1. Background
Respiratory syncytial virus (RSV) remains a major global health burden, posing a considerable threat to the health of children and the elderly. According to a meta-analysis [
1], RSV infection is extremely prevalent among children, accounting for 70% of all pediatric respiratory infections, and annually causing approximately 33 million cases of acute lower respiratory tract infections (LTRIs) in children aged <5 years worldwide, which cause an estimated 3.6 million hospitalizations due to RSV and approximately 26,300 deaths attributed to RSV-related hospitalizations. Concurrently, immunosenescence profoundly affects the immune systems of elderly individuals, in which antibody responses are less numerous and efficient compared to those of younger individuals, and dysregulation of cytokine production along with lymph node fibrosis, all of which increase their susceptibility to infectious diseases like RSV. For the elderly population, there were about 5.2 million cases of RSV-related acute respiratory infections (ARIs) among adults aged 60 and above worldwide, with approximately 470,000 hospitalizations, and 33,000 of these hospitalizations ended in death [
2]. In high-income countries, it is expected that by 2025, the number of RSV-ARI cases among individuals aged 65 and older could reach 10 million, with 800,000 requiring hospital treatment and 74,000 potentially leading to death in the hospital [
3].
The journey of RSV vaccine development has been filled with challenges and innovations. In the early 1960s, an attempt to develop a formalin-inactivated RSV (FI-RSV) vaccine was thwarted due to vaccine-enhanced disease (VED), which served as a cautionary tale for subsequent research [
4,
5,
6]. Since then, scientists have explored a variety of vaccine strategies to prevent severe disease caused by RSV. Advances in structural biology have revealed the potential of stable prefusion F protein subunit vaccines, a discovery that has injected new vitality into RSV vaccine development. Preclinical and clinical studies have shown that vaccine designs targeting the prefusion conformation of the RSV fusion (F) protein elicit stronger neutralizing antibody responses than the postfusion form [
7,
8].
Currently, the development of RSV vaccines is rapidly advancing. Several RSV candidate vaccines show good efficacy and safety in preventing RSV-related acute respiratory infections [
9]. For instance, the RSV prefusion F vaccine has demonstrated 68% efficacy in preventing RSV-related acute respiratory infections, 70% efficacy in preventing RSV-related lower respiratory infections that require medical intervention, and 87% efficacy in preventing severe RSV-related lower respiratory infections in elderly populations.
Despite the progress made in RSV vaccine development, there are still research gaps that need to be filled. The current research on the efficacy and safety of RSV vaccines in different populations is insufficient, particularly among infants [
10]. Moreover, further research is needed to assess the long-term protective effects and cost-effectiveness of RSV vaccines. Since the current RSV vaccines and antibodies are mainly based on the RSV prefusion protein (preF), researchers are also exploring the potential of other RSV non-fusion antigens as vaccine immunogens, such as glycoprotein G. This G protein serves as an attachment factor during RSV infection, interacting with receptors on target cells to facilitate viral entry and initiate infection [
11]. By developing subunit vaccines targeting the RSV G protein, researchers aim to elicit an immune response that neutralizes the virus before it can enter cells, thereby preventing infection. Promising results from preclinical studies have positioned the G protein as a viable candidate for subunit RSV vaccine development [
12,
13,
14].
Moreover, the use of a bacterially expressed non-glycosylated G protein-based vaccine candidate has demonstrated cross-protective efficacy against both homologous and heterologous RSV challenges in preclinical models, indicating its potential as a broadly protective vaccine candidate [
15].
The development of BARS13, a recombinant RSV G protein-based vaccine, represents a strategic approach to addressing key gaps in RSV vaccine research. Unlike other vaccines that focus on the F protein, BARS13 leverages the non-glycosylated G protein, a critical attachment factor in RSV infection, which plays a pivotal role in the virus’s ability to enter host cells. The RSV attachment (G) glycoprotein is a 298-amino acid surface protein heavily glycosylated with up to 35 N-linked glycans, which mask most linear epitopes and limit antibody recognition [
14]. Unlike the prefusion F protein, which is the target of licensed RSV vaccines, the G protein is structurally unique and lacks a well-defined neutralizing domain. However, a conserved region known as the “cysteine noose” has been implicated in receptor binding and can elicit neutralizing antibodies. The recombinant G protein used in BARS13 is non-glycosylated and expressed in E. coli, preserving the cysteine noose structure and avoiding glycan-mediated immune evasion. By targeting the G protein, BARS13 offers a novel mechanism of action aimed at neutralizing the virus at the point of entry, potentially offering broader protection against both homologous and heterologous RSV strains [
16].
