A Novel Carbohydrate Fatty-Acid Monosulphate Ester, Squalane-in-Water Adjuvant Is Safe and Enhances Inactivated Influenza Vaccine Immunogenicity in Older Adults
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Study Vaccine
2.3. Study Procedures
2.4. Endpoints
2.5. Statistical Analysis
3. Results
3.1. Study Population Demographics
3.2. Reactogenicity
3.3. Safety
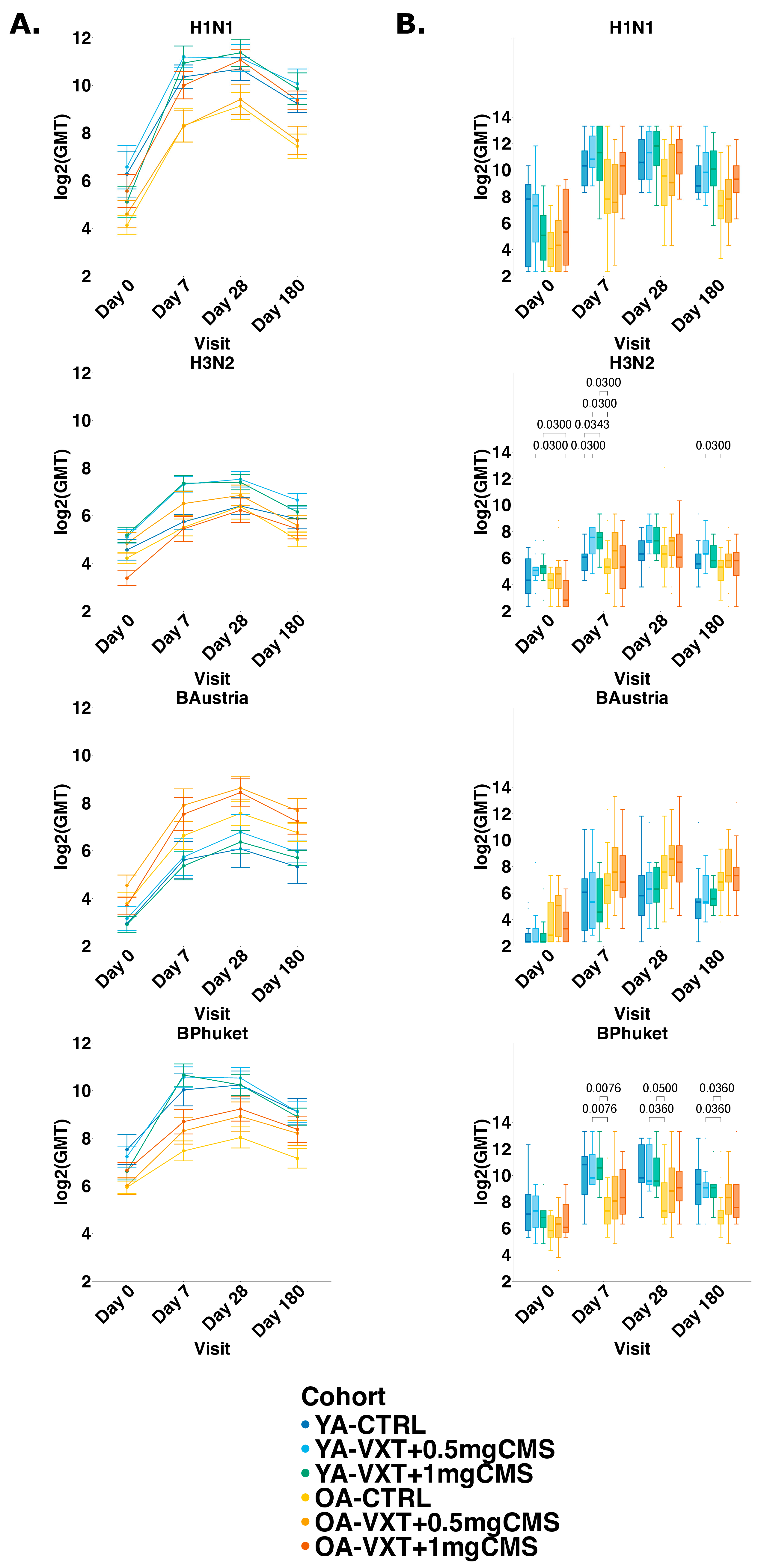
3.4. Hemagglutination Inhibition Titers
3.5. Seroprotection and Seroconversion
3.6. Microneutralization Titers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Influenza (Seasonal) Factsheet. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 15 June 2025).
