Strategies to Increase Vaccinations in Adult Cancer Patients: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Selection Process
2.4. Data Collection Process and Data Items
2.5. Study Risk of Bias Assessment
2.6. Synthesis Methods
2.7. Effect Measures, Reporting Bias Assessment and Certainty Assessment
3. Results
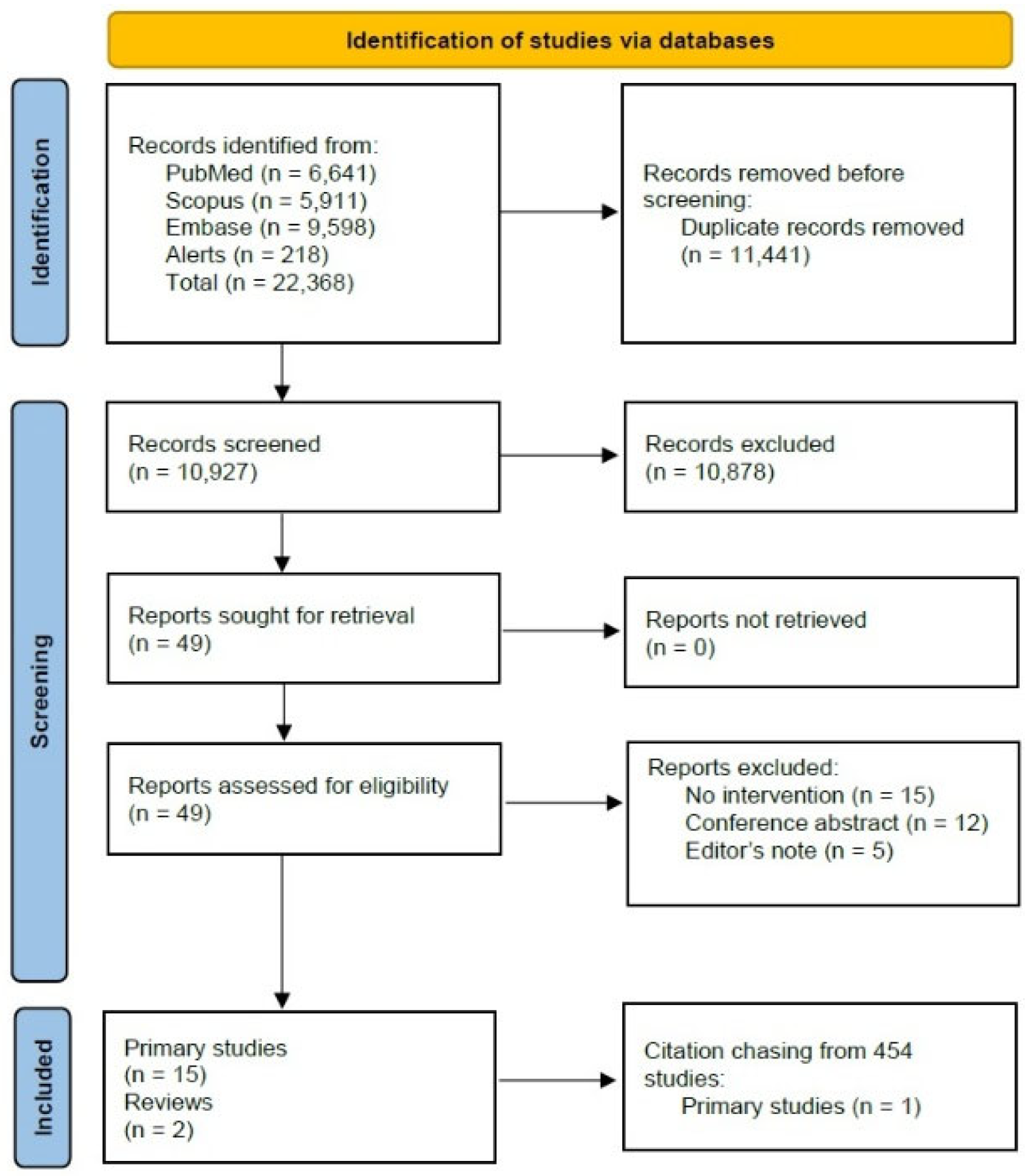
3.1. Study Selection
3.2. Study Characteristics
3.3. Results Synthesis
3.3.1. Educational Materials and Campaigns
3.3.2. Reminders
3.3.3. Patient Counselling
3.4. Risk of Bias in Studies and Certainty of Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APRN | Advanced Practice Nurse Practitioner |
| ASCO | American Society of Clinical Oncology |
| EMR | Electronic Medical Record |
| GP | General Practitioner |
| HCP | Healthcare Professional |
| PICO | Population, Intervention, Comparison, Outcome |
| PRISMA | Preferred Reporting Items for Systematic reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| RCT | Randomized Controlled Trial |
References
- International Agency for Research on Cancer Cancer Today. Absolute Numbers, Incidence, Both Sexes, in 2022. Available online: https://gco.iarc.fr/today/en/dataviz/pie?mode=population&group_populations=0&types=0&populations=900 (accessed on 30 July 2025).
