Identification of T-Cell Epitopes and Vaccine Development for African Swine Fever Virus
Abstract
1. Present State of ASFV Vaccine Research
2. T-Cell Immunity in ASFV Infection
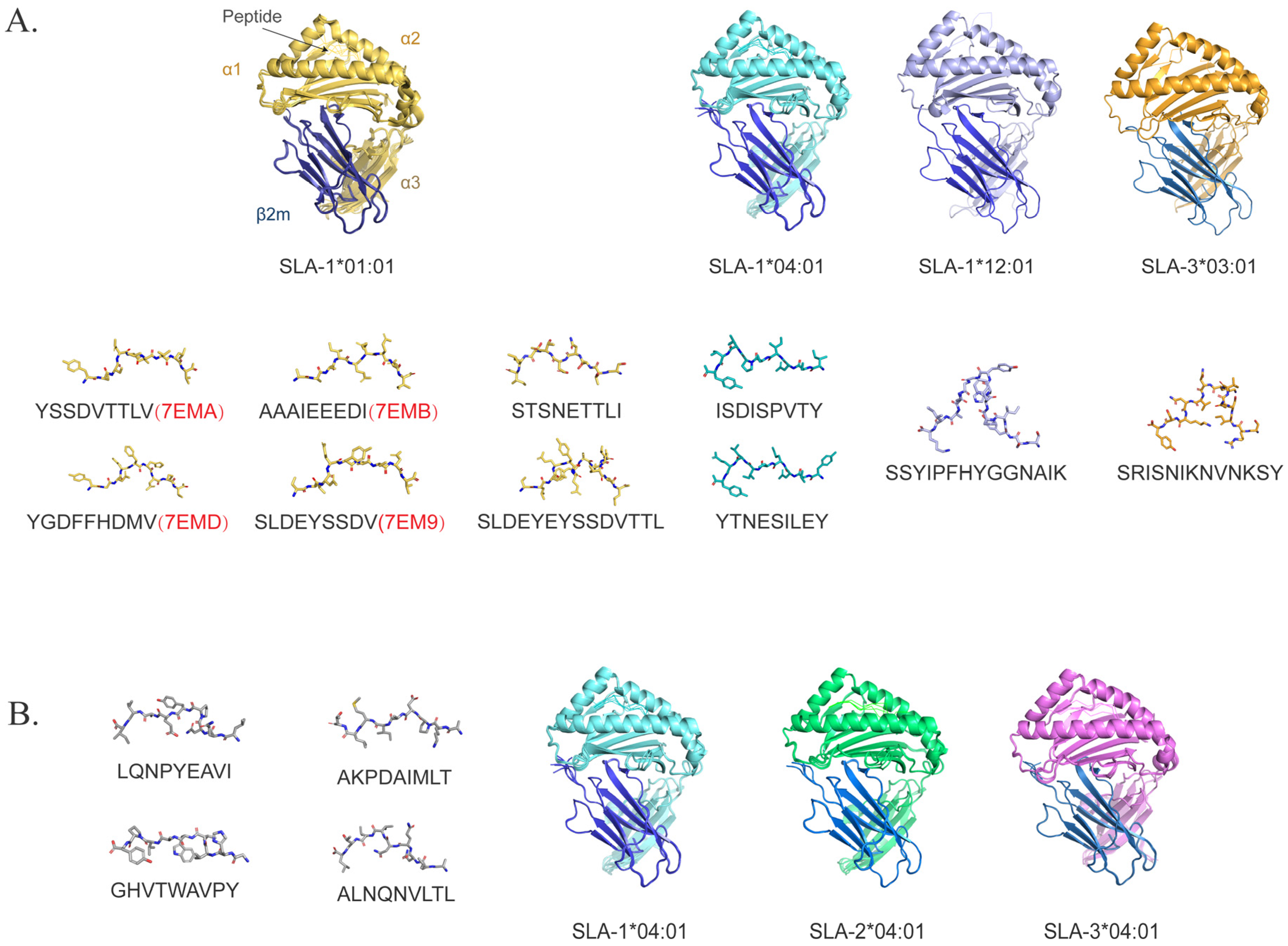
3. Identification of ASFV T-Cell Epitopes
| Proteins | Amino Acid Sequence | SLA | Predicting Tools | Experimental Animal | Experimental Methods | Cite |
|---|---|---|---|---|---|---|
| F317L | 246SRRSLVNPWT255 | / | IEDB; NetMHCpan-4.1 | Mouse | IFN-γ ELISpot | [69] |
| C129R | 53LQNPYEAVI61; 81GHVTWAVPY89; 97AKPDAIMLT105; 116ALNQNVLTL124 | SLA-1*0401 SLA-2*0401 SLA-3*0401 | IEDB; NetMHCpan-4.1 | Mouse | IFN-γ ELISpot | [70] |
| pp220 | 161LTHGLRAEY169; 859KSMAAKIFI867; 1363HIDKNIIQY1371; 1463RVFSRLVFY1471 | / | NetMHCpan-2.8 | Pig | IFN-γ ELISpot | [71] |
| CD2v | 150YTNESILEY158 | SLA-1*0401 | NetMHCpan-4.0 | Mouse | IFN-γ ELISpot | [72] |
| CD2v | 160WNNSNINNFT169 | / | ABCpred | Mouse | IFN-γ ELISpot | [73] |
| CD2v | 179STSNETTLI187 | SLA-1*0101 | NetMHCpan-4.0 | Pig | Refold in vitro | [76] |
| CD2v | 116SVDSPTITY124; 155TNGDILNYY163 | / | TAPREG | Pig | IFN-γ ELISpot | [74] |
| CD2v | 33INSETEGIFWNFYNNTFNTIATCGKKN59; 93TYQLVYSRNRINYTINLLLPVTSPIIT119; 297PLNPSPPPKPCPPPKPCPPPKPCPPPK323; 337YSPPKPLPSIPLLPNIPPLSTQNISLI363 | / | IEDB | Pig | IFN-γ ELISpot | [78] |
| MGF100-1L | 68LQMAPGGSYFITDNMTEEF86 | / | NetMHCpan-3.0 | Pig | IFN-γ ELISpot | [75] |
| MGF505-7R | 334NSTLVIRI341 | / | / | Pig | MHC-IAC; IFN-γ ELISpot | [75] |
| A238L | 81DKDGNSALHYL91 | / | / | Pig | MHC-IAC; IFN-γ ELISpot | [75] |
| p54 | 60AAAIEEEDI68 | SLA-1*0101 | NetMHCpan-4.0 | Pig | Refold in vitro | [76] |
| p72 | 522ISDISPVTY530 | SLA-1*0401 | NetMHCpan-4.0 | Mouse | IFN-γ ELISpot | [72] |
| p72 | 97YGDFFHDMV105; 199SLDEYSSDV207; 203YSSDVTTLV211 | SLA-1*0101 | NetMHCpan-4.0 | Pig | Tetramers | [76] |
| p72 | 199SLDEYSSDVTTL210 | SLA-1*0101 | IEDB | Pig | Tetramers | [77] |
| p72 | 28SRISNIKNVNKSY40 | SLA-3*0301 | ||||
| p72 | 559SSYIPFHYGGNAIK572 | SLA-1*1201 | ||||
| EP153R | 112SFLNLTKLYHHHSHYWVNYSLNNNNYSV138; 144KYNLNRKKSHYTDLLFICS162 | / | IEDB | Pig | IFN-γ ELISpot | [78] |
| pp220 | IADAINQEF; FLNKSTQAY; QIYKTLLEY; SLYPTQFDY | SLA-1*0401 | NetMHCcon; IEDB; ToxinPred; GalaxyPepDock; GalaxyRefine- Complex; PRODIGY; MDWeb | / | / | [79] |
| pp62 | GTDLYQSAM; FINSTDFLY; STDFLYTAI | SLA-1*0401 | / | / | ||
| G1211R | AADDTTCYY | / | IEDB: NetMHCpanEL 4.1 | Mouse: Pig | / | [80] |
| MPIDIHEVRY | ||||||
| CP2475L | HIDKNIIQY | |||||
| CP204L | VVFHAGSLY | |||||
| CP530R | YSDPETVHSY | |||||
| NP1450L | ILDLIRLQY | |||||
| D339L | SVYHVQEEL | |||||
| D1133L | VPAKPEHLY | |||||
| D345L | HIDGTYLGY | |||||
| P1192R | MPVYQELGY | |||||
| H359L | IPDISFVGY | |||||
| E423R | SEYKQYNEF | |||||
| Q706L | IVDEAHNLF | |||||
| E248R | FIADAISAV | |||||
| I329L | ISFSNNNTY |
4. Challenges in ASFV T-Cell Epitope Identification
4.1. SLA Polymorphism
4.2. The Limited Prediction Tools
4.3. Experimental Methods
5. ASFV T-Cell Epitope Vaccine
5.1. Lessons from Other Vaccine Platforms
5.2. mRNA Vaccines
5.3. Delivery Systems
5.4. Current Status and Prospects of ASFV Epitope-Based Vaccines
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Juszkiewicz, M.; Walczak, M.; Woźniakowski, G.; Podgórska, K. African swine fever: Transmission, spread, and control through biosecurity and disinfection, including Polish trends. Viruses 2023, 15, 2275. [Google Scholar] [CrossRef]
- Dixon, L.K.; Sun, H.; Roberts, H. African swine fever. Antivir. Res. 2019, 165, 34–41. [Google Scholar] [CrossRef]
- Liu, S.; Luo, Y.; Wang, Y.; Li, S.; Zhao, Z.; Bi, Y.; Sun, J.; Peng, R.; Song, H.; Zhu, D.; et al. Cryo-EM structure of the African swine fever virus. Cell Host Microbe 2019, 26, 836–843. [Google Scholar] [CrossRef]
- Chang, H.; Hua, Q.Y.; Duan, G. The advancement on molecular biology of African swine fever virus. Microbiol. China 2007, 34, 572–575. [Google Scholar]
- Wang, G.; Xie, M.; Wu, W.; Chen, Z. Structures and functional diversities of ASFV proteins. Viruses 2021, 13, 2124. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhao, D.; Wang, J.; Zhang, Y.; Wang, M.; Gao, Y.; Li, F.; Wang, J.; Bu, Z.; Rao, Z.; et al. Architecture of African swine fever virus and implications for viral assembly. Science 2019, 366, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kang, W.; Yang, W.; Zhang, J.; Li, D.; Zheng, H. Structure of African swine fever virus and associated molecular mechanisms underlying infection and immunosuppression: A review. Front. Immunol. 2021, 12, 715582. [Google Scholar] [CrossRef]
- Zhang, X.; Xiang, Y.; Dunigan, D.D.; Klose, T.; Chipman, P.R.; Van Etten, J.L.; Rossmann, M.G. Three-dimensional structure and function of the Paramecium bursaria chlorella virus capsid. Proc. Natl. Acad. Sci. USA 2011, 108, 14837–14842. [Google Scholar] [CrossRef] [PubMed]
- Blome, S.; Franzke, K.; Beer, M. African swine fever—A review of current knowledge. Virus Res. 2020, 287, 198099. [Google Scholar] [CrossRef]
