Role of the Virome in Vaccine-Induced Immunization
Abstract
1. Introduction
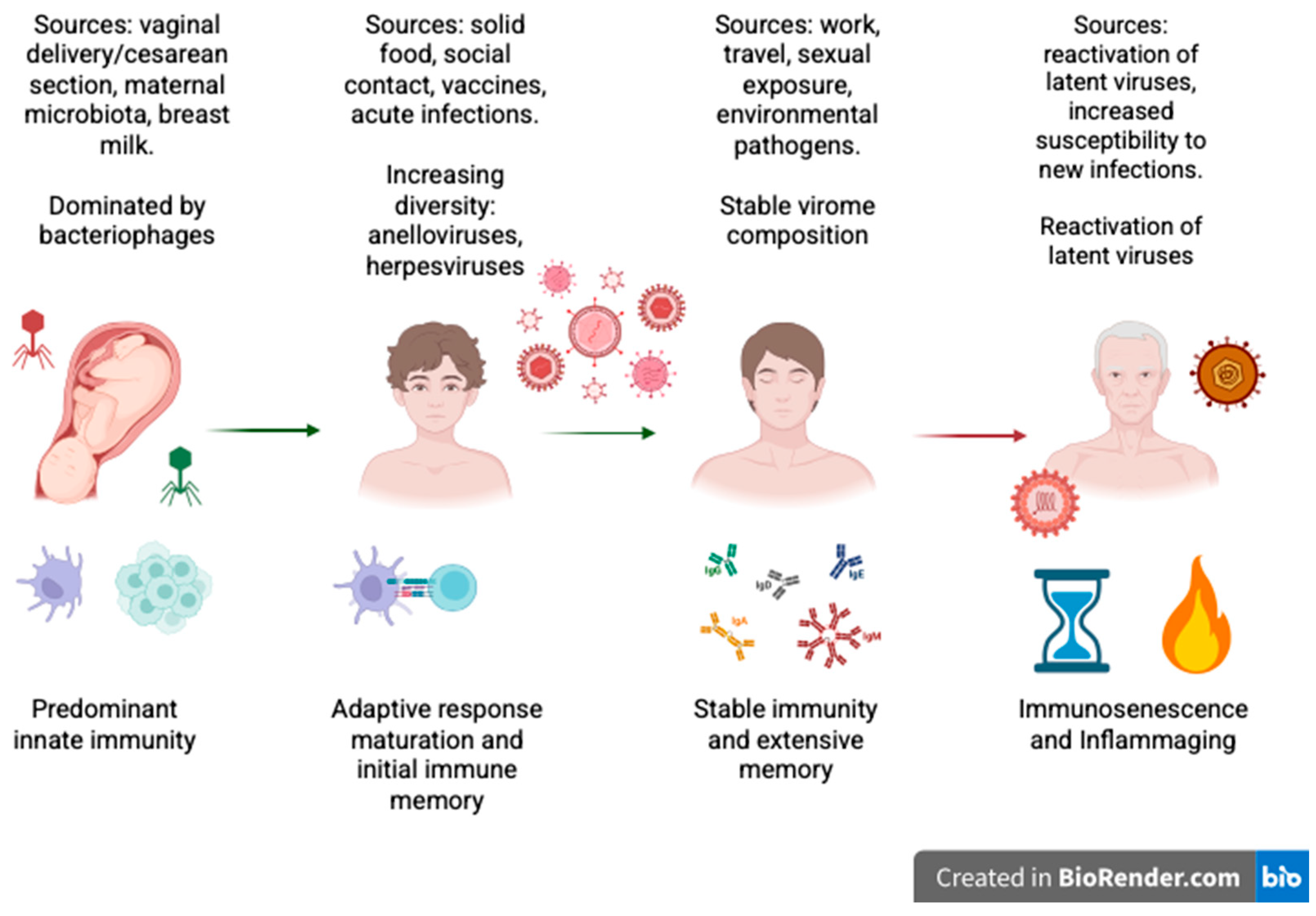
2. Historical Overview: From the Microbiome to the Virome
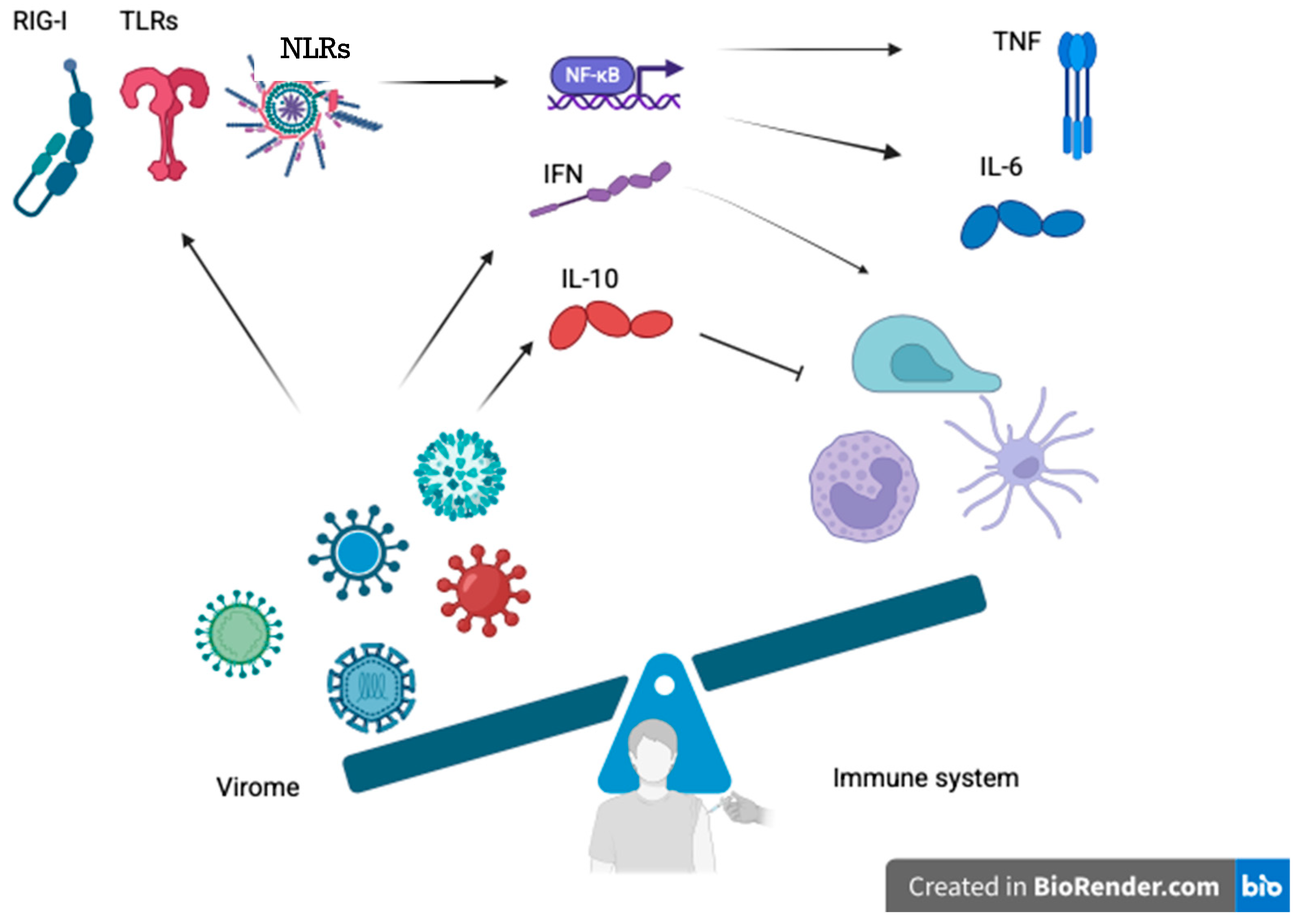
3. Virome-Mediated Modulation of Innate and Adaptive Immunity
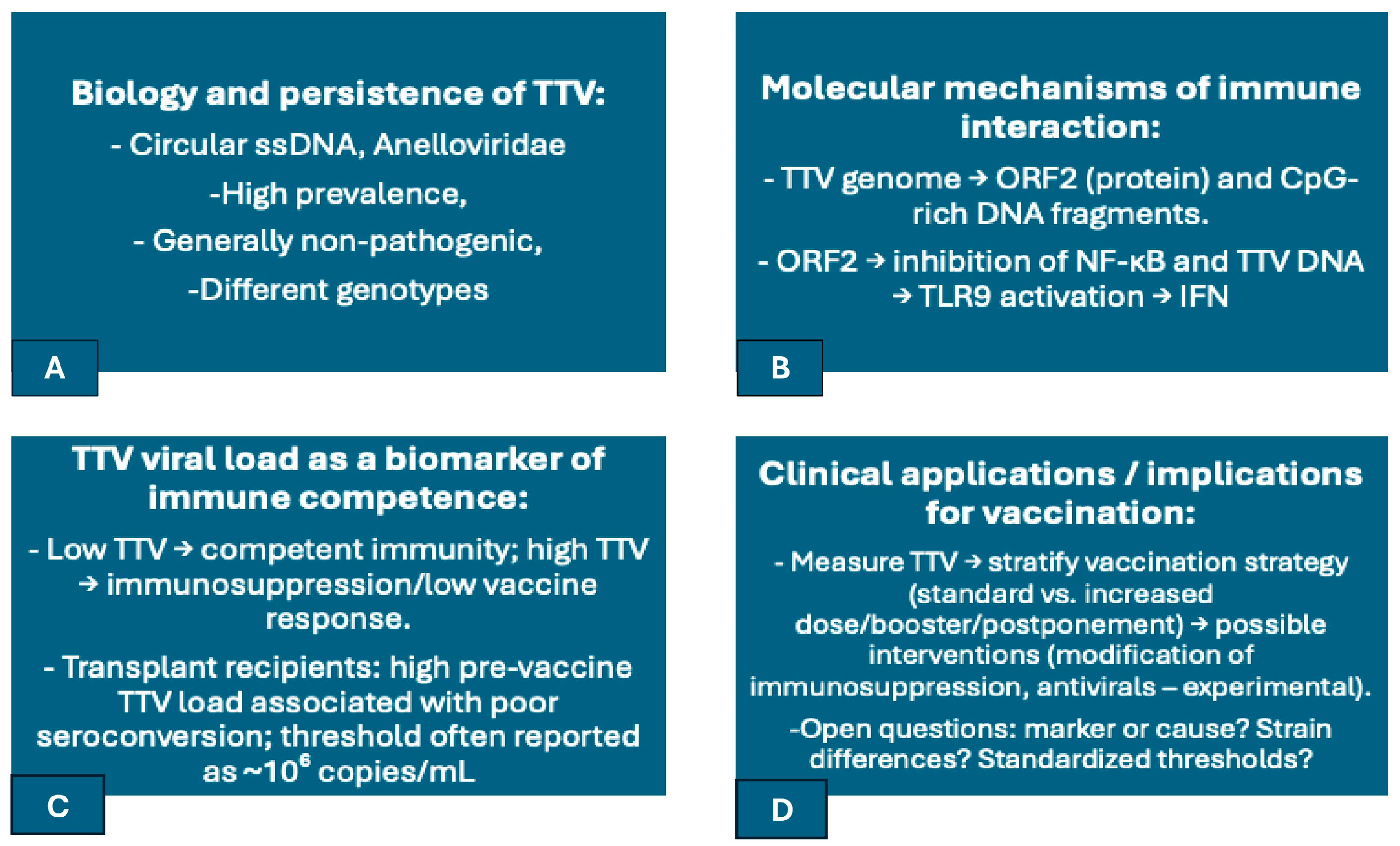
4. Torque Teno Virus: A Sentinel of Immune Status
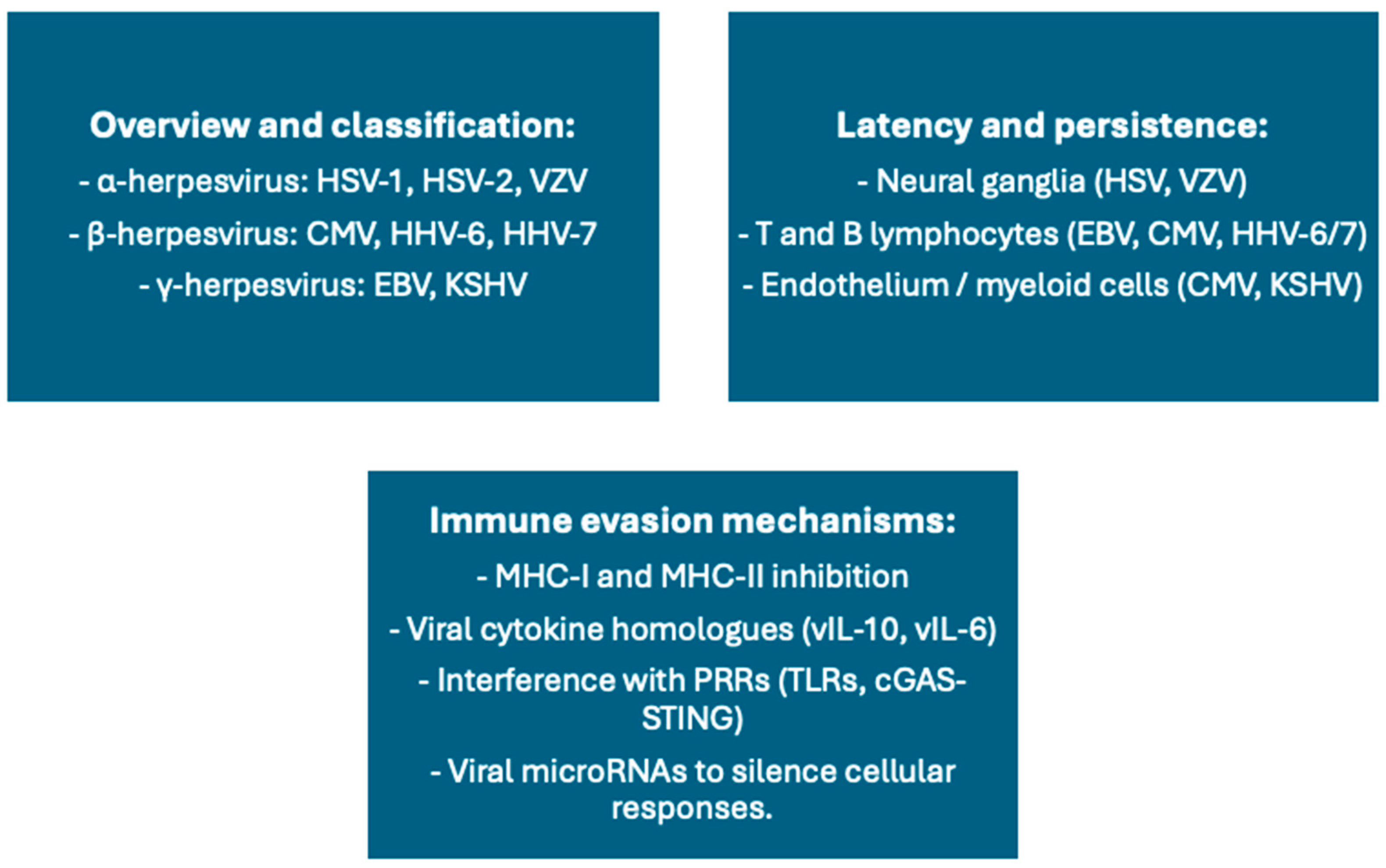
5. Latent Herpesviruses and Immune Modulation of Vaccination
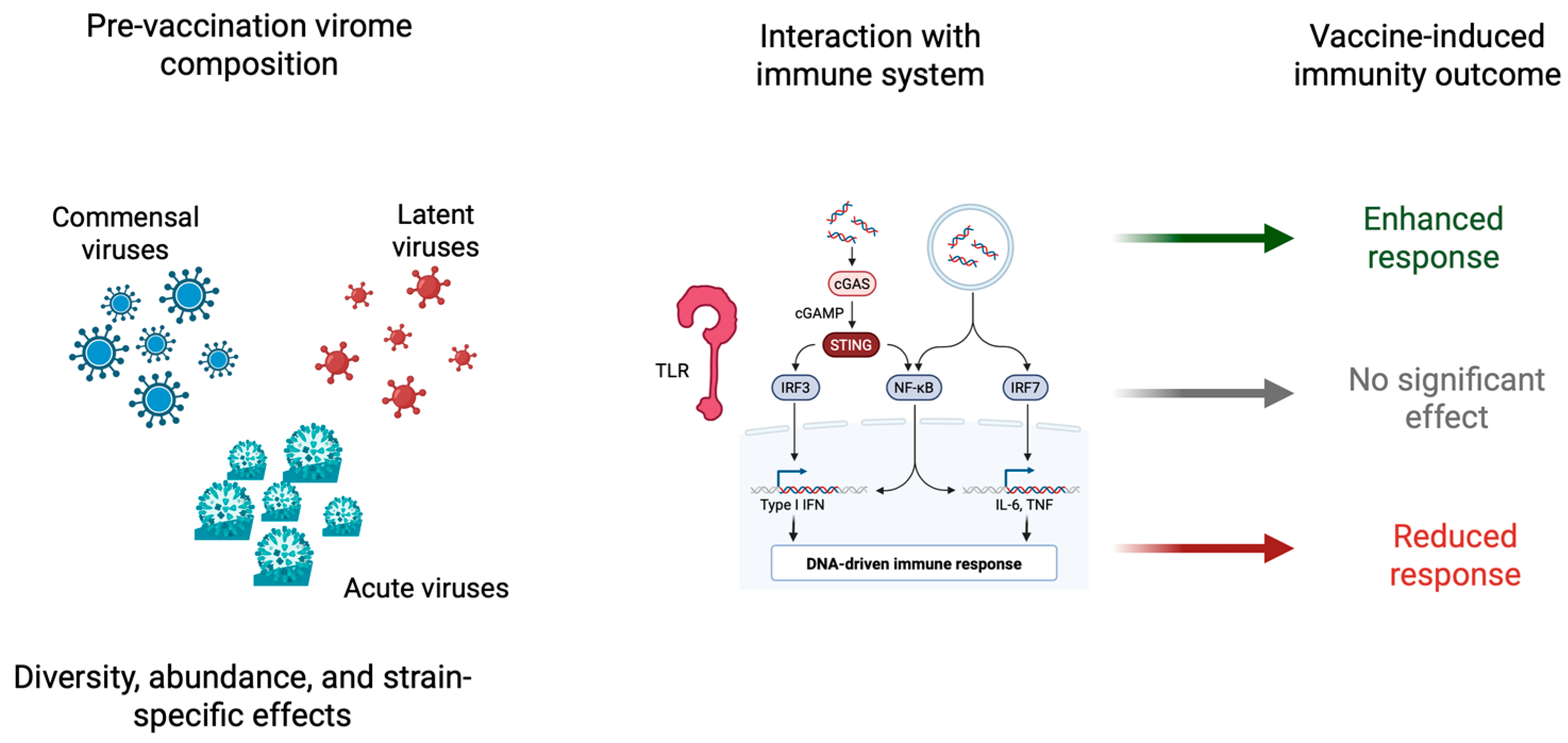
6. Other Commensal Viruses and Vaccine Responses
7. Bacteriophage-Based Vaccines and Their Immunogenic Potential
8. Clinical Implications: Transplant and Immunocompromised Patients
9. Future Perspectives: Virome-Aware Precision Vaccinology
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ardura-Garcia, C.; Curtis, N.; Zimmermann, P. Systematic Review of the Impact of Intestinal Microbiota on Vaccine Responses. npj Vaccines 2024, 9, 254. [Google Scholar] [CrossRef]
- Freer, G.; Maggi, F.; Pifferi, M.; Di Cicco, M.E.; Peroni, D.G.; Pistello, M. The Virome and Its Major Component, Anellovirus, a Convoluted System Molding Human Immune Defenses and Possibly Affecting the Development of Asthma and Respiratory Diseases in Childhood. Front. Microbiol. 2018, 9, 686. [Google Scholar] [CrossRef]
- Pardi, N.; Weissman, D. Development of Vaccines and Antivirals for Combating Viral Pandemics. Nat. Biomed. Eng. 2020, 4, 1128–1133. [Google Scholar] [CrossRef]
- Shkoporov, A.N.; Hill, C. Bacteriophages of the Human Gut: The “Known Unknown” of the Microbiome. Cell Host Microbe 2019, 25, 195–209. [Google Scholar] [CrossRef]
- Norman, J.M.; Handley, S.A.; Baldridge, M.T.; Droit, L.; Liu, C.Y.; Keller, B.C.; Kambal, A.; Monaco, C.L.; Zhao, G.; Fleshner, P.; et al. Disease-Specific Alterations in the Enteric Virome in Inflammatory Bowel Disease. Cell 2015, 160, 447–460. [Google Scholar] [CrossRef]
- Liang, G.; Bushman, F.D. The Human Virome: Assembly, Composition and Host Interactions. Nat. Rev. Microbiol. 2021, 19, 514–527. [Google Scholar] [CrossRef]
- Kaczorowska, J.; van der Hoek, L. Human Anelloviruses: Diverse, Omnipresent and Commensal Members of the Virome. FEMS Microbiol. Rev. 2020, 44, 305–313. [Google Scholar] [CrossRef]
- McGovern, K.E.; Sonar, S.A.; Watanabe, M.; Coplen, C.P.; Bradshaw, C.M.; Nikolich, J.Ž. The Aging of the Immune System and Its Implications for Transplantation. GeroScience 2023, 45, 1383–1400. [Google Scholar] [CrossRef]
- Abbas, A.A.; Taylor, L.J.; Dothard, M.I.; Leiby, J.S.; Fitzgerald, A.S.; Khatib, L.A.; Collman, R.G.; Bushman, F.D. Redondoviridae, a Family of Small, Circular DNA Viruses of the Human Oro-Respiratory Tract Associated with Periodontitis and Critical Illness. Cell Host Microbe 2019, 25, 719–729.e4. [Google Scholar] [CrossRef]
- Shkoporov, A.N.; Khokhlova, E.V.; Stephens, N.; Hueston, C.; Seymour, S.; Hryckowian, A.J.; Scholz, D.; Ross, R.P.; Hill, C. Long-Term Persistence of crAss-like Phage crAss001 Is Associated with Phase Variation in Bacteroides Intestinalis. BMC Biol. 2021, 19, 163. [Google Scholar] [CrossRef]
- Forster, S.C.; Clare, S.; Beresford-Jones, B.S.; Harcourt, K.; Notley, G.; Stares, M.D.; Kumar, N.; Soderholm, A.T.; Adoum, A.; Wong, H.; et al. Identification of Gut Microbial Species Linked with Disease Variability in a Widely Used Mouse Model of Colitis. Nat. Microbiol. 2022, 7, 590–599. [Google Scholar] [CrossRef]
- Griffin, D.E. Why Does Viral RNA Sometimes Persist after Recovery from Acute Infections? PLoS Biol. 2022, 20, e3001687. [Google Scholar] [CrossRef]
- Ng, C.T.; Snell, L.M.; Brooks, D.G.; Oldstone, M.B.A. Networking at the Level of Host Immunity: Immune Cell Interactions during Persistent Viral Infections. Cell Host Microbe 2013, 13, 652–664. [Google Scholar] [CrossRef]
- Rascovan, N.; Duraisamy, R.; Desnues, C. Metagenomics and the Human Virome in Asymptomatic Individuals. Annu. Rev. Microbiol. 2016, 70, 125–141. [Google Scholar] [CrossRef]
- Phan, T.G.; da Costa, A.C.; del Valle Mendoza, J.; Bucardo-Rivera, F.; Nordgren, J.; O’Ryan, M.; Deng, X.; Delwart, E. The Fecal Virome of South and Central American Children with Diarrhea Includes Small Circular DNA Viral Genomes of Unknown Origin. Arch. Virol. 2016, 161, 959–966. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Kim, M.-S.; Kim, E.; Cheon, J.H.; Lee, Y.-S.; Kim, Y.; Lee, S.-H.; Seo, S.-U.; Shin, S.-H.; Choi, S.S.; et al. Enteric Viruses Ameliorate Gut Inflammation via Toll-like Receptor 3 and Toll-like Receptor 7-Mediated Interferon-β Production. Immunity 2016, 44, 889–900. [Google Scholar] [CrossRef]