The innovation of using a non-glycosylated form of the G protein with its low dose of CsA lies in its capacity to induce a strong antibody response while avoiding the complexities associated with vaccine-enhanced disease (VED) seen in the early RSV vaccines [
4,
5]. This non-glycosylated form improves the stability of the antigen and simplifies the manufacturing process, offering cost-effective scalability for widespread vaccine distribution. CsA was included in the BARS13 formulation at a sub-immunosuppressive dose (1000× lower than clinical immunosuppressive dosing) based on prior preclinical studies [
15]. In this context, CsA acts as an immunomodulator, enhancing humoral immunity by promoting regulatory T cell (Treg) responses, which mitigate vaccine-enhanced disease (VED) while preserving antigen-specific antibody production. Preclinical studies have demonstrated that this approach not only enhances immunogenicity but also avoids the immune evasion mechanisms often facilitated by the glycosylated forms of viral proteins [
14], making BARS13 a promising candidate for durable protection.
Moreover, BARS13 fills a critical gap in vaccine development for older adults, a population historically underserved by the existing RSV vaccine efforts. The elderly are disproportionately affected by RSV due to age-related immune decline, and the effectiveness of the existing vaccines is often limited by pre-existing RSV immunity and weaker vaccine-induced responses in this demographic [
17]. By focusing on the G protein, BARS13 has the potential to overcome these challenges, as initial studies have shown it induces a robust humoral response even in individuals with prior RSV exposure.
Recently, we conducted the first Phase 1 clinical study to preliminarily evaluate the safety and immune response of a non-glycosylated G protein plus the immunomodulator CsA as an RSV vaccine in healthy adults aged 18 to 45 [
18]. The results showed that no serious adverse events occurred in volunteers after vaccination at different dosage levels. Concurrently, the vaccine induced a strong humoral immune response, with an increase in RSV-specific antibody concentrations [
18]. As a promising candidate for the development of a subunit RSV vaccine, we have further conducted phase II clinical studies in elderly populations to assess its safety and immune response.
2. Methods
2.1. Study Design and Procedures
In this phase II clinical trial (NCT04681833), a randomized, double-blind, placebo-controlled, dose-ranging study was implemented to evaluate the safety, tolerability, and immunogenicity of the respiratory syncytial virus (RSV) vaccine candidate BARS13, which contained purified RSV G recombinant protein and a formulated Cyclosporine A (CsA) sterile solution. This study targeted healthy elderly participants, aged between 60 and 80 years.
Participants were randomly assigned to receive the BARS13 vaccine or placebo, with Cohort 1 consisting of 40 participants and Cohort 2 also comprising 40 participants, both at a 3:1 ratio of vaccine to placebo. Cohort 3 included 45 participants at a 2:1 ratio. Injections were administered on Days 1 and 29 to all cohorts, with Cohort 3 receiving an additional dose on Day 57. The single dosage for Cohort 1 was 10 μg of rRSV G protein plus 10 μg of CsA, whereas Cohorts 2 and 3 received 20 μg of each component.
The trial, conducted from May 2021 to January 2024 across two Australian sites, included three dosing cohorts with a total of 125 participants. Cohorts 1 and 2 received two injections on Days 1 and 29, while Cohort 3 received additional injections on Day 57. This study was approved by the Human Research Ethics Committee (HREC), adhering to the Declaration of Helsinki and ICH GCP guidelines, and informed consent was obtained from participants prior to their involvement. A Safety Review Committee monitored participant safety throughout the trial.
The primary endpoints of this study were focused on the safety and tolerability of BARS13, monitoring the incidence and severity of local and systemic adverse events post-vaccination. The secondary endpoints involved measuring the levels of IgG antibodies specific for the RSV G protein using an enzyme-linked immunosorbent assay (ELISA), offering insights into the vaccine’s immunogenicity.
