Participation in a Voluntary Blood Donation Program as an Opportunity to Assess and Enhance Tetanus Immunity in Adult Blood Donors with an Outdated or Unknown Vaccination Status
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Characteristics of the Study Population and Assessment of Tetanus Immunity Among Adult Blood Donors
3.2. Effectiveness of a Single Tetanus Booster Dose Administered Many Years After the Last Vaccination
4. Discussion
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adult Vaccinations: Needs and Opportunities. Opening Report. MZdrowie: Warsaw; Karpacz. 2023. Available online: https://www.mzdrowie.pl/medycyna/raport-o-szczepieniach-doroslych-potrzebna-strategia (accessed on 15 May 2025).
- Mirska, B.; Zenczak, M.; Nowis, K.; Stolarek, I.; Podkowiński, J.; Rakoczy, M.; Marcinkowska-Swojak, M.; Koralewska, N.; Zmora, P.; Lenartowicz Onyekaa, E.; et al. The landscape of the COVID-19 pandemic in Poland emerging from epidemiological and genomic data. Sci. Rep. 2024, 14, 14416. [Google Scholar] [CrossRef]
- Sowa, P.; Kiszkiel, Ł.; Laskowski, P.P.; Alimowski, M.; Szczerbiński, Ł.; Paniczko, M.; Moniuszko-Malinowska, A.; Kamiński, K. COVID-19 Vaccine Hesitancy in Poland-Multifactorial Impact Trajectories. Vaccines 2021, 9, 876. [Google Scholar] [CrossRef]
- Kuchar, E.; Antczak, A.; Skoczyńska, A.; Fal, A.; Wysocki, J.; Walusiak-Skorupa, J.; Czajkowska-Malinowska, M.; Mastalerz-Migas, A.; Flisiak, R.; Nitsch-Osuch, A. Pneumococcal vaccination among adults—Updated Polish recommendations. Fam. Med. Prim. Care Rev. 2022, 24, 285–291. [Google Scholar] [CrossRef]
- Kuchar, E.; Rudnicka, L.; Kocot-Kępska, M.; Nitsch-Osuch, A.; Rejdak, K.; Wysocki, J.; Biesiada, A.; Ledwoch, J.; Wawrzuta, D.; Mastalerz-Migas, A.; et al. Herpes zoster vaccination—Recommendations of the group of experts of the Polish Society of Vaccinology, the Polish Society of Family Medicine, the Polish Society of Dermatology, the Polish Association for the Study of Pain and the Polish Neurological Society. Med. Prakt. 2023, 5, 64–72. [Google Scholar]
- Nitsch-Osuch, A.; Antczak, A.; Barczyk, A.; Czupryniak, L.; Grabowski, M.; Kupczyk, M.; Ledwoch, J.; Mastalerz-Migas, A.; Sutkowski, M.; Szymański, F.M.; et al. Rekomendacje grupy ekspertów w zakresie szczepień przeciw wirusowi RS osób dorosłych. Recommendations of the Expert Panel on Vaccination Against Respiratory Syncytial Virus (RSV) in Adults. Lek. POZ 2023, 9, 301–308. [Google Scholar]
- National Immunization Program. 2025. Available online: https://dziennikmz.mz.gov.pl/DUM_MZ/2024/93/akt.pdf (accessed on 15 May 2025).
- Rosiek, A.; Nieradkiewicz, A.; Lachert, E.; Goczyńska, P.; Lasocka, J.; Mikołowska, A.; Łętowska, M.; Antoniewicz-Papis, J. Blood transfusion service in Poland in 2023. J. Transfus. Med. 2024, 17, 147–167. [Google Scholar] [CrossRef]
- Liang, J.L.; Tiwari, T.; Moro, P.; Messonnier, N.E.; Reingold, A.; Sawyer, M.; Clark, T.A. Prevention of Pertussis, Tetanus, and Diphtheria with Vaccines in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2018, 67, 1–44. [Google Scholar] [CrossRef]
- Rumik, A.; Paradowska-Stankiewicz, I. Tetanus in Poland in 2020–2022. Przegl Epidemiol. 2024, 78, 439–446. [Google Scholar] [CrossRef]
- Infectious Diseases and Poisonings in Poland 2023—National Institute of Public Health NIH—National Research Institute. Available online: https://wwwold.pzh.gov.pl/oldpage/epimeld/2023/Ch_2023.pdf (accessed on 15 May 2025).