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X. Immunosenescence: Molecular mechanisms and diseases. Signal Transduct. Target. Ther. 2023, 8, 200. [Google Scholar] [CrossRef]
- Rodrigues, L.P.; Teixeira, V.R.; Alencar-Silva, T.; Simonassi-Paiva, B.; Pereira, R.W.; Pogue, R.; Carvalho, J.L. Hallmarks of aging and immunosenescence: Connecting the dots. Cytokine Growth Factor Rev. 2021, 59, 9–21. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- World Health Organization. Recommended Composition of Influenza Virus Vaccines for Use in the 2022–2023 Nothern Hemisphere Influenza Season; World Health Organization: Geneva, Switzerland, 25 February 2022. [Google Scholar]
- Boraschi, D.; Italiani, P. Immunosenescence and vaccine failure in the elderly: Strategies for improving response. Immunol. Lett. 2014, 162 Pt B, 346–353. [Google Scholar] [CrossRef]
- Weinberger, B.; Herndler-Brandstetter, D.; Schwanninger, A.; Weiskopf, D.; Grubeck-Loebenstein, B. Biology of immune responses to vaccines in elderly persons. Clin. Infect. Dis. 2008, 46, 1078–1084. [Google Scholar] [CrossRef]
- Nichol, K.L.; Nordin, J.D.; Nelson, D.B.; Mullooly, J.P.; Hak, E. Effectiveness of influenza vaccine in the community-dwelling elderly. N. Engl. J. Med. 2007, 357, 1373–1381. [Google Scholar] [CrossRef]
- Veroniki, A.A.; Thirugnanasampanthar, S.S.; Konstantinidis, M.; Dourka, J.; Ghassemi, M.; Neupane, D.; Khan, P.; Nincic, V.; Corry, M.; Robson, R.; et al. Trivalent and quadrivalent seasonal influenza vaccine in adults aged 60 and older: A systematic review and network meta-analysis. BMJ Evid.-Based Med. 2024, 29, 239–254. [Google Scholar] [CrossRef]
- Cortese, M.; Hagan, T.; Rouphael, N.; Wu, S.-Y.; Xie, X.; Kazmin, D.; Wimmers, F.; Gupta, S.; van der Most, R.; Coccia, M.; et al. System vaccinology analysis of predictors and mechanisms of antibody response durability to multiple vaccines in humans. Nat. Immunol. 2025, 26, 116–130. [Google Scholar] [CrossRef]
- Aspinall, R.; Lang, P.O. Vaccine responsiveness in the elderly: Best practice for the clinic. Expert Rev. Vaccines 2014, 13, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Sullivan, M.; Narvaez, C.F.; Holmes, T.H.; Furman, D.; Zheng, N.-Y.; Nishtala, M.; Wrammert, J.; Smith, K.; James, J.A.; et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J. Clin. Investig. 2011, 121, 3109–3119. [Google Scholar] [CrossRef]
