Therapeutic Vaccines for Non-Communicable Diseases: Global Progress and China’s Deployment Pathways
Abstract
1. Introduction
2. Methodology
3. Existing NCD Vaccines
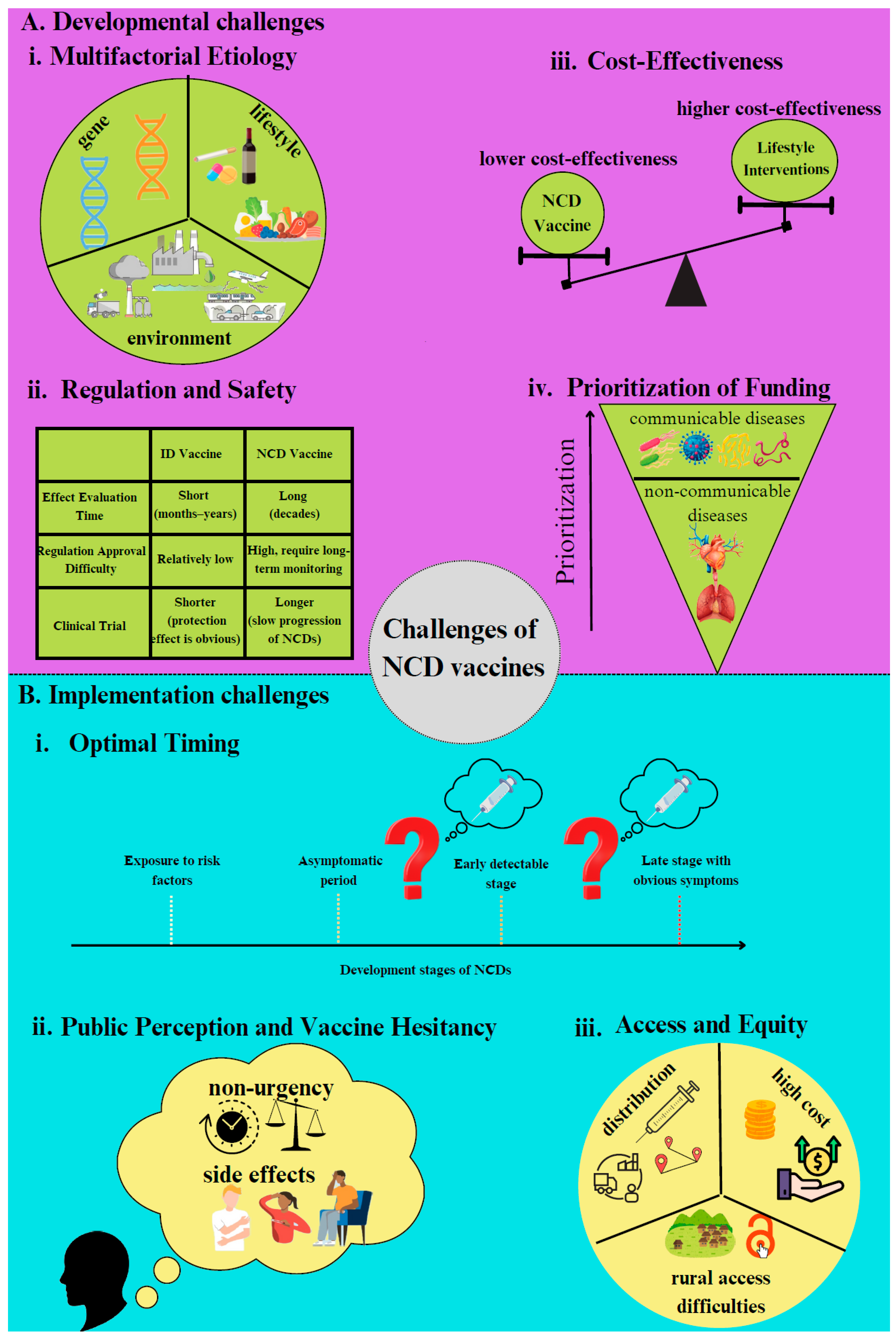
4. Challenges to NCD Vaccine Development and Implementation
5. Developmental Challenges
6. Implementation Challenges
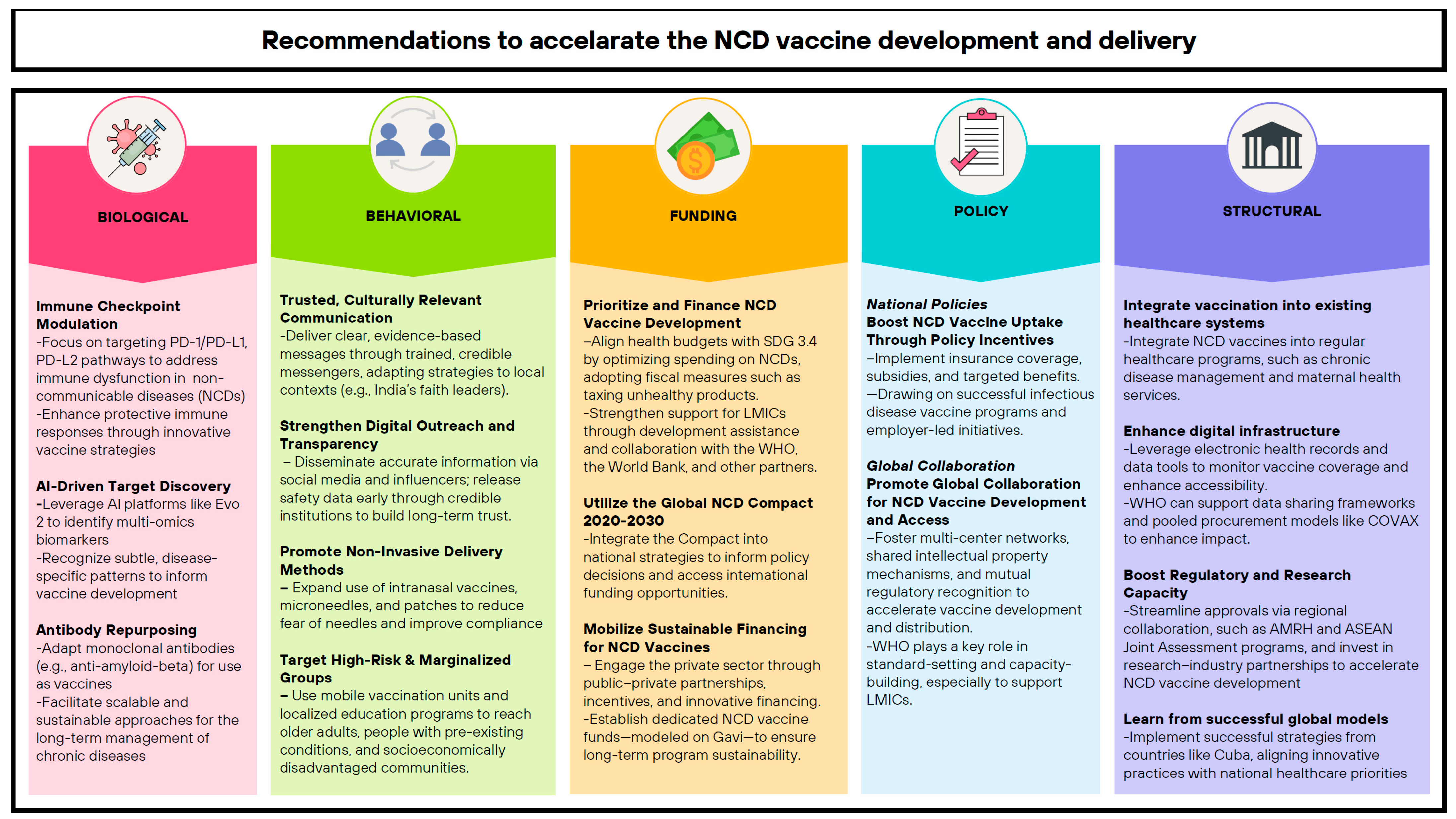
7. Recommendations
7.1. Biological
7.2. Non-Biological
Behavioral
7.3. Funding
7.4. Policy
7.4.1. National Policies
7.4.2. Global Collaboration
7.5. Structural
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noncommunicable Diseases. Available online: https://www.who.int/health-topics/noncommunicable-diseases (accessed on 26 August 2024).
- Institute for Health Metrics and Evaluation. GBD Compare. Available online: http://vizhub.healthdata.org/gbd-compare (accessed on 28 August 2024).
- Institute for Health Metrics and Evaluation. Global Burden of Disease 2021 Findings from the GBD 2021 Study. 2021. Available online: https://www.healthdata.org/sites/default/files/2024-05/GBD_2021_Booklet_FINAL_2024.05.16.pdf (accessed on 28 August 2024).
