, 1535–1544. [Google Scholar] [CrossRef]
- Das, P.; Varun, C.N.; Ashwini, M.A.; Kesavan, M.; Ravi, V.; Desai, A. Immunological memory in infected and exposure naïve individuals one year post SARS-CoV-2 vaccination. Indian J. Med. Res. 2025, 161, 287–297. [Google Scholar] [CrossRef]
- Suntronwong, N.; Assawakosri, S.; Klinfueng, S.; Duangchinda, T.; Chantima, W.; Pakchotanon, P.; Nilyanimit, P.; Vichaiwattana, P.; Aeemjinda, R.; Wongsrisang, L.; et al. Age-associated SARS-CoV-2 immune responses provide population immunity over four years since the COVID-19 pandemic. Sci. Rep. 2025, 15, 23183. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Z.; Gan, M.; Cui, Z.; Wu, B.; Feng, S.; Xu, J.; Cao, J.; Wen, Y.; Wang, Z.; et al. Dynamics of crossed acquired immunity against SARS-CoV-2 variants: From vaccine to hybrid immunity in China. J. Med. Virol. 2025, 97, e70487. [Google Scholar] [CrossRef] [PubMed]
- Pradenas, E.; Urrea, V.; Marfil, S.; Pidkova, T.; Aguilar-Gurrieri, C.; Abancó, F.; Mateu, L.; Chamorro, A.; Grau, E.; Trigueros, M.; et al. Recurrent waning of anti-SARS-CoV-2 neutralizing antibodies despite multiple antigen exposures. J. Transl. Med. 2025, 23, 783. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, R.; Gupta, D.; Paul, J.S.; Babji, S.; Syed, C.; Saravanan, P.; Kumar, A.; Immanuel, S.; Gandhi, V.; Eswaran, A.; et al. Monitoring of antibodies against SARS-CoV-2 over two years and characterization of immune responses following Omicron infection in a South Indian community cohort. Sci. Rep. 2025, 15, 24756. [Google Scholar] [CrossRef]
- Nasab, S.D.S.; Eniya, M.L.; Judith, A.; Clasen, F.; Faith, B.; Poongulali, S.; Gita, J.B.; Ashok, C.; Raghavi, V.; Vedavalli, S.; et al. Detection and consistency of mucosal fluid T lymphocyte phenotypes and their relationship with blood, age and gender. J. Immunol. Methods 2024, 532, 113731. [Google Scholar] [CrossRef]
- National Institutes of Health (US). Coronavirus Disease 2019 (COVID-19) Treatment Guidelines [Internet]; National Institutes of Health (US): Bethesda, MD, USA, 2021. [Google Scholar]
- Jia, T.; Wang, F.; Chen, Y.; Liao, G.; Xu, Q.; Chen, J.; Wu, J.; Li, N.; Wang, L.; Yuan, L.; et al. Expanded immune imprinting and neutralization spectrum by hybrid immunization following breakthrough infections with SARS-CoV-2 variants after three-dose vaccination. J. Infect. 2024, 89, 106362. [Google Scholar] [CrossRef] [PubMed]
