Presence of Vaccine-Induced Antibodies Against Leptospira spp. Complicates the Diagnosis of Leptospirosis by the Microscopic Agglutination Test
Abstract
1. Introduction
2. Materials and Methods
2.1. Dog Population
2.2. Microscopic Agglutination Test
2.3. Statistical Analysis
3. Results
3.1. Antibody Levels Before and After Revaccination
3.2. Vaccine-Associated Adverse Events
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sykes, J.E.; Haake, D.A.; Gamage, C.D.; Mills, W.Z.; Nally, J.E. A global one health perspective on leptospirosis in humans and animals. J. Am. Vet. Med. Assoc. 2022, 260, 1589–1596. [Google Scholar] [CrossRef]
- Vincent, A.T.; Schiettekatte, O.; Goarant, C.; Neela, V.K.; Bernet, E.; Thibeaux, R.; Ismail, N.; Mohd Khalid, M.K.N.; Amran, F.; Masuzawa, T.; et al. Revisiting the taxonomy and evolution of pathogenicity of the genus Leptospira through the prism of genomics. PLoS Negl. Trop. Dis. 2019, 13, e0007270. [Google Scholar] [CrossRef]
- Johnson, R.C.; Rogers, P. Differentiation of Pathogenic and Saprophytic Leptospires with 8-Azaguanine. J. Bacteriol. 1964, 88, 1618–1623. [Google Scholar] [CrossRef]
- Sykes, J.E.; Reagan, K.L.; Nally, J.E.; Galloway, R.L.; Haake, D.A. Role of Diagnostics in Epidemiology, Management, Surveillance, and Control of Leptospirosis. Pathogens 2022, 11, 395. [Google Scholar] [CrossRef]
- Jansen, A.; Schoneberg, I.; Frank, C.; Alpers, K.; Schneider, T.; Stark, K. Leptospirosis in Germany, 1962–2003. Emerg. Infect. Dis. 2005, 11, 1048–1054. [Google Scholar] [CrossRef]
- Klaasen, H.L.; Molkenboer, M.J.; Vrijenhoek, M.P.; Kaashoek, M.J. Duration of immunity in dogs vaccinated against leptospirosis with a bivalent inactivated vaccine. Vet. Microbiol. 2003, 95, 121–132. [Google Scholar] [CrossRef]
- Klaasen, H.L.; van der Veen, M.; Molkenboer, M.J.; Sutton, D. A novel tetravalent Leptospira bacterin protects against infection and shedding following challenge in dogs. Vet. Rec. 2013, 172, 181. [Google Scholar] [CrossRef] [PubMed]