- Nichols, D.B.; De Martini, W.; Cottrell, J. Poxviruses Utilize Multiple Strategies to Inhibit Apoptosis. Viruses 2017, 9, 215. [Google Scholar] [CrossRef]
- Barton, E.S.; White, D.W.; Cathelyn, J.S.; Brett-McClellan, K.A.; Engle, M.; Diamond, M.S.; Miller, V.L.; Virgin, H.W. Herpesvirus Latency Confers Symbiotic Protection from Bacterial Infection. Nature 2007, 447, 326–329. [Google Scholar] [CrossRef]
- Miwa, K.; Asano, M.; Horai, R.; Iwakura, Y.; Nagata, S.; Suda, T. Caspase 1-Independent IL-1β Release and Inflammation Induced by the Apoptosis Inducer Fas Ligand. Nat. Med. 1998, 4, 1287–1292. [Google Scholar] [CrossRef]
- Monaco, C.L.; Gootenberg, D.B.; Zhao, G.; Handley, S.A.; Ghebremichael, M.S.; Lim, E.S.; Lankowski, A.; Baldridge, M.T.; Wilen, C.B.; Flagg, M.; et al. Altered Virome and Bacterial Microbiome in Human Immunodeficiency Virus-Associated Acquired Immunodeficiency Syndrome. Cell Host Microbe 2016, 19, 311–322. [Google Scholar] [CrossRef]
- Li, L.; Deng, X.; Linsuwanon, P.; Bangsberg, D.; Bwana, M.B.; Hunt, P.; Martin, J.N.; Deeks, S.G.; Delwart, E. AIDS Alters the Commensal Plasma Virome. J. Virol. 2013, 87, 10912–10915. [Google Scholar] [CrossRef]
- Maggi, F.; Pifferi, M.; Tempestini, E.; Lanini, L.; De Marco, E.; Fornai, C.; Andreoli, E.; Presciuttini, S.; Vatteroni, M.L.; Pistello, M.; et al. Correlation between Torque Tenovirus Infection and Serum Levels of Eosinophil Cationic Protein in Children Hospitalized for Acute Respiratory Diseases. J. Infect. Dis. 2004, 190, 971–974. [Google Scholar] [CrossRef]
- Mahmud, R.; Tamanna, S.K.; Akter, S.; Mazumder, L.; Akter, S.; Hasan, R.; Acharjee, M.; Esti, I.Z.; Islam, S.; Shihab, M.R.; et al. Role of Bacteriophages in Shaping Gut Microbial Community. Gut Microbes 2024, 16, 2390720. [Google Scholar] [CrossRef]
- Logan, A.C.; Jacka, F.N.; Craig, J.M.; Prescott, S.L. The Microbiome and Mental Health: Looking Back, Moving Forward with Lessons from Allergic Diseases. Clin. Psychopharmacol. Neurosci. 2016, 14, 131–147. [Google Scholar] [CrossRef]
- Hannigan, G.D.; Duhaime, M.B.; Koutra, D.; Schloss, P.D. Biogeography and Environmental Conditions Shape Bacteriophage-Bacteria Networks across the Human Microbiome. PLoS Comput. Biol. 2018, 14, e1006099. [Google Scholar] [CrossRef]
- Sundaresan, B.; Shirafkan, F.; Ripperger, K.; Rattay, K. The Role of Viral Infections in the Onset of Autoimmune Diseases. Viruses 2023, 15, 782. [Google Scholar] [CrossRef]
- Lynn, D.J.; Benson, S.C.; Lynn, M.A.; Pulendran, B. Modulation of Immune Responses to Vaccination by the Microbiota: Implications and Potential Mechanisms. Nat. Rev. Immunol. 2022, 22, 33–46. [Google Scholar] [CrossRef]
- Jamieson, A.M. Influence of the Microbiome on Response to Vaccination. Hum. Vaccin. Immunother. 2015, 11, 2329–2331. [Google Scholar] [CrossRef]
- Cianci, R.; Caldarelli, M.; Brani, P.; Bosi, A.; Ponti, A.; Giaroni, C.; Baj, A. Cytokines Meet Phages: A Revolutionary Pathway to Modulating Immunity and Microbial Balance. Biomedicines 2025, 13, 1202. [Google Scholar] [CrossRef]
- Li, S.; Xie, Y.; Yu, C.; Zheng, C.; Xu, Z. The Battle between Host Antiviral Innate Immunity and Immune Evasion by Cytomegalovirus. Cell Mol. Life Sci. 2024, 81, 341. [Google Scholar] [CrossRef]
- La Rosa, C.; Diamond, D.J. The Immune Response to Human CMV. Future Virol. 2012, 7, 279–293. [Google Scholar] [CrossRef]
- Neil, J.A.; Cadwell, K. The Intestinal Virome and Immunity. J. Immunol. 2018, 201, 1615–1624. [Google Scholar] [CrossRef]
- Mertowska, P.; Smolak, K.; Mertowski, S.; Grywalska, E. Immunomodulatory Role of Interferons in Viral and Bacterial Infections. Int. J. Mol. Sci. 2023, 24, 10115. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, S.; Yang, L.; Cui, S.; Pan, W.; Jackson, R.; Zheng, Y.; Rongvaux, A.; Sun, Q.; Yang, G.; et al. Nlrp6 Regulates Intestinal Antiviral Innate Immunity. Science 2015, 350, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Dubois, H.; van Loo, G.; Wullaert, A. Nucleic Acid Induced Interferon and Inflammasome Responses in Regulating Host Defense to Gastrointestinal Viruses. Int. Rev. Cell Mol. Biol. 2019, 345, 137–171. [Google Scholar] [CrossRef]
- Taks, E.J.M.; Moorlag, S.J.C.F.M.; Netea, M.G.; van der Meer, J.W.M. Shifting the Immune Memory Paradigm: Trained Immunity in Viral Infections. Annu. Rev. Virol. 2022, 9, 469–489. [Google Scholar] [CrossRef]