- Shrotri, M.; Navaratnam, A.M.D.; Nguyen, V.; Byrne, T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.L.E.; Patel, P.; Kovar, J.; et al. Spike-antibody waning after second vaccination of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–387. [Google Scholar] [CrossRef]
- Hall, V.J.; Foulkes, S.; Saci, A.; Andrews, N.; Oguti, B.; Charlett, A.; Wellington, E.; Stowe, J.; Gillson, N.; Atti, A.; et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): A prospective, multicentre, cohort study. Lancet 2021, 397, 1725–1735. [Google Scholar] [CrossRef]
- Seyedi, S.; Sottile, S.; Abedini, M.; Boffetta, P.; Violante, F.S.; Lodi, V.; De Palma, G.; Sala, E.; Mauro, M.; Rui, F.; et al. Antibody kinetics of immunological memory in SARS-CoV-2-vaccinated healthcare workers—The orchestra project. Vaccines 2025, 13, 611. [Google Scholar] [CrossRef]
- Kaneko, N.; Kuo, H.; Boucau, J.; Farmer, J.R.; Allard-Chamard, H.; Mahajan, V.S.; Piechocka-Trocha, A.; Lefteri, K.; Osborn, M.; Bals, J.; et al. Loss of Bcl-6-expressing T follicular helper cells and germinal centers in COVID-19. Cell 2021, 183, 143–157. [Google Scholar] [CrossRef]
- Haralambieva, I.H.; Monroe, J.M.; Ovsyannikova, I.G.; E Grill, D.; A Poland, G.; Kennedy, R.B. Distinct homologous and variant-specific memory B-cell and antibody response over time after severe acute respiratory syndrome coronavirus 2 messenger RNA vaccination. J. Infect. Dis. 2022, 26, 23–31. [Google Scholar] [CrossRef]
- Garziano, M.; Fiestas, M.C.; Vanetti, C.; Strizzi, S.; Murno, M.L.; Clerici, M.; Biasin, M. SARS-CoV-2 natural infection but not vaccine-induced immunity elicits cross-reactivity to OC43. Heliyon 2024, 10, e37928. [Google Scholar] [CrossRef]
- Sano, K.; Miyakawa, K.; Kato, H.; Kimura, Y.; Goto, A.; Ryo, A.; Watanabe, S.; Hasegawa, H. Neutralizing antibody evasion of SARS-CoV-2 JN.1 derivatives KP.3, KP.3.1.1, LB.1 and XEC. Vaccine 2025, 62, 127472. [Google Scholar] [CrossRef]
- Schmitt, M.M.; Rossler, A.; Netzl, A.; Knabl, L.; Falch, A.; Neckermann, P.; Bermejo-Jambrina, M.; Borena, W.; Singh, G.; Krammer, F.; et al. Exposure to two antigenically distinct SARS-CoV-2 variants broadens neutralization patterns. Vaccine 2025, 62, 127459. [Google Scholar] [CrossRef]
- Brazer, N.; Morris, M.K.; Servellita, V.; Oseguera, M.; Sumimoto, N.; Saldhi, P.; Foresythe, A.; Nguyen, J.; Wadford, D.A.; Hanson, C.; et al. Differential immunity induced by Omicron sublineages in naïve and vaccine breakthrough infections. Sci. Rep. 2025, 15, 23718. [Google Scholar] [CrossRef]
