Freeze-Drying of mRNA-LNPs Vaccines: A Review
Abstract
1. Introduction
2. Stability of mRNA Vaccines
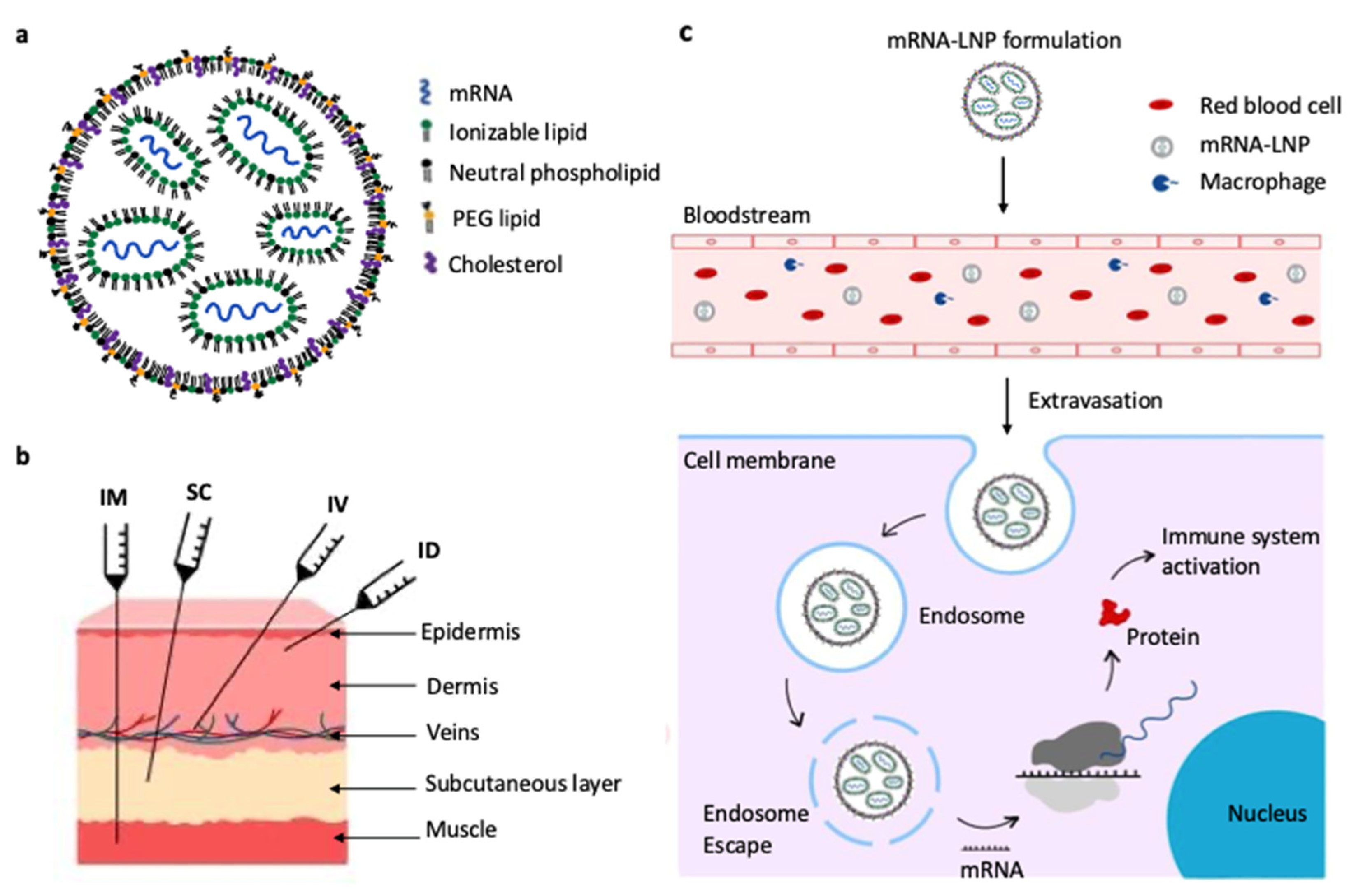
2.1. Structure and Delivery Mechanism of mRNA Vaccines
2.2. Stabilizing mRNA Vaccines Through Freeze-Drying
2.3. Challenges During Freeze-Drying of mRNA Vaccines
3. Formulations
3.1. Influence of Lipid Composition
3.2. Stabilizers
3.2.1. Sugars
3.2.2. Sugar Alcohols
3.2.3. Amino Acids
3.3. Influence of pH and Buffer
3.4. Impact of Reconstitution Buffer
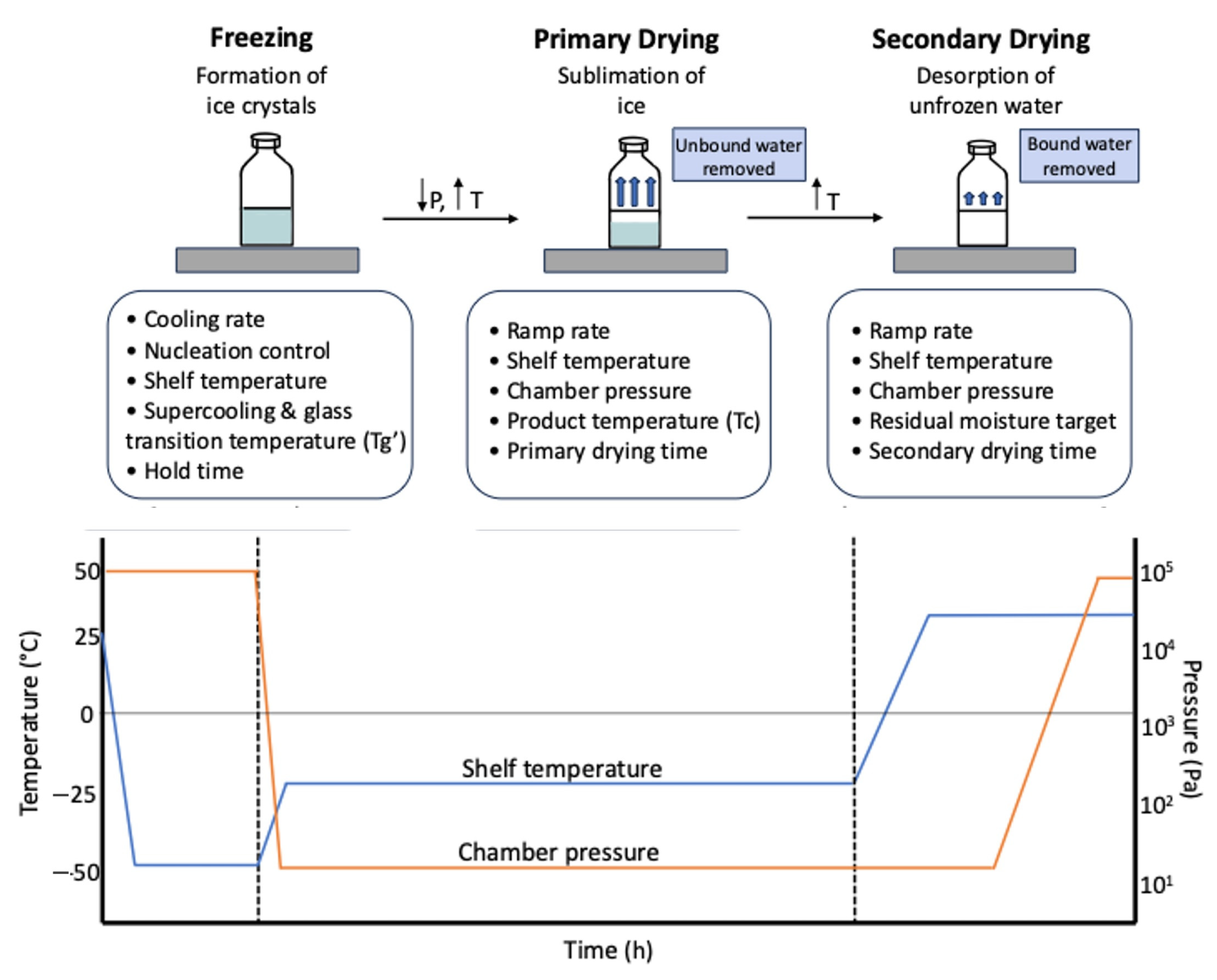
4. Lyophilization Process Development and Intensification
5. Critical Process Parameters (CPPs) and Critical Quality Attributes (CQAs)
5.1. Critical Process Parameters (CPPs)
5.2. Critical Quality Attributes (CQAs)
6. Analytics for the Freeze-Drying Study
7. Conclusions
8. Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, N.-N.; Li, X.-F.; Deng, Y.-Q.; Zhao, H.; Huang, Y.-J.; Yang, G.; Huang, W.-J.; Gao, P.; Zhou, C.; Zhang, R.-R.; et al. A Thermostable mRNA Vaccine against COVID-19. Cell 2020, 182, 1271–1283.e16. [Google Scholar] [CrossRef]