- Kang, K.S.; Lee, N.; Shin, M.S.; Kim, S.D.; Yu, Y.; Mohanty, S.; Belshe, R.B.; Montgomery, R.R.; Shaw, A.C.; Kang, I. An altered relationship of influenza vaccine-specific IgG responses with T cell immunity occurs with aging in humans. Clin. Immunol. 2013, 147, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.; Hong, M.S.; Nolasco, H.; Park, S.H.; Dan, J.M.; Choi, J.-Y.; Craft, J. Age-associated change in the frequency of memory CD4+ T cells impairs long term CD4+ T cell responses to influenza vaccine. J. Immunol. 2004, 173, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Platenburg, P.P.L.; Deschamps, F.; Jung, J.; Leonard, C.; Rusconi, S.; Kumar, S.M.; Sulaiman, S.; de Waal, L.; Hilgers, L.A.T. Carbohydrate fatty acid monosulphate ester is a potent adjuvant for low-dose seasonal influenza vaccines. Vaccine 2023, 41, 6980–6990. [Google Scholar] [CrossRef] [PubMed]
- D’onofrio, V.; Porrez, S.; Jacobs, B.; Alhatemi, A.; De Boever, F.; Waerlop, G.; Michels, E.; Vanni, F.; Manenti, A.; Leroux-Roels, G.; et al. Safety and Immunogenicity of a Carbohydrate Fatty Acid Monosulphate Ester Adjuvant Combined with a Low-Dose Quadrivalent Split-Virion Inactivated Influenza Vaccine: A Randomised, Observer-Blind, Active-Controlled, First-in-Human, Phase 1 Study. Vaccines 2024, 12, 1036. [Google Scholar] [CrossRef]
- US Food and Drug Administration, Center for Biologics Evaluation and Research. Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials; US Food and Drug Administration, Center for Biologics Evaluation and Research: Silver Spring, MD, USA, 2007.
- Ruiz-Palacios, G.M.; Leroux-Roels, G.; Beran, J.; Devaster, J.-M.; Esen, M.; Launay, O.; McElhaney, J.E.; van Essen, G.A.; Benoit, A.; Claeys, C.; et al. Immunogenicity of AS03-adjuvanted and non-adjuvanted trivalent inactivated influenza vaccines in elderly adults: A Phase 3, randomized trial and post-hoc correlate of protection analysis. Hum. Vaccines Immunother. 2016, 12, 3043–3055. [Google Scholar] [CrossRef]
- Rümke, H.C.; Richardus, J.H.; Rombo, L.; Pauksens, K.; Plaßmann, G.; Durand, C.; Devaster, J.-M.; Dewé, W.; Oostvogels, L. Selection of an adjuvant for seasonal influenza vaccine in elderly people: Modelling immunogenicity from a randomized trial. BMC Infect. Dis. 2013, 13, 348. [Google Scholar] [CrossRef]
- Couch, R.B.; Bayas, J.M.; Caso, C.; Mbawuike, I.N.; López, C.N.; Claeys, C.; El Idrissi, M.; Hervé, C.; Laupèze, B.; Oostvogels, L.; et al. Superior antigen-specific CD4+ T-cell response with AS03-adjuvantation of a trivalent influenza vaccine in a randomised trial of adults aged 65 and older. BMC Infect. Dis. 2014, 14, 425. [Google Scholar] [CrossRef]