- World Health Organization—Regional Office for the Eastern Mediterranean. WHO EMRO|Management|NCDs. Available online: http://www.emro.who.int/noncommunicable-diseases/management/index.html (accessed on 6 September 2024).
- Jamison, D.T.; Breman, J.G.; Measham, A.R.; Alleyne, G.; Claeson, M.; Evans, D.B.; Jha, P.; Mills, A.; Musgrove, P. Cost-Effective Strategies for Noncommunicable Diseases, Risk Factors, and Behaviors. In Priorities in Health; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2006. Available online: https://www.ncbi.nlm.nih.gov/books/NBK10246/ (accessed on 6 September 2024).
- Vetrano, D.L.; Rizzuto, D.; Calderón-Larrañaga, A.; Onder, G.; Welmer, A.-K.; Bernabei, R.; Marengoni, A.; Fratiglioni, L. Trajectories of functional decline in older adults with neuropsychiatric and cardiovascular multimorbidity: A Swedish cohort study. PLoS Med. 2018, 15, e1002503. [Google Scholar] [CrossRef]
- Omotayo, O.; Maduka, C.P.; Muonde, M.; Olorunsogo, T.O.; Ogugua, J.O. The rise of non-communicable diseases: A global health review of challenges and prevention strategies. Int. Med. Sci. Res. J. 2024, 4, 74–88. [Google Scholar] [CrossRef]
- WHO-NMH-NVI-18.8-eng.pdf. Available online: https://iris.who.int/bitstream/handle/10665/272534/WHO-NMH-NVI-18.8-eng.pdf (accessed on 7 September 2024).