2.2. Participants
In this phase II clinical trial, healthy elderly male and female participants aged between 60 and 80 years were enrolled. Individuals with stable chronic diseases were eligible for inclusion, but those with a history of severe allergies, immunosuppressive therapy, known HIV, HBV, or HCV infections, or those who had recently received other vaccines (except for influenza and SARS-CoV-2 [COVID-19] vaccines, which should not be administered within a 14-day interval of the study vaccine) or had previously received an investigational RSV vaccine were excluded. All participants were required to have a BMI not exceeding 40 kg/m2, normal ECG parameters, and stable blood pressure.
2.3. The Vaccine
The lyophilized powder of rRSV G protein (14 µg/vial) and CsA diluent (18 µg/0.9 mL/vial) was produced by Advaccine Biopharmaceuticals Suzhou Co., Ltd., located in Suzhou, China. The placebo was a formulation buffer (0.9 mL/vial) without active components. The rRSV G protein lyophilized powder was reconstituted with 0.7 mL of sterile CsA diluent solution as the active BARS13 vaccine and intramuscularly injected with 0.5 mL of reconstituted BARS13 vaccine per arm. The reconstituted vaccine remains stable for ≤4 h at 2–8 °C before intramuscular (IM) deltoid injection.
2.4. Safety Assessment
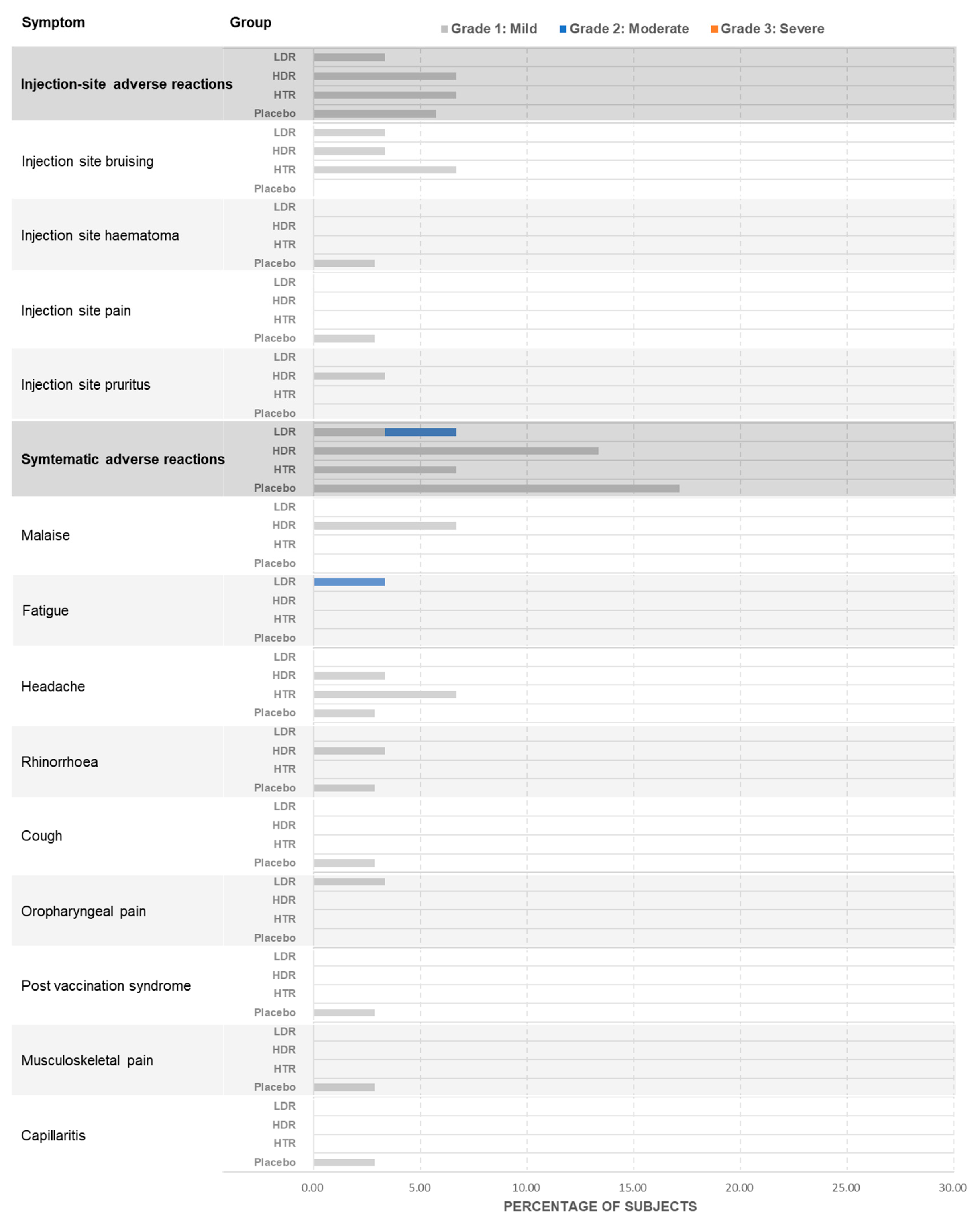
The safety endpoints in this study were designed to evaluate the vaccine’s safety profile, with a particular emphasis on the frequency and intensity of adverse events (AEs) associated with the vaccine. This involved monitoring both solicited AEs, such as local reactions like pain, tenderness, and erythema, as well as systemic reactions like fatigue and myalgia. In addition, unsolicited AEs were also captured for a period extending beyond the initial 7 days. The occurrence of AEs leading to withdrawal, serious adverse events (SAEs), clinically significant laboratory abnormalities, and treatment-emergent, clinically significant changes in vital signs and physical examinations were also systematically tracked. The AE Severity Grading Definitions were as follows: Grade 1 (mild): symptom is noticeable but easily tolerated; Grade 2 (moderate): discomfort sufficient to interfere with normal activities; Grade 3 (severe): incapacitating, prevents work or daily activities; Grade 4 (life-threatening): immediate risk of death, requires urgent intervention.
2.5. Immunogenicity Assessment