- Cowling, B.J.; Perera, R.A.P.M.; Valkenburg, S.A.; Leung, N.H.L.; Iuliano, A.D.; Tam, Y.H.; Wong, J.H.F.; Fang, V.J.; Li, A.P.Y.; So, H.C.; et al. Comparative Immunogenicity of Several Enhanced Influenza Vaccine Options for Older Adults: A Randomized, Controlled Trial. Clin. Infect. Dis. 2020, 71, 1704–1714. [Google Scholar] [CrossRef]
- Domnich, A.; de Waure, C. Comparative effectiveness of adjuvanted versus high-dose seasonal influenza vaccines for older adults: A systematic review and meta-analysis. Int. J. Infect. Dis. 2022, 122, 855–863. [Google Scholar] [CrossRef]
- Coleman, B.L.; Sanderson, R.; Haag, M.D.M.; McGovern, I. Effectiveness of the MF59-adjuvanted trivalent or quadrivalent seasonal influenza vaccine among adults 65 years of age or older, a systematic review and meta-analysis. Influenza Other Respir. Viruses 2021, 15, 813–823. [Google Scholar] [CrossRef]
- Gavazzi, G.; Fougère, B.; Hanon, O.; Leroux-Roels, I.; Brochot, E.; Blanchard, E.; Russell, C.A.; Paccalin, M.; Schwarz, T.F. Enhanced influenza vaccination for older adults in Europe: A review of the current situation and expert recommendations for the future. Expert Rev. Vaccines 2025, 24, 350–364. [Google Scholar] [CrossRef]
- Miller, M.S.; Montomoli, E.; Leshem, E.; Schotsaert, M.; Weinke, T.; Vicic, N.; Rudin, D. Seasonal influenza vaccines: Variability of immune responses to B lineage viruses. Hum. Vaccines Immunother. 2024, 20, 2421096. [Google Scholar] [CrossRef]
- Belongia, E.A.; Simpson, M.D.; King, J.P.; Sundaram, M.E.; Kelley, N.S.; Osterholm, M.T.; McLean, H.Q. Variable influenza vaccine effectiveness by subtype: A systematic review and meta-analysis of test-negative design studies. Lancet Infect. Dis. 2016, 16, 942–951. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Hottes, T.S.; De Serres, G.; Ward, B.J.; Janjua, N.Z.; Sabaiduc, S.; Chan, T.B.; Petric, M.P. Influenza Β/Victoria antigen induces strong recall of Β/Yamagata but lower Β/Victoria response in children primed with two doses of Β/Yamagata. Pediatr. Infect. Dis. J. 2011, 30, 833–839. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.T.; van der Most, R.; Lodaya, R.N.; Coccia, M.; Lofano, G. “World in motion”—Emulsion adjuvants rising to meet the pandemic challenges. NPJ Vaccines 2021, 6, 158. [Google Scholar] [CrossRef] [PubMed]
- Harandi, A.M. Systems analysis of human vaccine adjuvants. Semin. Immunol. 2018, 39, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Olafsdottir, T.; Lindqvist, M.; Harandi, A.M. Molecular signatures of vaccine adjuvants. Vaccine 2015, 33, 5302–5307. [Google Scholar] [CrossRef]