- Darrow, J.J.; Kesselheim, A.S. A New Wave of Vaccines for Non-Communicable Diseases: What Are the Regulatory Challenges? Food Drug Law J. 2015, 70, 243–258. [Google Scholar] [PubMed]
- Kashutina, M.; Fadeeva, I.; Zhernov, Y. World Experience in Immunization against Noncommunicable Diseases: Successes and Vectors for Further Development. Vaccines 2023, 11, 1286. [Google Scholar] [CrossRef]
- Plotkin, S.; Robinson, J.M.; Cunningham, G.; Iqbal, R.; Larsen, S. The complexity and cost of vaccine manufacturing—An overview. Vaccine 2017, 35, 4064–4071. [Google Scholar] [CrossRef] [PubMed]
- Leidner, A.J.; Murthy, N.; Chesson, H.W.; Biggerstaff, M.; Stoecker, C.; Harris, A.M.; Acosta, A.; Dooling, K.; Bridges, C.B. Cost-effectiveness of adult vaccinations: A systematic review. Vaccine 2019, 37, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Farmer, E.; Wu, T.C.; Hung, C.-F. Perspectives for therapeutic HPV vaccine development. J. Biomed. Sci. 2016, 23, 75. [Google Scholar] [CrossRef]
- Nakamaru, R.; Nakagami, H.; Rakugi, H.; Morishita, R. Future Directions of Therapeutic Vaccines for Chronic Diseases. Circ. J. 2020, 84, 1895–1902. [Google Scholar] [CrossRef]
- Tian, Y.; Hu, D.; Li, Y.; Yang, L. Development of therapeutic vaccines for the treatment of diseases. Mol. Biomed. 2022, 3, 40. [Google Scholar] [CrossRef]
- González, G.; Crombet, T.; Catalá, M.; Mirabal, V.; Hernández, J.C.; González, Y.; Marinello, P.; Guillén, G.; Lage, A. A novel cancer vaccine composed of human-recombinant epidermal growth factor linked to a carrier protein: Report of a pilot clinical trial. Ann. Oncol. 1998, 9, 431–435. [Google Scholar] [CrossRef]
- Li, T.; Qian, C.; Gu, Y.; Zhang, J.; Li, S.; Xia, N. Current progress in the development of prophylactic and therapeutic vaccines. Sci. China Life Sci. 2023, 66, 679–710. [Google Scholar] [CrossRef]
- Hernandez, M.; Ortiz, R.A.; Salomon, E.; Acosta, S.; Santiesteban, E.; Amador, R.M.; Mendoza, I.C.; Guerra, P.P.; Robaina, M.; Sanchez, C.; et al. Safety and efficacy of CIMAvax-EGF vaccine for the treatment of real-world non-small cell lung cancer patients. Integr. Clin. Med. 2021, 5, 1–5. Available online: https://www.oatext.com/safety-and-efficacy-of-cimavax-egf-vaccine-for-the-treatment-of-real-world-non-small-cell-lung-cancer-patients.php (accessed on 7 August 2025). [CrossRef]
- Flores Vega, Y.I.; Páramo González, D.L.; Alsina Sarmiento, S.C.; Alsina Tul, L.E.; Inguanzo Valdés, I.B.; Rodríguez Machado, J.; Elejalde Larrinaga, Á.; Flores Rodríguez, J.E.; Lamadrid García, J.; Corrales Otero, D.; et al. Survival of NSCLC Patients Treated with Cimavax-EGF as Switch Maintenance in the Real-World Scenario. J. Cancer 2023, 14, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.; Maglakelidze, M.; Andrić, Z.; Ryspayeva, D.; Bulat, I.; Nikolić, I.; Petrović, Z.; Chawla, T.; Nagarkar, R.; Garner-Spitzer, E.; et al. Phase II Trial of HER-Vaxx, a B-cell Peptide-Based Vaccine, in HER2-Overexpressing Advanced Gastric Cancer Patients Under Platinum-Based Chemotherapy (HERIZON). Clin. Cancer Res. 2024, 30, 4044–4054. [Google Scholar] [CrossRef]
- Koido, S.; Taguchi, J.; Shimabuku, M.; Kan, S.; Bito, T.; Misawa, T.; Ito, Z.; Uchiyama, K.; Saruta, M.; Tsukinaga, S.; et al. Dendritic cells pulsed with multifunctional Wilms’ tumor 1 (WT1) peptides combined with multiagent chemotherapy modulate the tumor microenvironment and enable conversion surgery in pancreatic cancer. J. Immunother. Cancer 2024, 12, e009765. [Google Scholar] [CrossRef]
- Nagai, K.; Adachi, T.; Harada, H.; Eguchi, S.; Sugiyama, H.; Miyazaki, Y. Dendritic Cell-based Immunotherapy Pulsed With Wilms Tumor 1 Peptide and Mucin 1 as an Adjuvant Therapy for Pancreatic Ductal Adenocarcinoma After Curative Resection: A Phase I/IIa Clinical Trial. Anticancer Res. 2020, 40, 5765–5776. [Google Scholar] [CrossRef]
- Harris, J.E.; Ryan, L.; Hoover, H.C.; Stuart, R.K.; Oken, M.M.; Benson, A.B.; Mansour, E.; Haller, D.G.; Manola, J.; Hanna, M.G. Adjuvant Active Specific Immunotherapy for Stage II and III Colon Cancer With an Autologous Tumor Cell Vaccine: Eastern Cooperative Oncology Group Study E5283. J. Clin. Oncol. 2000, 18, 148. [Google Scholar] [CrossRef]
- Braun, D.A.; Moranzoni, G.; Chea, V.; McGregor, B.A.; Blass, E.; Tu, C.R.; Vanasse, A.P.; Forman, C.; Forman, J.; Afeyan, A.B.; et al. A neoantigen vaccine generates antitumour immunity in renal cell carcinoma. Nature 2025, 639, 474–482. [Google Scholar] [CrossRef]
- Handy, C.E.; Antonarakis, E.S. Sipuleucel-T for the Treatment of Prostate Cancer: Novel Insights and Future Directions. Future Oncol. 2018, 14, 907–917. [Google Scholar] [CrossRef]
- Tissot, A.C.; Maurer, P.; Nussberger, J.; Sabat, R.; Pfister, T.; Ignatenko, S.; Volk, H.-D.; Stocker, H.; Müller, P.; Jennings, G.T.; et al. Effect of immunisation against angiotensin II with CYT006-AngQb on ambulatory blood pressure: A double-blind, randomised, placebo-controlled phase IIa study. Lancet 2008, 371, 821–827. [Google Scholar] [CrossRef]
- Harbi, M.H. Current usage of inclisiran for cardiovascular diseases: Overview of current clinical trials. Front. Pharmacol. 2025, 16, 1449712. [Google Scholar] [CrossRef] [PubMed]
- Cavelti-Weder, C.; Timper, K.; Seelig, E.; Keller, C.; Osranek, M.; Lässing, U.; Spohn, G.; Maurer, P.; Müller, P.; Jennings, G.T.; et al. Development of an Interleukin-1β Vaccine in Patients with Type 2 Diabetes. Mol. Ther. 2016, 24, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Han, W. Phase I/II Study of CAR-T Cell Therapy Targeting GPC3 in Patients with Treated Advanced GPC3-Positive Hepatocellular Carcinoma. clinicaltrials.gov; 2024 Oct. Report No.: NCT06641453. Available online: https://clinicaltrials.gov/study/NCT06641453 (accessed on 7 August 2025).