- Chivukula, S.; Plitnik, T.; Tibbitts, T.; Karve, S.; Dias, A.; Zhang, D.; Goldman, R.; Gopani, H.; Khanmohammed, A.; Sarode, A.; et al. Development of multivalent mRNA vaccine candidates for seasonal or pandemic influenza. NPJ Vaccines 2021, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Sittplangkoon, C.; Alameh, M.-G.; Weissman, D.; Lin, P.J.C.; Tam, Y.K.; Prompetchara, E.; Palaga, T. mRNA vaccine with unmodified uridine induces robust type I interferon-dependent anti-tumor immunity in a melanoma model. Front. Immunol. 2022, 13, 983000. [Google Scholar] [CrossRef] [PubMed]
- Qiu, K.; Duan, X.; Mao, M.; Song, Y.; Rao, Y.; Cheng, D.; Feng, L.; Shao, X.; Jiang, C.; Huang, H.; et al. mRNA-LNP vaccination-based immunotherapy augments CD8+ T cell responses against HPV-positive oropharyngeal cancer. NPJ Vaccines 2023, 8, 144. [Google Scholar] [CrossRef]
- Krienke, C.; Kolb, L.; Diken, E.; Streuber, M.; Kirchhoff, S.; Bukur, T.; Akilli-Öztürk, Ö.; Kranz, L.M.; Berger, H.; Petschenka, J.; et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science 2021, 371, 145–153. [Google Scholar] [CrossRef]
- Rizvi, F.; Everton, E.; Smith, A.R.; Liu, H.; Osota, E.; Beattie, M.; Tam, Y.; Pardi, N.; Weissman, D.; Gouon-Evans, V. Murine. Murine liver repair via transient activation of regenerative pathways in hepatocytes using lipid nanoparticle-complexed nucleoside-modified mRNA. Nat. Commun. 2021, 12, 613. [Google Scholar] [CrossRef]
- Perez-Garcia, C.G.; Diaz-Trelles, R.; Vega, J.B.; Bao, Y.; Sablad, M.; Limphong, P.; Chikamatsu, S.; Yu, H.; Taylor, W.; Karmali, P.P.; et al. Development of an mRNA replacement therapy for phenylketonuria. Mol. Ther. Nucleic Acids 2022, 28, 87–98. [Google Scholar] [CrossRef]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef]
- Dey, S.K.; Islam, R.; Islam, T.; Islam, S.; Hasan, N. Molecular Epidemiology of Influenza in Asia. East. J. Med. 2015, 19, 119–125. Available online: https://dergipark.org.tr/en/pub/ejm/issue/5369/72760 (accessed on 24 July 2025).
