A QS21+ CpG-Adjuvanted Rabies Virus G Subunit Vaccine Elicits Superior Humoral and Moderate Cellular Immunity
Abstract
1. Introduction
2. Materials and Methods
2.1. Vaccine Preparation
2.2. Animals and Immunization
2.3. Determination of Antibody Titers by Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Quantification of Rabies Virus-Neutralizing Antibodies Using Rapid Fluorescent Focus Inhibition Test (RFFIT)
2.5. Enzyme-Linked Immunospot Assay (ELISPOT) of Splenocytes
2.6. Cytokine Analysis
2.7. Flow Cytometry
2.8. Statistical Analysis
3. Results
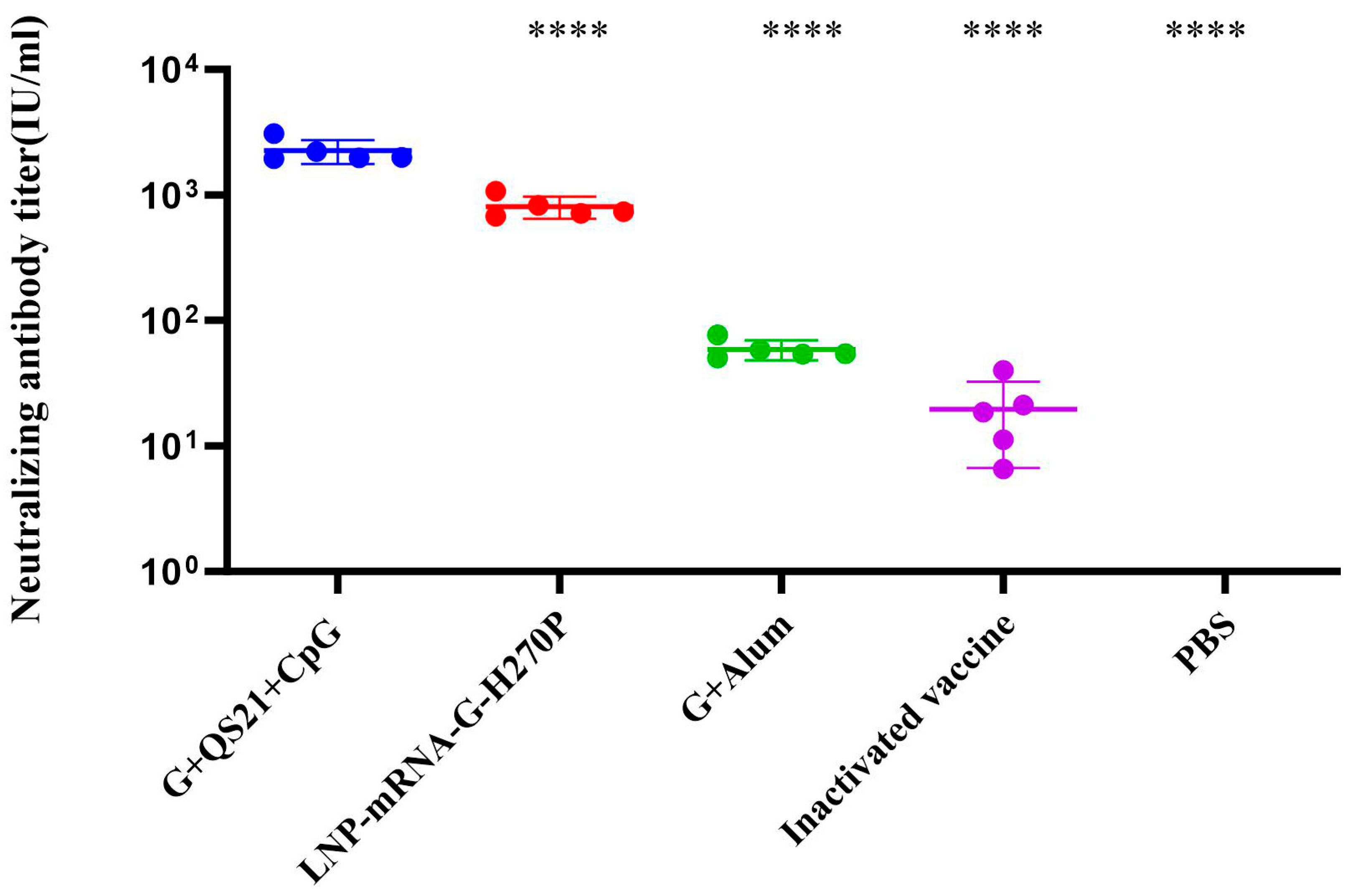
3.1. QS21+CpG Adjuvanted Vaccine Elicits Superior Humoral Immunity
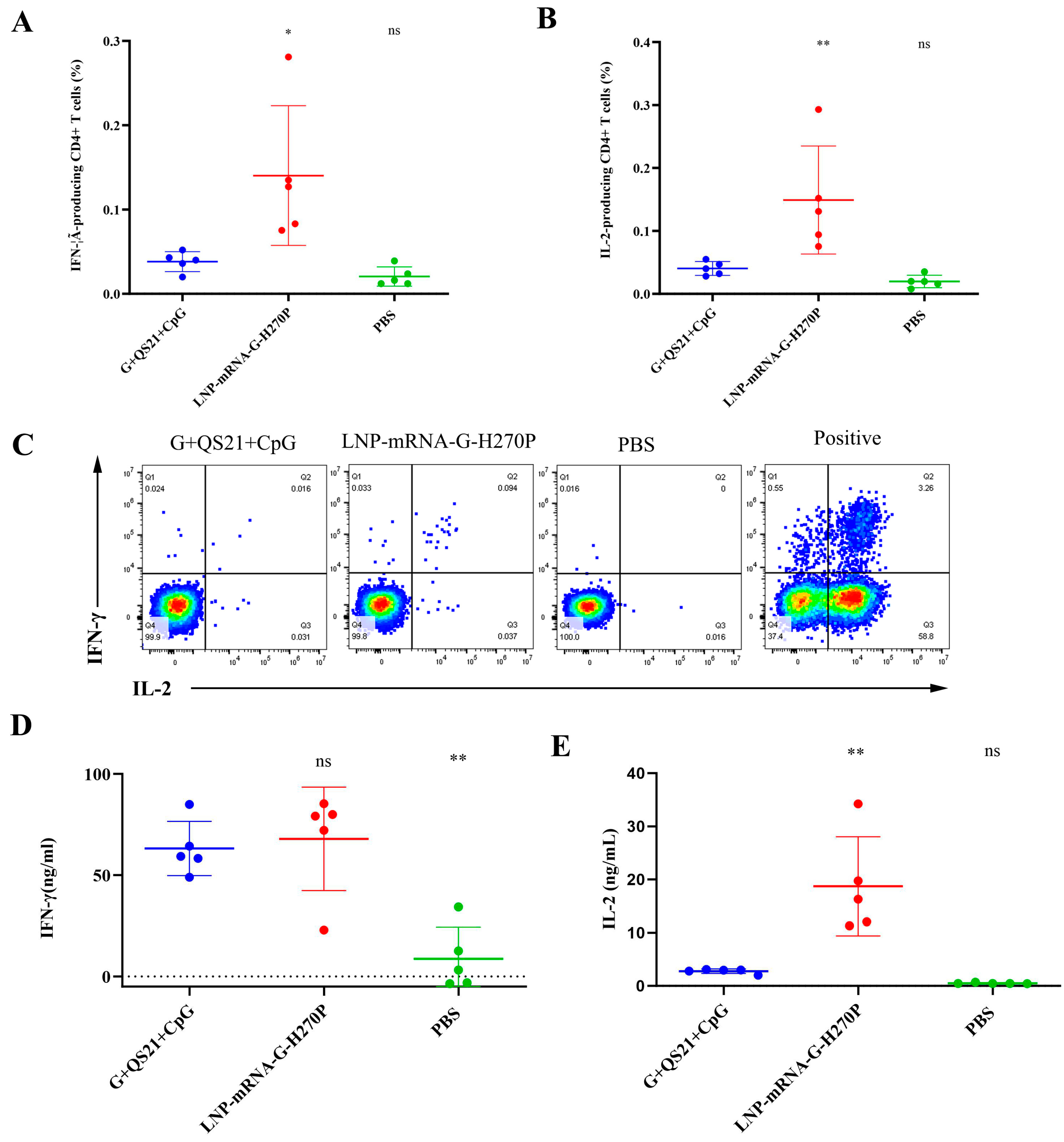
3.2. QS21+CpG Adjuvanted Vaccine Elicits Superior Cellular Immunity
3.3. Protective Efficacy Against Lethal Challenge in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hongtu, Q.; BoLi, L.; Jianguo, C.; Shusheng, P.; Ming, M. Immunogenicity of rabies virus G mRNA formulated with lipid nanoparticles and nucleic acid immunostimulators in mice. Vaccine 2023, 41, 7129–7137. [Google Scholar] [CrossRef]
- Wang, X.; Bao, M.; Wan, M.; Wei, H.; Wang, L.; Yu, H.; Zhang, X.; Yu, Y.; Wang, L. A CpG oligodeoxynucleotide acts as a potent adjuvant for inactivated rabies virus vaccine. Vaccine 2008, 26, 1893–1901. [Google Scholar] [CrossRef]
- Wang, P.-H.; Xing, L. The roles of rabies virus structural proteins in immune evasion and implications for vaccine development. Can. J. Microbiol. 2024, 70, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.; Schnell, M. New developments in rabies vaccination. Rev. Sci. Tech. 2018, 37, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Kaye, A.D.; Perilloux, D.M.; Field, E.; Orvin, C.A.; Zaheri, S.C.; Upshaw, W.C.; Behara, R.; Parker-Actlis, T.Q.; Kaye, A.M.; Ahmadzadeh, S. Rabies Vaccine for Prophylaxis and Treatment of Rabies: A Narrative Review. Cureus 2024, 16, e62429. [Google Scholar] [CrossRef]