- Sawada, Y.; Yoshikawa, T.; Ofuji, K.; Yoshimura, M.; Tsuchiya, N.; Takahashi, M.; Nobuoka, D.; Gotohda, N.; Takahashi, S.; Kato, Y.; et al. Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. OncoImmunology 2016, 5, e1129483. [Google Scholar] [CrossRef]
- National Cancer Institute (NCI). Phase I Study of GPC3 Targeted CAR-T Cell Therapy in Advanced GPC3 Expressing Hepatocellular Carcinoma (HCC). clinicaltrials.gov; 2025 May. Report No.: NCT05003895. Available online: https://clinicaltrials.gov/study/NCT05003895 (accessed on 7 August 2025).
- Imugene Limited. A Phase 1b/2 Open-Label Study with Randomization in Phase 2 of IMU-131 HER2/Neu Peptide Vaccine Plus Standard of Care Chemotherapy in Patients with HER2/Neu Overexpressing Metastatic or Advanced Adenocarcinoma of the Stomach or Gastroesophageal Junction. clinicaltrials.gov; 2025 Apr. Report No.: NCT02795988. Available online: https://clinicaltrials.gov/study/NCT02795988 (accessed on 7 August 2025).
- Ott, P. A Phase I Study Combining NeoVax, a Personalized NeoAntigen Cancer Vaccine, with Ipilimumab to Treat High-Risk Renal Cell Carcinoma. clinicaltrials.gov; 2025 Mar. Report No.: NCT02950766. Available online: https://clinicaltrials.gov/study/NCT02950766 (accessed on 7 August 2025).
- Smith, L. A Randomized, Double Blind, Placebo Controlled Phase 3 Trial of Immunotherapy with Autologous Antigen Presenting Cells Loading with PA2024 (Provenge(R), APC8015) in Men with Metastatic Androgen Independent Prostatic Adenocarcinoma. clinicaltrials.gov; 2010 Sep. Report No.: NCT00065442. Available online: https://clinicaltrials.gov/study/NCT00065442 (accessed on 7 August 2025).
- Study Details|Safety, Tolerability, Pharmacodynamic Effects and Preliminary Evidence for Efficacy of the Anti-Hypertension Vaccine CYT006-AngQb|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT00500786?term=NCT00500786&rank=1 (accessed on 7 August 2025).
- Drug Trials Snapshots: LEQVIO FDA. 2023. Available online: https://web.archive.org/web/20230728201951/https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots-leqvio (accessed on 7 August 2025).
- Cytos Biotechnology AG. A Two-Stage Randomized Placebo-Controlled Ascending Dose Phase I/IIa Study to Evaluate Safety, Tolerability, Pharmacodynamic Effects and Preliminary Efficacy of an Anti-Interleukin 1 Beta Vaccine (CYT013-IL1bQb) in Patients with Type 2 Diabetes Mellitus. clinicaltrials.gov; 2012 Feb. Report No.: NCT00924105. Available online: https://clinicaltrials.gov/study/NCT00924105 (accessed on 16 December 2024).
- Novartis Pharmaceuticals. A Randomized, Double-Blind, Placebo-Controlled, Two-Cohort, Parallel Group Study to Evaluate the Efficacy of CAD106 and CNP520 in Participants at Risk for the Onset of Clinical Symptoms of Alzheimer’s Disease. clinicaltrials.gov; 2021 Jul. Report No.: NCT02565511. Available online: https://clinicaltrials.gov/study/NCT02565511 (accessed on 7 August 2025).
- Mogi, M. Clinical study on angiotensin II vaccination—The first big step. Hypertens. Res. 2022, 45, 162–163. [Google Scholar] [CrossRef]
- Novartis Pharmaceuticals. Effectiveness of Inclisiran for Patients with Coronary Heart Disease in China: A Multicenter, Standard of Care-Controlled Pragmatic Randomized Trial. clinicaltrials.gov; 2025 Jul. Report No.: NCT06941792. Available online: https://clinicaltrials.gov/study/NCT06941792 (accessed on 7 August 2025).