- Kim, A.H.; Armah, G.; Dennis, F.; Wang, L.; Rodgers, R.; Droit, L.; Baldridge, M.T.; Handley, S.A.; Harris, V.C. Enteric Virome Negatively Affects Seroconversion Following Oral Rotavirus Vaccination in a Longitudinally Sampled Cohort of Ghanaian Infants. Cell Host Microbe 2022, 30, 110–123.e5. [Google Scholar] [CrossRef]
- Metzger, R.N.; Krug, A.B.; Eisenächer, K. Enteric Virome Sensing-Its Role in Intestinal Homeostasis and Immunity. Viruses 2018, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Gogokhia, L.; Buhrke, K.; Bell, R.; Hoffman, B.; Brown, D.G.; Hanke-Gogokhia, C.; Ajami, N.J.; Wong, M.C.; Ghazaryan, A.; Valentine, J.F.; et al. Expansion of Bacteriophages Is Linked to Aggravated Intestinal Inflammation and Colitis. Cell Host Microbe 2019, 25, 285–299.e8. [Google Scholar] [CrossRef] [PubMed]
- Piazzesi, A.; Pane, S.; Del Chierico, F.; Romani, L.; Campana, A.; Palma, P.; Putignani, L. The Pediatric Gut Bacteriome and Virome in Response to SARS-CoV-2 Infection. Front. Cell. Infect. Microbiol. 2024, 14, 1335450. [Google Scholar] [CrossRef]
- Kramná, L.; Kolářová, K.; Oikarinen, S.; Pursiheimo, J.-P.; Ilonen, J.; Simell, O.; Knip, M.; Veijola, R.; Hyöty, H.; Cinek, O. Gut Virome Sequencing in Children with Early Islet Autoimmunity. Diabetes Care 2015, 38, 930–933. [Google Scholar] [CrossRef]
- Maguire, C.; Wang, C.; Ramasamy, A.; Fonken, C.; Morse, B.; Lopez, N.; Wylie, D.; Melamed, E. Molecular Mimicry as a Mechanism of Viral Immune Evasion and Autoimmunity. Nat. Commun. 2024, 15, 9403. [Google Scholar] [CrossRef]
- Ison, M.G.; Grossi, P. Donor-Derived Infections in Solid Organ Transplantation. Am. J. Transplant. 2013, 13, 22–30. [Google Scholar] [CrossRef]
- Breznik, J.A.; Huynh, A.; Zhang, A.; Bilaver, L.; Bhakta, H.; Stacey, H.D.; Ang, J.C.; Bramson, J.L.; Nazy, I.; Miller, M.S.; et al. Cytomegalovirus Seropositivity in Older Adults Changes the T Cell Repertoire but Does Not Prevent Antibody or Cellular Responses to SARS-CoV-2 Vaccination. J. Immunol. 2022, 209, 1892–1905. [Google Scholar] [CrossRef]
- Dangi, T.; Sanchez, S.; Lew, M.H.; Visvabharathy, L.; Richner, J.; Koralnik, I.J.; Penaloza-MacMaster, P. Pre-Existing Immunity Modulates Responses to mRNA Boosters. bioRxiv 2022. bioRxiv:2022.06.27.497248. [Google Scholar]
- Brani, P.; Manzoor, H.Z.; Spezia, P.G.; Vigezzi, A.; Ietto, G.; Dalla Gasperina, D.; Minosse, C.; Bosi, A.; Giaroni, C.; Carcano, G.; et al. Torque Teno Virus: Lights and Shades. Viruses 2025, 17, 334. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Ye, L.; Fang, X.; Li, B.; Wang, Y.; Xiang, X.; Kong, L.; Wang, W.; Zeng, Y.; Ye, L.; et al. Torque Teno Virus (SANBAN Isolate) ORF2 Protein Suppresses NF-kappaB Pathways via Interaction with IkappaB Kinases. J. Virol. 2007, 81, 11917–11924. [Google Scholar] [CrossRef]
- Dal Lago, S.; Brani, P.; Ietto, G.; Dalla Gasperina, D.; Gianfagna, F.; Giaroni, C.; Bosi, A.; Drago Ferrante, F.; Genoni, A.; Manzoor, H.Z.; et al. Torque Teno Virus: A Promising Biomarker in Kidney Transplant Recipients. Int. J. Mol. Sci. 2024, 25, 7744. [Google Scholar] [CrossRef]
- Krieg, A.M. CpG Motifs in Bacterial DNA and Their Immune Effects. Annu. Rev. Immunol. 2002, 20, 709–760. [Google Scholar] [CrossRef]
- Rocchi, J.; Ricci, V.; Albani, M.; Lanini, L.; Andreoli, E.; Macera, L.; Pistello, M.; Ceccherini-Nelli, L.; Bendinelli, M.; Maggi, F. Torquetenovirus DNA Drives Proinflammatory Cytokines Production and Secretion by Immune Cells via Toll-like Receptor 9. Virology 2009, 394, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Antonelli, G.; Pistello, M.; Maggi, F. Torquetenovirus: The Human Virome from Bench to Bedside. Clin. Microbiol. Infect. 2016, 22, 589–593. [Google Scholar] [CrossRef]
- Bellon, M.; Nicot, C. Telomere Dynamics in Immune Senescence and Exhaustion Triggered by Chronic Viral Infection. Viruses 2017, 9, 289. [Google Scholar] [CrossRef]
- Freeman, M.L.; Mudd, J.C.; Shive, C.L.; Younes, S.-A.; Panigrahi, S.; Sieg, S.F.; Lee, S.A.; Hunt, P.W.; Calabrese, L.H.; Gianella, S.; et al. CD8 T-Cell Expansion and Inflammation Linked to CMV Coinfection in ART-Treated HIV Infection. Clin. Infect. Dis. 2016, 62, 392–396. [Google Scholar] [CrossRef]
- Bowyer, G.; Sharpe, H.; Venkatraman, N.; Ndiaye, P.B.; Wade, D.; Brenner, N.; Mentzer, A.; Mair, C.; Waterboer, T.; Lambe, T.; et al. Reduced Ebola Vaccine Responses in CMV+ Young Adults Is Associated with Expansion of CD57+KLRG1+ T Cells. J. Exp. Med. 2020, 217, e20200004. [Google Scholar] [CrossRef]
- Hess, K.L.; Jewell, C.M. Phage Display as a Tool for Vaccine and Immunotherapy Development. Bioeng. Transl. Med. 2019, 5, e10142. [Google Scholar] [CrossRef]