- Kobayashi, T.; Yamanaka, R.; Homma, J.; Tsuchiya, N.; Yajima, N.; Yoshida, S.; Tanaka, R. Tumor mRNA-loaded dendritic cells elicit tumor-specific CD8+ cytotoxic T cells in patients with malignant glioma. Cancer Immunol. Immunother. 2003, 52, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, M.; Schröder, A.; Scheel, B.; Hong, H.S.; Muth, A.; von Boehmer, L.; Zippelius, A.; Mayer, F.; Reck, M.; Atanackovic, D.; et al. A phase I/IIa study of the mRNA-based cancer immunotherapy CV9201 in patients with stage IIIB/IV non-small cell lung cancer. Cancer Immunol. Immunother. 2019, 68, 799–812. [Google Scholar] [CrossRef]
- Hernando, J.J.; Park, T.-W.; Fischer, H.-P.; Zivanovic, O.; Braun, M.; Pölcher, M.; Grünn, U.; Leutner, C.; Pötzsch, B.; Kuhn, W. Vaccination with dendritic cells transfected with mRNA-encoded folate-receptor-α for relapsed metastatic ovarian cancer. Lancet Oncol. 2007, 8, 451–454. [Google Scholar] [CrossRef]
- Xu, Z.; Xiao, Z.-X.; Wang, J.; Qiu, H.-W.; Cao, F.; Zhang, S.-Q.; Xu, Y.-D.; Lei, H.-Q.; Xia, H.; He, Y.-R.; et al. Novel mRNA adjuvant ImmunER enhances prostate cancer tumor-associated antigen mRNA therapy via augmenting T cell activity. Oncoimmunology 2024, 13, 2373526. [Google Scholar] [CrossRef]
- Van Tendeloo, V.F.; Van de Velde, A.; Van Driessche, A.; Cools, N.; Anguille, S.; Ladell, K.; Gostick, E.; Vermeulen, K.; Pieters, K.; Nijs, G.; et al. Induction of complete and molecular remissions in acute myeloid leukemia by Wilms tumor 1 antigen-targeted dendritic cell vaccination. Proc. Natl. Acad. Sci. USA 2010, 107, 13824–13829. [Google Scholar] [CrossRef]
- Golubovskaya, V.; Sienkiewicz, J.; Sun, J.; Huang, Y.; Hu, L.; Zhou, H.; Harto, H.; Xu, S.; Berahovich, R.; Bodmer, W.; et al. mRNA-Lipid Nanoparticle (LNP) Delivery of Humanized EpCAM-CD3 Bispecific Antibody Significantly Blocks Colorectal Cancer Tumor Growth. Cancers 2023, 15, 2860. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hashiba, K.; Taguchi, M.; Sakamoto, S.; Otsu, A.; Maeda, Y.; Ebe, H.; Okazaki, A.; Harashima, H.; Sato, Y. Overcoming thermostability challenges in mRNA–lipid nanoparticle systems with piperidine-based ionizable lipids. Commun. Biol. 2024, 7, 556. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Roni, M.A. Challenges of Storage and Stability of mRNA-Based COVID-19 Vaccines. Vaccines 2021, 9, 1033. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.; Hitti, C.; Fulber, J.P.C.; Khan, M.F.H.; Perumal, A.S.; Kamen, A.A. Preliminary Evaluation of Formulations for Stability of mRNA-LNPs Through Freeze-Thaw Stresses and Long-Term Storage. Biol. Life Sci. 2025. [Google Scholar] [CrossRef]
- Young, R.E.; Hofbauer, S.I.; Riley, R.S. Overcoming the challenge of long-term storage of mRNA-lipid nanoparticle vaccines. Mol. Ther. 2022, 30, 1792–1793. [Google Scholar] [CrossRef]
- Hansen, L.J.J.; Daoussi, R.; Vervaet, C.; Remon, J.-P.; De Beer, T.R.M. Freeze-drying of live virus vaccines: A review. Vaccine 2015, 33, 5507–5519. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Res. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Ai, L.; Li, Y.; Zhou, L.; Yao, W.; Zhang, H.; Hu, Z.; Han, J.; Wang, W.; Wu, J.; Xu, P.; et al. Lyophilized mRNA-lipid nanoparticle vaccines with long-term stability and high antigenicity against SARS-CoV-2. Cell Discov. 2023, 9, 9. [Google Scholar] [CrossRef]