- Cheever, M.A.; Higano, C.S. PROVENGE (Sipuleucel-T) in Prostate Cancer: The First FDA-Approved Therapeutic Cancer Vaccine. Clin. Cancer Res. 2011, 17, 3520–3526. [Google Scholar] [CrossRef]
- Anassi, E.; Ndefo, U.A. Sipuleucel-T (Provenge) Injection. Pharm. Ther. 2011, 36, 197–202. [Google Scholar]
- Uyldegroot, C.; Vermorken, J.; Hannajr, M.; Verboom, P.; Groot, M.; Bonsel, G.; Meijer, C.; Pinedo, H. Immunotherapy with autologous tumor cell-BCG vaccine in patients with colon cancer: A prospective study of medical and economic benefits. Vaccine 2005, 23, 2379–2387. [Google Scholar] [CrossRef]
- Papi, A. COPD increases the risk of squamous histological subtype in smokers who develop non-small cell lung carcinoma. Thorax 2004, 59, 679–681. [Google Scholar] [CrossRef]
- Young, R.P.; Duan, F.; Chiles, C.; Hopkins, R.J.; Gamble, G.D.; Greco, E.M.; Gatsonis, C.; Aberle, D. Airflow Limitation and Histology Shift in the National Lung Screening Trial. The NLST-ACRIN Cohort Substudy. Am. J. Respir. Crit. Care Med. 2015, 192, 1060–1067. [Google Scholar] [CrossRef]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.-L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Saly, D.L.; Eswarappa, M.S.; Street, S.E.; Deshpande, P. Renal Cell Cancer and Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2021, 28, 460–468.e1. [Google Scholar] [CrossRef]
- Lewicki, S.; Bałan, B.J.; Stelmasiak, M.; Radomska-Leśniewska, D.M.; Szymański, Ł.; Rios-Turek, N.; Bień-Kalinowska, J.; Szarpak, Ł.; Hajduk, B. Immunological Insights and Therapeutic Advances in COPD: Exploring Oral Bacterial Vaccines for Immune Modulation and Clinical Improvement. Vaccines 2025, 13, 107. [Google Scholar] [CrossRef]
- Azegami, T.; Nakayama, T.; Hayashi, K.; Hishikawa, A.; Yoshimoto, N.; Nakamichi, R.; Itoh, H. Vaccination Against Receptor for Advanced Glycation End Products Attenuates the Progression of Diabetic Kidney Disease. Diabetes 2021, 70, 2147–2158. [Google Scholar] [CrossRef]
- Dec, A.; Niemiec, A.; Wojciechowska, E.; Maligłówka, M.; Bułdak, Ł.; Bołdys, A.; Okopień, B. Inclisiran—A Revolutionary Addition to a Cholesterol-Lowering Therapy. Int. J. Mol. Sci. 2023, 24, 6858. [Google Scholar] [CrossRef] [PubMed]
- Soffer, D.; Stoekenbroek, R.; Plakogiannis, R. Small interfering ribonucleic acid for cholesterol lowering—Inclisiran. J. Clin. Lipidol. 2022, 16, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Chellappan, D.K.; Bhandare, R.R.; Shaik, A.B.; Prasad, K.; Suhaimi, N.A.A.; Yap, W.S.; Das, A.; Banerjee, P.; Ghosh, N.; Guith, T.; et al. Vaccine for Diabetes—Where Do We Stand? Int. J. Mol. Sci. 2022, 23, 9470. [Google Scholar] [CrossRef]
- Amend, D. A Randomized, Blinded, Placebo Controlled, Safety and Pharmacodynamic Study of BHT-3021 with Open Label Cross-Over in Subjects with Type I Diabetes Mellitus. clinicaltrials.gov; 2011 Jun. Report No.: NCT00453375. Available online: https://clinicaltrials.gov/study/NCT00453375 (accessed on 9 August 2025).
- Roep, B.O.; Solvason, N.; Gottlieb, P.A.; Abreu, J.R.F.; Harrison, L.C.; Eisenbarth, G.S.; Yu, L.; Leviten, M.; Hagopian, W.A.; Buse, J.B.; et al. Plasmid-Encoded Proinsulin Preserves C-Peptide While Specifically Reducing Proinsulin-Specific CD8+ T Cells in Type 1 Diabetes. Sci. Transl. Med. 2013, 5, 191ra82. Available online: https://www.science.org/doi/10.1126/scitranslmed.3006103 (accessed on 9 August 2025). [CrossRef] [PubMed]
- Peters, R.; Ee, N.; Peters, J.; Beckett, N.; Booth, A.; Rockwood, K.; Anstey, K.J. Common risk factors for major noncommunicable disease, a systematic overview of reviews and commentary: The implied potential for targeted risk reduction. Ther. Adv. Chronic Dis. 2019, 10, 2040622319880392. [Google Scholar] [CrossRef]
- Bays, H.E.; Taub, P.R.; Epstein, E.; Michos, E.D.; Ferraro, R.A.; Bailey, A.L.; Kelli, H.M.; Ferdinand, K.C.; Echols, M.R.; Weintraub, H.; et al. Ten things to know about ten cardiovascular disease risk factors. Am. J. Prev. Cardiol. 2021, 5, 100149. [Google Scholar] [CrossRef]
- Ardisson Korat, A.V.; Willett, W.C.; Hu, F.B. Diet, Lifestyle, and Genetic Risk Factors for Type 2 Diabetes: A Review from the Nurses’ Health Study, Nurses’ Health Study 2, and Health Professionals’ Follow-Up Study. Curr. Nutr. Rep. 2014, 3, 345–354. [Google Scholar] [CrossRef]
- National Institute on Aging. Alzheimer’s Disease Genetics Fact Sheet. Available online: https://www.nia.nih.gov/health/alzheimers-causes-and-risk-factors/alzheimers-disease-genetics-fact-sheet (accessed on 16 January 2025).
- Pena, D.L.; Aurelian, J.; Grigore, M.; Hodorogea, A.S.; Weiss, E.; Bădilă, E.; Ilieșiu, A.M.; Balahura, A.M. Emerging therapeutic frontiers in hypertension management. Front. Cardiovasc. Med. 2025, 12, 1550181. [Google Scholar] [CrossRef]
- Riviere, M.; Langbaum, J.B.; Turner, R.S.; Rinne, J.O.; Sui, Y.; Cazorla, P.; Ricart, J.; Meneses, K.; Caputo, A.; Tariot, P.N.; et al. Effects of the active amyloid beta immunotherapy CAD106 on PET measurements of amyloid plaque deposition in cognitively unimpaired APOE ε4 homozygotes. Alzheimers Dement. 2024, 20, 1839–1850. [Google Scholar] [CrossRef]
- Carazo, S.; Talbot, D.; Boulianne, N.; Brisson, M.; Gilca, R.; Deceuninck, G.; Brousseau, N.; Drolet, M.; Ouakki, M.; Sauvageau, C.; et al. Single-Dose mRNA Vaccine Effectiveness Against SARS-CoV-2 in Healthcare Workers Extending 16 Weeks Post-Vaccination: A Test-Negative Design from Quebec, Canada 2021. Available online: http://medrxiv.org/lookup/doi/10.1101/2021.07.19.21260445 (accessed on 5 August 2025).