- Wall, N.; Godlee, A.; Geh, D.; Jones, C.; Faustini, S.; Harvey, R.; Penn, R.; Chanouzas, D.; Nightingale, P.; O’Shea, M.; et al. Latent Cytomegalovirus Infection and Previous Capsular Polysaccharide Vaccination Predict Poor Vaccine Responses in Older Adults, Independent of Chronic Kidney Disease. Clin. Infect. Dis. 2021, 73, e880–e889. [Google Scholar] [CrossRef]
- McElhaney, J.E.; Garneau, H.; Camous, X.; Dupuis, G.; Pawelec, G.; Baehl, S.; Tessier, D.; Frost, E.H.; Frasca, D.; Larbi, A.; et al. Predictors of the Antibody Response to Influenza Vaccination in Older Adults with Type 2 Diabetes. BMJ Open Diabetes Res. Care 2015, 3, e000140. [Google Scholar] [CrossRef]
- Lecuit, M.; Eloit, M. The Human Virome: New Tools and Concepts. Trends Microbiol. 2013, 21, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.N.; Southall, E.; Daon, Y.; Lovell-Read, F.A.; Iwami, S.; Thompson, C.P.; Obolski, U. The Impact of Cross-Reactive Immunity on the Emergence of SARS-CoV-2 Variants. Front. Immunol. 2022, 13, 1049458. [Google Scholar] [CrossRef] [PubMed]
- Maggi, F.; Bendinelli, M. Human Anelloviruses and the Central Nervous System. Rev. Med. Virol. 2010, 20, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Roberto, P.; Cinti, L.; Napoli, A.; Paesani, D.; Riveros Cabral, R.J.; Maggi, F.; Garofalo, M.; Pretagostini, R.; Centofanti, A.; Carillo, C.; et al. Torque Teno Virus (TTV): A Gentle Spy Virus of Immune Status, Predictive Marker of Seroconversion to COVID-19 Vaccine in Kidney and Lung Transplant Recipients. J. Med. Virol. 2023, 95, e28512. [Google Scholar] [CrossRef]
- Rodrigo, E.; González-López, E.; Ocejo-Vinyals, J.G.; Pasache, E.; García-Majado, C.; López Del Moral, C.; García-Santiago, A.; Benito-Hernández, A.; Francia, M.V.; Ruiz, J.C. Exploring Net Immunosuppressive Status with Torque Teno Virus Viral Load in Kidney Transplant Recipients with High Molecular Injury. J. Clin. Med. 2025, 14, 2417. [Google Scholar] [CrossRef]
- Kincaid, R.P.; Panicker, N.G.; Lozano, M.M.; Sullivan, C.S.; Dudley, J.P.; Mustafa, F. MMTV Does Not Encode Viral MicroRNAs but Alters the Levels of Cancer-Associated Host MicroRNAs. Virology 2018, 513, 180–187. [Google Scholar] [CrossRef]
- Solis, M.; Benotmane, I.; Gallais, F.; Caillard, S.; Fafi-Kremer, S. Torque Teno Virus Viral Load Predicts SARS-CoV-2 Vaccine Response in Kidney Transplant Recipients. J. Med. Virol. 2023, 95, e28936. [Google Scholar] [CrossRef]
- Gallais, F.; Renaud-Picard, B.; Solis, M.; Laugel, E.; Soulier, E.; Caillard, S.; Kessler, R.; Fafi-Kremer, S. Torque Teno Virus DNA Load as a Predictive Marker of Antibody Response to a Three-Dose Regimen of COVID-19 mRNA-Based Vaccine in Lung Transplant Recipients. J. Heart Lung Transplant. 2022, 41, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Graninger, M.; Stumpf, J.; Bond, G.; Görzer, I.; Springer, D.N.; Kessel, F.; Kröger, H.; Frank, K.; Tonn, T.; Hugo, C.; et al. Prediction of Humoral and Cellular Immune Response to COVID-19 mRNA Vaccination by TTV Load in Kidney Transplant Recipients and Hemodialysis Patients. J. Clin. Virol. 2023, 162, 105428. [Google Scholar] [CrossRef] [PubMed]
- Sylwester, A.W.; Mitchell, B.L.; Edgar, J.B.; Taormina, C.; Pelte, C.; Ruchti, F.; Sleath, P.R.; Grabstein, K.H.; Hosken, N.A.; Kern, F.; et al. Broadly Targeted Human Cytomegalovirus-Specific CD4+ and CD8+ T Cells Dominate the Memory Compartments of Exposed Subjects. J. Exp. Med. 2005, 202, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Royston, L.; Isnard, S.; Lin, J.; Routy, J.-P. Cytomegalovirus as an Uninvited Guest in the Response to Vaccines in People Living with HIV. Viruses 2021, 13, 1266. [Google Scholar] [CrossRef]
- van den Berg, S.P.H.; Warmink, K.; Borghans, J.A.M.; Knol, M.J.; van Baarle, D. Effect of latent cytomegalovirus infection on the antibody response to influenza vaccination: A systematic review and meta-analysis. Med. Microbiol. Immunol. 2019, 208, 305–321. [Google Scholar] [CrossRef]
- Haq, K.; Fulop, T.; Tedder, G.; Gentleman, B.; Garneau, H.; Meneilly, G.S.; Kleppinger, A.; Pawelec, G.; McElhaney, J.E. Cytomegalovirus Seropositivity Predicts a Decline in the T Cell But Not the Antibody Response to Influenza in Vaccinated Older Adults Independent of Type 2 Diabetes Status. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1163–1170. [Google Scholar] [CrossRef]
- Chanouzas, D.; Sagmeister, M.; Faustini, S.; Nightingale, P.; Richter, A.; Ferro, C.J.; Morgan, M.D.; Moss, P.; Harper, L. Subclinical Reactivation of Cytomegalovirus Drives CD4+CD28null T-Cell Expansion and Impaired Immune Response to Pneumococcal Vaccination in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. J. Infect. Dis. 2019, 219, 234–244. [Google Scholar] [CrossRef]