- Klaasen, H.L.; van der Veen, M.; Sutton, D.; Molkenboer, M.J. A new tetravalent canine leptospirosis vaccine provides at least 12 months immunity against infection. Vet. Immunol. Immunopathol. 2014, 158, 26–29. [Google Scholar] [CrossRef]
- Sant’Anna da Costa, R.; Di Azevedo, M.I.N.; Dos Santos Baptista Borges, A.L.; Aymee, L.; Martins, G.; Lilenbaum, W. Effect of Vaccination against Leptospira on Shelter Asymptomatic Dogs Following a Long-Term Study. Animals 2022, 12, 1788. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, P.; Martin, V.; Grousson, D.; Sanquer, A.; Gueguen, S.; Lebreux, B. One-Year Duration of Immunity in Dogs for Leptospira Interrogans Serovar Icterohaemorrhagiae After Vaccination. Intern. J. Appl. Res. Vet. Med. 2012, 10, 305–310. [Google Scholar]
- Day, M.J.; Horzinek, M.C.; Schultz, R.D.; Squires, R.A.; Vaccination Guidelines Group of the World Small Animal Veterinary, A. WSAVA Guidelines for the vaccination of dogs and cats. J. Small. Anim. Pract. 2016, 57, e1–e45. [Google Scholar] [CrossRef]
- Schuller, S.; Francey, T.; Hartmann, K.; Hugonnard, M.; Kohn, B.; Nally, J.E.; Sykes, J. European consensus statement on leptospirosis in dogs and cats. J. Small. Anim. Pract. 2015, 56, 159–179. [Google Scholar] [CrossRef]
- Sykes, J.E.; Francey, T.; Schuller, S.; Stoddard, R.A.; Cowgill, L.D.; Moore, G.E. Updated ACVIM consensus statement on leptospirosis in dogs. J. Vet. Intern. Med. 2023, 37, 1966–1982. [Google Scholar] [CrossRef]
- Francey, T.; Schweighauser, A.; Reber, A.; Schuller, S. Evaluation of changes in the epidemiology of leptospirosis in dogs after introduction of a quadrivalent antileptospiral vaccine in a highly endemic area. J. Vet. Intern. Med. 2020, 34, 2405–2417. [Google Scholar] [CrossRef]
- Martin, L.E.; Wiggans, K.T.; Wennogle, S.A.; Curtis, K.; Chandrashekar, R.; Lappin, M.R. Vaccine-associated Leptospira antibodies in client-owned dogs. J. Vet. Intern. Med. 2014, 28, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.A.; Heseltine, J.C.; Creevy, K.E. The Evaluation of the Diagnostic Value of a PCR Assay When Compared to a Serologic Micro-Agglutination Test for Canine Leptospirosis. Front. Vet. Sci. 2022, 9, 815103. [Google Scholar] [CrossRef]