- Okoli, G.N.; Lam, O.L.T.; Abdulwahid, T.; Neilson, C.J.; Mahmud, S.M.; Abou-Setta, A.M. Seasonal Influenza Vaccination among Cancer Patients: A Systematic Review and Meta-Analysis of the Determinants. Curr. Probl. Cancer 2021, 45, 100646. [Google Scholar] [CrossRef] [PubMed]
- Kunisaki, K.M.; Janoff, E.N. Influenza in Immunosuppressed Populations: A Review of Infection Frequency, Morbidity, Mortality, and Vaccine Responses. Lancet Infect. Dis. 2009, 9, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, Y.; Yu, K.; Yang, Y.; Wang, X.; Yang, X.; Qian, J.; Liu, Z.-X.; Wu, B. Fatal Infections Among Cancer Patients: A Population-Based Study in the United States. Infect. Dis. Ther. 2021, 10, 871–895. [Google Scholar] [CrossRef] [PubMed]
- Elhadi, M.; Khaled, A.; Msherghi, A. Infectious Diseases as a Cause of Death among Cancer Patients: A Trend Analysis and Population-Based Study of Outcome in the United States Based on the Surveillance, Epidemiology, and End Results Database. Infect. Agent. Cancer 2021, 16, 72. [Google Scholar] [CrossRef]
- Homsi, J.; Walsh, D.; Panta, R.; Lagman, R.; Nelson, K.A.; Longworth, D.L. Infectious Complications of Advanced Cancer. Support. Care Cancer 2000, 8, 487–492. [Google Scholar] [CrossRef]
- Lopez, A.; Mariette, X.; Bachelez, H.; Belot, A.; Bonnotte, B.; Hachulla, E.; Lahfa, M.; Lortholary, O.; Loulergue, P.; Paul, S.; et al. Vaccination Recommendations for the Adult Immunosuppressed Patient: A Systematic Review and Comprehensive Field Synopsis. J. Autoimmun. 2017, 80, 10–27. [Google Scholar] [CrossRef]
- Kirkwood, M.K.; Aggarwal, C.; Bruinooge, S.S.; Williams, J.H.; Kaltenbaugh, M.; Ramos, N.; Gralow, J.R.; Garrett-Mayer, E. Differential Survival Among Patients With Cancer by COVID-19 Vaccination Status: An Analysis of the ASCO COVID-19 Registry. JCO Oncol. Adv. 2024, 1, e2400034. [Google Scholar] [CrossRef]
- Bersanelli, M.; Verzoni, E.; Cortellini, A.; Giusti, R.; Calvetti, L.; Ermacora, P.; Di Napoli, M.; Catino, A.; Guadalupi, V.; Guaitoli, G.; et al. Impact of Influenza Vaccination on Survival of Patients with Advanced Cancer Receiving Immune Checkpoint Inhibitors (INVIDIa-2): Final Results of the Multicentre, Prospective, Observational Study. eClinicalMedicine 2023, 61, 102044. [Google Scholar] [CrossRef]
- Li, C.; Chen, L.; Lin, H.; Lee, M.; Hung, S.; Lai, C.; Huang, L.; Yu, B.; Hsu, F.; Chiou, W. Impact of 23-valent Pneumococcal Polysaccharide Vaccination on the Frequency of Pneumonia-related Hospitalization and Survival in Elderly Patients with Prostate Cancer: A Seven-year Nationwide Matched Cohort Study. Cancer 2021, 127, 124–136. [Google Scholar] [CrossRef]
- Salem, A.; La, E.M.; Curran, D.; Patterson, B.J.; Carrico, J.; Lorenc, S.; Hicks, K.A.; Poston, S.; Carpenter, C.F. Cost-Effectiveness of Recombinant Zoster Vaccine for the Prevention of Herpes Zoster in Hematopoietic Stem Cell Transplant Recipients and Other Immunocompromised Adults in the United States. Pharmacoecon. Open 2023, 7, 975–985. [Google Scholar] [CrossRef]
- Cho, B.-H.; Stoecker, C.; Link-Gelles, R.; Moore, M.R. Cost-Effectiveness of Administering 13-Valent Pneumococcal Conjugate Vaccine in Addition to 23-Valent Pneumococcal Polysaccharide Vaccine to Adults with Immunocompromising Conditions. Vaccine 2013, 31, 6011–6021. [Google Scholar] [CrossRef] [PubMed]