- European Centre for Disease Prevention and Control Tetanus. In Annual Epidemiological Report for 2022; ECDC: Stockholm, Sweden, 2024. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/TETA_AER_2022_Report%20FINAL.pdf (accessed on 31 May 2025).
- Gałązka, A.; Tomaszunas-Błaszczyk, J.; Tężec, w; Kostrzewski, J.; Magdzik, W.; Naruszewicz-Lesiuk, D. Infectious Diseases and Their Control in Polish Territories in the 20th Century; PZWL: Warsaw, Poland, 2001. [Google Scholar]
- Galazka, A.; Kardymowicz, B. Tetanus incidence and immunity in Poland. Eur. J. Epidemiol. 1989, 5, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewska, A. Diphtheria and tetanus immunity of blood donors. Przegl Epidemiol. 1997, 51, 25–27. (In Polish) [Google Scholar] [PubMed]
- Eslamifar, A.; Ramezani, A.; Banifazl, M.; Sofian, M.; Mahdaviani, F.A.; Yaghmaie, F.; Aghakhani, A. Immunity to diphtheria and tetanus among blood donors in Arak, central province of Iran. Iran. J. Microbiol. 2014, 6, 190–193. [Google Scholar] [PubMed] [PubMed Central]
- Martin, S.; Giss, A.; Ackermann, B.; Russer, S.; Inderwisch, U.; Howe, J.; Wichmann, M.; Weinauer, F. Assessment of the tetanus immune status in plasma donors of the Blood Donor Service of the Bavarian Red Cross. Dtsch. Med. Wochenschr. 2005, 130, 1810–1813. (In German) [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Lau, W.; Thipphawong, J.; Kasenda, M.; Xie, F.; Bevilacqua, J. Diphtheria and tetanus immunity among blood donors in Toronto. CMAJ 1997, 156, 985–990. [Google Scholar] [PubMed] [PubMed Central]
- Karabay, O.; Ozkardes, F.; Tamer, A.; Karaarslan, K. Tetanus immunity in nursing home residents of Bolu, Turkey. BMC Public Health 2005, 5, 5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kjeldsen, K.; Simonsen, O.; Heron, I. Immunity against diphtheria and tetanus in the age group 30–70 years. Scand. J. Infect. Dis. 1988, 20, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Yamamoto, A.; Komiya, T.; Takeshita, N.; Takahashi, M. Seroprevalence of tetanus toxoid antibody and booster vaccination efficacy in Japanese travelers. J. Infect. Chemother. 2014, 20, 35–37. [Google Scholar] [CrossRef]
- Afzali, H.; Sharif, M.R.; Mousavi, S. Determination of Tetanus Antibody Levels in Trauma Patients Referred To Shahid Beheshti Hospital in Kashan, Iran, 2014. Arch. Trauma. Res. 2015, 4, e30687. [Google Scholar] [CrossRef]
- Janout, V.; Matouskova, I.; Machova, L.; Cizek, L.; Janoutova, G.; Hoskova, J. Protection against tetanus in the aged people in the Czech Republic--cross-sectional study. Arch. Gerontol. Geriatr. 2005, 40, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Gergen, P.J.; McQuillan, G.M.; Kiely, M.; Ezzati-Rice, T.M.; Sutter, R.W.; Virella, G. A population-based serologic survey of immunity to tetanus in the United States. N. Engl. J. Med. 1995, 332, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Cheng, X.; Wei, S.; Yuan, L.; Chen, C.; Yao, K. Decline of serologic immunity to diphtheria, tetanus and pertussis with age suggested a full life vaccination in mainland China. Hum. Vaccin. Immunother. 2021, 17, 1757–1762. [Google Scholar] [CrossRef]
- Bampoe, V.D.; Brown, N.; Deng, L.; Schiffer, J.; Jia, L.T.; Epperson, M.; Gorantla, Y.; Park, S.H.; Ao, J.; Acosta, A.M.; et al. Serologic Immunity to Tetanus in the United States, National Health and Nutrition Examination Survey, 2015–2016. Clin. Infect. Dis. 2024, 78, 470–475. [Google Scholar] [CrossRef]
- Małecka, I.; Wysocki, J.; Mastalerz-Migas, A.; Biesiada, A.; Neumann-Podczaska, A.; Kokoszka-Paszkot, J.; Targowski, T. Influenza Vaccination in the Elderly Population in Poland—Summary of the 2024/2025 Season. The Urgent Need for Action in Influenza Prevention Among Older Adults—Joint Statement of Scientific Societies: The Polish Society of Vaccinology, the Polish Society of Family Medicine, and the Polish Gerontological Society. Available online: https://ptwakc.org.pl/2025/05/24/szczepienia-przeciwko-grypie-w-populacji-osob-starszych-w-polsce-podsumowanie-sezonu-2024-2025/ (accessed on 15 May 2025).
