Humoral Response to the Third Dose of SARS-CoV-2 Vaccine Among Dialysis Patients: A Breakthrough Infection Case–Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Case–Control Study Design
2.3. SARS-CoV-2 Antibody Testing
2.4. Sample Size
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jager, K.J.; Kramer, A.; Chesnaye, N.C.; Couchoud, C.; Sánchez-Álvarez, J.E.; Garneata, L.; Collart, F.; Hemmelder, M.H.; Ambühl, P.; Kerschbaum, J.; et al. Results from the ERA-EDTA Registry Indicate a High Mortality Due to COVID-19 in Dialysis Patients and Kidney Transplant Recipients across Europe. Kidney Int. 2020, 98, 1540–1548. [Google Scholar] [CrossRef]
- El Karoui, K.; De Vriese, A.S. COVID-19 in Dialysis: Clinical Impact, Immune Response, Prevention, and Treatment. Kidney Int. 2022, 101, 883–894. [Google Scholar] [CrossRef]
- Windpessl, M.; Bruchfeld, A.; Anders, H.-J.; Kramer, H.; Waldman, M.; Renia, L.; Ng, L.F.P.; Xing, Z.; Kronbichler, A. COVID-19 Vaccines and Kidney Disease. Nat. Rev. Nephrol. 2021, 17, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Yap, D.Y.-H.; Fong, C.H.-Y.; Zhang, X.; Ip, J.D.; Chan, W.-M.; Chu, A.W.-H.; Chen, L.-L.; Zhao, Y.; Chan, B.P.-C.; Luk, K.S.; et al. Humoral and Cellular Immunity against Different SARS-CoV-2 Variants in Patients with Chronic Kidney Disease. Sci. Rep. 2023, 13, 19932. [Google Scholar] [CrossRef]
- Frantzen, L.; Thibeaut, S.; Moussi-Frances, J.; Indreies, M.; Kiener, C.; Saingra, Y.; Santini, J.; Stroumza, P.; El-Haik, Y.; Cavaillé, G. COVID-19 Vaccination in Haemodialysis Patients: Good Things Come in Threes…. Nephrol. Dial. Transplant. 2021, 36, 1947–1949. [Google Scholar] [CrossRef]
- Liao, B.-H.; Platen, L.; Grommes, M.; Cheng, C.-C.; Holzmann-Littig, C.; Christa, C.; Haller, B.; Kappler, V.; Bester, R.; Werz, M.L.; et al. SARS-CoV-2 Neutralization Capacity in Hemodialysis Patients with and without a Fifth Vaccination with the Updated Comirnaty Original/Omicron BA.4-5 Vaccine. Vaccines 2024, 12, 308. [Google Scholar] [CrossRef]
- Robert, T.; Lano, G.; Giot, M.; Fourié, T.; de Lamballeri, X.; Jehel, O.; Bouchouareb, D.; Brunet, P.; Ninove, L.; Burtey, S. Humoral Response after SARS-CoV-2 Vaccination in Patients Undergoing Maintenance Haemodialysis: Loss of Immunity, Third Dose and Non-Responders. Nephrol. Dial. Transplant. 2022, 37, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Einbinder, Y.; Perl, J.; Nacasch, N.; Bnaya, A.; Shavit, L.; Erez, D.; Shashar, M.; Halperin, T.; Grupper, A.; Benchetrit, S.; et al. Humoral Response and SARS-CoV-2 Infection Risk following the Third and Fourth Doses of the BNT162b2 Vaccine in Dialysis Patients. Am. J. Nephrol. 2022, 53, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Shashar, M.; Nacasch, N.; Grupper, A.; Benchetrit, S.; Halperin, T.; Erez, D.; Rozenberg, I.; Shitrit, P.; Sela, Y.; Wand, O.; et al. Humoral Response to Pfizer BNT162b2 Vaccine Booster in Maintenance Hemodialysis Patients. Am. J. Nephrol. 2022, 53, 207–214. [Google Scholar] [CrossRef]