- Cao, H.; Li, H.; Luan, N.; Zhang, H.; Lin, K.; Hu, J.; Song, J.; Liu, C. A rabies mRNA vaccine with H270P mutation in its glycoprotein induces strong cellular and humoral immunity. Vaccine 2024, 42, 1116–1121. [Google Scholar] [CrossRef]
- Lin, H.; Perrin, P. Influence of aluminum adjuvant to experimental rabies vaccine. J. Exp. Clin. Virol. 1999, 13, 133–135. [Google Scholar]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef]
- Han, R.; Wang, T.; Cheng, X.; Bing, J.; Li, J.; Deng, Y.; Shan, X.; Zhang, X.; Wang, D.; Sun, S. Immune Responses and Protection Profiles in Mice Induced by Subunit Vaccine Candidates Based on the Extracellular Domain Antigen of Respiratory Syncytial Virus G Protein Combined with Different Adjuvants. Vaccines 2024, 12, 686. [Google Scholar] [CrossRef]
- Kuo, T.-Y.; Lin, M.-Y.; Coffman, R.L.; Campbell, J.D.; Traquina, P.; Lin, Y.-J.; Liu, L.T.-C.; Cheng, J.; Wu, Y.-C.; Wu, C.-C. Development of CpG-adjuvanted stable prefusion SARS-CoV-2 spike antigen as a subunit vaccine against COVID-19. Sci. Rep. 2020, 10, 20085. [Google Scholar] [CrossRef] [PubMed]
- Maddeppungeng, M.; Nurdin, A.; Nency, Y.M.; Sekartini, R.; Medise, B.E.; Soedjatmiko, S.; Massi, M.N.; Darma, S.; Darussalam, A.H.E.; Ramadhani, N. Safety and immunogenicity of a SARS-CoV-2 recombinant protein subunit vaccine adjuvanted with Alum + CpG 1018 in healthy Indonesian adults: A multicenter, randomized, comparative, observer-blind, placebo-controlled phase 2 study. Hum. Vaccines Immunother. 2024, 20, 2429231. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, Y.; Meng, C.; Fan, M.; Guo, J.; Li, J.; Jing, Z.; Wang, P.; Li, R.; Feng, Z. A novel polypeptide vaccine and adjuvant formulation of EV71. Pathog. Dis. 2021, 79, ftab057. [Google Scholar] [CrossRef]
- Isaacs, A.; Li, Z.; Cheung, S.T.; Wijesundara, D.K.; McMillan, C.L.; Modhiran, N.; Young, P.R.; Ranasinghe, C.; Watterson, D.; Chappell, K.J. Adjuvant selection for influenza and RSV prefusion subunit vaccines. Vaccines 2021, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Peng, M.; Zhu, S.; Wu, Y.; Ji, R.; Shen, C. Screening of efficient adjuvants for the RBD-based subunit vaccine of SARS-CoV-2. Vaccines 2023, 11, 713. [Google Scholar] [CrossRef]