- Olander, R.M.; Auranen, K.; Härkänen, T.; Leino, T. High tetanus and diphtheria antitoxin concentrations in Finnish adults–time for new booster recommendations? Vaccine 2009, 27, 5295–5298. [Google Scholar] [CrossRef]
- Bulletin—Vaccinations in Poland in 2023. Available online: https://wwwold.pzh.gov.pl/oldpage/epimeld/2023/Sz_2023.pdf (accessed on 20 May 2025).
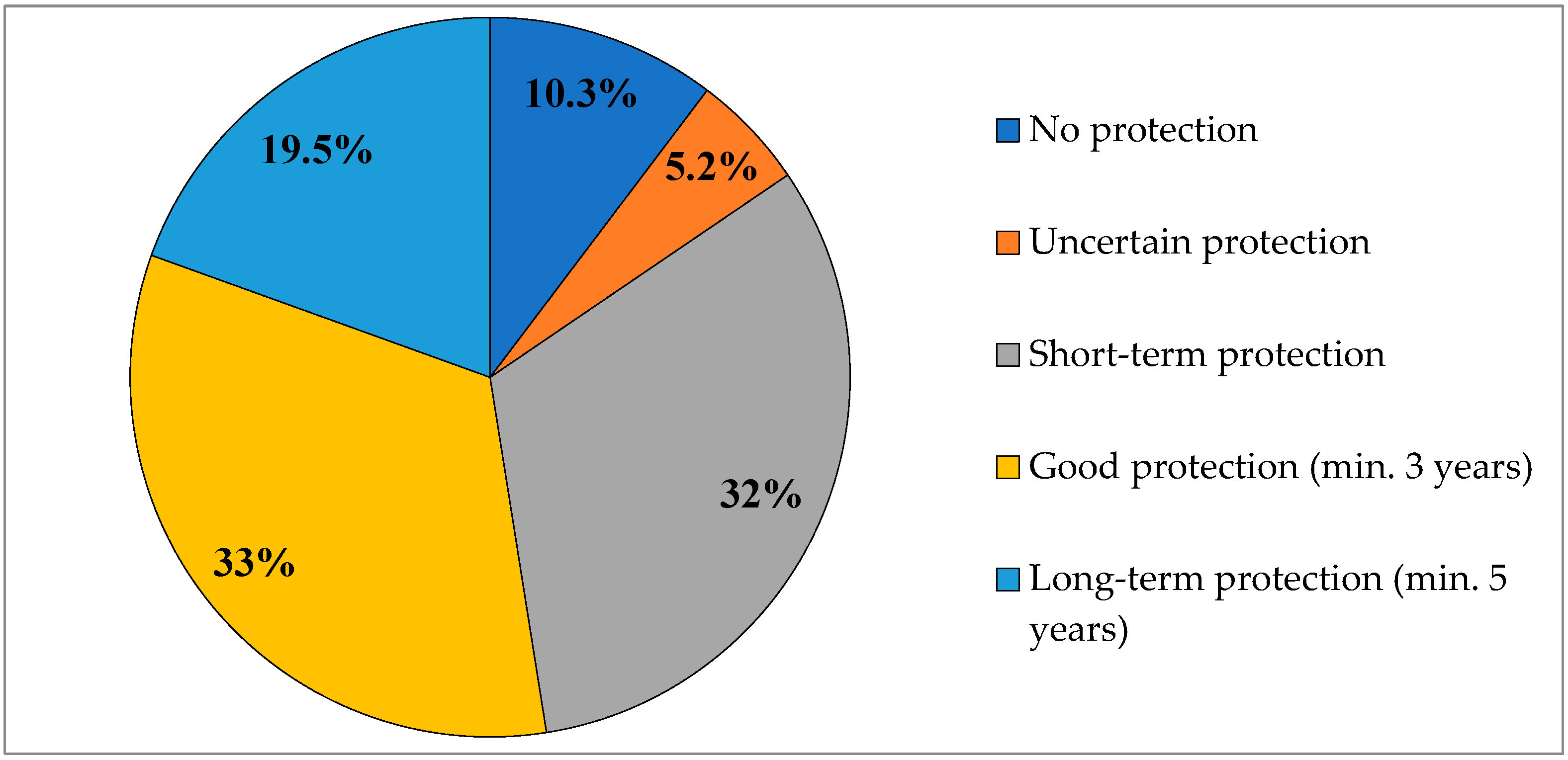
| Anti-Tetanus IgG Antibody Level [IU/mL] | Manufacturer’s Interpretation | Post-Vaccination Immunity Status |
|---|---|---|
| <0.01 | No protection; primary immunization required | Non-protective |
| 0.01–0.1 | Uncertain post-vaccination protection; next dose recommended in 4–8 weeks | Non-protective |
| >0.1–0.5 | Short-term protective immunity present; booster vaccination recommended | Protective |
| >0.5–1.0 | Protective immunity present; next booster recommended in 3 years | Protective |
| 1.0–5.0 | Long-term protective immunity present; next booster in 5 years | Protective |
| >5.0 | Long-term protective immunity present; next booster in 8 years | Protective |
| Variable | Immune N (%) | Non-Immune N (%) | Chi2 p-Value | |
|---|---|---|---|---|
| Sex | Female | 16 (76.2) | 5 (23.8) | NS |
| Male | 66 (86.8) | 10 (13.2) | ||
| Place of residence | Urban | 67 (83.7) | 13 (16.2) | NS |
| Rural | 13 (86.7) | 2 (13.3) | ||
| Education level | Primary + secondary | 57 (87.7) | 8 (12.3) | NS |
| Higher | 22 (75.9) | 7 (24.1) | ||
| Additional vaccination | Yes | 16 (94.1) | 1 (5.9) | NS |
| No | 60 (81.1) | 14 (18.9) | ||
| Authors | Country | Population | Age Range (Years) | Proportion Immune (%) |
|---|---|---|---|---|
| Zakrzewska, 1997 [15] | Poland | Blood donors | 20–59 | 100 |
| Eslamifar et al., 2014 [16] | Iran | Blood donors | 18–71 | 96 (92 in >50 age group) |
| Martin et al., 2005 [17] | Germany | Blood donors | 18–64 | 96.3 (92 in ≥50 age group) |
| Yuan et al., 1997 [18] | Canada | Blood donors | 20–70 | 82.5 |