- Andrés, G.; Simón-Mateo, C.; Viñuela, E. Assembly of African swine fever virus: Role of polyprotein pp220. J. Virol. 1997, 71, 2331–2341. [Google Scholar] [CrossRef]
- Alejo, A.; Matamoros, T.; Guerra, M.; Andrés, G. A proteomic atlas of the African swine fever virus particle. J. Virol. 2018, 92, 01293-18. [Google Scholar] [CrossRef] [PubMed]
- Angulo, A.; Viñuela, E.; Alcamí, A. Inhibition of African swine fever virus binding and infectivity by purified recombinant virus attachment protein p12. J. Virol. 1993, 67, 5463–5471. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, W.; Qiu, Z.; Li, Y.; Fan, J.; Wu, K.; Li, X.; Zhao, M.; Ding, H.; Fan, S.; et al. African swine fever virus: A review. Life 2022, 12, 1255. [Google Scholar] [CrossRef]
- Nogal, M.L.; González de Buitrago, G.; Rodríguez, C.; Cubelos, B.; Carrascosa, A.L.; Salas, M.L.; Revilla, Y. African swine fever virus IAP homologue inhibits caspase activation and promotes cell survival in mammalian cells. J. Virol. 2001, 75, 2535–2543. [Google Scholar] [CrossRef]
- Shi, J.; Liu, W.; Zhang, M.; Sun, J.; Xu, X. The A179L gene of African swine fever virus suppresses virus-induced apoptosis but enhances necroptosis. Viruses 2021, 13, 2490. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yang, Y.; Luo, Y.; Chen, X.; Gong, T.; Wu, D.; Feng, Y.; Zheng, X.; Wang, H.; Zhang, G.; et al. African swine fever virus envelope glycoprotein CD2v interacts with host CSF2RA to regulate the JAK2-STAT3 pathway and inhibit apoptosis to facilitate virus replication. J. Virol. 2023, 97, 01889-22. [Google Scholar] [CrossRef]
- Li, J.; Song, J.; Kang, L.; Huang, L.; Zhou, S.; Hu, L.; Zheng, J.; Li, C.; Zhang, X.; He, X.; et al. pMGF505-7R determines pathogenicity of African swine fever virus infection by inhibiting IL-1β and type I IFN production. PLoS Pathog. 2021, 17, 1009733. [Google Scholar] [CrossRef]
- Zhuo, Y.; Guo, Z.; Ba, T.; Zhang, C.; He, L.; Zeng, C.; Dai, H. African swine fever virus MGF360-12L inhibits type I interferon production by blocking the interaction of importin α and NF-κB signaling pathway. Virol. Sin. 2021, 36, 176–186. [Google Scholar] [CrossRef]
- Huang, Z.; Cao, H.; Zeng, F.; Lin, S.; Chen, J.; Luo, Y.; You, J.; Kong, C.; Mai, Z.; Deng, J.; et al. African swine fever virus MGF505-7R interacts with interferon regulatory factor 9 to evade the type I interferon signaling pathway and promote viral replication. J. Virol. 2023, 97, 01977-22. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, Z.; Feng, T.; Ma, Z.; Xue, Q.; Wu, P.; Li, P.; Li, S.; Yang, F.; Cao, W.; et al. African swine fever virus E120R protein inhibits interferon beta production by interacting with IRF3 to block its activation. J. Virol. 2021, 95, 00824-21. [Google Scholar] [CrossRef]
- Chen, Q.; Li, L.; Guo, S.; Liu, Z.; Liu, L.; Tan, C.; Chen, H.; Wang, X. African swine fever virus pA104R protein acts as a suppressor of type I interferon signaling. Front. Microbiol. 2023, 14, 1169699. [Google Scholar] [CrossRef]
- Huang, L.; Chen, W.; Liu, H.; Xue, M.; Dong, S.; Liu, X.; Feng, C.; Cao, S.; Ye, G.; Zhou, Q.; et al. African swine fever virus HLJ/18 CD2v suppresses type I IFN production and IFN-stimulated genes expression through negatively regulating cGMP-AMP synthase-STING and IFN signaling pathways. J. Immunol. 2023, 210, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zhang, C.; Chen, N.; Meurens, F.; Zhu, J.; Zheng, W. How does African swine fever virus evade the cGAS-STING pathway? Pathogens 2024, 13, 957. [Google Scholar] [CrossRef]
- Dodantenna, N.; Ranathunga, L.; Chathuranga, W.A.G.; Weerawardhana, A.; Cha, J.W.; Subasinghe, A.; Gamage, N.; Haluwana, D.K.; Kim, Y.; Jheong, W.; et al. African swine fever virus EP364R and C129R target cyclic GMP-AMP to inhibit the cGAS-STING signaling pathway. J. Virol. 2022, 96, 01022-22. [Google Scholar] [CrossRef]
- Granja, A.G.; Nogal, M.L.; Hurtado, C.; Del Aguila, C.; Carrascosa, A.L.; Salas, M.L.; Fresno, M.; Revilla, Y. The viral protein A238L inhibits TNF-alpha expression through a CBP/p300 transcriptional coactivators pathway. J. Immunol. 2006, 176, 451–462. [Google Scholar] [CrossRef]
- Dixon, L.K.; Islam, M.; Nash, R.; Reis, A.L. African swine fever virus evasion of host defences. Virus Res. 2019, 266, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, K.; Li, T.; Zhao, G.; Zhou, S.; Li, H.; Li, J.; Weng, C. Screening of PRRSV- and ASFV-encoded proteins involved in the inflammatory response using a porcine iGLuc reporter. J. Virol. Methods 2020, 285, 113958. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, D.; He, S.; Cao, Z.; Li, W.; Jiang, F.; Shi, Y.; Hao, Y.; Wei, X.; Wang, Q.; et al. Immune cell early activation, apoptotic kinetic, and T-cell functional impairment in domestic pigs after ASFV CADC_HN09 strain infection. Front. Microbiol. 2024, 15, 1328177. [Google Scholar] [CrossRef]