- Bensouna, I.; Caudwell, V.; Kubab, S.; Acquaviva, S.; Pardon, A.; Vittoz, N.; Bozman, D.-F.; Hanafi, L.; Faucon, A.-L.; Housset, P. SARS-CoV-2 Antibody Response After a Third Dose of the BNT162b2 Vaccine in Patients Receiving Maintenance Hemodialysis or Peritoneal Dialysis. Am. J. Kidney Dis. 2022, 79, 185–192.e1. [Google Scholar] [CrossRef]
- Agur, T.; Zingerman, B.; Ben-Dor, N.; Alkeesh, W.; Steinmetz, T.; Rachamimov, R.; Korzets, A.; Rozen-Zvi, B.; Herman-Edelstein, M. Humoral Response to the Third Dose of BNT162b2 COVID-19 Vaccine among Hemodialysis Patients. Nephron 2023, 147, 185–192. [Google Scholar] [CrossRef]
- Murt, A.; Dinc, H.O.; Altiparmak, M.R.; Yalin, S.F.; Yadigar, S.; Parmaksiz, E.; Kocazeybek, B.; Pekpak, M.; Ataman, M.R. Waning of SARS-CoV-2 Vaccine-Induced Immune Response over 6 Months in Peritoneal Dialysis Patients and the Role of a Booster Dose in Maintaining Seropositivity. Nephron 2022, 146, 559–563. [Google Scholar] [CrossRef]
- Ma, B.M.; Tam, A.R.; Chan, K.W.; Hung, I.F.N.; Tang, S.C.W.; Chan, T.M.; Yap, D.Y.-H. Immunogenicity and Safety of the Three-Dose COVID-19 Vaccine Regimen in Patients Receiving Renal Replacement Therapy: A Systematic Review and Meta-Analysis. Kidney Dis. 2024, 10, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Ponce, P.; Peralta, R.; Felix, C.; Pinto, C.; Pinto, B.; Matos, J.F. Vaccination against SARS-CoV-2 in Haemodialysis Patients: Spike’s Ab Response and the Influence of BMI and Age. Int. J. Environ. Res. Public Health 2022, 19, 10091. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, B.; Soler, M.J.; Ortiz, A.; de Sequera, P. Lessons from SENCOVAC: A Prospective Study Evaluating the Response to SARS-CoV-2 Vaccination in the CKD Spectrum. Nefrología 2023, 43, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.H.; Kireta, S.; Leedham, E.; Russ, G.R.; Coates, P.T. Uremia Impairs Monocyte and Monocyte-Derived Dendritic Cell Function in Hemodialysis Patients. Kidney Int. 2007, 72, 1138–1148. [Google Scholar] [CrossRef]
- Mink, S.; Fraunberger, P. Anti-SARS-CoV-2 Antibody Testing: Role and Indications. J. Clin. Med. 2023, 12, 7575. [Google Scholar] [CrossRef]
- Italian Ministry of Health. Circolare del Ministero della Salute No. 45886 del 8 Ottobre. 2021. Available online: https://3.flcgil.stgy.it/files/pdf/20211011/circolare-ministeriale-45886-dell-8-ottobre-2021-aggiornamento-indicazioni-sulla-somministrazione-di-dosi-addizionali-e-di-dosi-booster-nell-ambito-della-campagna-di-vaccinazione-anti-sars-cov-2-covid-19.pdf (accessed on 15 July 2025).
- Piano strategico nazionale dei vaccini per la prevenzione delle infezioni da SARS-CoV-2. Raccomandazioni ad interim sui gruppi Tatget della vaccinazione anti SARS-CoV-2/COVID-19. GU Serie Generale n.72 del 24-03-2021, 10 March 2021.