| YA-CTRL (N = 12) | YA-VXT + 0.5 mg CMS (N = 12) | YA-VXT + 1 mg CMS (N = 12) | OA-CTRL (N = 16) | OA-VXT + 0.5 mg CMS (N = 16) | OA-VXT + 1 mg CMS (N = 16) | Total (N = 84) | ||
|---|---|---|---|---|---|---|---|---|
| Age (years (SD)) | 28.9 (9.0) | 28.3 (8.6) | 27.8 (7.7) | 63.6 (2.6) | 66.9 (6.2) | 67.2 (5.0) | 49.8 (19.8) | |
| Gender (n, %) | Female | 8 (66.7%) | 7 (58.3%) | 8 (66.7%) | 8 (50.0%) | 8 (50.0%) | 9 (56.3%) | 48 (57.1%) |
| Male | 4 (33.3%) | 5 (41.7%) | 4 (33.3%) | 8 (50.0%) | 8 (50.0%) | 7 (43.8%) | 36 (42.9%) | |
| Race (n,%) | White | 12 (100.0%) | 12 (100.0%) | 10 (83.3%) | 16 (100.0%) | 16 (100.0%) | 15 (93.8%) | 81 (96.4%) |
| Asian | 0 (0.0%) | 0 (0.0%) | 2 (16.7%) | 0 (0.0%) | 0 (0.0%) | 1 (6.3%) | 3 (3.6%) | |
| Weight (kg, (SD)) | 72.37 (10.19) | 71.99 (13.21) | 66.51 (10.21) | 77.55 (11.47) | 74.38 (7.92) | 75.04 (15.60) | 73.36 (11.88) | |
| BMI (kg/m2 (SD)) | 24.90 (3.26) | 25.76 (4.69) | 22.23 (2.69) | 24.96 (2.54) | 25.14 (2.24) | 26.24 (3.82) | 24.95 (3.39) |
| YA-CTRL (N = 12) | YA-VXT + 0.5 mg CMS (N = 12) | YA-VXT + 1 mg CMS (N = 12) | OA-CTRL (N = 16) | OA-VXT + 0.5 mg CMS (N = 16) | OA-VXT + 1 mg CMS (N = 16) | Total (N = 84) | |
|---|---|---|---|---|---|---|---|
| Any (nP, %, nE) | 8 (66.7%) 12 | 11 (91.7%) 20 | 11 (91.7%) 39 | 11 (68.8%) 20 | 11 (68.8%) 16 | 13 (81.3%) 29 | 65 (77.4%) 136 |
| Any severe AEs (Grade 3 or higher, (nP, %, nE)) | 1 (8.3%) 2 | 0 (0.0%) 0 | 1 (8.3%) 2 | 0 (0.0%) 0 | 2 (12.5%) 2 | 1 (6.3%) 1 | 5 (6.0%) 7 |
| Probably related (nP, %, nE) | 1 (8.3%) 1 | 4 (33.3%) 5 | 6 (50.0%) 7 | 2 (12.5%) 3 | 0 (0.0%) 0 | 4 (25.0%) 7 | 17 (20.2%) 23 |
| Definitely related (nP, %, nE) | 1 (8.3%) 1 | 5 (41.7%) 7 | 10 (83.3%) 22 | 1 (6.3%) 1 | 1 (6.3%) 1 | 8 (50.0%) 10 | 26 (31.0%) 42 |
| Severe and related * AEs (nP, %, nE) | 0 (0.0%) 0 | 0 (0.0%) 0 | 1 (8.3%) 2 | 0 (0.0%) 0 | 0 (0.0%) 0 | 0 (0.0%) 0 | 1 (1.2%) 2 |
| H3N2 | H1N1 | B/Austria | B/Phuket | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| YA | Visit | Statistic | CTRL | VXT + 0.5 mg CMS | VXT + 1 mg CMS | CTRL | VXT + 0.5 mg CMS | VXT + 1 mg CMS | CTRL | VXT + 0.5 mg CMS | VXT + 1 mg CMS | CTRL | VXT + 0.5 mg CMS | VXT + 1 mg CMS |
| Day 0 | GMT | 21.8 | 17.3 | 41.2 | 31.7 | 30.0 | 12.2 | 5.3 | 6.3 | 5.6 | 15.4 | 11.6 | 7.9 | |
| 95% CI | 9.3–51.3 | 7.1–42.3 | 20.6–82.3 | 11.3–88.9 | 11.9–75.5 | 6.5–23.1 | 4.7–6.0 | 3.8–10.5 | 4.6–6.8 | 6.9–34.6 | 6.4–20.8 | 4.8–13.1 | ||
| Day 7 | GMT | 106.8 | 310.9 | 415.0 | 293.4 | 439.7 | 479.5 | 36.7 | 37.8 | 49.0 | 84.8 | 97.9 | 109.9 | |