- Zhu, J.J.; Ramanathan, P.; Bishop, E.A.; O’Donnell, V.; Gladue, D.P.; Borca, M.V. Mechanisms of African swine fever virus pathogenesis and immune evasion inferred from gene expression changes in infected swine macrophages. PLoS ONE 2019, 14, 0223955. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, C.; Bustos, M.J.; Granja, A.G.; de León, P.; Sabina, P.; López-Viñas, E.; Gómez-Puertas, P.; Revilla, Y.; Carrascosa, A.L. The African swine fever virus lectin EP153R modulates the surface membrane expression of MHC class I antigens. Arch. Virol. 2011, 156, 219–234. [Google Scholar] [CrossRef]
- Forman, A.J.; Wardley, R.C.; Wilkinson, P.J. The immunological response of pigs and guinea pigs to antigens of African swine fever virus. Arch. Virol. 1982, 74, 91–100. [Google Scholar] [CrossRef]
- Stone, S.S.; Hess, W.R. Antibody response to inactivated preparations of African swine fever virus in pigs. Am. J. Vet. Res. 1967, 28, 475–481. [Google Scholar] [PubMed]
- Blome, S.; Gabriel, C.; Beer, M. Modern adjuvants do not enhance the efficacy of an inactivated African swine fever virus vaccine preparation. Vaccine 2014, 32, 3879–3882. [Google Scholar] [CrossRef]
- Cadenas-Fernández, E.; Sánchez-Vizcaíno, J.M.; van den Born, E.; Kosowska, A.; van Kilsdonk, E.; Fernández-Pacheco, P.; Gallardo, C.; Arias, M.; Barasona, J.A. High doses of inactivated African swine fever virus are safe, but do not confer protection against a virulent challenge. Vaccines 2021, 9, 242. [Google Scholar] [CrossRef]
- Pikalo, J.; Porfiri, L.; Akimkin, V.; Roszyk, H.; Pannhorst, K.; Kangethe, R.T.; Wijewardana, V.; Sehl-Ewert, J.; Beer, M.; Cattoli, G.; et al. Vaccination with a gamma irradiation-inactivated African swine fever virus is safe but does not protect against a challenge. Front. Immunol. 2022, 13, 832264. [Google Scholar] [CrossRef]
- Gómez-Puertas, P.; Rodríguez, F.; Oviedo, J.M.; Ramiro-Ibáñez, F.; Ruiz-Gonzalvo, F.; Alonso, C.; Escribano, J.M. Neutralizing antibodies to different proteins of African swine fever virus inhibit both virus attachment and internalization. J. Virol. 1996, 70, 5689–5694. [Google Scholar] [CrossRef]
- Escribano, J.M.; Galindo, I.; Alonso, C. Antibody-mediated neutralization of African swine fever virus: Myths and facts. Virus Res. 2013, 173, 101–109. [Google Scholar] [CrossRef]
- Cadenas-Fernández, E.; Sánchez-Vizcaíno, J.M.; Kosowska, A.; Rivera, B.; Mayoral-Alegre, F.; Rodríguez-Bertos, A.; Yao, J.; Bray, J.; Lokhandwala, S.; Mwangi, W.; et al. Adenovirus-vectored African swine fever virus antigens cocktail is not protective against virulent Arm07 isolate in Eurasian wild boar. Pathogens 2020, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Liu, J.; Wang, L.; Fan, S.; Li, Z.; Li, Y.; Yi, L.; Ding, H.; Zhao, M.; Chen, J. Current state of global African swine fever vaccine development under the prevalence and transmission of ASF in China. Vaccines 2020, 8, 531. [Google Scholar] [CrossRef]
- Neilan, J.G.; Zsak, L.; Lu, Z.; Burrage, T.G.; Kutish, G.F.; Rock, D.L. Neutralizing antibodies to African swine fever virus proteins p30, p54, and p72 are not sufficient for antibody-mediated protection. Virology 2004, 319, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, C.; Soler, A.; Rodze, I.; Nieto, R.; Cano-Gómez, C.; Fernandez-Pinero, J.; Arias, M. Attenuated and non-haemadsorbing (non-HAD) genotype II African swine fever virus (ASFV) isolated in Europe, Latvia 2017. Transbound. Emerg. Dis. 2019, 66, 1399–1404. [Google Scholar] [CrossRef]
- Gil, S.; Sepúlveda, N.; Albina, E.; Leitão, A.; Martins, C. The low-virulent African swine fever virus (ASFV/NH/P68) induces enhanced expression and production of relevant regulatory cytokines (IFNalpha, TNFalpha and IL12p40) on porcine macrophages in comparison to the highly virulent ASFV/L60. Arch. Virol. 2008, 153, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Boinas, F.S.; Hutchings, G.H.; Dixon, L.K.; Wilkinson, P.J. Characterization of pathogenic and non-pathogenic African swine fever virus isolates from Ornithodoros erraticus inhabiting pig premises in Portugal. J. Gen. Virol. 2004, 85, 2177–2187. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, D.; He, X.; Liu, R.; Wang, Z.; Zhang, X.; Li, F.; Shan, D.; Chen, H.; Zhang, J. A seven-gene-deleted African swine fever virus is safe and effective as a live attenuated vaccine in pigs. Sci. China Life Sci. 2020, 63, 623–634. [Google Scholar] [CrossRef]
- Mulumba-Mfumu, L.K.; Goatley, L.C.; Saegerman, C.; Takamatsu, H.H.; Dixon, L.K. Immunization of African indigenous pigs with attenuated genotype I African swine fever virus OURT88/3 induces protection against challenge with virulent strains of genotype I. Transbound. Emerg. Dis. 2016, 63, 12303. [Google Scholar] [CrossRef]
- Sánchez-Cordón, P.J.; Chapman, D.; Jabbar, T.; Reis, A.L.; Goatley, L.; Netherton, C.L.; Taylor, G.; Montoya, M.; Dixon, L. Different routes and doses influence protection in pigs immunised with the naturally attenuated African swine fever virus isolate OURT88/3. Antivir. Res. 2017, 138, 1–8. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, S.; Zhang, H.; Qin, Z.; Shan, H.; Cai, X. Vaccines for African swine fever: An update. Front. Microbiol. 2023, 14, 1139494. [Google Scholar] [CrossRef] [PubMed]