- Miller, M.D.; Annis, K.M.; Lappin, M.R.; Lunn, K.F. Variability in results of the microscopic agglutination test in dogs with clinical leptospirosis and dogs vaccinated against leptospirosis. J. Vet. Intern. Med. 2011, 25, 426–432. [Google Scholar] [CrossRef]
- Fraune, C.K.; Schweighauser, A.; Francey, T. Evaluation of the diagnostic value of serologic microagglutination testing and a polymerase chain reaction assay for diagnosis of acute leptospirosis in dogs in a referral center. J. Am. Vet. Med. Assoc. 2013, 242, 1373–1380. [Google Scholar] [CrossRef]
- Troia, R.; Balboni, A.; Zamagni, S.; Frigo, S.; Magna, L.; Perissinotto, L.; Battilani, M.; Dondi, F. Prospective evaluation of rapid point-of-care tests for the diagnosis of acute leptospirosis in dogs. Vet. J. 2018, 237, 37–42. [Google Scholar] [CrossRef]
- Lizer, J.; Grahlmann, M.; Hapke, H.; Velineni, S.; Lin, D.; Kohn, B. Evaluation of a rapid IgM detection test for diagnosis of acute leptospirosis in dogs. Vet. Rec. 2017, 180, 517. [Google Scholar] [CrossRef] [PubMed]
- Gloor, C.I.; Schweighauser, A.; Francey, T.; Rodriguez-Campos, S.; Vidondo, B.; Bigler, B.; Schuller, S. Diagnostic value of two commercial chromatographic “patient-side” tests in the diagnosis of acute canine leptospirosis. J. Small Anim. Pract. 2017, 58, 154–161. [Google Scholar] [CrossRef]
- Kodjo, A.; Calleja, C.; Loenser, M.; Lin, D.; Lizer, J. A Rapid In-Clinic Test Detects Acute Leptospirosis in Dogs with High Sensitivity and Specificity. Biomed. Res. Int. 2016, 2016, 3760191. [Google Scholar] [CrossRef]
- Gendron, K.; Christe, A.; Walter, S.; Schweighauser, A.; Francey, T.; Doherr, M.G.; Lang, J. Serial CT features of pulmonary leptospirosis in 10 dogs. Vet. Rec. 2014, 174, 169. [Google Scholar] [CrossRef]
- Grosenbaugh, D.A.; Pardo, M.C. Fifteen-month duration of immunity for the serovar Grippotyphosa fraction of a tetravalent canine leptospirosis vaccine. Vet. Rec. 2018, 182, 665. [Google Scholar] [CrossRef]