- He, L.; Sun, B.; Guo, Y.; Yan, K.; Liu, D.; Zang, Y.; Jiang, C.; Zhang, Y.; Kong, W. Immune response of C57BL/6J mice to herpes zoster subunit vaccines formulated with nanoemulsion-based and liposome-based adjuvants. Int. Immunopharmacol. 2021, 101, 108216. [Google Scholar] [CrossRef] [PubMed]
- Luan, N.; Cao, H.; Wang, Y.; Lin, K.; Liu, C. Ionizable lipid nanoparticles enhanced the synergistic adjuvant effect of CpG ODNs and QS21 in a varicella zoster virus glycoprotein E subunit vaccine. Pharmaceutics 2022, 14, 973. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, X.; Cheng, J.; Li, Y.; Luan, N.; Hu, J.; Liang, B.; Zhang, H.; Gao, D.; Lei, Z. A QS21+ CpG-Adjuvanted Trivalent HSV-2 Vaccine and Trivalent HSV-2 mRNA Vaccine Induce a Strong Immune Response, Protect Against HSV-2 Infection, and Cross-Protect Against HSV-1 Infection in Mice. Vaccines 2025, 13, 497. [Google Scholar] [CrossRef]
- Lin, K.; Cao, H.; Luan, N.; Wang, Y.; Hu, J.; Liu, C. Comparison of the Immune Effects of an mRNA Vaccine and a Subunit Vaccine against Herpes Zoster Administered by Different Injection Methods. Vaccines 2023, 11, 1003. [Google Scholar] [CrossRef]
- Luan, N.; Cao, H.; Wang, Y.; Lin, K.; Liu, C. LNP-CpG ODN-adjuvanted varicella-zoster virus glycoprotein E induced comparable levels of immunity with Shingrix in VZV-primed mice. Virol. Sin. 2022, 37, 731–739. [Google Scholar] [CrossRef]
- Hu, J.; Cao, H.; Luan, N.; Zhang, X.; Liang, B.; Gao, D.; Lei, Z.; Bi, Y.; Liu, C. Herpes simplex virus 1 fusion glycoprotein B H516P prefusion mutation had no effect on vaccine immunogenicity. Vaccine 2025, 57, 127241. [Google Scholar] [CrossRef]
- Yu, P.; Lv, X.; Shen, X.; Tang, Q.; Liang, G. Establishment and preliminary application of a rapid fluorescent focus inhibition test (RFFIT) for rabies virus. Virol. Sin. 2013, 28, 223–227. [Google Scholar] [CrossRef]