The immunogenicity assessment was conducted to evaluate the immune response to the vaccinations. Serum samples from participants were collected at predefined timepoints and analyzed for antibodies against the recombinant non-glycosylated G protein (IgG against rRSV G protein) using validated ELISA protocols. For Cohorts 1 and 2, samples were collected on Days 1, 29, 57, and 85, while Cohort 3 provided additional samples on Day 113. The assay was performed by a validated method, with results presented as geometric mean concentration values and the proportion of participants showing an antibody concentration increase post-vaccination.
A quantitative enzyme-linked immunosorbent assay (ELISA) was used to assess the concentrations of G protein-specific IgG antibodies. Plates were coated with rRSV protein G before being blocked. A standard RSV IgG serum (NIBSC, London, UK, Cat No.: 16/284) was serially diluted to set as the standard curve. Human serum samples were diluted (1 in 2000) and placed on the plate for a 1 h incubation. Following washing, goat anti-human IgG (H + L) peroxidase-labeled IgG antibodies (Invitrogen, Carlsbad, CA, USA, Catalog No.: 31410) were added to the plate for a further 1 h incubation, followed by additional washes. TMB (Sigma-Aldrich, St. Louis, MO, USA, Catalog No.: T0440) and a stop solution were used to create a colorimetric signal. An ELISA plate reader (SpectraMax VersaMax, Molecular Devices, Sunnyvale, CA, USA) was used to read the signal. The signal produced was proportional to the amount of analyte present and interpolated from the calibration curve presented on each plate. The concentrations of anti-G protein IgG antibodies in the samples were calculated from the calibration curves (4 PL curve fitting with 1/Y weighting factor).
2.6. Statistical Analysis
The safety population and the immunogenicity population were statistically separated. The safety population included all participants who received the investigational treatment, and the data were used to describe the demographic characteristics, baseline features, and safety/tolerability endpoints. The immunogenicity population comprised participants who received any dose of the treatment and completed at least one post-baseline immunological assessment, with data used primarily for immunogenicity endpoints.
The descriptive statistical methods were used to summarize the demographic variables and participants’ adherence and exposure to the study vaccine, presenting these by treatment group. Adverse events were categorized by using MedDRA coding (v24.0), and a comprehensive summary was compiled, including severity and causality assessments, as well as the incidence and severity of injection site reactions.