- Swetha, K.; Kotla, N.G.; Tunki, L.; Jayaraj, A.; Bhargava, S.K.; Hu, H.; Bonam, S.R.; Kurapati, R. Recent Advances in the Lipid Nanoparticle-Mediated Delivery of mRNA Vaccines. Vaccines 2023, 11, 658. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Granot, Y.; Peer, D. Delivering the right message: Challenges and opportunities in lipid nanoparticles-mediated modified mRNA therapeutics—An innate immune system standpoint. Semin. Immunol. 2017, 34, 68–77. [Google Scholar] [CrossRef]
- AboulFotouh, K.; Southard, B.; Dao, H.M.; Xu, H.; Moon, C.; Iii, R.O.W.; Cui, Z. Effect of lipid composition on RNA-Lipid nanoparticle properties and their sensitivity to thin-film freezing and drying. Int. J. Pharm. 2024, 650, 123688. [Google Scholar] [CrossRef]
- Cheng, X.; Lee, R.J. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv. Drug Deliv. Rev. 2016, 99, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Eygeris, Y.; Gupta, M.; Sahay, G. Self-assembled mRNA vaccines. Adv. Drug Deliv. Rev. 2021, 170, 83–112. [Google Scholar] [CrossRef] [PubMed]
- Hajj, K.A.; Whitehead, K.A. Tools for translation: Non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2017, 2, 17056. [Google Scholar] [CrossRef]
- Wang, T.; Yu, T.; Li, W.; Liu, Q.; Sung, T.-C.; Higuchi, A. Design and lyophilization of mRNA-encapsulating lipid nanoparticles. Int. J. Pharm. 2024, 662, 124514. [Google Scholar] [CrossRef]
- Zhao, P.; Hou, X.; Yan, J.; Du, S.; Xue, Y.; Li, W.; Xiang, G.; Dong, Y. Long-term storage of lipid-like nanoparticles for mRNA delivery. Bioact. Mater. 2020, 5, 358–363. [Google Scholar] [CrossRef]
- Wang, J.; Ding, Y.; Chong, K.; Cui, M.; Cao, Z.; Tang, C.; Tian, Z.; Hu, Y.; Zhao, Y.; Jiang, S. Recent Advances in Lipid Nanoparticles and Their Safety Concerns for mRNA Delivery. Vaccines 2024, 12, 1148. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, M.; Nawaz, M.; Papadimitriou, A.; Angerfors, A.; Camponeschi, A.; Na, M.; Hölttä, M.; Skantze, P.; Johansson, S.; Sundqvist, M.; et al. Linkage between endosomal escape of LNP-mRNA and loading into EVs for transport to other cells. Nat. Commun. 2019, 10, 4333. [Google Scholar] [CrossRef]
- Zeng, C.; Zhang, C.; Walker, P.G.; Dong, Y. Formulation and Delivery Technologies for mRNA Vaccines. Curr. Top. Microbiol. Immunol. 2022, 440, 71. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Trenkenschuh, E.; Friess, W. Freeze-drying of nanoparticles: How to overcome colloidal instability by formulation and process optimization. Eur. J. Pharm. Biopharm. 2021, 165, 345–360. [Google Scholar] [CrossRef]
- Mahmud, A.K.M.F.; Rahman, K.M.Z.; Dey, S.K.; Islam, T.; Talukder, A.A. Genome Annotation and Comparative Genomics of ORF Virus. Adv. Microbiol. 2014, 04, 1117–1131. [Google Scholar] [CrossRef]
- Packer, M.; Gyawali, D.; Yerabolu, R.; Schariter, J.; White, P. A novel mechanism for the loss of mRNA activity in lipid nanoparticle delivery systems. Nat. Commun. 2021, 12, 6777. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Ruppl, A.; Kiesewetter, D.; Köll-Weber, M.; Lemazurier, T.; Süss, R.; Allmendinger, A. Formulation screening of lyophilized mRNA-lipid nanoparticles. Int. J. Pharm. 2025, 671, 125272. [Google Scholar] [CrossRef]