- Biacchesi, S.; Skiadopoulos, M.H.; Yang, L.; Murphy, B.R.; Collins, P.L.; Buchholz, U.J. Rapid human metapneumovirus microneutralization assay based on green fluorescent protein expression. J. Virol. Methods 2005, 128, 192–197. [Google Scholar] [CrossRef]
- Plotkin, S.A. Updates on immunologic correlates of vaccine-induced protection. Vaccine 2020, 38, 2250–2257. [Google Scholar] [CrossRef]
- Moyle, P.M.; Toth, I. Modern subunit vaccines: Development, components, and research opportunities. ChemMedChem 2013, 8, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Mahajan, P.; Singh, N.K.; Gupta, A.; Aggarwal, R.; Rappuoli, R.; Johri, A.K. New-age vaccine adjuvants, their development, and future perspective. Front. Immunol. 2023, 14, 1043109. [Google Scholar] [CrossRef] [PubMed]
- Didierlaurent, A.M.; Laupèze, B.; Di Pasquale, A.; Hergli, N.; Collignon, C.; Garçon, N. Adjuvant system AS01: Helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 2017, 16, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Marty-Roix, R.; Vladimer, G.I.; Pouliot, K.; Weng, D.; Buglione-Corbett, R.; West, K.; MacMicking, J.D.; Chee, J.D.; Wang, S.; Lu, S. Identification of QS-21 as an inflammasome-activating molecular component of saponin adjuvants. J. Biol. Chem. 2016, 291, 1123–1136. [Google Scholar] [CrossRef]
- Faber, M.; Bette, M.; Preuss, M.A.; Pulmanausahakul, R.; Rehnelt, J.; Schnell, M.J.; Dietzschold, B.; Weihe, E. Overexpression of tumor necrosis factor alpha by a recombinant rabies virus attenuates replication in neurons and prevents lethal infection in mice. J. Virol. 2005, 79, 15405–15416. [Google Scholar] [CrossRef]
- Damodar, T.; Mani, R.S.; Prathyusha, P. Utility of rabies neutralizing antibody detection in cerebrospinal fluid and serum for ante-mortem diagnosis of human rabies. PLoS Negl. Trop. Dis. 2019, 13, e0007128. [Google Scholar] [CrossRef]
- Expert, C. WHO Expert Consultation on rabies. Wkly. Epidemiol. Rec. 2010, 85, 309–320. [Google Scholar]