For the immunogenicity, the RSV G protein-specific IgG antibody concentrations were obtained with the calculated geometric mean concentrations (GMCs) and tracking changes over time. The response criteria and calculated response rates were defined at each timepoint.
4. Discussion
This phase II study evaluates the safety, tolerability, and immunogenicity of BARS13 in healthy elderly males and females aged 60 to 80. Consistent with prior Phase I findings, this phase II trial confirms the favorable safety profile and strong immunogenicity of BARS13.
The findings demonstrate that the BARS13 vaccine is well-tolerated among elderly participants, with no serious adverse events reported across the dosage groups. Participants in all dosage groups demonstrated good tolerance to the vaccine, with no serious adverse events (SAEs) observed. All treatment-emergent adverse events (TEAEs) were mild to moderate and not clearly related to vaccination. These TEAEs mainly included local reactions such as pain and tenderness, as well as systemic reactions such as headache and fatigue, which were usually transient and resolved within 24 to 48 h. Furthermore, no clinically significant vital sign changes related to vaccination were recorded during this study, providing additional assurance for the safety of BARS13.
Immunogenicity assessments revealed that after vaccination with BARS13, there was a significant increase in the levels of IgG antibodies against the RSV G protein in the participants. This increase was dose-dependent, meaning that as the vaccine dose increased, the observed immune response was stronger. In the low-dose group (Cohort 1), the geometric mean concentration (GMC) of IgG steadily rose from before vaccination to Day 85, indicating that the vaccine successfully induced an immune response. In the high-dose groups (Cohort 2 and Cohort 3), the observed increase in the IgG GMC was even more pronounced, indicating that increasing the vaccine dose and frequency of administration can enhance the immune response.
The elderly population is frequently susceptible to respiratory syncytial virus (RSV) infections, which can pose a challenge to the efficacy of RSV vaccines [
3]. This is mostly attributed to the presence of pre-existing antibodies that neutralize the vaccine antigens and, hence, impede the vaccination’s effectiveness. Developing an effective respiratory syncytial virus (RSV) vaccination for this particular age range presents a significant hurdle. Furthermore, pre-existing medical disorders and the concurrent use of medications may exacerbate the challenges associated with the effectiveness of the vaccine in older populations. Additionally, the immune systems of older individuals tend to weaken over time, making it more difficult for vaccines to generate a strong and lasting immune response. This further emphasizes the need for the careful evaluation and consideration of the dosing escalation and frequency to ensure the optimal effectiveness of an RSV vaccine in older populations. An analysis of the anti-RSV-G IgG antibody baseline revealed that subjects with a history of environmental exposure to RSV infections exhibited a significantly elevated baseline level of this antibody. To eliminate inconsistency among individuals, we measured the quantities of anti-G IgG. These concentrations were reported in international units per microliter (IU/mL), along with 95% confidence intervals for each treatment group.
The administration of two doses of low-dose BARS13 (10 µg) via injection induced significant anti-G IgG antibodies at Week 4 after vaccination and more than 60% of the participants exhibited responsiveness. The cohorts administered a high dosage of BARS13 (20 µg) had sustained production of antibodies at both Weeks 4 and 8. Furthermore, these antibodies exhibited an enhanced response towards BARS13. An additional dose of BARS13 led to a substantial boost in antibody production, surpassing baseline levels at every measured timepoint, highlighting the benefit of repeated dosing. This demonstrated a dose-dependent increase pattern from the low-dose to high-dose cohorts, as evidenced by the rise from baseline to post-vaccination. As evidenced by the antibody levels in Cohort 3, which increased further after the second and third doses, the boost dose also played an important role in sustaining high levels in the antibody response.
These results position BARS13 as a competitive candidate among the current RSV vaccines, particularly due to its unique targeting of the G protein rather than the more commonly studied F protein [
18]. While many recent efforts have focused on the prefusion F protein, which has been shown to elicit potent neutralizing antibodies, BARS13 targets the G protein, providing a complementary mechanism of action. By preventing viral attachment and entry into host cells, BARS13 addresses a critical phase of RSV infection, potentially offering a broader spectrum of protection. The dose-dependent antibody responses observed in this study highlight BARS13’s potential to induce robust immunity, even in the elderly, a population that has historically shown diminished responses to vaccines targeting the F protein. This distinction may prove advantageous in populations with pre-existing RSV immunity, where the G protein’s immunogenicity may not be as influenced by previous exposure as the F protein is.