| 95% CI | 49.0–232.7 | 161.7–597.7 | 216.3–796.0 | 136.7–629.7 | 235.1–822.3 | 195.0–1179.0 | 17.2–78.3 | 12.3–116.0 | 22.9–104.7 | 31.5–227.8 | 53.5–179.2 | 65.5–184.5 | ||
| GMR | 4.9 | 18.0 | 10.1 | 9.2 | 14.7 | 39.2 | 6.9 | 6.0 | 8.7 | 5.5 | 8.5 | 13.8 | ||
| 95% CI | 2.7–8.9 | 6.0–53.5 | 3.9–25.9 | 2.5–34.0 | 4.3–49.8 | 11.6–132.5 | 3.3–14.5 | 2.0–18.1 | 4.4–17.3 | 2.0–15.1 | 3.3–21.8 | 6.5–29.4 | ||
| Day 28 | GMT | 201.6 | 465.8 | 415.0 | 339.0 | 538.2 | 349.0 | 53.4 | 49.0 | 47.6 | 82.3 | 127.0 | 92.4 | |
| 95% CI | 99.6–407.9 | 221.8–978.3 | 220.9–779.8 | 165.0–696.6 | 257.7–1123.9 | 183.2–664.8 | 19.6–145.4 | 18.0–133.1 | 25.8–87.7 | 31.0–218.9 | 67.0–240.6 | 61.7–138.4 | ||
| GMR | 9.2 | 26.9 | 10.1 | 10.7 | 18.0 | 28.5 | 10.1 | 7.8 | 8.5 | 5.3 | 11.0 | 11.6 | ||
| 95% CI | 4.1–21.0 | 8.2–88.7 | 4.5–22.4 | 3.1–37.1 | 4.0–80.5 | 11.0–73.7 | 3.8–26.7 | 2.7–22.1 | 4.7–15.3 | 2.2–13.1 | 3.9–30.8 | 6.0–22.5 | ||
| Day 180 | GMT | 123.4 | 179.6 | 213.6 | 174.5 | 232.9 | 151.0 | 21.2 | 15.0 | 17.3 | 49.0 | 49.0 | 35.6 | |
| 95% CI | 56.2–270.8 | 94.1–342.6 | 110.8–411.6 | 76.9–395.9 | 133.2–407.4 | 68.9–331.0 | 8.9–50.3 | 7.2–31.2 | 10.0–30.0 | 19.3–124.3 | 22.9–104.7 | 19.8–64.2 | ||
| GMR | 5.7 | 10.4 | 5.2 | 5.5 | 7.8 | 12.3 | 4.0 | 2.4 | 3.1 | 3.2 | 4.2 | 4.5 | ||
| 95% CI | 2.5–12.6 | 3.7–29.0 | 2.9–9.3 | 1.9–16.3 | 2.3–26.7 | 5.1–29.6 | 1.8–9.1 | 1.2–4.9 | 1.8–5.3 | 1.7–6.1 | 1.6–11.5 | 2.3–8.7 | ||
| OA | Visit | Statistic | CTRL | VXT + 0.5 mg CMS | VXT + 1 mg CMS | CTRL | VXT + 0.5 mg CMS | VXT + 1 mg CMS | CTRL | VXT + 0.5 mg CMS | VXT + 1 mg CMS | CTRL | VXT + 0.5 mg CMS | VXT + 1 mg CMS |
| Day 0 | GMT | 11.2 | 24.8 | 12.2 | 10.5 | 10.9 | 20.9 | 9.5 | 12.7 | 7.2 | 6.2 | 7.2 | 8.1 | |
| 95% CI | 6.9–18.3 | 9.0–68.6 | 6.7–22.0 | 5.6–19.7 | 6.1–19.6 | 9.5–45.7 | 5.5–16.7 | 6.6–24.5 | 4.8–10.9 | 5.1–7.4 | 5.0–10.4 | 5.4–12.1 | ||
| Day 7 | GMT | 83.8 | 182.2 | 174.5 | 89.8 | 81.8 | 275.0 | 81.9 | 182.2 | 68.7 | 18.2 | 29.5 | 20.4 | |
| 95% CI | 37.7–186.0 | 77.7–427.4 | 64.1–474.9 | 35.2–229.1 | 38.3–174.5 | 149.9–504.5 | 45.7–146.6 | 93.8–353.8 | 25.2–187.3 | 10.0–33.1 | 14.0–62.4 | 9.6–43.4 | ||
| GMR | 7.5 | 7.3 | 14.4 | 8.6 | 7.5 | 13.2 | 8.6 | 14.4 | 9.5 | 3.0 | 4.1 | 2.5 | ||
| 95% CI | 3.5–16.0 | 2.7–19.7 | 6.3–32.5 | 4.0–18.6 | 3.6–15.5 | 5.5–31.4 | 4.6–16.0 | 5.7–36.4 | 3.7–24.6 | 1.7–5.1 | 2.1–8.0 | 1.2–5.2 | ||
| Day 28 | GMT | 121.3 | 252.2 | 334.2 | 145.9 | 97.2 | 364.4 | 127.0 | 212.0 | 99.3 | 24.1 | 39.1 | 31.5 | |