- Diep, N.V.; Duc, N.V.; Ngoc, N.T.; Dang, V.X.; Tiep, T.N.; Nguyen, V.D.; Than, T.T.; Maydaniuk, D.; Goonewardene, K.; Ambagala, A.; et al. Genotype II live-attenuated ASFV vaccine strains unable to completely protect pigs against the emerging recombinant ASFV genotype I/II strain in Vietnam. Vaccines 2024, 12, 1114. [Google Scholar] [CrossRef]
- Krug, P.W.; Holinka, L.G.; O’Donnell, V.; Reese, B.; Sanford, B.; Fernandez-Sainz, I.; Gladue, D.P.; Arzt, J.; Rodriguez, L.; Risatti, G.R.; et al. The progressive adaptation of a Georgian isolate of African swine fever virus to vero cells leads to a gradual attenuation of virulence in swine corresponding to major modifications of the viral genome. J. Virol. 2015, 89, 2324–2332. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.; Han, Y.; Pan, L.; Yang, J.; Sun, M.; Zhou, P.; Sun, Y.; Bi, Y.; Qiu, H.J. Adaptation of African swine fever virus to HEK293T cells. Transbound. Emerg. Dis. 2021, 68, 2853–2866. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cordón, P.J.; Jabbar, T.; Chapman, D.; Dixon, L.K.; Montoya, M. Absence of long-term protection in domestic pigs immunized with attenuated African swine fever virus isolate OURT88/3 or BeninΔMGF correlates with increased levels of regulatory T cells and interleukin-10. J. Virol. 2020, 94, 00350-20. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.L.; Goatley, L.C.; Jabbar, T.; Lopez, E.; Rathakrishnan, A.; Dixon, L.K. Deletion of the gene for the type I interferon inhibitor I329L from the attenuated African swine fever virus OURT88/3 strain reduces protection induced in pigs. Vaccines 2020, 8, 262. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, Z.; Li, Y.; Song, Y.; Di, D.; Liu, J.; Gong, L.; Chen, Z.; Wu, J.; Ye, Z. Evaluation of an I177L gene-based five-gene-deleted African swine fever virus as a live attenuated vaccine in pigs. Emerg. Microbes Infect. 2023, 12, 2148560. [Google Scholar] [CrossRef]
- Hooper, G.L.; Netherton, C.L.; Wright, E. Cell entry mechanisms of African swine fever virus. Virology 2024, 600, 110277. [Google Scholar] [CrossRef]
- Gao, P.; Zhou, L.; Wu, J.; Weng, W.; Wang, H.; Ye, M.; Qu, Y.; Hao, Y.; Zhang, Y.; Ge, X. Riding apoptotic bodies for cell-cell transmission by African swine fever virus. Proc. Natl. Acad. Sci. USA 2023, 120, 2309506120. [Google Scholar] [CrossRef]
- Yang, X.; Sun, E.; Zhai, H.; Wang, T.; Wang, S.; Gao, Y.; Hou, Q.; Guan, X.; Li, S.; Li, L.F. The antibodies against the A137R protein drive antibody-dependent enhancement of African swine fever virus infection in porcine alveolar macrophages. Emerg. Microbes Infect. 2024, 13, 2377599. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, T.; Luo, R.; Lu, Z.; Lan, J.; Sun, Y.; Fu, Q.; Qiu, H.J. Genetic variations of African swine fever virus: Major challenges and prospects. Viruses 2024, 16, 913. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, H.; Zhang, Y.; Luan, J.; Wang, H. Progress in African swine fever vector vaccine development. Int. J. Mol. Sci. 2025, 26, 921. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Camós, L.; López, E.; Rodríguez, F. African swine fever vaccines: A promising work still in progress. Porc. Health Manag. 2020, 6, 17. [Google Scholar] [CrossRef]
- Ivanov, V.; Efremov, E.E.; Novikov, B.V.; Balyshev, V.M.; Tsibanov, S.Z.; Kalinovsky, T.; Kolbasov, D.V.; Niedzwiecki, A.; Rath, M. Vaccination with viral protein-mimicking peptides postpones mortality in domestic pigs infected by African swine fever virus. Mol. Med. Rep. 2011, 4, 395–401. [Google Scholar] [CrossRef]
- Gaudreault, N.N.; Madden, D.W.; Wilson, W.C.; Trujillo, J.D.; Richt, J.A. African swine fever virus: An emerging DNA arbovirus. Front. Vet. Sci. 2020, 7, 215. [Google Scholar] [CrossRef]
- Oura, C.A.L.; Denyer, M.S.; Takamatsu, H.; Parkhouse, R.M.E. In vivo depletion of CD8+ T lymphocytes abrogates protective immunity to African swine fever virus. J. Gen. Virol. 2005, 85, 2445–2450. [Google Scholar] [CrossRef]
- Takamatsu, H.H.; Denyer, M.S.; Lacasta, A.; Stirling, C.M.; Argilaguet, J.M.; Netherton, C.L.; Oura, C.A.; Martins, C.; Rodríguez, F. Cellular immunity in ASFV responses. Virus Res. 2013, 173, 110–121. [Google Scholar] [CrossRef]
- Wardley, R.C.; Wilkinson, P.J. Lymphocyte responses to African swine fever virus infection. Res. Vet. Sci. 1980, 28, 185–189. [Google Scholar] [CrossRef]
- Scholl, T.; Lunney, J.K.; Mebus, C.A.; Duffy, E.; Martins, C.L. Virus-specific cellular blastogenesis and interleukin-2 production in swine after recovery from African swine fever. Am. J. Vet. Res. 1989, 50, 1781–1786. [Google Scholar] [CrossRef]
- Revilla, Y.; Pena, L.; Viñuela, E. Interferon-gamma production by African swine fever virus-specific lymphocytes. Scand. J. Immunol. 1992, 35, 225–230. [Google Scholar] [CrossRef]
- Martins, C.L.; Lawman, M.J.; Scholl, T.; Mebus, C.A.; Lunney, J.K. African swine fever virus specific porcine cytotoxic T cell activity. Arch. Virol. 1993, 129, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chen, W.; Jiang, C.; Zhang, X.; Sun, Y.; Liu, R.; Wang, J.; Yang, D.; Zhao, D.; Bu, Z.; et al. Host responses to live-attenuated ASFV (HLJ/18-7GD). Viruses 2022, 14, 2003. [Google Scholar] [CrossRef] [PubMed]