- Smith, C.; Moraka, N.O.; Ibrahim, M.; Moyo, S.; Mayondi, G.; Kammerer, B.; Leidner, J.; Gaseitsiwe, S.; Li, S.; Shapiro, R.; et al. Human Immunodeficiency Virus Exposure but Not Early Cytomegalovirus Infection Is Associated with Increased Hospitalization and Decreased Memory T-Cell Responses to Tetanus Vaccine. J. Infect. Dis. 2020, 221, 1167–1175. [Google Scholar] [CrossRef]
- Falconer, O.; Newell, M.-L.; Jones, C.E. The Effect of Human Immunodeficiency Virus and Cytomegalovirus Infection on Infant Responses to Vaccines: A Review. Front. Immunol. 2018, 9, 328. [Google Scholar] [CrossRef]
- Sanz-Ramos, M.; Manno, D.; Kapambwe, M.; Ndumba, I.; Musonda, K.G.; Bates, M.; Chibumbya, J.; Siame, J.; Monze, M.; Filteau, S.; et al. Reduced Poliovirus Vaccine Neutralising-Antibody Titres in Infants with Maternal HIV-Exposure. Vaccine 2013, 31, 2042–2049. [Google Scholar] [CrossRef]
- Cicin-Sain, L.; Brien, J.D.; Uhrlaub, J.L.; Drabig, A.; Marandu, T.F.; Nikolich-Zugich, J. Cytomegalovirus Infection Impairs Immune Responses and Accentuates T-Cell Pool Changes Observed in Mice with Aging. PLoS Pathog. 2012, 8, e1002849. [Google Scholar] [CrossRef]
- Pallikkuth, S.; De Armas, L.R.; Pahwa, R.; Rinaldi, S.; George, V.K.; Sanchez, C.M.; Pan, L.; Dickinson, G.; Rodriguez, A.; Fischl, M.; et al. Impact of Aging and HIV Infection on Serologic Response to Seasonal Influenza Vaccination. AIDS 2018, 32, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Parmigiani, A.; Alcaide, M.L.; Freguja, R.; Pallikkuth, S.; Frasca, D.; Fischl, M.A.; Pahwa, S. Impaired Antibody Response to Influenza Vaccine in HIV-Infected and Uninfected Aging Women Is Associated with Immune Activation and Inflammation. PLoS ONE 2013, 8, e79816. [Google Scholar] [CrossRef] [PubMed]
- Miller-Kittrell, M.; Sparer, T.E. Feeling Manipulated: Cytomegalovirus Immune Manipulation. Virol. J. 2009, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Golden-Mason, L.; Rosen, H.R. Natural Killer Cells: Primary Target for Hepatitis C Virus Immune Evasion Strategies? Liver Transplant. 2006, 12, 363–372. [Google Scholar] [CrossRef]
- Zampino, R.; Marrone, A.; Restivo, L.; Guerrera, B.; Sellitto, A.; Rinaldi, L.; Romano, C.; Adinolfi, L.E. Chronic HCV Infection and Inflammation: Clinical Impact on Hepatic and Extra-Hepatic Manifestations. World J. Hepatol. 2013, 5, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Vimali, J.; Yong, Y.K.; Murugesan, A.; Govindaraj, S.; Raju, S.; Balakrishnan, P.; Larsson, M.; Velu, V.; Shankar, E.M. Human Immunodeficiency Virus–Human Pegivirus Coinfected Individuals Display Functional Mucosal-Associated Invariant T Cells and Follicular T Cells Irrespective of PD-1 Expression. Viral Immunol. 2024, 37, 240–250. [Google Scholar] [CrossRef]
- Bhattarai, N.; Stapleton, J.T. GB Virus C: The Good Boy Virus? Trends Microbiol. 2012, 20, 124–130. [Google Scholar] [CrossRef]
- Duerkop, B.A.; Hooper, L.V. Resident Viruses and Their Interactions with the Immune System. Nat. Immunol. 2013, 14, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Molyneaux, P.L.; Mallia, P.; Cox, M.J.; Footitt, J.; Willis-Owen, S.A.G.; Homola, D.; Trujillo-Torralbo, M.-B.; Elkin, S.; Kon, O.M.; Cookson, W.O.C.; et al. Outgrowth of the Bacterial Airway Microbiome after Rhinovirus Exacerbation of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2013, 188, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.C.; Armah, G.; Fuentes, S.; Korpela, K.E.; Parashar, U.; Victor, J.C.; Tate, J.; de Weerth, C.; Giaquinto, C.; Wiersinga, W.J.; et al. Significant Correlation Between the Infant Gut Microbiome and Rotavirus Vaccine Response in Rural Ghana. J. Infect. Dis. 2017, 215, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.J.; Kwon, J.; Kim, S.G.; Lee, S.-J. The Biotechnological Application of Bacteriophages: What to Do and Where to Go in the Middle of the Post-Antibiotic Era. Microorganisms 2023, 11, 2311. [Google Scholar] [CrossRef]
- Moingeon, P.; de Taisne, C.; Almond, J. Delivery Technologies for Human Vaccines. Br. Med. Bull. 2002, 62, 29–44. [Google Scholar] [CrossRef]
- González-Mora, A.; Hernández-Pérez, J.; Iqbal, H.M.N.; Rito-Palomares, M.; Benavides, J. Bacteriophage-Based Vaccines: A Potent Approach for Antigen Delivery. Vaccines 2020, 8, 504. [Google Scholar] [CrossRef]