| 95% CI | 65.7–223.6 | 119.8–530.6 | 144.4–773.4 | 70.0–304.0 | 51.7–183.0 | 243.3–545.8 | 67.0–240.7 | 124.0–362.5 | 46.8–211.1 | 13.4–43.3 | 17.8–86.3 | 15.1–65.9 | ||
| GMR | 10.8 | 10.2 | 27.5 | 13.9 | 8.9 | 17.4 | 13.3 | 16.7 | 13.7 | 3.9 | 5.4 | 3.9 | ||
| 95% CI | 5.9–19.8 | 4.0–25.7 | 13.7–55.1 | 7.1–27.4 | 4.7–16.8 | 8.1–37.4 | 6.1–28.8 | 7.6–36.9 | 7.0–27.1 | 2.2–6.8 | 2.7–10.9 | 1.9–7.9 | ||
| Day 180 | GMT | 40.9 | 110.6 | 190.3 | 40.9 | 40.9 | 149.9 | 54.0 | 87.7 | 46.5 | 10.7 | 21.4 | 18.3 | |
| 95% CI | 24.5–68.5 | 56.1–217.9 | 78.8–459.6 | 21.9–76.6 | 21.2–79.1 | 96.7–232.4 | 28.5–102.4 | 51.9–148.4 | 22.2–97.4 | 6.5–17.5 | 10.0–45.7 | 7.7–43.8 | ||
| GMR | 3.6 | 6.1 | 15.7 | 3.9 | 3.6 | 7.2 | 5.7 | 6.5 | 6.4 | 1.7 | 2.9 | 2.3 | ||
| 95% CI | 2.4–5.7 | 3.2–11.7 | 7.3–33.7 | 2.0–7.7 | 2.1–6.0 | 3.5–14.6 | 2.7–11.6 | 2.9–14.6 | 3.6–11.5 | 1.1–2.7 | 1.6–5.3 | 1.1–4.8 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Onofrio, V.; Jacobs, B.; Alhatemi, A.; De Gussem, S.; Verstraete, M.; Porrez, S.; Willems, A.; De Boever, F.; Waerlop, G.; Leroux-Roels, G.; et al. A Novel Carbohydrate Fatty-Acid Monosulphate Ester, Squalane-in-Water Adjuvant Is Safe and Enhances Inactivated Influenza Vaccine Immunogenicity in Older Adults. Vaccines 2025, 13, 922. https://doi.org/10.3390/vaccines13090922
D’Onofrio V, Jacobs B, Alhatemi A, De Gussem S, Verstraete M, Porrez S, Willems A, De Boever F, Waerlop G, Leroux-Roels G, et al. A Novel Carbohydrate Fatty-Acid Monosulphate Ester, Squalane-in-Water Adjuvant Is Safe and Enhances Inactivated Influenza Vaccine Immunogenicity in Older Adults. Vaccines. 2025; 13(9):922. https://doi.org/10.3390/vaccines13090922
Chicago/Turabian StyleD’Onofrio, Valentino, Bart Jacobs, Azhar Alhatemi, Simon De Gussem, Marjolein Verstraete, Sharon Porrez, Anthony Willems, Fien De Boever, Gwenn Waerlop, Geert Leroux-Roels, and et al. 2025. "A Novel Carbohydrate Fatty-Acid Monosulphate Ester, Squalane-in-Water Adjuvant Is Safe and Enhances Inactivated Influenza Vaccine Immunogenicity in Older Adults" Vaccines 13, no. 9: 922. https://doi.org/10.3390/vaccines13090922
APA StyleD’Onofrio, V., Jacobs, B., Alhatemi, A., De Gussem, S., Verstraete, M., Porrez, S., Willems, A., De Boever, F., Waerlop, G., Leroux-Roels, G., Michels, E., Vanni, F., Manenti, A., Platenburg, P. P., Hilgers, L., & Leroux-Roels, I. (2025). A Novel Carbohydrate Fatty-Acid Monosulphate Ester, Squalane-in-Water Adjuvant Is Safe and Enhances Inactivated Influenza Vaccine Immunogenicity in Older Adults. Vaccines, 13(9), 922. https://doi.org/10.3390/vaccines13090922