- Kim, B.; Hosn, R.R.; Remba, T.; Yun, D.; Li, N.; Abraham, W.; Melo, M.B.; Cortes, M.; Li, B.; Zhang, Y.; et al. Optimization of storage conditions for lipid nanoparticle-formulated self-replicating RNA vaccines. J. Control. Release 2023, 353, 241–253. [Google Scholar] [CrossRef]
- Muramatsu, H.; Lam, K.; Bajusz, C.; Laczkó, D.; Karikó, K.; Schreiner, P.; Martin, A.; Lutwyche, P.; Heyes, J.; Pardi, N. Lyophilization provides long-term stability for a lipid nanoparticle-formulated, nucleoside-modified mRNA vaccine. Mol. Ther. 2022, 30, 1941–1951. [Google Scholar] [CrossRef]
- Li, W.; Wang, T.; Chen, J.; Guo, M.; Ling, L.; Higuchi, A. Application of Saccharide Cryoprotectants in the Freezing or Lyophilization Process of Lipid Nanoparticles Encapsulating Gene Drugs for Regenerative Medicine. Regen. Med. Dent. 2024, 1, 3. [Google Scholar] [CrossRef]
- Alejo, T.; Toro-Córdova, A.; Fernández, L.; Rivero, A.; Stoian, A.M.; Pérez, L.; Navarro, V.; Martínez-Oliván, J.; de Miguel, D. Comprehensive Optimization of a Freeze-Drying Process Achieving Enhanced Long-Term Stability and In Vivo Performance of Lyophilized mRNA-LNPs. Int. J. Mol. Sci. 2024, 25, 10603. [Google Scholar] [CrossRef]
- Shirane, D.; Tanaka, H.; Sakurai, Y.; Taneichi, S.; Nakai, Y.; Tange, K.; Ishii, I.; Akita, H. Development of an Alcohol Dilution–Lyophilization Method for the Preparation of mRNA-LNPs with Improved Storage Stability. Pharmaceutics 2023, 15, 1819. [Google Scholar] [CrossRef]
- Meulewaeter, S.; Nuytten, G.; Cheng, M.H.; De Smedt, S.C.; Cullis, P.R.; De Beer, T.; Lentacker, I.; Verbeke, R. Continuous freeze-drying of messenger RNA lipid nanoparticles enables storage at higher temperatures. J. Control. Release 2023, 357, 149–160. [Google Scholar] [CrossRef]
- Klepzig, L.S.; Juckers, A.; Knerr, P.; Harms, F.; Strube, J. Digital twin for lyophilization by process modeling in manufacturing of biologics. Processes 2020, 8, 1325. [Google Scholar] [CrossRef]
- Islam, T.; Diba, F.; Miah, R.; Siddiqa, A.; Azmuda, N.; Nahar, S.; Adnan, N.; Dey, S.K.; Talukder, A.A. Optimization of Acetic Acid Production Rate by Thermotolerant Acetobacter spp. Adv. Microbiol. 2017, 7, 749–759. [Google Scholar] [CrossRef]
- Luo, W.-C.; Beringhs, A.O.; Kim, R.; Zhang, W.; Patel, S.M.; Bogner, R.H.; Lu, X. Impact of formulation on the quality and stability of freeze-dried nanoparticles. Eur. J. Pharm. Biopharm. 2021, 169, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Kafetzis, K.N.; Papalamprou, N.; McNulty, E.; Thong, K.X.; Sato, Y.; Mironov, A.; Purohit, A.; Welsby, P.J.; Harashima, H.; Yu-Wai-Man, C.; et al. The Effect of Cryoprotectants and Storage Conditions on the Transfection Efficiency, Stability, and Safety of Lipid-Based Nanoparticles for mRNA and DNA Delivery. Adv. Healthc. Mater. 2023, 12, 2203022. [Google Scholar] [CrossRef] [PubMed]
- Flood, A.; Estrada, M.; McAdams, D.; Ji, Y.; Chen, D. Development of a Freeze-Dried, Heat-Stable Influenza Subunit Vaccine Formulation. PLoS ONE 2016, 11, e0164692. [Google Scholar] [CrossRef]
- Zhang, L.; More, K.R.; Ojha, A.; Jackson, C.B.; Quinlan, B.D.; Li, H.; He, W.; Farzan, M.; Pardi, N.; Choe, H. Effect of mRNA-LNP components of two globally-marketed COVID-19 vaccines on efficacy and stability. NPJ Vaccines 2023, 8, 156. [Google Scholar] [CrossRef]
- Lball, R.; Bajaj, P.; Whitehead, K.A. Achieving long-term stability of lipid nanoparticles: Examining the effect of pH, temperature, and lyophilization. Int. J. Nanomed. 2017, 12, 305–315. [Google Scholar] [CrossRef]