- Avritscher, E.B.C.; Cooksley, C.D.; Geraci, J.M.; Bekele, B.N.; Cantor, S.B.; Rolston, K.V.; Elting, L.S. Cost-effectiveness of Influenza Vaccination in Working-age Cancer Patients. Cancer 2007, 109, 2357–2364. [Google Scholar] [CrossRef] [PubMed]
- Robin, C.; Beckerich, F.; Cordonnier, C. Immunization in Cancer Patients: Where We Stand. Pharmacol. Res. 2015, 92, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, M.; Bohlke, K.; Baptiste, D.M.; Dunleavy, K.; Fueger, A.; Jones, L.; Kelkar, A.H.; Law, L.Y.; LeFebvre, K.B.; Ljungman, P.; et al. Vaccination of Adults With Cancer: ASCO Guideline. J. Clin. Oncol. 2024, 42, 1699–1721. [Google Scholar] [CrossRef]
- Commission of the European Communities. Council Recommendation on Seasonal Influenza Vaccination; Commission of the European Communities: Brussels, Belgium, 2009. [Google Scholar]
- Amdisen, L.; Pedersen, L.; Abildgaard, N.; Benn, C.S.; Rørth, M.; Cronin-Fenton, D.; Sørup, S. The Coverage of Influenza Vaccination and Predictors of Influenza Non-Vaccination in Danish Cancer Patients: A Nationwide Register-Based Cohort Study. Vaccine 2024, 42, 1690–1697. [Google Scholar] [CrossRef]
- Chang, A.; Ellingson, M.K.; Flowers, C.R.; Bednarczyk, R.A. Influenza Vaccination Rates Among Patients With a History of Cancer: Analysis of the National Health Interview Survey. Open Forum Infect. Dis. 2021, 8, ofab198. [Google Scholar] [CrossRef]
- Doornekamp, L.; van Leeuwen, L.; van Gorp, E.; Voeten, H.; Goeijenbier, M. Determinants of Vaccination Uptake in Risk Populations: A Comprehensive Literature Review. Vaccines 2020, 8, 480. [Google Scholar] [CrossRef]
- Doherty, M.; Schmidt-Ott, R.; Santos, J.I.; Stanberry, L.R.; Hofstetter, A.M.; Rosenthal, S.L.; Cunningham, A.L. Vaccination of Special Populations: Protecting the Vulnerable. Vaccine 2016, 34, 6681–6690. [Google Scholar] [CrossRef]
- Chun, J.Y.; Kim, S.I.; Park, E.Y.; Park, S.-Y.; Koh, S.-J.; Cha, Y.; Yoo, H.J.; Joung, J.Y.; Yoon, H.M.; Eom, B.W.; et al. Cancer Patients’ Willingness to Take COVID-19 Vaccination: A Nationwide Multicenter Survey in Korea. Cancers 2021, 13, 3883. [Google Scholar] [CrossRef]
- Malik, A.A.; Ahmed, N.; Shafiq, M.; Elharake, J.A.; James, E.; Nyhan, K.; Paintsil, E.; Melchinger, H.C.; Team, Y.B.I.; Malik, F.A.; et al. Behavioral Interventions for Vaccination Uptake: A Systematic Review and Meta-Analysis. Health Policy 2023, 137, 104894. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Calabrese, S.C.; Lo Moro, G.; Basile, A.; Golzio, F.; Scaioli, G.; Bert, F.; Siliquini, R. Strategies to Improve Vaccine Adherence in Adult Oncology Patients: A Systematic Review. In Proceedings of the Atti 57° Congresso Nazionale Società Italiana Igiene, Medicina Preventiva e Sanità Pubblica (SItI), Palermo, Italy, 23–26 October 2024; p. E490. [Google Scholar]
- Lefebvre, C.; Glanville, J.; Briscoe, S.; Featherstone, R.; Littlewood, A.; Metzendorf, M.-I.; Noel-Storr, A.; Paynter, R.; Rader, T.; Thomas, J.; et al. Chapter 4: Searching for and Selecting Studies. In Cochrane Handbook for Systematic Reviews of Interventions version 6.5.1 Cochrane; Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., Welch, V., Eds.; John Wiley & Sons: Chichester, UK, 2019; Available online: https://www.cochrane.org/authors/handbooks-and-manuals/handbook/current/chapter-04#section-4-7 (accessed on 3 September 2025).