- Menniti-Ippolito, F.; Mele, A.; Da Cas, R.; De Masi, S.; Chiarotti, F.; Fabiani, M.; Baglio, G.; Traversa, G.; Colavita, F.; Castilletti, C.; et al. Safety and Efficacy of COVID-19 Vaccines in Patients on Dialysis: A Multicentre Cohort Study in Italy. J. Nephrol. 2023, 36, 2013–2022. [Google Scholar] [CrossRef]
- Ciabattini, A.; Pettini, E.; Fiorino, F.; Polvere, J.; Lucchesi, S.; Coppola, C.; Costagli, S.; Pastore, G.; Sicuranza, A.; Tozzi, M.; et al. Longitudinal Immunogenicity Cohort Study of SARS-CoV-2 MRNA Vaccines across Individuals with Different Immunocompromising Conditions: Heterogeneity in the Immune Response and Crucial Role of Omicron-Adapted Booster Doses. eBioMedicine 2025, 113, 105577. [Google Scholar] [CrossRef]
- Cossmann, A.; Hoffmann, M.; Stankov, M.V.; Lürken, K.; Morillas Ramos, G.; Kempf, A.; Nehlmeier, I.; Pöhlmann, S.; Behrens, G.M.N.; Dopfer-Jablonka, A. Immune Responses Following BNT162b2 XBB.1.5 Vaccination in Patients on Haemodialysis in Germany. Lancet Infect. Dis. 2024, 24, e145–e146. [Google Scholar] [CrossRef]
- Case, J.B.; Jain, S.; Suthar, M.S.; Diamond, M.S. SARS-CoV-2: The Interplay Between Evolution and Host Immunity. Annu. Rev. Immunol. 2025, 43, 29–55. [Google Scholar] [CrossRef] [PubMed]
- De Masi, S.; Da Cas, R.; Menniti Ippolito, F.; Baglio, G.; Zoccali, C.; Chiarotti, F.; Fabiani, M.; Colavita, F.; Castilletti, C.; Salomone, M.; et al. Impact of COVID-19 vaccines in patients on hemodialysis: An Italian multicentre cohort study. J. Nephrol. 2024, 37, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Kedzierski, L.; Chua, B.Y.; Mayo, M.; Lonzi, C.; Rigas, V.; Middleton, B.F.; McQuilten, H.A.; Rowntree, L.C.; Allen, L.F.; et al. Robust and Prototypical Immune Responses toward COVID-19 Vaccine in First Nations Peoples Are Impacted by Comorbidities. Nat. Immunol. 2023, 24, 966–978. [Google Scholar] [CrossRef] [PubMed]