- Goulart, L.R.; de S Santos, P. Strategies for Vaccine Design Using Phage Display-Derived Peptides. In Vaccine Design: Methods and Protocols, Volume 2: Vaccines for Veterinary Diseases; Thomas, S., Ed.; Springer: New York, NY, USA, 2016; pp. 423–435. ISBN 978-1-4939-3389-1. [Google Scholar]
- Eriksson, F.; Tsagozis, P.; Lundberg, K.; Parsa, R.; Mangsbo, S.M.; Persson, M.A.A.; Harris, R.A.; Pisa, P. Tumor-Specific Bacteriophages Induce Tumor Destruction through Activation of Tumor-Associated Macrophages. J. Immunol. 2009, 182, 3105–3111. [Google Scholar] [CrossRef]
- Clark, J.R.; Bartley, K.; Jepson, C.D.; Craik, V.; March, J.B. Comparison of a Bacteriophage-Delivered DNA Vaccine and a Commercially Available Recombinant Protein Vaccine against Hepatitis B. FEMS Immunol. Med. Microbiol. 2011, 61, 197–204. [Google Scholar] [CrossRef]
- Hashemi, H.; Bamdad, T.; Jamali, A.; Pouyanfard, S.; Mohammadi, M.G. Evaluation of Humoral and Cellular Immune Responses against HSV-1 Using Genetic Immunization by Filamentous Phage Particles: A Comparative Approach to Conventional DNA Vaccine. J. Virol. Methods 2010, 163, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Iwagami, Y.; Casulli, S.; Nagaoka, K.; Kim, M.; Carlson, R.I.; Ogawa, K.; Lebowitz, M.S.; Fuller, S.; Biswas, B.; Stewart, S.; et al. Lambda Phage-Based Vaccine Induces Antitumor Immunity in Hepatocellular Carcinoma. Heliyon 2017, 3, e00407. [Google Scholar] [CrossRef]
- Bazan, J.; Całkosiński, I.; Gamian, A. Phage Display—A Powerful Technique for Immunotherapy: 1. Introduction and Potential of Therapeutic Applications. Hum. Vaccines Immunother. 2012, 8, 1817–1828. [Google Scholar] [CrossRef]
- Peabody, D.S.; Peabody, J.; Bradfute, S.B.; Chackerian, B. RNA Phage VLP-Based Vaccine Platforms. Pharmaceuticals 2021, 14, 764. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid Nanoparticles for mRNA Delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Jepson, C.D.; March, J.B. Bacteriophage Lambda Is a Highly Stable DNA Vaccine Delivery Vehicle. Vaccine 2004, 22, 2413–2419. [Google Scholar] [CrossRef]
- Górski, A.; Międzybrodzki, R.; Borysowski, J.; Dąbrowska, K.; Wierzbicki, P.; Ohams, M.; Korczak-Kowalska, G.; Olszowska-Zaremba, N.; Łusiak-Szelachowska, M.; Kłak, M.; et al. Chapter 2—Phage as a Modulator of Immune Responses: Practical Implications for Phage Therapy. In Advances in Virus Research; Łobocka, M., Szybalski, W., Eds.; Bacteriophages, Part B; Academic Press: New York, NY, USA, 2012; Volume 83, pp. 41–71. [Google Scholar]
- Clark, J.R.; March, J.B. Bacteriophage-Mediated Nucleic Acid Immunisation. FEMS Immunol. Med. Microbiol. 2004, 40, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, K.; Meshram, S. The Evolution of Phage Therapy: A Comprehensive Review of Current Applications and Future Innovations. Cureus 2024, 16, e70414. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Takakusagi, Y.; Ru, B. Editorial: Phage Display: Technique and Applications. Front. Microbiol. 2022, 13, 1097661. [Google Scholar] [CrossRef]
- Fujiki, J.; Schnabl, B. Phage Therapy: Targeting Intestinal Bacterial Microbiota for the Treatment of Liver Diseases. JHEP Rep. 2023, 5, 100909. [Google Scholar] [CrossRef]
- Faruk, O.; Jewel, Z.A.; Bairagi, S.; Rasheduzzaman, M.; Bagchi, H.; Tuha, A.S.M.; Hossain, I.; Bala, A.; Ali, S. Phage Treatment of Multidrug-Resistant Bacterial Infections in Humans, Animals, and Plants: The Current Status and Future Prospects. Infect. Med. 2025, 4, 100168. [Google Scholar] [CrossRef]
- Mohan, A.; Saxena, H.M. Effect of Phage Targeting Therapy of Brucellosis on Host Antibody Response in Cattle. Phage 2020, 1, 223–229. [Google Scholar] [CrossRef]
- Xu, H.; Bao, X.; Wang, Y.; Xu, Y.; Deng, B.; Lu, Y.; Hou, J. Engineering T7 Bacteriophage as a Potential DNA Vaccine Targeting Delivery Vector. Virol. J. 2018, 15, 49. [Google Scholar] [CrossRef]
- Pérez-Massón, B.; Quintana-Pérez, Y.; Tundidor, Y.; Pérez-Martínez, D.; Castro-Martínez, C.; Pupo-Meriño, M.; Orosa, I.; Relova-Hernández, E.; Villegas, R.; Guirola, O.; et al. Studying SARS-CoV-2 Interactions Using Phage-Displayed Receptor Binding Domain as a Model Protein. Sci. Rep. 2024, 14, 712. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Pan, P.; Ye, J.-J.; Zhang, Q.-L.; Zhang, X.-Z. Hybrid M13 Bacteriophage-Based Vaccine Platform for Personalized Cancer Immunotherapy. Biomaterials 2022, 289, 121763. [Google Scholar] [CrossRef]
- Eckerle, I.; Rosenberger, K.D.; Zwahlen, M.; Junghanss, T. Serologic Vaccination Response after Solid Organ Transplantation: A Systematic Review. PLoS ONE 2013, 8, e56974. [Google Scholar] [CrossRef] [PubMed]