- Gusenbauer, M.; Haddaway, N.R. Which Academic Search Systems Are Suitable for Systematic Reviews or Meta-analyses? Evaluating Retrieval Qualities of Google Scholar, PubMed, and 26 Other Resources. Res. Synth. Methods 2020, 11, 181–217. [Google Scholar] [CrossRef] [PubMed]
- Pranckutė, R. Web of Science (WoS) and Scopus: The Titans of Bibliographic Information in Today’s Academic World. Publications 2021, 9, 12. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Barker, T.H.; Habibi, N.; Aromataris, E.; Stone, J.C.; Leonardi-Bee, J.; Sears, K.; Hasanoff, S.; Klugar, M.; Tufanaru, C.; Moola, S.; et al. The Revised JBI Critical Appraisal Tool for the Assessment of Risk of Bias for Quasi-Experimental Studies. JBI Evid. Synth. 2024, 22, 378–388. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; deBeer, H. GRADE Guidelines: 1. Introduction—GRADE Evidence Profiles and Summary of Findings Tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Butow, P.; Shaw, J.; Bartley, N.; Milch, V.; Sathiaraj, R.; Turnbull, S.; Der Vartanian, C. Vaccine Hesitancy in Cancer Patients: A Rapid Review. Patient Educ. Couns. 2023, 111, 107680. [Google Scholar] [CrossRef]
- Vanderpool, R.C.; Gaysynsky, A.; Chou, W.-Y.S.; Tonorezos, E.S. Using Behavioral Science to Address COVID-19 Vaccine Hesitancy Among Cancer Survivors: Communication Strategies and Research Opportunities. J. Behav. Med. 2023, 46, 366–376. [Google Scholar] [CrossRef]
- Kiderlen, T.R.; Trostdorf, K.; Delmastro, N.; Salomon, A.; Scholz, C.W.; Späth-Schwalbe, E.; Mansmann, V.; Roll, S.; Reinwald, M.; de Wit, M. Controlled Non-randomised before–after Study Evaluating the Impact of a Focused Recommendation Card on Vaccination Rates of Oncological Patients—The Easy Vaccination in Oncology (EVO) Strategy. Eur. J. Cancer Care 2022, 31, e13725. [Google Scholar] [CrossRef]
- Kiderlen, T.R.; Trostdorf, K.; Delmastro, N.; Salomon, A.; Scholz, C.W.; Späth-Schwalbe, E.; Mansmann, V.; Roll, S.; Reinwald, M.; de Wit, M. Raising Immunization Rates among Cancer Patients. Dtsch. Arztebl. Int. 2022, 119, 466–467. [Google Scholar] [CrossRef]
- Rivière, P.; Penel, N.; Faure, K.; Marie, G.; Najem, A.; Rivière, M.-K.; Panaget, S. Effect of Medical Staff Training on Vaccination Coverage in Outpatients with Cancer: An Interventional Multicenter before-and-after Study. Vaccine X 2023, 13, 100261. [Google Scholar] [CrossRef] [PubMed]
- Sitte, J.; Frentiu, E.; Baumann, C.; Rousseau, H.; May, T.; Bronowicki, J.; Peyrin-Biroulet, L.; Lopez, A. Vaccination for Influenza and Pneumococcus in Patients with Gastrointestinal Cancer or Inflammatory Bowel Disease: A Prospective Cohort Study of Methods for Improving Coverage. Aliment. Pharmacol. Ther. 2019, 49, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Toleman, M.S.; Herbert, K.; McCarthy, N.; Church, D.N. Vaccination of Chemotherapy Patients—Effect of Guideline Implementation. Support. Care Cancer 2016, 24, 2317–2321. [Google Scholar] [CrossRef]
- Narinx, J.; Houbiers, M.; Seidel, L.; Beguin, Y. Adherence to Sars-CoV2 Vaccination in Hematological Patients. Front. Immunol. 2022, 13, 994311. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.-T.; Sidorkiewicz, S.; Péan, C.; Ravaud, P. Impact of an Interactive Web Tool on Patients’ Intention to Receive COVID-19 Vaccination: A before-and-after Impact Study among Patients with Chronic Conditions in France. BMC Med. Inf. Decis. Mak. 2021, 21, 228. [Google Scholar] [CrossRef]