- Huth, L.; Schäfer, L.; Almanzar, G.; Lupoli, G.; Bischof, M.; Wratil, P.R.; Stövesand, T.; Drechsler, C.; Keppler, O.T.; Prelog, M. Immunologic Effect of Bivalent MRNA Booster in Patients Undergoing Hemodialysis. N. Engl. J. Med. 2023, 388, 950–952. [Google Scholar] [CrossRef]
- Tometten, I.; Brandt, T.; Schlotz, M.; Stumpf, R.; Landmann, S.; Kantauskaite, M.; Lamberti, J.; Hillebrandt, J.; Müller, L.; Kittel, M.; et al. Comparison of Immune Responses to SARS-CoV-2 Spike Following Omicron Infection or Omicron BA.4/5 Vaccination in Kidney Transplant Recipients. Front. Immunol. 2025, 15, 1476294. [Google Scholar] [CrossRef]
- Matusali, G.; Colavita, F.; Lapa, D.; Meschi, S.; Bordi, L.; Piselli, P.; Gagliardini, R.; Corpolongo, A.; Nicastri, E.; Antinori, A.; et al. SARS-CoV-2 Serum Neutralization Assay: A Traditional Tool for a Brand-New Virus. Viruses 2021, 13, 655. [Google Scholar] [CrossRef]
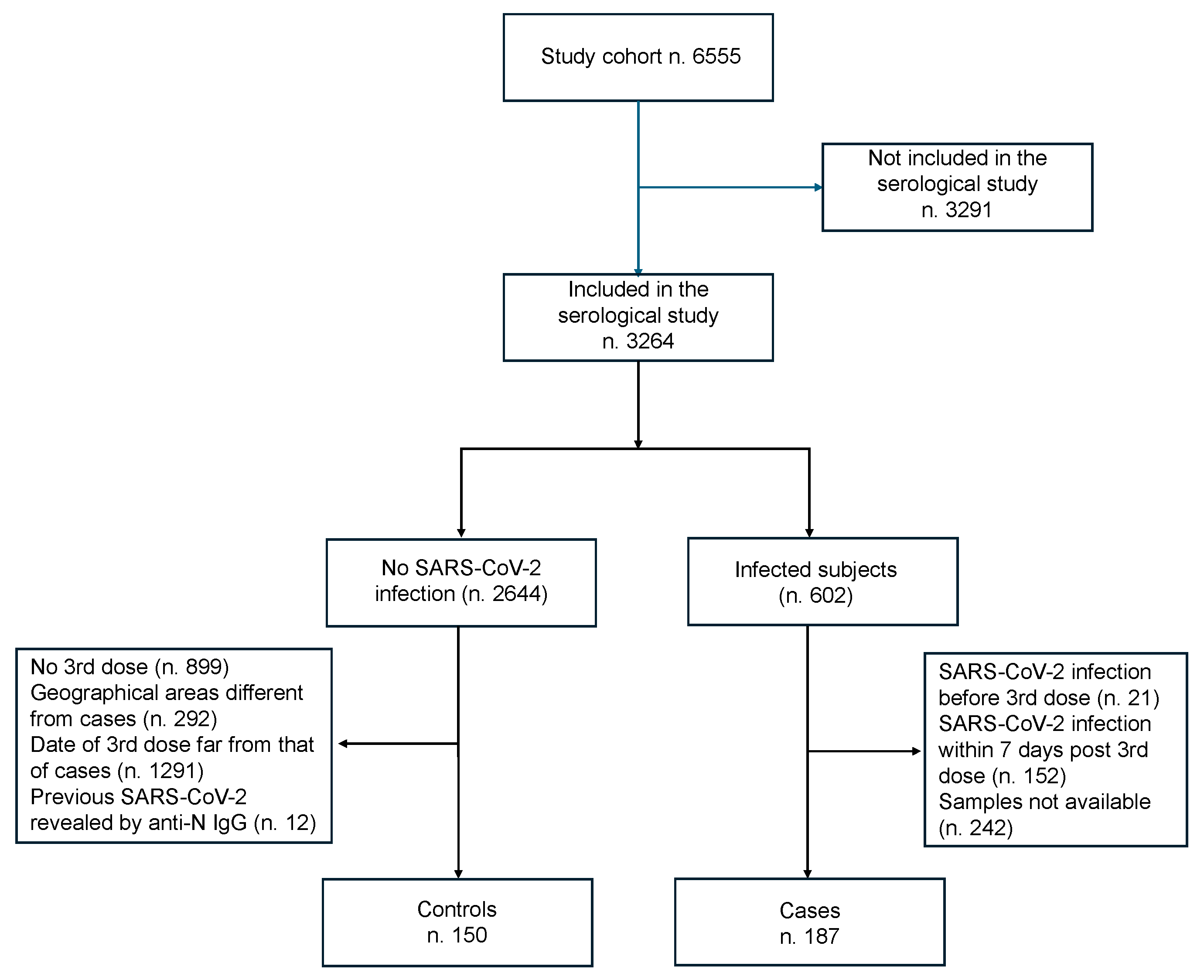
| Cases | Controls | p | Cohort Study [20] | ||
|---|---|---|---|---|---|
| Total | 187 | 150 | 6555 | ||
| No. of clinical centers | 22 | 23 | 120 | ||
| Age | mean (SD) median (min; max) | 64 (15) 66 (22; 88) | 68 (14) 70 (30; 96) | 0.023 | 68 (14) 70 (18; 99) |
| Male sex | n (%) | 119 (64) | 91 (60) | 0.573 | 4254 (65) |
| Heart disease | n (%) | 86 (46) | 49 (32) | 0.014 | 2668 (41) |
| Diabetes | n (%) | 58 (31) | 28 (19) | 0.012 | 1821 (28) |
| Arterial hypertension | n (%) | 139 (74) | 93 (62) | 0.013 | 4381 (67) |
| Pulmonary diseases | n (%) | 28 (15.0) | 17 (11.3) | 0.338 | 682 (10.4) |
| Autoimmune diseases | n (%) | 17 (9) | 4 (3) | 0.021 | 352 (5) |
| Neoplasia | n (%) | 29 (16) | 37 (25) | 0.040 | 1134 (17) |
| Cerebrovascular diseases | n (%) | 22 (11.8) | 10 (6.6) | 0.135 | 677 (10) |
| Comorbidities | mean (SD) median (min; max) | 3.0 (1.7) 3 (0; 8) | 2.5 (1.8) 2 (0; 8) | 0.012 | 2.8 (2.1) 3 (0; 8) |
| Comorbidities ≥ 2 | n (%) | 152 (81.3) | 110 (72.9) | 0.068 | 5921 (90) |