- Kotton, C.N.; Kumar, D.; Caliendo, A.M.; Huprikar, S.; Chou, S.; Danziger-Isakov, L.; Humar, A.; The Transplantation Society International CMV Consensus Group. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-Organ Transplantation. Transplantation 2018, 102, 900–931. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Chen, P.; Du, J.; Wang, L.; Lu, N.; Sun, J.; Qilong, X.; Wang, Y.; Dou, L.; Liu, D.-H. Adoptive Transfer of CMV-Specific TCR-T Cells for the Treatment of CMV Infection after Haploidentical Hematopoietic Stem Cell Transplantation. J. Immunother. Cancer 2024, 12, e007735. [Google Scholar] [CrossRef]
- Tarancon-Diez, L.; Carrasco, I.; Montes, L.; Falces-Romero, I.; Vazquez-Alejo, E.; Jiménez de Ory, S.; Dapena, M.; Iribarren, J.A.; Díez, C.; Ramos-Ruperto, L.; et al. Torque Teno Virus: A Potential Marker of Immune Reconstitution in Youths with Vertically Acquired HIV. Sci. Rep. 2024, 14, 24691. [Google Scholar] [CrossRef]
- Reese, T.A.; Bi, K.; Kambal, A.; Filali-Mouhim, A.; Beura, L.K.; Bürger, M.C.; Pulendran, B.; Sekaly, R.-P.; Jameson, S.C.; Masopust, D.; et al. Sequential Infection with Common Pathogens Promotes Human-like Immune Gene Expression and Altered Vaccine Response. Cell Host Microbe 2016, 19, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Kernbauer, E.; Ding, Y.; Cadwell, K. An Enteric Virus Can Replace the Beneficial Function of Commensal Bacteria. Nature 2014, 516, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.D.; Ishikawa, F.; Greiner, D.L. Humanized Mice in Translational Biomedical Research. Nat. Rev. Immunol. 2007, 7, 118–130. [Google Scholar] [CrossRef]
- Münz, C. Humanized Mouse Models for Epstein Barr Virus Infection. Curr. Opin. Virol. 2017, 25, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Schnittman, S.R.; Hunt, P.W. CMV and Persistent Immune Activation in HIV. Curr. Opin. HIV AIDS 2021, 16, 168–176. [Google Scholar] [CrossRef]
- Lin, A.; Flynn, J.; DeRespiris, L.; Figgins, B.; Griffin, M.; Lau, C.; Proli, A.; Devlin, S.M.; Cho, C.; Tamari, R.; et al. Letermovir for Prevention of Cytomegalovirus Reactivation in Haploidentical and Mismatched Adult Donor Allogeneic Hematopoietic Cell Transplantation with Post-Transplantation Cyclophosphamide for Graft-versus-Host Disease Prophylaxis. Transplant. Cell. Ther. 2021, 27, 85.e1–85.e6. [Google Scholar] [CrossRef]
- Gredmark, S.; Söderberg-Nauclér, C. Human Cytomegalovirus Inhibits Differentiation of Monocytes into Dendritic Cells with the Consequence of Depressed Immunological Functions. J. Virol. 2003, 77, 10943–10956. [Google Scholar] [CrossRef]
- Redeker, A.; Arens, R. Improving Adoptive T Cell Therapy: The Particular Role of T Cell Costimulation, Cytokines, and Post-Transfer Vaccination. Front. Immunol. 2016, 7, 345. [Google Scholar] [CrossRef]
- Maple, P.A.C. Cytomegalovirus and Epstein-Barr Virus Associations with Neurological Diseases and the Need for Vaccine Development. Vaccines 2020, 8, 35. [Google Scholar] [CrossRef]
- Wommack, K.E.; Bhavsar, J.; Polson, S.W.; Chen, J.; Dumas, M.; Srinivasiah, S.; Furman, M.; Jamindar, S.; Nasko, D.J. VIROME: A Standard Operating Procedure for Analysis of Viral Metagenome Sequences. Stand. Genom. Sci. 2012, 6, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Rees-Spear, C.; McCoy, L.E. Vaccine Responses in Ageing and Chronic Viral Infection. Oxf. Open Immunol. 2021, 2, iqab007. [Google Scholar] [CrossRef] [PubMed]
- Bravi, B. Development and Use of Machine Learning Algorithms in Vaccine Target Selection. npj Vaccines 2024, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- El Arab, R.A.; Alkhunaizi, M.; Alhashem, Y.N.; Al Khatib, A.; Bubsheet, M.; Hassanein, S. Artificial Intelligence in Vaccine Research and Development: An Umbrella Review. Front. Immunol. 2025, 16, 1567116. [Google Scholar] [CrossRef] [PubMed]
- Šustić, M.; Cokarić Brdovčak, M.; Lisnić, B.; Materljan, J.; Juranić Lisnić, V.; Rožmanić, C.; Indenbirken, D.; Hiršl, L.; Busch, D.H.; Brizić, I.; et al. Memory CD8 T Cells Generated by Cytomegalovirus Vaccine Vector Expressing NKG2D Ligand Have Effector-Like Phenotype and Distinct Functional Features. Front. Immunol. 2021, 12, 681380. [Google Scholar] [CrossRef]
| Virus Family | Genome | Site of Replication/Persistence | Commensal or Pathogenic | Reference |
|---|---|---|---|---|
| Anelloviridae | Circular ssDNA | Blood, lymphoid tissues; lifelong persistence | Commensal (non-pathogenic) | [7] |
| Herpesviridae | Linear dsDNA | Latent in neurons, epithelial cells, lymphocytes | Pathogenic or immunomodulatory | [8] |