- Kelkar, A.H.; Blake, J.A.; Cherabuddi, K.; Cornett, H.; McKee, B.L.; Cogle, C.R. Vaccine Enthusiasm and Hesitancy in Cancer Patients and the Impact of a Webinar. Healthcare 2021, 9, 351. [Google Scholar] [CrossRef]
- McGinnis, J.M.; Jones, R.; Hillis, C.; Kokus, H.; Thomas, H.; Thomas, J.; Alyafi, M.; Bernard, L.; Eiriksson, L.R.; Elit, L.M.; et al. A Pneumococcal Pneumonia and Influenza Vaccination Quality Improvement Program for Women Receiving Chemotherapy for Gynecologic Cancers at a Major Tertiary Cancer Centre. Gynecol. Oncol. 2021, 161, 236–243. [Google Scholar] [CrossRef]
- Church, E.C.; Banks, R.; Wilson, B.; Arfons, L.; Perez, F.; Jump, R. Improving Pneumococcal Vaccine Uptake in Veterans with Chronic Lymphocytic Leukemia through a Virtual Clinic. Curr. Oncol. 2018, 25, 95–98. [Google Scholar] [CrossRef]
- Delacruz, W.; Terrazzino, S.; Osswald, M.; Payne, C.; Haney, B. Implementing a Multidisciplinary Approach to Enhance Compliance With Guideline-Recommended Prechemotherapy Pneumococcal Vaccination in a Military-Based Medical Oncology Practice. J. Oncol. Pr. 2017, 13, e966–e971. [Google Scholar] [CrossRef]
- Grivas, P.D.; Devata, S.; Khoriaty, R.; Boonstra, P.S.; Ruch, J.; McDonnell, K.; Hernandez-Aya, L.; Wilfong, J.; Smerage, J.; Ison, M.G.; et al. Low-Cost Intervention to Increase Influenza Vaccination Rate at a Comprehensive Cancer Center. J. Cancer Educ. 2017, 32, 871–877. [Google Scholar] [CrossRef]
- Nipp, R.D.; Ruddy, M.; Fuh, C.-X.; Zangardi, M.L.; Chio, C.; Kim, E.B.; Li, B.K.M.; Long, Y.; Blouin, G.C.; Lage, D.; et al. Pilot Randomized Trial of a Pharmacy Intervention for Older Adults with Cancer. Oncologist 2019, 24, 211–218. [Google Scholar] [CrossRef]
- Ganju, P.; Kalaiyarasi, J.P.; Karunakaran, P.; Veeraiah, S.; Mehra, N. COVID-19 Vaccine Uptake in Patients with Multiple Myeloma and AL Amyloidosis: A Cross-Sectional Observational Study from India. Indian J. Hematol. Blood Transfus. 2024, 40, 30–35. [Google Scholar] [CrossRef]
- Shapiro Ben David, S.; Shamai-Lubovitz, O.; Mourad, V.; Goren, I.; Cohen Iunger, E.; Alcalay, T.; Irony, A.; Greenfeld, S.; Adler, L.; Cahan, A. A Nationwide Digital Multidisciplinary Intervention Aimed at Promoting Pneumococcal Vaccination in Immunocompromised Patients. Vaccines 2023, 11, 1355. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, N.; Aktas, B.Y.; Gulmez, A.; Inkaya, A.C.; Bayraktar-Ekincioglu, A.; Kilickap, S.; Unal, S. Impact of Pharmacist-Led Educational Intervention on Pneumococcal Vaccination Rates in Cancer Patients: A Randomized Controlled Study. Support. Care Cancer 2023, 31, 194. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Regional Office for the Eastern Mediterranean. Health Education: Theoretical Concepts, Effective Strategies and Core Competencies: A Foundation Document to Guide Capacity Development of Health Educators. 2012. Available online: http://applications.emro.who.int/dsaf/EMRPUB_2012_EN_1362.pdf (accessed on 3 September 2025).