- Schuster, S.J.; Neelapu, S.S.; Gause, B.L.; Janik, J.E.; Muggia, F.M.; Gockerman, J.P.; Winter, J.N.; Flowers, C.R.; Nikcevich, D.A.; Sotomayor, E.M.; et al. Vaccination With Patient-Specific Tumor-Derived Antigen in First Remission Improves Disease-Free Survival in Follicular Lymphoma. J. Clin. Oncol. 2011, 29, 2787–2794. [Google Scholar] [CrossRef]
- Kemp, R.; Prasad, V. Surrogate endpoints in oncology: When are they acceptable for regulatory and clinical decisions, and are they currently overused? BMC Med. 2017, 15, 134. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Burgess, C.; Shendale, S.; Morgan, W.; Cw Hutubessy, R.; Measles–rubella Eradication Modelling Group; Jit, M.; Measles-rubella Eradication Modelling Group. Cost-effectiveness of measles and rubella elimination in low-income and middle-income countries. BMJ Glob. Health 2023, 8, e011526. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, Y.; Wang, J.; Che, X.; Du, J.; Zhang, X.; Gu, W.; Zhang, X.; Jiang, W. Cost-effectiveness of various immunization schedules with inactivated Sabin strain polio vaccine in Hangzhou, China. Front. Public Health 2022, 10, 990042. [Google Scholar] [CrossRef]
- Singh Thakur, J.; Nangia, R.; Singh, S. Progress and challenges in achieving noncommunicable diseases targets for the sustainable development goals. FASEB BioAdv. 2021, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Mehand, M.S.; Millett, P.; Al-Shorbaji, F.; Roth, C.; Kieny, M.P.; Murgue, B. World Health Organization Methodology to Prioritize Emerging Infectious Diseases in Need of Research and Development. Emerg. Infect. Dis. 2018, 24, e171427. Available online: http://wwwnc.cdc.gov/eid/article/24/9/17-1427_article.htm (accessed on 16 January 2025). [CrossRef]
- Lalani, H.S.; Nagar, S.; Sarpatwari, A.; Barenie, R.E.; Avorn, J.; Rome, B.N.; Kesselheim, A.S. US public investment in development of mRNA covid-19 vaccines: Retrospective cohort study. BMJ 2023, 380, e073747. [Google Scholar] [CrossRef]
- Financing NCDs|NCD Alliance. Available online: https://ncdalliance.org/explore-ncds/solutions/financing-ncds (accessed on 10 August 2025).
- Kong, L. China’s Medium-to-Long Term Plan for the Prevention and Treatment of Chronic Diseases (2017–2025) under the Healthy China Initiative. Chronic Dis. Transl. Med. 2017, 3, 135–137. [Google Scholar] [CrossRef]
- Tan, X.; Liu, X.; Shao, H. Healthy China 2030: A Vision for Health Care. Value Health Reg. Issues 2017, 12, 112–114. [Google Scholar] [CrossRef] [PubMed]
- China Cancer Vaccines Market Size & Outlook, 2020-2030. Available online: https://www.grandviewresearch.com/horizon/outlook/cancer-vaccines-market/china (accessed on 5 August 2025).
- China Cancer Immunotherapy Market Size & Outlook. Available online: https://www.grandviewresearch.com/horizon/outlook/cancer-immunotherapy-market/china (accessed on 5 August 2025).
- Lin, Y.; Fu, H. Challenges in improving non-communicable diseases management and achieving universal health coverage in China. Lancet Reg. Health—West. Pac. 2024, 44, 101007. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Alatise, O.I.; O’Connell, K.; Ogunleye, S.G.; Aderounmu, A.A.; Samson, M.L.; Wuraola, F.; Olasehinde, O.; Kingham, T.P.; Du, M. Healthcare utilisation, cancer screening and potential barriers to accessing cancer care in rural South West Nigeria: A cross-sectional study. BMJ Open 2021, 11, e040352. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.M.; Del Giudice, G.; Maggi, S. Adult vaccination as part of a healthy lifestyle: Moving from medical intervention to health promotion. Ann. Med. 2019, 51, 128–140. [Google Scholar] [CrossRef]
- Smith, L.E.; Webster, R.K.; Weinman, J.; Amlôt, R.; Yiend, J.; Rubin, G.J. Psychological factors associated with uptake of the childhood influenza vaccine and perception of post-vaccination side-effects: A cross-sectional survey in England. Vaccine 2017, 35, 1936–1945. [Google Scholar] [CrossRef]
- Yang, R.; Penders, B.; Horstman, K. Addressing Vaccine Hesitancy in China: A Scoping Review of Chinese Scholarship. Vaccines 2019, 8, 2. [Google Scholar] [CrossRef]
- Vora, A.; Di Pasquale, A.; Kolhapure, S.; Agrawal, A.; Agrawal, S. The need for vaccination in adults with chronic (noncommunicable) diseases in India—Lessons from around the world. Hum. Vaccines Immunother. 2022, 18, 2052544. [Google Scholar] [CrossRef]
- Zhang, Z. Survey and analysis on the resource situation of primary health care institutions in rural China. Front. Public Health 2024, 12, 1394527. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Q.; Li, C.; Meng, P.; Ding, L. Inequalities in non-communicable disease management in China and progress toward universal health coverage: An analysis of nationwide household survey data from 2004 to 2018. Lancet Reg. Health—West. Pac. 2024, 44, 100989. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- Hofmann, C.; Völkers, M.; Katus, H.A. Targeting coagulation in heart failure with preserved ejection fraction and cardiac fibrosis. Eur. Heart J. 2019, 40, 3333–3335. [Google Scholar] [CrossRef] [PubMed]
- Baruch, K.; Deczkowska, A.; Rosenzweig, N.; Tsitsou-Kampeli, A.; Sharif, A.M.; Matcovitch-Natan, O.; Kertser, A.; David, E.; Amit, I.; Schwartz, M. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease. Nat. Med. 2016, 22, 135–137. [Google Scholar] [CrossRef]
- Brixi, G.; Durrant, M.G.; Ku, J.; Poli, M.; Brockman, G.; Chang, D.; Gonzalez, G.A.; King, S.H.; Li, D.B.; Merchant, A.T.; et al. Genome modeling and design across all domains of life with Evo 2. bioRxiv 2025. [Google Scholar] [CrossRef]
- Malik, B.; Ghatol, A. Understanding How Monoclonal Antibodies Work. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK572118/ (accessed on 1 April 2025).