- Minke, J.M.; Bey, R.; Tronel, J.P.; Latour, S.; Colombet, G.; Yvorel, J.; Cariou, C.; Guiot, A.L.; Cozette, V.; Guigal, P.M. Onset and duration of protective immunity against clinical disease and renal carriage in dogs provided by a bi-valent inactivated leptospirosis vaccine. Vet. Microbiol. 2009, 137, 137–145. [Google Scholar] [CrossRef]
- Arbeitskreis kleine Haustiere der Standigen Impfkommission, V. Guidelines for the vaccination of small animals-6th edition. Tierarztl Prax Ausg K Kleintiere Heimtiere 2025, 53, 12–22. [Google Scholar] [CrossRef]
- Goris, M.G.; Hartskeerl, R.A. Leptospirosis serodiagnosis by the microscopic agglutination test. Curr. Protoc. Microbiol. 2014, 32, Unit 12E 15. [Google Scholar] [CrossRef]
- Teng, K.T.; Brodbelt, D.C.; Pegram, C.; Church, D.B.; O’Neill, D.G. Life tables of annual life expectancy and mortality for companion dogs in the United Kingdom. Sci. Rep. 2022, 12, 6415. [Google Scholar] [CrossRef]
- Montoya, M.; Morrison, J.A.; Arrignon, F.; Spofford, N.; Charles, H.; Hours, M.A.; Biourge, V. Life expectancy tables for dogs and cats derived from clinical data. Front. Vet. Sci. 2023, 10, 1082102. [Google Scholar] [CrossRef] [PubMed]
- Creevy, K.E.; Grady, J.; Little, S.E.; Moore, G.E.; Strickler, B.G.; Thompson, S.; Webb, J.A. 2019 AAHA Canine Life Stage Guidelines. J. Am. Anim. Hosp. Assoc. 2019, 55, 267–290. [Google Scholar] [CrossRef] [PubMed]
- Hartman, E.G.; van Houten, M.; Frik, J.F.; van der Donk, J.A. Humoral immune response of dogs after vaccination against leptospirosis measured by an IgM- and IgG-specific ELISA. Vet. Immunol. Immunopathol. 1984, 7, 245–254. [Google Scholar] [CrossRef]
- Andre-Fontaine, G. Diagnosis algorithm for leptospirosis in dogs: Disease and vaccination effects on the serological results. Vet. Rec. 2013, 172, 502. [Google Scholar] [CrossRef] [PubMed]
- Sonrier, C.; Branger, C.; Michel, V.; Ruvoen-Clouet, N.; Ganiere, J.P.; Andre-Fontaine, G. Evidence of cross-protection within Leptospira interrogans in an experimental model. Vaccine 2000, 19, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Andre-Fontaine, G.; Triger, L. MAT cross-reactions or vaccine cross-protection: Retrospective study of 863 leptospirosis canine cases. Heliyon 2018, 4, e00869. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, J.; Lemaitre, L.; Cariou, C.; Scotto, M.; Blain, C.; Oberli, F.; Cupillard, L.; Guigal, P.M. A canine vaccine against Leptospira serovars Icterohaemorrhagiae, Canicola and Grippotyphosa provides cross protection against Leptospira serovar Copenhageni. Vet. Immunol. Immunopathol. 2020, 219, 109985. [Google Scholar] [CrossRef]
- Barr, S.C.; McDonough, P.L.; Scipioni-Ball, R.L.; Starr, J.K. Serologic responses of dogs given a commercial vaccine against Leptospira interrogans serovar pomona and Leptospira kirschneri serovar grippotyphosa. Am. J. Vet. Res. 2005, 66, 1780–1784. [Google Scholar] [CrossRef]
- Midence, J.N.; Leutenegger, C.M.; Chandler, A.M.; Goldstein, R.E. Effects of recent Leptospira vaccination on whole blood real-time PCR testing in healthy client-owned dogs. J. Vet. Intern. Med. 2012, 26, 149–152. [Google Scholar] [CrossRef]
- Spiri, A.M.; Rodriguez-Campos, S.; Matos, J.M.; Glaus, T.M.; Riond, B.; Reusch, C.E.; Hofmann-Lehmann, R.; Willi, B. Clinical, serological and echocardiographic examination of healthy field dogs before and after vaccination with a commercial tetravalent leptospirosis vaccine. BMC Vet. Res. 2017, 13, 138. [Google Scholar] [CrossRef]
- Lang, R.W.; Morse, E.V. Serologic cross reactions among Leptospirae observed with sera from animals infected with Leptospira pomona. J. Immunol. 1959, 82, 471–476. [Google Scholar] [CrossRef]
- Jakel, V.; Konig, M.; Cussler, K.; Hanschmann, K.; Thiel, H.J. Factors influencing the antibody response to vaccination against rabies. Dev. Biol. 2008, 131, 431–437. [Google Scholar]
- Kennedy, L.J.; Lunt, M.; Barnes, A.; McElhinney, L.; Fooks, A.R.; Baxter, D.N.; Ollier, W.E. Factors influencing the antibody response of dogs vaccinated against rabies. Vaccine 2007, 25, 8500–8507. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.E.; Guptill, L.F.; Ward, M.P.; Glickman, N.W.; Faunt, K.K.; Lewis, H.B.; Glickman, L.T. Adverse events diagnosed within three days of vaccine administration in dogs. J. Am. Vet. Med. Assoc. 