- Cataldi, J.R.; Kerns, M.E.; O’Leary, S.T. Evidence-Based Strategies to Increase Vaccination Uptake: A Review. Curr. Opin. Pediatr. 2020, 32, 151–159. [Google Scholar] [CrossRef]
- Du, P.; Jin, S.; Lu, S.; Wang, L.; Ma, X.; Wang, J.; Huang, R.; Luo, Q.; Yang, S.; Feng, X. Strategies to Increase the Coverage of Influenza and Pneumonia Vaccination in Older Adults: A Systematic Review and Network Meta-Analysis. Age Ageing 2024, 53, afae035. [Google Scholar] [CrossRef] [PubMed]
- Bisset, K.A.; Paterson, P. Strategies for Increasing Uptake of Vaccination in Pregnancy in High-Income Countries: A Systematic Review. Vaccine 2018, 36, 2751–2759. [Google Scholar] [CrossRef]
- Morgan, J.L.; Baggari, S.R.; Chung, W.; Ritch, J.; McIntire, D.D.; Sheffield, J.S. Association of a Best-Practice Alert and Prenatal Administration With Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis Vaccination Rates. Obstet. Gynecol. 2015, 126, 333–337. [Google Scholar] [CrossRef]
- Ruffin, M.T.; Plegue, M.A.; Rockwell, P.G.; Young, A.P.; Patel, D.A.; Yeazel, M.W. Impact of an Electronic Health Record (EHR) Reminder on Human Papillomavirus (HPV) Vaccine Initiation and Timely Completion. J. Am. Board Fam. Med. 2015, 28, 324–333. [Google Scholar] [CrossRef]
- Monier, A.; Puyade, M.; Hernanz, M.P.G.; Bouchaert, P.; Leleu, X.; Tourani, J.M.; Roblot, F.; Rammaert, B. Observational Study of Vaccination in Cancer Patients: How Can. Vaccine Coverage Be Improved? Med. Mal. Infect. 2020, 50, 263–268. [Google Scholar] [CrossRef]
- Ogliastro, M.; Ferrari, A.; Sticchi, L.; Domnich, A.; Zappa, G.; Di Biagio, A.; Massaro, E.; Giribaldi, E.; Orsi, A. Effectiveness of a Counseling Intervention to Increase Vaccination Uptake among Men Who Have Sex with Men during the Mpox Outbreak. Vaccines 2024, 12, 751. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.L.; Biddell, C.B.; Rhodes, B.E.; Brewer, N.T. Provider Communication and HPV Vaccine Uptake: A Meta-Analysis and Systematic Review. Prev. Med. 2021, 148, 106554. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaikh, A.; Mahmoud, R.I.; Boukerdenna, H.; Muthu, N.; Aidyralieva, C.; Bellizzi, S. Counselling of Non-Communicable Diseases’ Patients for COVID-19 Vaccine Uptake in Jordan: Evaluating the Intervention. Vaccine 2022, 40, 6658–6663. [Google Scholar] [CrossRef] [PubMed]
- Lasagna, A.; Alessio, N.; Gambini, G.; Klersy, C.; Monaco, T.; Corallo, S.; Cicognini, D.; Pedrazzoli, P. Vaccine Hesitancy in Patients with Solid Tumors: A Cross-Sectional Single-Center Survey. BMC Public. Health 2024, 24, 2998. [Google Scholar] [CrossRef]
- Johnstone, K.; Cooper, J.; Smithson, J.; Glass, B. Perspectives of Healthcare Professionals on the Pharmacist’s Role in Delivering Vaccinations for Patients with Cancer: A Qualitative Study Using Role Theory. Int. J. Clin. Pharm. 2025. [Google Scholar] [CrossRef]
- Le, L.M.; Veettil, S.K.; Donaldson, D.; Kategeaw, W.; Hutubessy, R.; Lambach, P.; Chaiyakunapruk, N. The Impact of Pharmacist Involvement on Immunization Uptake and Other Outcomes: An Updated Systematic Review and Meta-Analysis. J. Am. Pharm. Assoc. 2022, 62, 1499–1513.e16. [Google Scholar] [CrossRef]
- Isenor, J.E.; Edwards, N.T.; Alia, T.A.; Slayter, K.L.; MacDougall, D.M.; McNeil, S.A.; Bowles, S.K. Impact of Pharmacists as Immunizers on Vaccination Rates: A Systematic Review and Meta-Analysis. Vaccine 2016, 34, 5708–5723. [Google Scholar] [CrossRef]
- Lo Moro, G.; Ferrara, M.; Langiano, E.; Accortanzo, D.; Cappelletti, T.; De Angelis, A.; Esposito, M.; Prinzivalli, A.; Sannella, A.; Sbaragli, S.; et al. Countering Vaccine Hesitancy: A Systematic Review of Interventions to Strengthen Healthcare Professionals’ Action. Eur. J. Public Health 2023, 33, 905–915. [Google Scholar] [CrossRef]
- Rand, C.M.; Humiston, S.G. Provider Focused Interventions to Improve Child and Adolescent Vaccination Rates. Acad. Pediatr. 2021, 21, S34–S39. [Google Scholar] [CrossRef]