- Zhu, X.; Chen, Z.; Shen, W.; Huang, G.; Sedivy, J.M.; Wang, H.; Ju, Z. Inflammation, epigenetics, and metabolism converge to cell senescence and ageing: The regulation and intervention. Signal Transduct. Target. Ther. 2021, 6, 245. [Google Scholar] [CrossRef]
- Raub, J.N. Knowledge, fear of the unknown, opinion, and the pandemic. Am. J. Health-Syst. Pharm. 2022, 79, 400–401. [Google Scholar] [CrossRef]
- Song, M.; Elson, J.; Nguyen, T.; Obasi, S.; Pintar, J.; Bastola, D. Exploring trust dynamics in health information systems: The impact of patients’ health conditions on information source preferences. Front. Public Health 2024, 12, 1478502. [Google Scholar] [CrossRef]
- Poland, C.M.; Ratishvili, T. Vaccine hesitancy and health care providers: Using the preferred cognitive styles and decision-making model and empathy tool to make progress. Vaccine X 2022, 11, 100174. [Google Scholar] [CrossRef]
- Banerjee, P.; Seth, R.; Dhaliwal, B.K.; Sullivan, A.; Qiayum, Y.; Thankachen, B.; Closser, S.; Shet, A. Vaccine acceptance in rural India: Engaging faith leaders as vaccine ambassadors. Front. Public Health 2022, 10, 979424. [Google Scholar] [CrossRef]
- In Northwestern Pakistan, Religious Leaders Convert the Vaccine-Hesitant. Available online: https://www.gavi.org/vaccineswork/northwestern-pakistan-religious-leaders-convert-vaccine-hesitant?utm_source=.com (accessed on 10 August 2025).
- Goobie, G.C. Social media impacts on the dissemination of health-related information and patient-physician relationships. Cover 2008, 35, 6. [Google Scholar]
- Kehagia, E.; Papakyriakopoulou, P.; Valsami, G. Advances in intranasal vaccine delivery: A promising non-invasive route of immunization. Vaccine 2023, 41, 3589–3603. [Google Scholar] [CrossRef]
- Lu, P.-J.; Hung, M.-C.; Srivastav, A.; Grohskopf, L.A.; Kobayashi, M.; Harris, A.M.; Dooling, K.L.; Markowitz, L.E.; Rodriguez-Lainz, A.; Williams, W.W. Surveillance of Vaccination Coverage Among Adult Populations—United States, 2018. MMWR Surveill Summ. 2021, 70, 33983910. [Google Scholar] [CrossRef]
- Global NCD Compact 2020–2030. Available online: https://www.who.int/initiatives/global-noncommunicable-diseases-compact-2020-2030 (accessed on 7 April 2025).
- Goal 3|Department of Economic and Social Affairs. Available online: https://sdgs.un.org/goals/goal3#targets_and_indicators (accessed on 7 April 2025).
- Understanding Results-Based Financing—Delivering Impact Through Verified Outcomes|GPRBA. Available online: https://www.gprba.org/who-we-are/results-based-financing (accessed on 10 August 2025).
- The Global Noncommunicable Diseases (NCD) Compact 2020-2030 (NCD Compact). Available online: https://cdn.who.int/media/docs/default-source/ncds/final_ncd-compact-(1).pdf?sfvrsn=d8895106_1 (accessed on 13 August 2025).
- Banatvala, N.; Bovet, P. Noncommunicable Diseases: A Compendium, 1st ed.; Routledge: London, UK, 2023; Available online: https://www.taylorfrancis.com/books/9781003306689 (accessed on 7 April 2025).
- Lob-Levyt, J. Contribution of the GAVI Alliance to improving health and reducing poverty. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2743–2747. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.S. Adult Immunization Policy in Korea. Infect. Chemother. 2023, 55, 317. [Google Scholar] [CrossRef]
- Richwine, C.J.; Dor, A.; Moghtaderi, A. Do Stricter Immunization Laws Improve Coverage? Evidence from the Repeal of Non-Medical Exemptions for School Mandated Vaccines; Report No.: W25847; National Bureau of Economic Research: Cambridge, MA, USA, 2019; p. w25847. Available online: http://www.nber.org/papers/w25847.pdf (accessed on 7 April 2025).