2005, 227, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Miotto, B.A.; Tozzi, B.F.; Penteado, M.S.; Guilloux, A.G.A.; Moreno, L.Z.; Heinemann, M.B.; Moreno, A.M.; Lilenbaum, W.; Hagiwara, M.K. Diagnosis of acute canine leptospirosis using multiple laboratory tests and characterization of the isolated strains. BMC Vet. Res. 2018, 14, 222. [Google Scholar] [CrossRef] [PubMed]
| Serogroup | Serovar | Reference Strain |
|---|---|---|
| Australis 1 | Australis | Ballico |
| Autumnalis | Autumnalis | Akiyami A |
| Ballum | Ballum | Mus 127 |
| Bataviae | Bataviae | Swart |
| Canicola 1 | Canicola | Hond Utrecht IV |
| Grippotyphosa 1 | Grippotyphosa | Moskva V |
| Icterohaemorrhagiae 1 | Icterohaemorrhagiae | RGA |
| Javanica | Javanica | Veldrat Batavia 46 |
| Pomona | Pomona | Pomona |
| Pyrogenes | Pyrogenes | Salinem |
| Sejroe | Hardjo | Hardjoprajitno |
| Tarassovi | Tarassovi | Perepelitsin |
| Serovars | Prevalence | Detectable Antibodies Against n Serovars | ||||
|---|---|---|---|---|---|---|
| n (%) | 95% CI 1 | 1 Serovar | 2 Serovars | 3 Serovars | 4 Serovars | |
| Vaccine serovars | 63/97 | 0.550–0.737 | 24/63 | 25/63 | 11/63 | 3/63 |
| (64.9%) | (38.1%) | (39.7%) | (17.5%) | (4.8%) | ||
| Non-vaccine serovars | 23/97 | 0.163–0.331 | 18/23 | 4/23 | 1/23 | - |
| (23.7%) | (78.3%) | (17.4%) | (4.3%) | |||
| Serovar | Prevalence | Antibody Titre Range | ||
|---|---|---|---|---|
| n (%) | 95% CI 1 | |||
| Vaccine serovars | Icterohaemorrhagiae | 30/63 (47.6%) | 0.358–0.597 | 10–40 |
| Grippotyphosa | 28/63 (44.4%) | 0.328–0.567 | 10–20 | |
| Canicola | 35/63 (55.5%) | 0.433–0.672 | 10–80 | |
| Australis | 26/63 (41.3%) | 0.300–0.536 | 10–20 | |
| Non-vaccine serovars | Autumnalis | 15/23 (65.2%) | 0.458–0.847 | 10–20 |
| Pomona | 4/23 (17.4%) | 0.020–0.330 | 10–40 | |
| Tarassovi | 4/23 (17.4%) | 0.020–0.330 | 10 | |
| Pyrogenes | 3/23 (13.0%) | −0.007–0.268 | 10 | |
| Javanica | 1/23 (4.3%) | −0.040–0.127 | 40 | |
| Ballum | 1/23 (4.3%) | −0.040–0.127 | 10 | |
| Bataviae | 0/23 (0.0%) | - | n.d. 2 | |
| Hardjo | 0/23 (0.0%) | - | n.d. |