- Aguolu, O.G.; Malik, A.A.; Ahmed, N.; Omer, S.B. Overcoming Vaccine Hesitancy for Future COVID-19 and HIV Vaccines: Lessons from Measles and HPV Vaccines. Curr. HIV/AIDS Rep. 2022, 19, 328–343. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
| First Author Publication Date | Country | Year of Study | Study Design | Target of the Intervention | Type of Cancer | Target VPD | Multi-Component Intervention |
|---|---|---|---|---|---|---|---|
| Toleman M.S. 2015 [38] | UK | 2012−2014 | Quasi-Experimental, Pre-post evaluation | HCP | Any | Flu, Pneum. | Yes |
| Grivas P.D. 2016 [45] | USA | 2011−2013 | Natural Experimental, Pre-post evaluation | HCP + Patients | Any | Flu | Yes |
| Delacruz W. 2017 [44] | USA | 2015−2016 | Quasi-Experimental, Pre-post evaluation | HCP | Any | Pneum. | Yes |
| Church E.C. 2018 [43] | USA | 2015 | Quasi-Experimental, Pre-post evaluation | HCP + Patients | Chronic Lymphocytic Leukemia | Pneum. | Yes |
| Nipp R.D. 2018 [46] | USA | 2017 | RCT | Patients | Breast, gastrointestinal, or lung cancer. | Flu, Pneum. | No |
| Sitte J. 2018 [37] | France | 2016−2017 | Quasi-Experimental, Pre-post evaluation | Patients | Gastrointestinal | Flu, Pneum. (Diphtheria-Tetanus-Poliomyelitis, Hepatitis b, HPV) | No |
| Kelkar A.H. 2021 [41] | USA | 2020−2021 | Quasi-Experimental, Pre-post evaluation | HCP + Patients | Any | COVID-19 | No |
| McGinnis J.M. 2021 [42] | Canada | 2019−2020 | Quasi-Experimental, Interrupted time series | HCP + Patients | Gynecologic | Flu, Pneum. | Yes |
| Tran V. 2021 [40] | France | 2021 | Quasi-Experimental, Pre-post evaluation | Patients | Any | COVID-19 | No |
| Kiderlen T.R. 2022 [34] | Germany | 2020−2021 | Quasi-Experimental, Pre-post evaluation | HCP | Any | Flu, Pneum., Herpes Zoster and Hepatitis B | No |
| Narinx J. 2022 [39] | Belgium | 2021 | Quasi-Experimental, Post evaluation | Patients | Hematologic | COVID-19 | Yes |
| Ganju P. 2023 [47] | India | 2021−2022 | Quasi-Experimental, Post evaluation | Patients | Multiple myeloma | COVID-19 | No |
| Ozdemir N. 2023 [49] | Turkey | 2019−2021 | RCT | Patients | Any | Pneum. | Yes |
| Rivière P. 2023 [36] | France | 2019−2020 | Quasi-Experimental, Pre-post evaluation | HCP | Any | Flu, Pneum. | Yes |
| Shapiro Ben David S. 2023 [48] | Israel | 2019−2021 | Quasi-Experimental, Pre-post evaluation | HCP + Patients | Any | Pneum. | Yes |
| First Author Publication Date | Patient Education | Patient Reminders | Patient Counselling | HCP Education | HCP Reminders | Other |
|---|---|---|---|---|---|---|
| Toleman M.S. 2015 [38] | X | Development of vaccination guidelines * Letter for primary care providers | ||||
| Grivas P.D. 2016 [45] | X | X * | X * | |||
| Delacruz W. 2017 [44] | X * | Recruitment of APRN to review vaccination status and prescribe vaccine if not done by oncologist | ||||
| Church E.C. 2018 [43] | X * | X * | Pact between virtual clinic and primary care providers | |||
| Nipp R.D. 2018 [46] | X * | |||||
| Sitte J. 2018 [37] | X * | |||||
| Kelkar A.H. 2021 [41] | X * | X * | ||||
| McGinnis J.M. 2021 [42] | X * | X * | In-house vaccination program; pre-printed prescriptions for prescribing physicians | |||
| Tran V. 2021 [40] | X * | |||||
| Kiderlen T.R. 2022 [34] | X * | |||||
| Narinx J. 2022 [39] | X * | X | ||||
| Ganju P. 2023 [47] | X * | |||||
| Ozdemir N. 2023 [49] | X | X * | ||||
| Rivière P. 2023 [36] | X * | Development of a vaccination protocol | ||||
| Shapiro Ben David S. 2023 [48] | X | X * | Preapproval for PCV13 is waived |
| First Author Publication Date | Primary Outcomes | Main Results |
|---|---|---|
| Toleman M.S. 2015 [38] | (1) Vaccination coverage: Flu (2) Vaccination coverage: Pneum. | (1) Flu: from 68.1% to 71.6% (p = 0.730) (2) Pneum. VC.: from 25% to 47% (p = 0.002) |
| Grivas P.D. 2016 [45] | Same day vaccination rate (Flu) (2 seasons) | Absolute increase in eligible adults vaccinated: 2.4% (p < 0.001) and 3.7% (p < 0.001) |