- Hühr, J.; Schäfer, A.; Schwaiger, T.; Zani, L.; Sehl, J.; Mettenleiter, T.C.; Blome, S.; Blohm, U. Impaired T-cell responses in domestic pigs and wild boar upon infection with a highly virulent African swine fever virus strain. Transbound. Emerg. Dis. 2020, 67, 3016–3032. [Google Scholar] [CrossRef]
- Huang, Y.; Zhai, W.; Wang, Z.; He, Y.; Tao, C.; Chu, Y.; Pang, Z.; Zhu, H.; Jia, H. Analysis of the immunogenicity of African swine fever F317L protein and screening of T cell epitopes. Animals 2024, 14, 1331. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Huang, Y.; He, Y.; Chu, Y.; Tao, C.; Pang, Z.; Wang, Z.; Zhu, H.; Jia, H. Immunogenicity analysis and identification of potential T-cell epitopes in C129R protein of African swine fever virus. Microorganisms 2024, 12, 1056. [Google Scholar] [CrossRef] [PubMed]
- Zajac, M.D.; Sangewar, N.; Lokhandwala, S.; Bray, J.; Sang, H.; McCall, J.; Bishop, R.P.; Waghela, S.D.; Kumar, R.; Kim, T.; et al. Adenovirus-vectored African swine fever virus pp220 induces robust antibody, IFN-γ, and CTL responses in pigs. Front. Vet. Sci. 2022, 9, 921481. [Google Scholar]
- Song, J.; Wang, M.; Zhou, L.; Tian, P.; Sun, Z.; Sun, J.; Wang, X.; Zhuang, G.; Jiang, D.; Wu, Y. A candidate nanoparticle vaccine comprised of multiple epitopes of the African swine fever virus elicits a robust immune response. J. Nanobiotechnol. 2023, 21, 424. [Google Scholar] [CrossRef]
- Song, J.; Wang, M.; Du, Y.; Wan, B.; Zhang, A.; Zhang, Y.; Zhuang, G.; Ji, P.; Wu, Y.; Zhang, G. Identification of a linear B-cell epitope on the African swine fever virus CD2v protein. Int. J. Biol. Macromol. 2023, 232, 123264. [Google Scholar] [CrossRef] [PubMed]
- Argilaguet, J.M.; Pérez-Martín, E.; Nofrarías, M.; Gallardo, C.; Accensi, F.; Lacasta, A.; Mora, M.; Ballester, M.; Galindo-Cardiel, I.; López-Soria, S.; et al. DNA vaccination partially protects against African swine fever virus lethal challenge in the absence of antibodies. PLoS ONE 2012, 7, e40942. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Camós, L.; López, E.; Navas, M.J.; Pina-Pedrero, S.; Accensi, F.; Correa-Fiz, F.; Park, C.; Carrascal, M.; Domínguez, J.; Salas, M.L.; et al. Identification of promiscuous African swine fever virus T-cell determinants using a multiple technical approach. Vaccines 2021, 9, 29. [Google Scholar] [CrossRef]
- Yue, C.; Xiang, W.; Huang, X.; Sun, Y.; Xiao, J.; Liu, K.; Sun, Z.; Qiao, P.; Li, H.; Gan, J.; et al. Mooring stone-like Arg114 pulls diverse bulged peptides: First insight into African swine fever virus-derived T cell epitopes presented by swine major histocompatibility complex class I. J. Virol. 2022, 96, 01378-21. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, H.; Fan, W.; He, L.; Chen, T.; Zhou, X.; Qi, Y.; Sun, L.; Hu, R.; Luo, T.; et al. Evaluation of cellular immunity with ASFV infection by swine leukocyte antigen (SLA)-peptide tetramers. Viruses 2021, 13, 2264. [Google Scholar] [CrossRef]
- Burmakina, G.; Malogolovkin, A.; Tulman, E.R.; Xu, W.; Delhon, G.; Kolbasov, D.; Rock, D.L. Identification of T-cell epitopes in African swine fever virus CD2v and C-type lectin proteins. J. Gen. Virol. 2019, 100, 259–265. [Google Scholar] [CrossRef]
- Herrera, L.R.M.; Bisa, E.P. In silico analysis of highly conserved cytotoxic T-cell epitopes in the structural proteins of African swine fever virus. Vet. World 2021, 14, 2625–2633. [Google Scholar] [CrossRef]
- Sun, L.; Niu, J.; Zhang, J.; Peng, Y.; Feng, X.; Huang, F.; Liu, J.; Li, S.; Chen, Z. Thermostable T cell multiepitope nanoparticle antigens inducing potent immune responses against the swine fever virus. ACS Infect. Dis. 2023, 9, 2358–2368. [Google Scholar] [CrossRef] [PubMed]
- Hammer, S.E.; Ho, C.S.; Ando, A.; Rogel-Gaillard, C.; Charles, M.; Tector, M.; Tector, A.J.; Lunney, J.K. Importance of the major histocompatibility complex (swine leukocyte antigen) in swine health and biomedical research. Annu. Rev. Anim. Biosci. 2020, 8, 171–198. [Google Scholar] [CrossRef]
- Pedersen, L.E.; Harndahl, M.; Rasmussen, M.; Lamberth, K.; Golde, W.T.; Lund, O.; Nielsen, M.; Buus, S. Porcine major histocompatibility complex (MHC) class I molecules and analysis of their peptide-binding specificities. Immunogenetics 2011, 63, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Li, S.; Wang, S.; Feng, G.; Xie, X.; Li, Z.; Zhang, N. Peptidomes and structures illustrate how SLA-I micropolymorphism influences the preference of binding peptide length. Front. Immunol. 2022, 13, 820881. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Wang, Y.; Wang, S.; Wang, X.; Wu, Y.; Li, Z.; Zhang, N.; Xia, C. Polymorphism and peptide-binding specificities of porcine major histocompatibility complex (MHC) class I molecules. Mol. Immunol. 2018, 93, 236–245. [Google Scholar] [CrossRef]