- Debnath, A.; Pathak, D.C.; D’silva, A.L.; Batheja, R.; Ramamurthy, N.; Vakharia, V.N.; Chellappa, M.M.; Dey, S. Newcastle disease virus vectored rabies vaccine induces strong humoral and cell mediated immune responses in mice. Vet. Microbiol. 2020, 251, 108890. [Google Scholar] [CrossRef] [PubMed]
- Mata-Haro, V.; Cekic, C.; Martin, M.; Chilton, P.M.; Casella, C.R.; Mitchell, T.C. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science 2007, 316, 1628–1632. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Yu, P.; Zhu, W. Development of mRNA rabies vaccines. Hum. Vaccines Immunother. 2024, 20, 2382499. [Google Scholar] [CrossRef] [PubMed]




| Vaccine Group | mRNA (μg/dose) | RABV-G (μg/dose) | QS21 (μg/dose) | CpG (μg/dose) | Alum (mg/dose) | Inactivated Virus (≥2.5 IU/dose) |
|---|---|---|---|---|---|---|
| G+QS21+CpG | - | 5 | 5 | 10 | - | - |
| LNP-mRNA-G-H270P | 15.4 | - | - | - | - | - |
| G+Alum | - | 5 | - | - | 1 | - |
| Inactivated vaccine | - | - | - | - | - | 1/6 |
| PBS | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, H.; Li, H.; Liu, W.; Luan, N.; Hu, J.; Kong, M.; Song, J.; Liu, C. A QS21+ CpG-Adjuvanted Rabies Virus G Subunit Vaccine Elicits Superior Humoral and Moderate Cellular Immunity. Vaccines 2025, 13, 887. https://doi.org/10.3390/vaccines13080887
Cao H, Li H, Liu W, Luan N, Hu J, Kong M, Song J, Liu C. A QS21+ CpG-Adjuvanted Rabies Virus G Subunit Vaccine Elicits Superior Humoral and Moderate Cellular Immunity. Vaccines. 2025; 13(8):887. https://doi.org/10.3390/vaccines13080887
Chicago/Turabian StyleCao, Han, Hui Li, Wenzhi Liu, Ning Luan, Jingping Hu, Meijun Kong, Jie Song, and Cunbao Liu. 2025. "A QS21+ CpG-Adjuvanted Rabies Virus G Subunit Vaccine Elicits Superior Humoral and Moderate Cellular Immunity" Vaccines 13, no. 8: 887. https://doi.org/10.3390/vaccines13080887
APA StyleCao, H., Li, H., Liu, W., Luan, N., Hu, J., Kong, M., Song, J., & Liu, C. (2025). A QS21+ CpG-Adjuvanted Rabies Virus G Subunit Vaccine Elicits Superior Humoral and Moderate Cellular Immunity. Vaccines, 13(8), 887. https://doi.org/10.3390/vaccines13080887