| Concurrent therapies * | mean (SD) median (min; max) | 7.5 (3.4) 7 (0; 18) | 6.8 (4.0) 7 (0; 16) | 0.360 | 7.0 (3.7) 7 (0; 22) |
| Concurrent therapies * ≥ 5 | n (%) | 153 (81.8) | 112 (74.2) | 0.110 | 5136 (78.4) |
| Time (days) between second dose and T3 | mean (SD) median (min; max) | 142.9 (8.1) 142 (57; 167) | 146.6 (12.8) 143 (116; 199) | 0.050 | |
| Time (days) between third dose and T4 | mean (SD) median (min; max) | 36.7 (20.5) 31 (9; 170) | 73.0 (45.8) 72 (11; 196) | <0.001 |
| Cases (187) | Controls (150) | p ** | ||
|---|---|---|---|---|
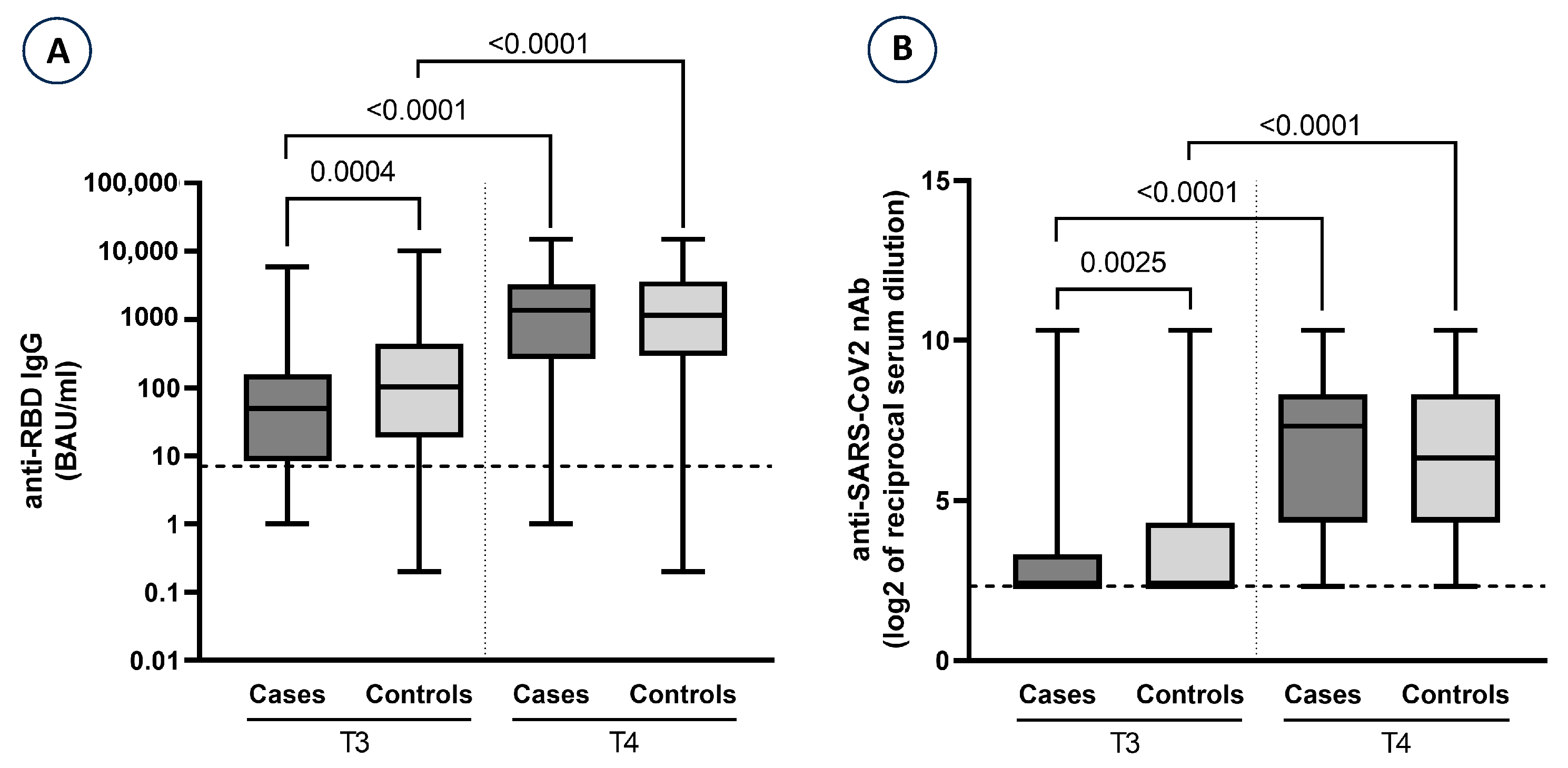
| Anti-S/RBD-IgG § pre-third dose (T3) | mean (SD) median (min; max) | 235.0 (686.5) 49.6 (0; 5939.5) | 705.5 (1676.0) 102.1 (0.2; 10,173.5) | 0.0004 |
| Anti-S/RBD-IgG § post-third dose (T4) | mean (SD) median (min; max) | 2291.7 (2761.6) 1363.9 (0; 15,000) | 2454.8 (3174.2) 1142.6 (0.2; 15,000) | 0.9527 |
| log2 nAb * pre-third dose (T3) | mean (SD) median (min; max) | 3.02 (1.56) 2.32 (2.32; 10.32) | 3.79 (2.40) 2.32 (2.32; 10.32) | 0.0025 |
| log2 nAb * post-third dose (T4) | mean (SD) median (min; max) | 6.23 (2.50) 7.32 (2.32; 10.32) | 6.27 (2.60) 6.32 (2.32; 10.32) | 0.9002 |
| Anti-S/RBD-IgG | log2 nAb | |||
|---|---|---|---|---|
| Unadjusted OR (95%CI) | Adjusted OR (95%CI) | Adjusted OR (95%CI) | ||
| Age (years) | <60 | 1 | 1 | 1 |
| ≥60 | 0.69 (0.37–1.28) | 0.87 (0.43–1.76) | 0.85 (0.40–1.80) | |
| Sex | Male | 1 | 1 | 1 |
| Female | 0.87 (0.46–1.65) | 0.88 (0.43–1.78) | 0.95 (0.45–2.00) | |
| Anti-S/RBD-IgG pre-third dose (100 units) (T3) | 0.97 (0.93–1.00) | |||
| Anti-S/RBD-IgG post-third dose (100 units) (T4) | 0.99 (0.93–1.00) | |||
| log2 nAb pre-third dose * | 0.81 (0.73–0.90) | |||
| log2 nAb post-third dose * | 0.96 (0.83–1.11) | |||
| Diabetes | No | 1 | 1 | 1 |
| Yes | 2.00 (1.10–3.70) | 1.82 (1.10–3.02) | 1.97 (1.17–3.32) | |
| Autoimmune diseases | No | 1 | 1 | 1 |
| Yes | 3.50 (1.40–9.00) | 3.02 (1.15–7.91) | 2.90 (1.19–7.08) | |