- Zhang, N.; Qi, J.; Feng, S.; Gao, F.; Liu, J.; Pan, X.; Chen, R.; Li, Q.; Chen, Z.; Li, X.; et al. Crystal structure of swine major histocompatibility complex class I SLA-1 0401 and identification of 2009 pandemic swine-origin influenza A H1N1 virus cytotoxic T lymphocyte epitope peptides. J. Virol. 2011, 85, 11709–11724. [Google Scholar] [CrossRef]
- Fan, S.; Wu, Y.; Wang, S.; Wang, Z.; Jiang, B.; Liu, Y.; Liang, R.; Zhou, W.; Zhang, N.; Xia, C.; et al. Structural and biochemical analyses of swine major histocompatibility complex class I complexes and prediction of the epitope map of important influenza A virus strains. J. Virol. 2016, 90, 6625–6641. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, N.; Wei, X.; Jiang, Y.; Chen, R.; Li, Q.; Liang, R.; Zhang, L.; Ma, L.; Xia, C.; et al. Illumination of PRRSV cytotoxic T lymphocyte epitopes by the three-dimensional structure and peptidome of swine lymphocyte antigen class I (SLA-I). Front. Immunol. 2020, 11, 2995. [Google Scholar] [CrossRef]
- Nielsen, M.; Lundegaard, C.; Blicher, T.; Lamberth, K.; Harndahl, M.; Justesen, S.; Røder, G.; Peters, B.; Sette, A.; Lund, O.; et al. NetMHCpan, a method for quantitative predictions of peptide binding to any HLA-A and -B locus protein of known sequence. PLoS ONE 2007, 2, e796. [Google Scholar] [CrossRef]
- Tung, C.W.; Ziehm, M.; Kämper, A.; Kohlbacher, O.; Ho, S.Y. POPISK: T-cell reactivity prediction using support vector machines and string kernels. BMC Bioinform. 2011, 12, 446. [Google Scholar] [CrossRef]
- Saethang, T.; Hirose, O.; Kimkong, I.; Tran, V.A.; Dang, X.T.; Nguyen, L.A.; Le, T.K.; Kubo, M.; Yamada, Y.; Satou, K. PAAQD: Predicting immunogenicity of MHC class I binding peptides using amino acid pairwise contact potentials and quantum topological molecular similarity descriptors. J. Immunol. Methods 2013, 387, 293–302. [Google Scholar] [CrossRef]
- Sturniolo, T.; Bono, E.; Ding, J.; Raddrizzani, L.; Tuereci, O.; Sahin, U.; Braxenthaler, M.; Gallazzi, F.; Protti, M.P.; Sinigaglia, F.; et al. Generation of tissue-specific and promiscuous HLA ligand databases using DNA microarrays and virtual HLA class II matrices. Nat. Biotechnol. 1999, 17, 555–561. [Google Scholar] [CrossRef]
- Rammensee, H.; Bachmann, J.; Emmerich, N.P.; Bachor, O.A.; Stevanović, S. SYFPEITHI: Database for MHC ligands and peptide motifs. Immunogenetics 1999, 50, 213–219. [Google Scholar] [CrossRef]
- O’Donnell, T.J.; Rubinsteyn, A.; Laserson, U. MHCflurry 2.0: Improved pan-allele prediction of MHC class I-presented peptides by incorporating antigen processing. Cell Syst. 2020, 11, 42–48. [Google Scholar] [CrossRef]
- Racle, J.; Gfeller, D. How to predict binding specificity and ligands for new MHC-II alleles with MixMHC2pred. Methods Mol. Biol. 2024, 2809, 215–235. [Google Scholar] [PubMed]
- Song, X.; Li, Y.; Wu, H.; Qiu, H.; Sun, Y. T-cell epitope-based vaccines: A promising strategy for prevention of infectious diseases. Vaccines 2024, 12, 1181. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, J.; Papangelopoulos, N.; Zajonc, D.M.; Peters, B.; Sette, A.; Bourne, P.E. IEDB-3D: Structural data within the immune epitope database. Nucleic Acids Res. 2011, 39, D1164–D1170. [Google Scholar] [CrossRef]
- Mendes, M.; Mahita, J.; Blazeska, N.; Greenbaum, J.; Ha, B.; Wheeler, K.; Wang, J.; Shackelford, D.; Sette, A.; Peters, B. IEDB-3D 2.0: Structural data analysis within the Immune Epitope Database. Protein Sci. 2023, 32, 4605. [Google Scholar] [CrossRef]
- Yan, Z.; Kim, K.; Kim, H.; Ha, B.; Gambiez, A.; Bennett, J.; de Almeida Mendes, M.F.; Trevizani, R.; Mahita, J.; Richardson, E.; et al. Next-generation IEDB tools: A platform for epitope prediction and analysis. Nucleic Acids Res. 2024, 52, W526–W532. [Google Scholar] [CrossRef] [PubMed]
- Hoof, I.; Peters, B.; Sidney, J.; Pedersen, L.E.; Sette, A.; Lund, O.; Buus, S.; Nielsen, M. NetMHCpan, a method for MHC class I binding prediction beyond humans. Immunogenetics 2009, 61, 1–13. [Google Scholar] [CrossRef]
- Karosiene, E.; Rasmussen, M.; Blicher, T.; Lund, O.; Buus, S.; Nielsen, M. NetMHCIIpan-3.0, a common pan-specific MHC class II prediction method including all three human MHC class II isotypes, HLA-DR, HLA-DP and HLA-DQ. Immunogenetics 2013, 65, 711–724. [Google Scholar] [CrossRef]
- Nielsen, M.; Andreatta, M. NetMHCpan-3.0; improved prediction of binding to MHC class I molecules integrating information from multiple receptor and peptide length datasets. Genome Med. 2016, 8, 33. [Google Scholar] [CrossRef]
- Reynisson, B.; Alvarez, B.; Paul, S.; Peters, B.; Nielsen, M. NetMHCpan-4.1 and NetMHCIIpan-4.0: Improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Res. 2020, 48, W449–W454. [Google Scholar] [CrossRef]
- Zhao, W.; Sher, X. Systematically benchmarking peptide-MHC binding predictors: From synthetic to naturally processed epitopes. PLoS Comput. Biol. 2018, 14, 1006457. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Li, F.; Leier, A.; Marquez-Lago, T.T.; Giam, K.; Croft, N.P.; Akutsu, T.; Smith, A.I.; Li, J.; Rossjohn, J.; et al. A comprehensive review and performance evaluation of bioinformatics tools for HLA class I peptide-binding prediction. Brief. Bioinform. 2020, 21, 1119–1135. [Google Scholar] [CrossRef] [PubMed]