- Thomas, S.; Smatti, M.K.; Alkhatib, H.A.M.; Tayyar, Y.; Nizar, M.; Zedan, H.T.; Ouhtit, A.; Althani, A.A.; Nasrallah, G.K.; Yassine, H.M. Antibody-dependent enhancement of SARS-COV-2, the impact of variants and vaccination. Hum. Vaccin. Immunother. 2025, 21, 2505356. [Google Scholar] [CrossRef]
- Koutsakos, M.; Ellebedy, A.H. Immunological imprinting: Understanding COVID-19. Immunity 2023, 56, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Aydillo, T.; Rombauts, A.; Stadlbauer, D.; Aslam, S.; Abelenda-Alonso, G.; Escalera, A.; Amanat, F.; Jiang, K.; Krammer, F.; Carratala, J.; et al. Immunological imprinting of the antibody response in COVID-19 patients. Nat. Commun. 2021, 12, 3781. [Google Scholar] [CrossRef] [PubMed]
- Ota, K.; Sakai, H.; Sasaki, D.; Mitsumoto-Kaseida, F.; Sakamoto, K.; Kosai, K.; Hasegawa, H.; Takazono, T.; Izumikawa, K.; Mukae, H.; et al. Rapid increase in salivary IgA and broad recognition of spike protein following SARS-CoV-2 vaccination. Virus. Res. 2024, 339, 199294. [Google Scholar] [CrossRef] [PubMed]
- Seow, J.; Jefferson, G.C.E.; Keegan, M.D.; Yau, Y.; Snell, L.B.; Doores, K.J.; Fox, J. Profiling serum immunodominance following SARS-CoV-2 primary and breakthrough infections reveals distinct variant-specific epitope usage and immune imprinting. PLoS Pathog. 2024, 20, e1012724. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.J.; Hudda, M.T.; Pallett, S.; Groppelli, E.; Boariu, E.; Finardi, N.F.; Wake, R.; Sofat, N.; Biddle, K.; Koushesh, S.; et al. Mucosal immune responses to SARS-CoV-2 infection and COVID-19 vaccination. Vaccine 2025, 56, 127175. [Google Scholar] [CrossRef] [PubMed]
- Akeel, S.; Almazrooa, S.; Jazzar, A.; Sindi, A.M.; Farsi, N.J.; Binmadi, N.; Badkok, R.; Aljohani, M.; AlFarabi, S. Detection of specific immunoglobulins in the saliva of patients with mild COVID-19. Cureus 2024, 16, e52113. [Google Scholar] [CrossRef] [PubMed]
- Bladh, O.; Aguilera, K.; Marking, U.; Kihlgren, M.; Norin, N.G.; Smed-Sörensen, A.; Chen, M.S.; Klingström, J.; Blom, K.; Russell, M.W.; et al. Comparison of SARS-CoV-2 spike-specific IgA and IgG in nasal secretions, saliva and serum. Front. Immunol. 2024, 15, 1346749. [Google Scholar] [CrossRef]
- Schmidt, V.A.; Stevens, V.R.; Rivieccio, M.; Sikar-Gang, A.; Simonetti, K.; Gunasekera, D.; Esfandiari, J.; Lyashchenko, K.P. Rapid detection of systemic and mucosal antibody responses to COVID-19 infection or vaccination. Int. Immunopharmacol. 2025, 153, 114512. [Google Scholar] [CrossRef] [PubMed]
| COVID-19-Naïve (CNG) | COVID-19-Exposed (CEG) | CNG Breakthrough Infections (BTIs) | CEG Reinfections (RIs) | |