| Polyomaviridae | Circular dsDNA | Kidney, urinary tract, CNS | Mostly commensal; opportunistic | [11] |
| Papillomaviridae | Circular dsDNA | Skin and mucosal epithelia | Both commensal and oncogenic types | [11] |
| Parvoviridae | Linear ssDNA | Blood, bone marrow | Potentially pathogenic | [11] |
| Redondoviridae | Circular ssDNA | Oropharyngeal and respiratory tract | Commensal (elevated in disease) | [5] |
| CrAss-like phages | Linear dsDNA | Gut mucosa and lumen; infect Bacteroidota spp. | Commensal (dominant phage family) | [4] |
| Microviridae, Siphoviridae, etc. | ssDNA or dsDNA (phages) | Gut; infect gut-resident bacteria | Commensal | [4] |
| Endogenous retroviruses | Integrated DNA | Human genome (germline-encoded) | Mostly inactive/commensal | [11] |
| Virus | Family/Genus | Key Characteristics | Role in Vaccine Immunity | Notes/References |
|---|---|---|---|---|
| TTV | Anelloviridae Alphatorquevirus | Circular ssDNA; ubiquitous, non-pathogenic | Inverse marker of immune competence; high TTV load correlates with poor vaccine response in transplant and HIV+ patients | Clinically relevant; extensively cited [30,31,33,34] |
| CMV | Herpesviridae Betaherpesvirus | dsDNA latency; lifelong infection | Alters vaccine responses, particularly in the elderly and immunocompromised; can impair CD8+ responses and T-cell repertoire | CMV seropositivity reduces vaccine efficacy [42,43,44,45,46,47], |
| EBV | Herpesviridae Gammaherpesvirus | Latent in B cells; widespread | Modulates B-cell dynamics; potential impact on long-term humoral memory | Suggested in B-cell vaccination studies [44,48,49] |
| HHV-6 | Herpesviridae Betaherpesvirus | Prevalent latent virus; neurotropic potential | Possible impact on vaccine efficacy (e.g., HBV, polio); underexplored | Mechanistic roles unclear [49,50] |
| BK/JC | Polyomaviridae | dsDNA; latent in the urinary tract | Used as a surrogate marker of immunosuppression in transplant recipients | Potential biomarker in immunized hosts [43] |
| GB Virus C (Pegivirus) | Flaviviridae Pegivirus | ssRNA; non-pathogenic, persistent in blood | Modulates T-cell activation and cytokine balance; protective in HIV co-infection | Immunomodulatory in co-infection models [51,52] |
| Norovirus (murine/human) | Caliciviridae | ssRNA; enteric, persistent in mice and humans | Promotes intestinal interferon signaling; model for virome–immune homeostasis | Shown in murine vaccine priming [53,54] |
| Murine CMV/γHV-68 | Herpesviridae | Mouse models of latent herpesvirus infection | Boosts baseline IFN-γ and antimicrobial resistance; impacts vaccine recall responses | Experimental models of latent infection [5,6,8,55] |
| Bacteriophages | Various | Highly abundant on mucosal surfaces; infect commensal bacteria | Indirectly modulates the immune response via microbiome shaping; alters vaccine response through metabolite and barrier effects | Key component of mucosal virome [22,25,56,57] |
| Study/Project | Target | Phage Type | Status | Notes | References |
|---|---|---|---|---|---|
| Phage-based vaccine for Brucella abortus | Brucellosis (zoonosis) | M13 | Phase I (animal model) | Induces Th1 immune response | [105] |
| Bacteriophage as DNA vaccine vector | Influenza | Filamentous (fd) | Phase I (mouse model) | Elicits strong immunogenicity | [106] |
| Phage display vaccine for SARS-CoV-2 | COVID-19 | RNA phage + spike epitopes | Preclinical | Is immunogenic in mice | [107] |
| Phage-based cancer vaccine (neoantigen-M13) | Melanoma/solid tumors | Modified M13 | Preclinical (animals) | Induces anti-tumor T-cell response | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cianci, R.; Caldarelli, M.; Brani, P.; Bosi, A.; Ponti, A.; Giaroni, C.; Baj, A. Role of the Virome in Vaccine-Induced Immunization. Vaccines 2025, 13, 895. https://doi.org/10.3390/vaccines13090895
Cianci R, Caldarelli M, Brani P, Bosi A, Ponti A, Giaroni C, Baj A. Role of the Virome in Vaccine-Induced Immunization. Vaccines. 2025; 13(9):895. https://doi.org/10.3390/vaccines13090895
Chicago/Turabian StyleCianci, Rossella, Mario Caldarelli, Paola Brani, Annalisa Bosi, Alessandra Ponti, Cristina Giaroni, and Andreina Baj. 2025. "Role of the Virome in Vaccine-Induced Immunization" Vaccines 13, no. 9: 895. https://doi.org/10.3390/vaccines13090895
APA StyleCianci, R., Caldarelli, M., Brani, P., Bosi, A., Ponti, A., Giaroni, C., & Baj, A. (2025). Role of the Virome in Vaccine-Induced Immunization. Vaccines, 13(9), 895. https://doi.org/10.3390/vaccines13090895