- Tadros, D.M.; Racle, J.; Gfeller, D. Predicting MHC-I ligands across alleles and species: How far can we go? Genome Med. 2025, 17, 25. [Google Scholar] [CrossRef]
- Schaap-Johansen, A.L.; Vujović, M.; Borch, A.; Hadrup, S.R.; Marcatili, P. T cell epitope prediction and its application to immunotherapy. Front. Immunol. 2021, 12, 712488. [Google Scholar] [CrossRef]
- Gutiérrez, A.H.; Martin, W.D.; Bailey-Kellogg, C.; Terry, F.; Moise, L.; De Groot, A.S. Development and validation of an epitope prediction tool for swine (PigMatrix) based on the pocket profile method. BMC Bioinform. 2015, 16, 290. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- McMaster, B.; Thorpe, C.; Ogg, G.; Deane, C.M.; Koohy, H. Can AlphaFold’s breakthrough in protein structure help decode the fundamental principles of adaptive cellular immunity? Nat. Methods 2024, 21, 766–776. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, L.; Wei, X.; Qu, Z.; Du, L.; Wang, S.; Zhang, N. Amino acid insertion in Bat MHC-I enhances complex stability and augments peptide presentation. Commun. Biol. 2024, 7, 586. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, S.; Du, L.; Hu, D.; Chen, X.; Zheng, H.; Ding, H.; Chen, S.; Zhang, L.; Zhang, N. The impact of micropolymorphism in Anpl-UAA on structural stability and peptide presentation. Int. J. Biol. Macromol. 2024, 267, 131665. [Google Scholar] [CrossRef]
- Akache, B.; McCluskie, M.J. The quantification of antigen-specific T cells by IFN-γ ELISpot. Methods Mol. Biol. 2021, 2183, 525–536. [Google Scholar] [PubMed]
- Portugal, R. ELISpot assay for the detection of ASFV-specific interferon-gamma (IFN-γ)-producing cells. Methods Mol. Biol. 2022, 2503, 169–178. [Google Scholar]
- Pymm, P.; Illing, P.T.; Ramarathinam, S.H.; O’Connor, G.M.; Hughes, V.A.; Hitchen, C.; Price, D.A.; Ho, B.K.; McVicar, D.W.; Brooks, A.G.; et al. MHC-I peptides get out of the groove and enable a novel mechanism of HIV-1 escape. Nat. Struct. Mol. Biol. 2017, 24, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Ramarathinam, S.H.; Gras, S.; Alcantara, S.; Yeung, A.W.S.; Mifsud, N.A.; Sonza, S.; Illing, P.T.; Glaros, E.N.; Center, R.J.; Thomas, S.R.; et al. Identification of native and posttranslationally modified HLA-B*57:01-restricted HIV envelope derived epitopes using immunoproteomics. Proteomics 2018, 18, 201700253. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, Q.; He, Y.; Zheng, Y.; Sha, H.; Li, G.; Kong, W.; Liao, J.; Zhao, M. Research Progress on the Development of Porcine Reproductive and Respiratory Syndrome Vaccines. Vet. Sci. 2023, 10, 491. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Yu, S.; Wang, W.; Chen, W.; Wang, X.; Wu, K.; Li, X.; Fan, S.; Ding, H.; Yi, L.; et al. Development of Foot-and-Mouth Disease Vaccines in Recent Years. Vaccines 2022, 10, 1817. [Google Scholar] [CrossRef]
- Petro-Turnquist, E.; Pekarek, M.J.; Weaver, E.A. Swine influenza A virus: Challenges and novel vaccine strategies. Front. Cell. Infect. Microbiol. 2024, 14, 1336013. [Google Scholar] [CrossRef]
- Heine, A.; Juranek, S.; Brossart, P. Clinical and immunological effects of mRNA vaccines in malignant diseases. Mol. Cancer 2021, 20, 52. [Google Scholar] [CrossRef]
- Gote, V.; Bolla, P.K.; Kommineni, N.; Butreddy, A.; Nukala, P.K.; Palakurthi, S.S.; Khan, W. A comprehensive review of mRNA vaccines. Int. J. Mol. Sci. 2023, 24, 2700. [Google Scholar] [CrossRef]
- Mochida, Y.; Uchida, S. mRNA vaccine designs for optimal adjuvanticity and delivery. RNA Biol. 2024, 21, 422–448. [Google Scholar] [CrossRef]
- Arevalo, C.P.; Bolton, M.J.; Le Sage, V.; Ye, N.; Furey, C.; Muramatsu, H.; Alameh, M.G.; Pardi, N.; Drapeau, E.M.; Parkhouse, K.; et al. A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes. Science 2022, 378, 899–904. [Google Scholar] [CrossRef]
- Crunkhorn, S. mRNA vaccine for Lyme disease prevention. Nat. Rev. Drug Discov. 2022, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fan, B.; Song, X.; Gao, J.; Guo, R.; Yi, C.; He, Z.; Hu, H.; Jiang, J.; Zhao, L.; et al. PEDV-spike-protein-expressing mRNA vaccine protects piglets against PEDV challenge. mBio 2024, 15, 02958-23. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xiao, L.; Chen, Z.; Fan, L.; Wang, W.; Guo, R.; He, Z.; Hu, H.; Jiang, J.; Zhao, L.; et al. A spike-based mRNA vaccine that induces durable and broad protection against porcine deltacoronavirus in piglets. J. Virol. 2024, 98, 00535-24. [Google Scholar] [CrossRef]
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 mRNA vaccine development. Signal Transduct. Target. Ther. 2022, 7, 94. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, Y.; Wang, L.; Zhao, X.; Wang, L.; Qiu, X.; Yang, X.; Zhu, W.; Lv, L.; Kang, Y.; et al. Advancing vaccine development: Evaluation of a mannose-modified lipid nanoparticle-based candidate for African swine fever p30 mRNA vaccine eliciting robust immune response in mice. Int. J. Biol. Macromol. 2024, 270, 132432. [Google Scholar] [CrossRef] [PubMed]