| Karabay et al., 2005 [19] | Turkey | General (nursing home residents) | 60–≥76 | 15.4 |
| Kjeldsen et al., 1988 [20] | Denmark | General | 30–70 | 49 |
| Mizuno et al., 2014 [21] | Japan | General | 20–≥50 | 76 |
| Afzali et al., 2015 [22] | Iran | General (trauma unit patients) | 40.9 ± 3.7 | 87.3 |
| Janout et al., 2005 [23] | Czech Republic | General | 60–≥90 | 90.9 |
| Gergen et al., 1995 [24] | United States | General | ≥6 | 69.7 (~50 in ≥50 age group) |
| Liu et al., 2021 [25] | China | General | 1 day—89 | 54.4 (<10 in >40 age group) |
| Bampoe et al., 2024 [26] | United States | General | ≥6 | 93.8 (>90 in 50–69 age group) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tkaczyszyn, K.; Szymczyk-Nużka, M.; Szenborn, L. Participation in a Voluntary Blood Donation Program as an Opportunity to Assess and Enhance Tetanus Immunity in Adult Blood Donors with an Outdated or Unknown Vaccination Status. Vaccines 2025, 13, 884. https://doi.org/10.3390/vaccines13080884
Tkaczyszyn K, Szymczyk-Nużka M, Szenborn L. Participation in a Voluntary Blood Donation Program as an Opportunity to Assess and Enhance Tetanus Immunity in Adult Blood Donors with an Outdated or Unknown Vaccination Status. Vaccines. 2025; 13(8):884. https://doi.org/10.3390/vaccines13080884
Chicago/Turabian StyleTkaczyszyn, Katarzyna, Małgorzata Szymczyk-Nużka, and Leszek Szenborn. 2025. "Participation in a Voluntary Blood Donation Program as an Opportunity to Assess and Enhance Tetanus Immunity in Adult Blood Donors with an Outdated or Unknown Vaccination Status" Vaccines 13, no. 8: 884. https://doi.org/10.3390/vaccines13080884
APA StyleTkaczyszyn, K., Szymczyk-Nużka, M., & Szenborn, L. (2025). Participation in a Voluntary Blood Donation Program as an Opportunity to Assess and Enhance Tetanus Immunity in Adult Blood Donors with an Outdated or Unknown Vaccination Status. Vaccines, 13(8), 884. https://doi.org/10.3390/vaccines13080884