Furthermore, the use of the non-glycosylated form of the G protein in BARS13 differentiates it from other RSV vaccines and offers potential benefits in terms of vaccine stability and safety. The avoidance of glycosylation could minimize the risk of vaccine-enhanced disease (VED), a concern that has lingered since the failure of the early RSV vaccines. By circumventing this risk, BARS13 could offer a safer profile in vulnerable populations, particularly the elderly, who are at increased risk for severe RSV infections [
19].
A prior clinical study revealed that the importance of dosage was established in a dose-ranging investigation of extracted and purified F, G, and M antigens from RSV A viruses through intramuscular injection at doses of 100, 50, or 25 µg to the elderly population. The antibody levels against RSV F and G proteins were found to be equal when administering doses of 25 and 50 µg of the vaccine. However, a considerably greater antibody response was achieved after administering a dose of 100 µg of the vaccine in comparison to the 25 and 50 µg doses [
20]. These findings support the need for dose optimization in elderly populations, where immune senescence may require higher antigen exposure to achieve protective immunity. Further studies are needed to determine the optimal dosage for any protection against RSV. These results are significant, as they indicate that the immune response to RSV can be enhanced by increasing the vaccine dosage. This is particularly important for the elderly population, as they are more susceptible to severe RSV infections. The findings warrant further investigation into the safety and efficacy of higher vaccine doses and could potentially lead to the development of a more effective vaccine against RSV in older adults. Dose selection, as observed in this current study, is pivotal for G protein-based vaccines in the elderly population. The elderly population often has weaker immune responses to vaccines, making it necessary to administer higher dosages to achieve optimal protection against RSV. Additionally, our research suggests that dosing selection is critical in G protein-based vaccines for older adults, as it directly impacts the vaccine’s efficacy and effectiveness. These findings underscore the need for more research and development in this area to improve the immunity and health outcomes of the elderly population [
21].
Despite promising findings, there are several limitations that should be acknowledged. First, the immunogenicity follow-up period was relatively short, limiting the ability to assess the long-term durability of the immune response. Future studies should extend the follow-up period to determine whether the antibody responses elicited by BARS13 are sustained over time and provide long-term protection against RSV. Second, the sample size, while sufficient for detecting short-term safety and immunogenicity signals, may limit the generalizability of the findings, particularly regarding rare adverse events or variability in immune responses across more diverse populations. A larger, more ethnically diverse cohort would strengthen the conclusions and provide a clearer understanding of BARS13’s efficacy across different demographics. Moreover, while our ELISA included a standard neutralizing antibody control from the NIBSC, it did not directly measure viral neutralization. We are currently optimizing a primary human airway epithelial cell-based neutralization assay for RSV G-specific antibodies, which we plan to include in future studies. Due to the lack of a validated cell line-based neutralization assay for G protein-based vaccines, we were unable to perform this assay in the current trial.
Additionally, the absence of direct comparisons to other RSV vaccines in this study prevents a full assessment of how BARS13’s safety and immunogenicity profile stacks up against those targeting the prefusion F protein. Future studies could consider head-to-head trials to evaluate the clinical advantages and immunological differences between G protein-based and F protein-based vaccines, particularly in elderly and high-risk populations. Lastly, the cost-effectiveness of BARS13 has yet to be evaluated, and given the global burden of RSV, especially in low- and middle-income countries, future studies should assess whether BARS13 can be produced and distributed at a competitive cost compared to other RSV vaccines.
Overall, this phase II study in older adults showed different dose levels of the recombinant G protein-based investigational RSV vaccine to be safe, well-tolerated, and highly immunogenic and responsive in adults 60–80 years of age. Further research and development in this area is crucial in order to determine the optimal dosage and administration schedule of the recombinant G protein-based investigational RSV vaccine for maximum effectiveness in elderly individuals. Additionally, studying the long-term effects of the vaccine and its potential impact on reducing hospitalizations and mortality rates among older adults will provide valuable insights into improving their overall health outcomes. Ultimately, investing in the continued development and implementation of the recombinant G protein-based RSV vaccine has the potential to significantly enhance the immunity and well-being of the elderly population.