|---|---|---|---|---|
| Overall (N = 205) | 111 (54) | 94 (46) | 49 (44) | 25 (27) |
| Gender | ||||
| Males (N = 56) | 33 (59) | 23 (41) | 21 (64) | 11 (48) |
| Females (N = 149) | 78 (52) | 71 (48) | 28 (36) | 14 (20) |
| p value | 0.4 | 0.007 | 0.008 | |
| Age (years) | ||||
| 20–44 (N = 140) | 75 (54) | 65 (46) | 33 (44) | 13 (20) |
| 45–60 (N = 51) | 28 (55) | 23 (45) | 12 (43) | 9 (39) |
| ≥61 (N = 14) | 8 (57) | 6 (43) | 4 (50) | 3 (50) |
| p value | 1.0 | 0.9 | 0.08 | |
| Time Points | CNG (N) | CEG (N) | BTI (N) | RI (N) |
|---|---|---|---|---|
| Median anti-SARS-CoV-2 spike Ig antibodies (U/mL) | ||||
| 1d-1m | 84 (111) | 10,152 (94) | ND | ND |
| 2d-1m | 856 (95) | 6986 (83) | ND | ND |
| 2d-3m | 341 (48) | 5372 (56) | 4258 (45) | 7188 (25) |
| 2d-6m | 153 (42) | 3056 (50) | 2386 (46) | 11,096 (25) |
| Mean IL-10 (pg/mL) | ||||
| 1d-1m | 11 (24) | 20 (36) | ND | ND |
| 2d-1m | 24 (24) | 36 (36) | ND | ND |
| Mean IL-17 (pg/mL) | ||||
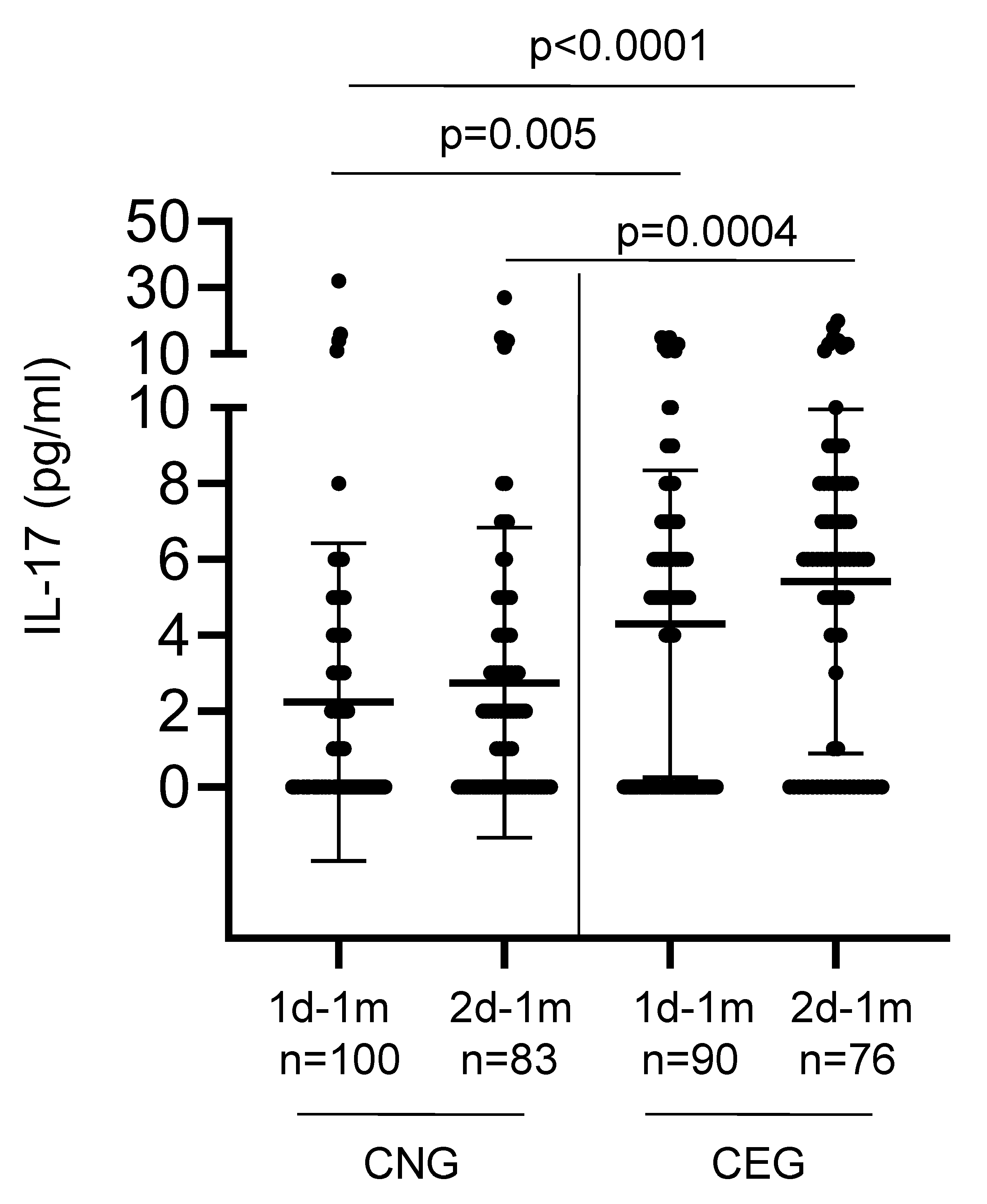
| 1d-1m | 2.24 (100) | 4.30 (90) | ND | ND |
| 2d-1m | 2.75 (83) | 5.42 (76) | ND | ND |
| Mean IL-21 (pg/mL) | ||||
| 1d-1m | 39 (100) | 60 (90) | ND | ND |
| 2d-1m | 46 (83) | 53 (76) | ND | ND |
| 3–6 months after second vaccine dose | ||||
| Mean IL-7 (pg/mL) | 10 (47) | 12 (54) | 18 (39) | 14 (22) |
| Mean IL-15 (pg/mL) | 21 (47) | 19 (54) | 23 (39) | 12 (22) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kannian, P.; Eniya, M.L.; Mahanathi, P.; Gracemary, A.; Kumarasamy, N.; Challacombe, S.J. Impact of COVID-19 on Mucosal Immunity and Antibody Responses in COVID Vaccinees. Vaccines 2025, 13, 967. https://doi.org/10.3390/vaccines13090967
Kannian P, Eniya ML, Mahanathi P, Gracemary A, Kumarasamy N, Challacombe SJ. Impact of COVID-19 on Mucosal Immunity and Antibody Responses in COVID Vaccinees. Vaccines. 2025; 13(9):967. https://doi.org/10.3390/vaccines13090967
Chicago/Turabian StyleKannian, Priya, Muruganantham Lillimary Eniya, Pasuvaraj Mahanathi, Arul Gracemary, Nagalingeswaran Kumarasamy, and Stephen J. Challacombe. 2025. "Impact of COVID-19 on Mucosal Immunity and Antibody Responses in COVID Vaccinees" Vaccines 13, no. 9: 967. https://doi.org/10.3390/vaccines13090967
APA StyleKannian, P., Eniya, M. L., Mahanathi, P., Gracemary, A., Kumarasamy, N., & Challacombe, S. J. (2025). Impact of COVID-19 on Mucosal Immunity and Antibody Responses in COVID Vaccinees. Vaccines, 13(9), 967. https://doi.org/10.3390/vaccines13090967