- Azimi, T.; Heller, J.; Latkovic, T.; Sabow, A. Getting to Work: Employers’ Role in COVID-19 Vaccination; McKinsey & Company: New York, NY, USA, 2021. [Google Scholar]
- Bollyky, T.J.; Templin, T.; Cohen, M.; Dieleman, J.L. Lower-Income Countries That Face The Most Rapid Shift In Noncommunicable Disease Burden Are Also The Least Prepared. Health Aff. 2017, 36, 1866–1875. [Google Scholar] [CrossRef]
- Blaschke, T.F.; Lumpkin, M.; Hartman, D. The World Health Organization Prequalification Program and Clinical Pharmacology in 2030. Clin. Pharmacol. Ther. 2020, 107, 68–71. [Google Scholar] [CrossRef]
- Anderson, T.C.; Masters, N.B.; Guo, A.; Shepersky, L.; Leidner, A.J.; Lee, G.M.; Kotton, C.N.; Dooling, K.L. Use of Recombinant Zoster Vaccine in Immunocompromised Adults Aged ≥19 Years: Recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022, 71, 80–84. [Google Scholar] [CrossRef] [PubMed]
- How Community-Led Solutions Are Strengthening Immunisation in Pakistan. Available online: https://www.unicef.org/media/93781/file/gavi-unicef-digital-technology-immunization-2021.pdf (accessed on 13 August 2025).
- COVAX. Available online: https://www.who.int/initiatives/act-accelerator/covax (accessed on 10 August 2025).
- ASEAN-Joint-Assessment-Procedure-for-Pharmaceutical-Products-Information-for-applicants-22-March-2022.pdf. Available online: https://asean.org/wp-content/uploads/2022/05/ASEAN-Joint-Assessment-Procedure-for-Pharmaceutical-Products-Information-for-applicants-22-March-2022.pdf (accessed on 10 August 2025).
| By DALYs | By Death | |||||||
|---|---|---|---|---|---|---|---|---|
| Rank | China | DALYs % | Global | DALYs % | China | Death % | Global | Death % |
| 1 | Stroke | 13.23% | Ischemic heart disease | 6.55% | Stroke | 22.16% | Ischemic heart disease | 13.25% |
| 2 | Ischemic heart disease | 8.87% | Stroke | 5.57% | Ischemic heart disease | 16.73% | Stroke | 10.68% |
| 3 | COPD | 5.88% | COPD | 2.77% | COPD | 10.99% | COPD | 5.48% |
| 4 | Tracheal, bronchus, and lung cancer | 4.7% | Diabetes mellitus | 2.73% | Tracheal, bronchus, and lung cancer | 6.95% | Tracheal, bronchus, and lung cancer | 2.97% |
| 5 | Age-related and other hearing loss | 3.07% | Low back pain | 2.42% | Alzheimer’s disease and other dementias | 4.22% | Alzheimer’s disease and other dementias | 2.88% |
| 6 | Diabetes mellitus | 2.9% | Depression disorders | 1.95% | Stomach cancer | 3.8% | Diabetes mellitus | 2.44% |
| 7 | Low back pain | 2.8% | Congenital birth defects | 1.82% | Hypertensive heart disease | 2.81% | Chronic kidney disease | 2.25% |
| 8 | Stomach cancer | 2.64% | Headache disorders | 1.65% | Esophageal cancer | 2.53% | Cirrhosis and other chronic liver diseases | 2.1% |
| 9 | Alzheimer’s disease and other dementias | 2.5% | Tracheal, bronchus, and lung cancer | 1.62% | Colon and rectum cancer | 2.35% | Hypertensive heart disease | 1.96% |
| 10 | Depression disorders | 1.95% | Cirrhosis and other chronic liver diseases | 1.61% | Chronic kidney disease | 1.75% | Colon and rectum cancer | 1.54% |
| NCD | Example | Stages in Other Countries | Stages in China |
|---|---|---|---|
| Lung cancer | CimaVax-EGF (Cuba) | Approved for Market 1 | Clinical trial 2 |
| Liver cancer | GPC3 (Japan) | Phase I/Phase II 3 | Phase I/II |
| Gastric cancer | HER-Vaxx (Austria) | Phase Ib/II 4 (Terminated) | Phase Ib/II 4 |
| Pancreatic cancer | CellgramDC-WT1 (Japan) | Phase I/II 5 | NA |
| Colorectal cancer | OncoVAX (United States) | Phase III 6 | NA |
| Kidney cancer | NeoVax (United States) | Phase I 7 | NA |
| Prostate cancer | Sipuleucel-T (United States) | Approved for Market 8 | NA |
| Hypertension | CYT006-AngQb (Switzerland) | Phase IIa 9 (Discontinued) | NA |
| Dyslipidemia | Inclisiran (Switzerland) | Approved for Market 10 | Approved for market 10 |
| Diabetes mellitus | CYT013-IL1bQb (Switzerland) | Phase I/IIa 11 | NA |
| Alzheimer’s disease | CAD106 | Phase II/III 12 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Lyu, X.; Kam, Y.-W. Therapeutic Vaccines for Non-Communicable Diseases: Global Progress and China’s Deployment Pathways. Vaccines 2025, 13, 881. https://doi.org/10.3390/vaccines13080881
Huang Y, Lyu X, Kam Y-W. Therapeutic Vaccines for Non-Communicable Diseases: Global Progress and China’s Deployment Pathways. Vaccines. 2025; 13(8):881. https://doi.org/10.3390/vaccines13080881
Chicago/Turabian StyleHuang, Yifan, Xiaohang Lyu, and Yiu-Wing Kam. 2025. "Therapeutic Vaccines for Non-Communicable Diseases: Global Progress and China’s Deployment Pathways" Vaccines 13, no. 8: 881. https://doi.org/10.3390/vaccines13080881
APA StyleHuang, Y., Lyu, X., & Kam, Y.-W. (2025). Therapeutic Vaccines for Non-Communicable Diseases: Global Progress and China’s Deployment Pathways. Vaccines, 13(8), 881. https://doi.org/10.3390/vaccines13080881