| Delacruz W. 2017 [44] | Vaccination coverage (Pneum.) | From 6.3% to 45.5% (p < 0.001) |
| Church E.C. 2018 [43] | Vaccination coverage (Pneum.) | Within 180 days, 62% received the vaccine. Significant difference in time to vaccination between pre and post-virtual clinic periods (log-rank test, p < 0.01). |
| Nipp R.D. 2018 [46] | (1) Vaccination coverage: Flu (2) Vaccination coverage: Pneum. | (1) Flu: intervention vs. control: 31.0% vs. 0.0% (p < 0.001) (2) Pneum.: intervention vs. control: 37.9% vs. 0.0% (p < 0.001) |
| Sitte J. 2018 [37] | Vaccination coverage (Pneum.) | From 10.1% to 87.5% (<0.001) |
| Kelkar A.H. 2021 [41] | (1) COVID-19 Vaccine intention (2) Changes in Beliefs and Perspectives on Vaccines against COVID-19 | (1) From 71% to 82.5% (no p-value) (2) Increased agreeance with belief statements about vaccine effectiveness; vaccine safety; vaccine acceptance if recommended by a doctor; extra effort to receive a vaccine; and encouraging their family, friends, co-workers, and community to receive a vaccine. (no descriptive data) (p < 0.05) |
| McGinnis J.M. 2021 [42] | (1) Vaccination coverage: Flu (2) Vaccination coverage: Pneum. | (1) Flu: from 36% to a monthly mean of 67% (no p-value) (2) Pneum. VC.: from 5% to a monthly mean of 61% (no p-value) |
| Tran V. 2021 [40] | COVID-19 Vaccine intention | Among the cancer patients who did not intend to be vaccinated at baseline, 18.0% changed their mind (p-value) |
| Kiderlen T.R. 2022 [34] | Vaccination coverage (Pneum.) | Intervention vs. control: VC increase +21.5pp vs. −5.8pp (OR 4.94, 95% CI 1.76–13.83, p = 0.002) |
| Narinx J. 2022 [39] | Vaccination coverage (COVID-19) | Vaccination rates: 88.9% among patients of the intervention group; 76.3% in the general population (control) (Standardized Incidence ratio: 1.17; 95%CI 1.12−1.22, p < 0.001) |
| Ganju P. 2023 [47] | Vaccination coverage (COVID-19) | At least one dose: 86% (2 doses: 67%) (no comparisons, no p-value) |
| Ozdemir N. 2023 [49] | (1) Pneum. vaccination knowledge (higher score = higher number of correct answers) (2) Vaccination Attitudes Examination (VAX) score (higher score = higher negative attitude) (3) Vaccination coverage (Pneum.) | (1) Intervention vs. control: median 10 (IQR = 3) vs. 8 (IQR = 4) (p < 0.001) (2) Intervention vs. control: mean 33.09 (SD = 7) vs. 36.07 (SD = 6.5) (p = 0.007) (3) Intervention vs. control: 20.2% vs. 6.1% (p = 0.003) |
| Rivière P. 2023 [36] | (1) Vaccination coverage: Flu (2) Vaccination coverage: Pneum. | (1) Flu VC: from 42.6% to 55.1% (p = 0.08) (2) Pneum. VC: from 11.8% to 15.4%, (p =1) |
| Shapiro Ben David S. 2023 [48] | Vaccination coverage (Pneum.) | PCV13: from 11.9% to 52% (p < 0.001) PPSV23: from 39.4% to 57.1% (p < 0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Moro, G.; Golzio, F.; Calabrese, S.C.; Scaioli, G.; Basile, A.; Siliquini, R.; Bert, F. Strategies to Increase Vaccinations in Adult Cancer Patients: A Systematic Review. Vaccines 2025, 13, 964. https://doi.org/10.3390/vaccines13090964
Lo Moro G, Golzio F, Calabrese SC, Scaioli G, Basile A, Siliquini R, Bert F. Strategies to Increase Vaccinations in Adult Cancer Patients: A Systematic Review. Vaccines. 2025; 13(9):964. https://doi.org/10.3390/vaccines13090964
Chicago/Turabian StyleLo Moro, Giuseppina, Federica Golzio, Sara Claudia Calabrese, Giacomo Scaioli, Alessandro Basile, Roberta Siliquini, and Fabrizio Bert. 2025. "Strategies to Increase Vaccinations in Adult Cancer Patients: A Systematic Review" Vaccines 13, no. 9: 964. https://doi.org/10.3390/vaccines13090964
APA StyleLo Moro, G., Golzio, F., Calabrese, S. C., Scaioli, G., Basile, A., Siliquini, R., & Bert, F. (2025). Strategies to Increase Vaccinations in Adult Cancer Patients: A Systematic Review. Vaccines, 13(9), 964. https://doi.org/10.3390/vaccines13090964