| Neoplasia | No | 1 | 1 | 1 |
| Yes | 0.55 (0.33–0.93) | 0.46 (0.21–1.01) | 0.44 (0.19–1.00) | |
| Time (days) between second dose and T3 | 0.96 (0.91–1.01) | 0.97 (0.94–1.00) | 0.97 (0.94–1.00) | |
| Time (days) between third dose and T4 | 0.97 (0.95–0.99) | 0.97 (0.95–0.99) | 0.97 (0.95–0.99) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colavita, F.; Castilletti, C.; Matusali, G.; Accordini, S.; De Masi, S.; Da Cas, R.; Gianesini, N.; Baglio, G.; Francalancia, M.; Traversa, G.; et al. Humoral Response to the Third Dose of SARS-CoV-2 Vaccine Among Dialysis Patients: A Breakthrough Infection Case–Control Study. Vaccines 2025, 13, 935. https://doi.org/10.3390/vaccines13090935
Colavita F, Castilletti C, Matusali G, Accordini S, De Masi S, Da Cas R, Gianesini N, Baglio G, Francalancia M, Traversa G, et al. Humoral Response to the Third Dose of SARS-CoV-2 Vaccine Among Dialysis Patients: A Breakthrough Infection Case–Control Study. Vaccines. 2025; 13(9):935. https://doi.org/10.3390/vaccines13090935
Chicago/Turabian StyleColavita, Francesca, Concetta Castilletti, Giulia Matusali, Silvia Accordini, Salvatore De Masi, Roberto Da Cas, Natasha Gianesini, Giovanni Baglio, Massimo Francalancia, Giuseppe Traversa, and et al. 2025. "Humoral Response to the Third Dose of SARS-CoV-2 Vaccine Among Dialysis Patients: A Breakthrough Infection Case–Control Study" Vaccines 13, no. 9: 935. https://doi.org/10.3390/vaccines13090935
APA StyleColavita, F., Castilletti, C., Matusali, G., Accordini, S., De Masi, S., Da Cas, R., Gianesini, N., Baglio, G., Francalancia, M., Traversa, G., Chiarotti, F., Meschi, S., Bianco, E., Salomone, M., Mele, A., Messa, P., Zoccali, C., Menniti Ippolito, F., & the COVIDVaxDia Study Group. (2025). Humoral Response to the Third Dose of SARS-CoV-2 Vaccine Among Dialysis Patients: A Breakthrough Infection Case–Control Study. Vaccines, 13(9), 935. https://doi.org/10.3390/vaccines13090935