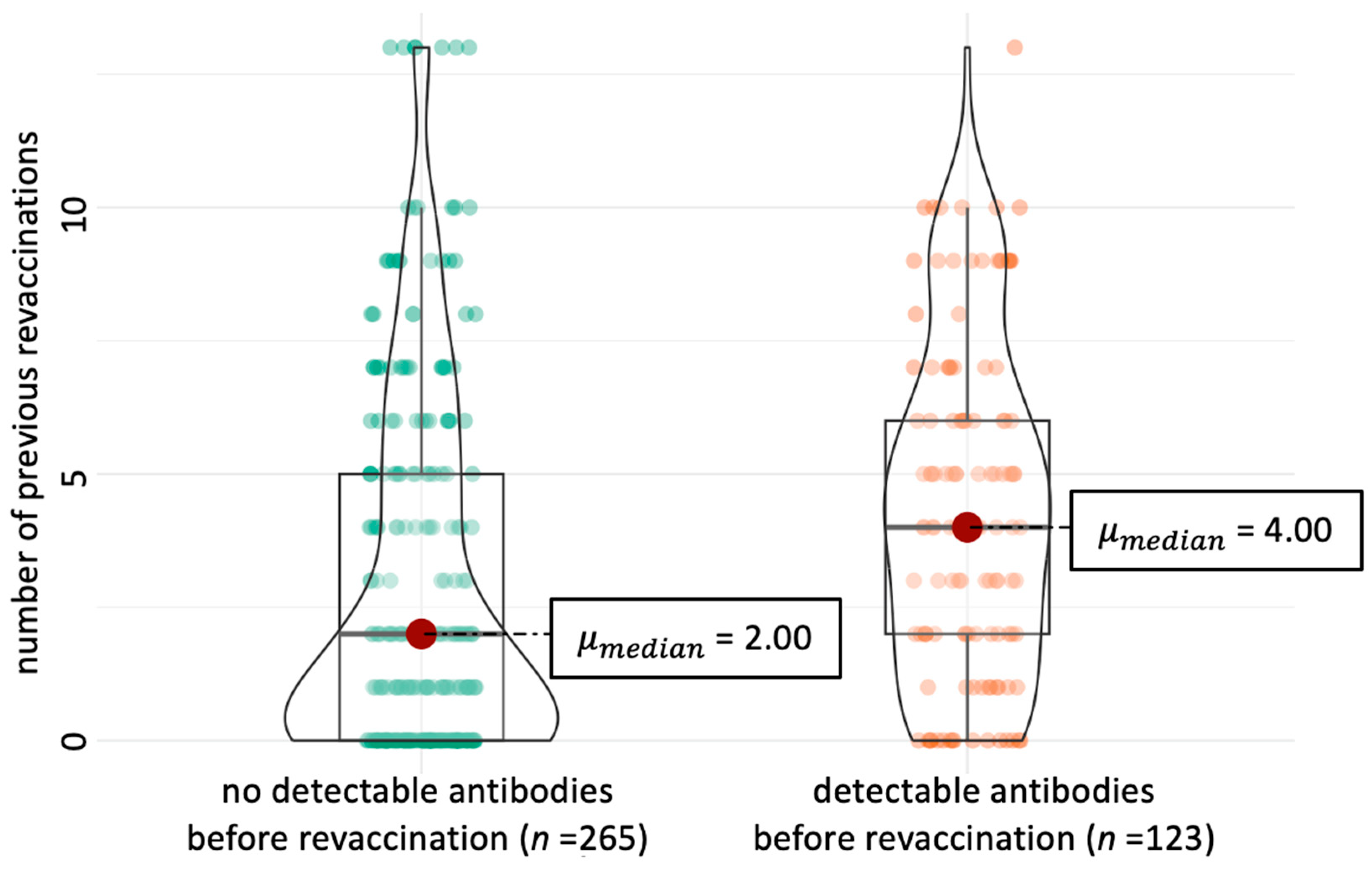
| Variable | Category | Prevalence | Dogs with Pre-Revaccination Antibodies 1 | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|---|---|---|
| p-Value | Odds Ratio | 95% CI 3 | p-Value | Odds Ratio | 95% CI | ||||
| Previous re-vaccinations 2 | primary immunisation | 27/97 | 11/27 | Ref. value 4 | - | - | Ref. value | - | - |
| 1 to <3 vaccinations | 25/97 | 15/25 | 0.443 | 0.502 | 0.165–1.530 | 0.482 | 1.940 | 0.640–5.890 | |
| 3 to <6 vaccinations | 21/97 | 19/21 | 0.001 | 0.144 | 0.046–0.449 | 0.001 | 5.840 | 1.880–18.100 | |
| 6 to <10 vaccinations | 19/97 | 16/19 | 0.003 | 0.215 | 0.068–0.068 | 0.002 | 4.740 | 1.510–14.900 | |
| 10 vaccinations | 5/97 | 4/5 | 0.327 | 0.298 | 0.052–1.723 | 0.802 | 2.060 | 0.350–12.300 | |
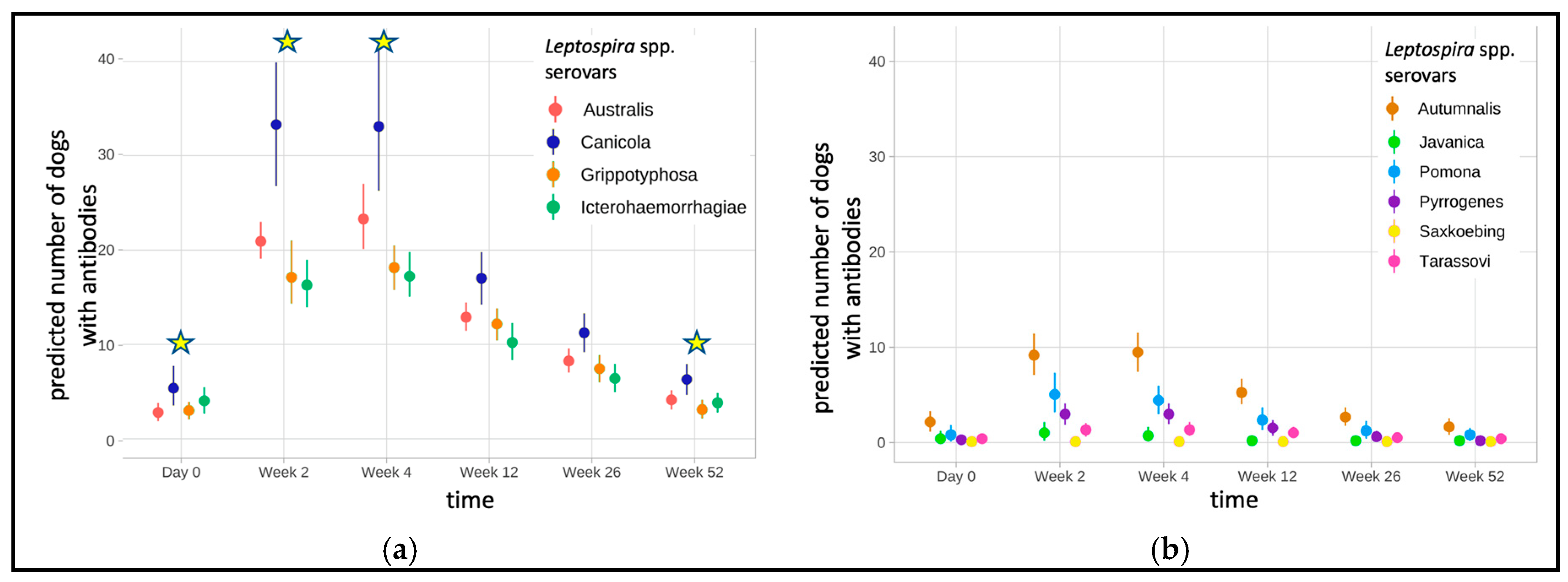
| Serovars | Number of Dogs (Prevalence, %, CI 1) | ||||||
|---|---|---|---|---|---|---|---|
| Overall | Week 0 | Week 2 | Week 4 | Week 12 | Week 26 | Week 52 | |
| Vaccine serovars | 97/97 (100.0%) | 63/97 (64.9%) (0.555–0.744) | 97/97 (100.0%) | 97/97 (100.0%) | 97/97 (100.0%) | 92/97 (94.8%) (0.904–0.992) | 71/97 (73.2%) (0.644–0.820) |
| Non-vaccine serovars | 75/97 (77.3%) (0.690–0.857) | 23/75 (23.7%) (0.202–0.411) | 3/75 (4.0%) (−0.004–0.084) | 22/75 (29.3%) (0.190–0.396) | 20/75 (26.7%) (0.166–0.367) | 10/75 (13.3%) (0.056–0.210) | 20/75 (26.7%) (0.166–0.367) |
| Time Point | Vaccine Serovars 1 (95% CI 2) | Non-Vaccine Serovars 1 (95% CI) | p-Value | Odds Ratio (95% CI) |
|---|---|---|---|---|
| Week 0 | 27.0% (0.213–0.336) | 3.3% (0.021–0.050) | 0.001 | 11.0 (6.96–17.2) |
| Week 2 | 96.7% (0.945–0.980) | 17.9% (0.139–0.227) | 0.001 | 132.7 (78.18–225.2) |
| Week 4 | 97.0% (0.950–0.982) | 19.1% (0.150–0.241) | 0.001 | 137.7 (79.64–238.1) |
| Week 12 | 86.5% (0.818–0.901) | 10.6% (0.079–0.141) | 0.001 | 53.7 (36.31–79.3) |
| Week 26 | 64.8% (0.576–0.715) | 5.0% (0.035–0.072) | 0.001 | 34.8 (23.25–52.2) |
| Week 52 | 35.0% (0.284–0.422) | 3.2% (0.020–0.048) | 0.001 | 16.5 (10.49–26.1) |
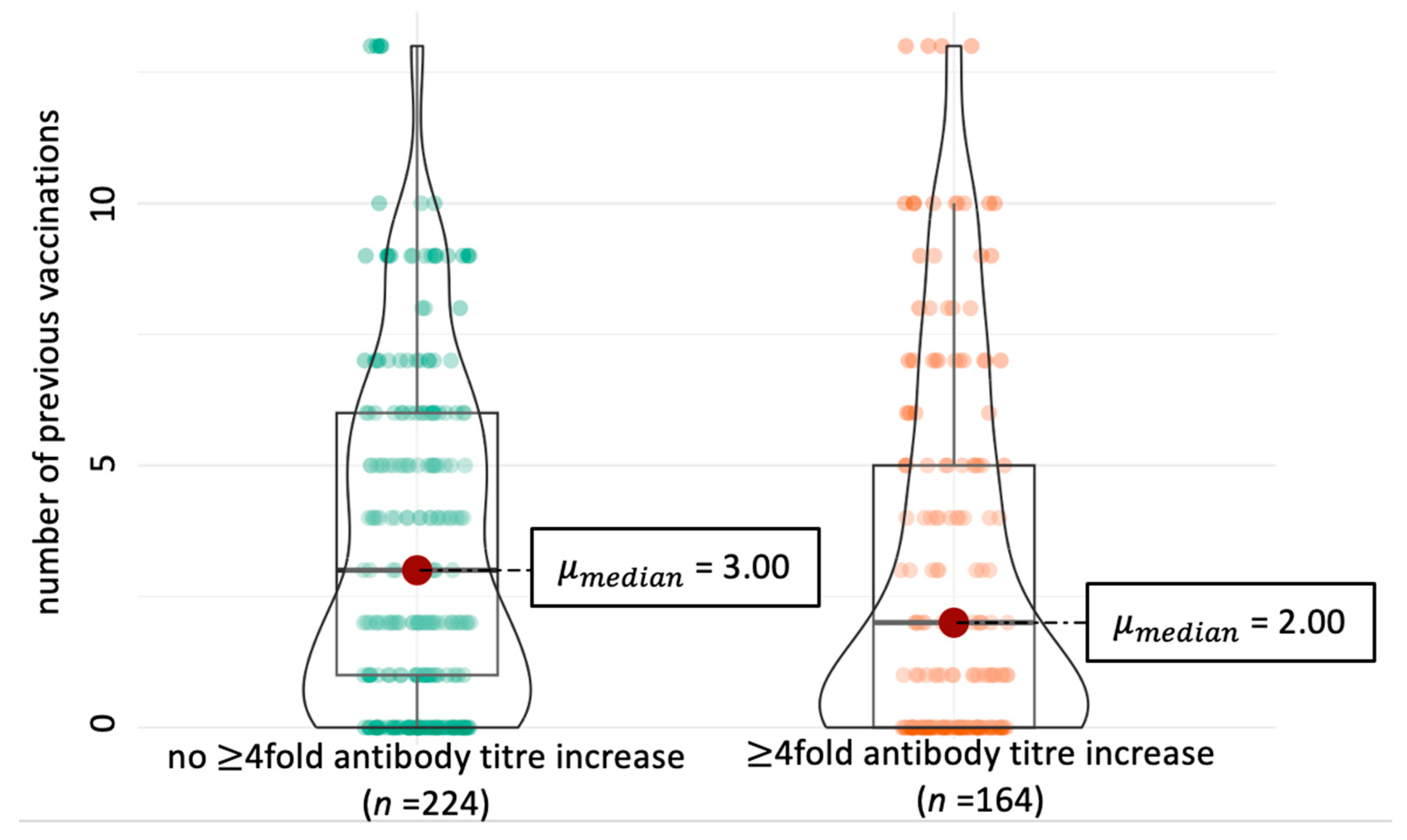