- Sira, E.M.J.S.; Fajardo, L.E.; Banico, E.C.; Odchimar, N.M.O.; Orosco, F.L. Design of a multiepitope pan-proteomic mRNA vaccine construct against African swine fever virus: A reverse vaccinology approach. Vet. Med. Int. 2025, 2025, 2638167. [Google Scholar] [CrossRef]
- Lim, H.X.; Lim, J.; Jazayeri, S.D.; Poppema, S.; Poh, C.L. Development of multi-epitope peptide-based vaccines against SARS-CoV-2. Biomed. J. 2021, 44, 18–30. [Google Scholar] [CrossRef]
- Purcell, A.W.; McCluskey, J.; Rossjohn, J. More than one reason to rethink the use of peptides in vaccine design. Nat. Rev. Drug Discov. 2007, 6, 404–414. [Google Scholar] [CrossRef]
- Skwarczynski, M.; Toth, I. Peptide-based synthetic vaccines. Chem. Sci. 2016, 7, 842–854. [Google Scholar] [CrossRef]
- Lü, J.M.; Wang, X.; Marin-Muller, C.; Wang, H.; Lin, P.H.; Yao, Q.; Chen, C. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev. Mol. Diagn. 2009, 9, 325–341. [Google Scholar] [CrossRef]
- Binjawadagi, B.; Dwivedi, V.; Manickam, C.; Ouyang, K.; Wu, Y.; Lee, L.J.; Torrelles, J.B.; Renukaradhya, G.J. Adjuvanted poly(lactic-co-glycolic) acid nanoparticle-entrapped inactivated porcine reproductive and respiratory syndrome virus vaccine elicits cross-protective immune response in pigs. Int. J. Nanomed. 2014, 9, 679–694. [Google Scholar]
- Kianfar, E. Protein nanoparticles in drug delivery: Animal protein, plant proteins and protein cages, albumin nanoparticles. J. Nanobiotechnol. 2021, 19, 159. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Wang, Q.; Li, C.; Zhou, Y.; Yao, X.; Xu, L.; Chang, Y.; Ding, F.; Sun, L.; Zhan, L.; et al. Self-assembled multiepitope nanovaccine provides long-lasting cross-protection against influenza virus. Adv. Healthc. Mater. 2024, 13, 202303531. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]


| Vaccine Platform | Antigen Structure | Efficacy/Protection | Biosafety Concerns |
|---|---|---|---|
| Inactivated | Whole virion, chemically or physically inactivated. | Poor to none. Fails to induce protective immunity even with modern adjuvants. | Safe but poor immunogenicity. |
| Subunit | Single or cocktail of recombinant structural proteins. | Inadequate. Partial reduction in viral load may occur, but fails to prevent clinical disease. | Generally safe. Limited immunogenicity. Potential for poor protective responses. |
| Natural attenuated | Genetically undefined attenuated virus. | Efficacy depends on dose, route, and host factors. | Residual virulence (fever, lesions, abortion). Potential for persistent infection and transmission. |
| Gene knockout attenuated | Virus with deletion of specific virulence genes. | Often strong homologous protection. Cross-protection is frequently inadequate. | Residual virulence, risk of reversion to virulence, and potential emergence of new strains. |
| Tool | Principle and Algorithm | Advantages | Limitations for ASFV/SLA |
|---|---|---|---|
| IEDB (IEDB.org, access on 6 June 2025) | Platform aggregating multiple methods (NetMHCpan, SMM, etc.). | Most widely used; provides consolidated results from various algorithms; includes 3D structural data. | Predictions are primarily based on HLA data; results from different algorithms can be inconsistent. |
| NetMHCpan (services.healthtech.dtu.dk, access on 6 June 2025) | Artificial neural network trained on MS-eluted peptide data. | Broad coverage of MHC-I alleles (including SLA); high accuracy for HLA; continuously updated. | Performance for SLA is still lower than for HLA due to less training data; limited number of SLA alleles covered. |
| MixMHCpred (mixmhc2pred.gfellerlab.org, access on 6 June 2025) | Motif deconvolution algorithm trained on MS-eluted data. | Performs well for alleles with similar binding motifs. | Limited number of SLA alleles are currently supported. |
| AlphaFold 2/3 (alphafoldserver.com, access on 6 June 2025) | Deep learning for predicting protein–peptide complex structures. | Provides structural insights into pSLA binding; not limited by allele-specific training data. | Predicts structure but not binding affinity score directly; requires expert interpretation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, W.; Yang, H.; Zhang, N. Identification of T-Cell Epitopes and Vaccine Development for African Swine Fever Virus. Vaccines 2025, 13, 955. https://doi.org/10.3390/vaccines13090955
Ni W, Yang H, Zhang N. Identification of T-Cell Epitopes and Vaccine Development for African Swine Fever Virus. Vaccines. 2025; 13(9):955. https://doi.org/10.3390/vaccines13090955
Chicago/Turabian StyleNi, Wanyi, Hanchun Yang, and Nianzhi Zhang. 2025. "Identification of T-Cell Epitopes and Vaccine Development for African Swine Fever Virus" Vaccines 13, no. 9: 955. https://doi.org/10.3390/vaccines13090955
APA StyleNi, W., Yang, H., & Zhang, N. (2025). Identification of T-Cell Epitopes and Vaccine Development for African Swine Fever Virus. Vaccines, 13(9), 955. https://doi.org/10.3390/vaccines13090955