| Variable | Category | Prevalence | 4-Fold Antibody Titre Increase 1 | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|---|---|---|
| p-Value | Odds Ratio | 95% CI 3 | p-Value | Odds Ratio | 95% CI | ||||
| Previous revaccinations 2 | primary immunisation | 27/97 | 25/27 | Ref. value 4 | - | - | Ref. value | - | - |
| 1 to <3 vaccinations | 25/97 | 19/25 | 0.289 | 1.889 | 0.777–4.590 | 0.439 | 0.580 | 0.240–1.390 | |
| 3 to <6 vaccinations | 21/97 | 17/21 | 0.152 | 2.190 | 0.856–5.610 | 0.159 | 0.470 | 0.190–1.170 | |
| 6 to <10 vaccinations | 19/97 | 14/19 | 0.051 | 2.664 | 0.996–7.130 | 0.058 | 0.390 | 0.150–1.020 | |
| 10 vaccinations | 5/97 | 5/5 | 0.912 | 0.609 | 0.126–2.950 | 0.999 | 1.050 | 0.220–5.150 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmitt, K.G.; Bergmann, M.; van der Linden, H.; Ahmed, A.A.; Straubinger, R.K.; Zablotski, Y.; Hartmann, K. Presence of Vaccine-Induced Antibodies Against Leptospira spp. Complicates the Diagnosis of Leptospirosis by the Microscopic Agglutination Test. Vaccines 2025, 13, 956. https://doi.org/10.3390/vaccines13090956
Schmitt KG, Bergmann M, van der Linden H, Ahmed AA, Straubinger RK, Zablotski Y, Hartmann K. Presence of Vaccine-Induced Antibodies Against Leptospira spp. Complicates the Diagnosis of Leptospirosis by the Microscopic Agglutination Test. Vaccines. 2025; 13(9):956. https://doi.org/10.3390/vaccines13090956
Chicago/Turabian StyleSchmitt, Katharina Gesa, Michèle Bergmann, Hans van der Linden, Ahmed A. Ahmed, Reinhard K. Straubinger, Yury Zablotski, and Katrin Hartmann. 2025. "Presence of Vaccine-Induced Antibodies Against Leptospira spp. Complicates the Diagnosis of Leptospirosis by the Microscopic Agglutination Test" Vaccines 13, no. 9: 956. https://doi.org/10.3390/vaccines13090956
APA StyleSchmitt, K. G., Bergmann, M., van der Linden, H., Ahmed, A. A., Straubinger, R. K., Zablotski, Y., & Hartmann, K. (2025). Presence of Vaccine-Induced Antibodies Against Leptospira spp. Complicates the Diagnosis of Leptospirosis by the Microscopic Agglutination Test. Vaccines, 13(9), 956. https://doi.org/10.3390/vaccines13090956