- Robinson, E.; MacDonald, K.D.; Slaughter, K.; McKinney, M.; Patel, S.; Sun, C.; Sahay, G. Lipid Nanoparticle-Delivered Chemically Modified mRNA Restores Chloride Secretion in Cystic Fibrosis. Mol. Ther. 2018, 26, 2034–2046. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, X.; Liu, F.; Xie, J.; Zhu, Q.; Tan, S. Trehalose in Biomedical Cryopreservation-Properties, Mechanisms, Delivery Methods, Applications, Benefits, and Problems. ACS Biomater. Sci. Eng. 2023, 9, 1190–1204. [Google Scholar] [CrossRef]
- Taylor, L.S.; Zografi, G. Sugar–polymer hydrogen bond interactions in lyophilized amorphous mixtures. J. Pharm. Sci. 1998, 87, 1615–1621. [Google Scholar] [CrossRef]
- Sola-Penna, M.; Meyer-Fernandes, J.R. Stabilization against Thermal Inactivation Promoted by Sugars on Enzyme Structure and Function: Why Is Trehalose More Effective Than Other Sugars? Arch. Biochem. Biophys. 1998, 360, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Roos, Y. Melting and glass transitions of low molecular weight carbohydrates. Carbohydr. Res. 1993, 238, 39–48. [Google Scholar] [CrossRef]
- Elbrink, K.; Van Hees, S.; Holm, R.; Kiekens, F. Optimization of the different phases of the freeze-drying process of solid lipid nanoparticles using experimental designs. Int. J. Pharm. 2023, 635, 122717. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Aikawa, O.; Tagami, T.; Ito, T.; Tahara, K.; Kawakami, S.; Ozeki, T. Stable and inhalable powder formulation of mRNA-LNPs using pH-modified spray-freeze drying. Int. J. Pharm. 2024, 665, 124632. [Google Scholar] [CrossRef]
- Henderson, M.I.; Eygeris, Y.; Jozic, A.; Herrera, M.; Sahay, G. Leveraging Biological Buffers for Efficient Messenger RNA Delivery via Lipid Nanoparticles. Mol. Pharm. 2022, 19, 4275–4285. [Google Scholar] [CrossRef] [PubMed]
- Blenke, E.O.; Örnskov, E.; Schöneich, C.; Nilsson, G.A.; Volkin, D.B.; Mastrobattista, E.; Almarsson, Ö.; Crommelin, D.J. The Storage and In-Use Stability of mRNA Vaccines and Therapeutics: Not A Cold Case. J. Pharm. Sci. 2023, 112, 386–403. [Google Scholar] [CrossRef]
- Kolhe, P.; Amend, E.; Singh, S.K. Impact of freezing on pH of buffered solutions and consequences for monoclonal antibody aggregation. Biotechnol. Prog. 2010, 26, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jia, L.; Xie, Y.; Ma, W.; Yan, Z.; Liu, F.; Deng, J.; Zhu, A.; Siwei, X.; Su, W.; et al. Lyophilization process optimization and molecular dynamics simulation of mRNA-LNPs for SARS-CoV-2 vaccine. NPJ Vaccines 2023, 8, 153. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yin, Q.; Yi, L.; Su, C.; Wen, Y.; Qiao, M.; Ju, Y.; Liu, Z.; Xiong, Y.; Liu, Z. Lyophilized monkeypox mRNA lipid nanoparticle vaccines with long-term stability and robust immune responses in mice. Hum. Vaccin. Immunother. 2025, 21, 2477384. [Google Scholar] [CrossRef]
- Suzuki, Y.; Miyazaki, T.; Muto, H.; Kubara, K.; Mukai, Y.; Watari, R.; Sato, S.; Kondo, K.; Tsukumo, S.-I.; Yasutomo, K.; et al. Design and lyophilization of lipid nanoparticles for mRNA vaccine and its robust immune response in mice and nonhuman primates. Mol. Ther. Nucleic Acids 2022, 30, 226. [Google Scholar] [CrossRef] [PubMed]
- Gulati, G.K.; Simpson, A.C.; MacMillen, Z.; Krieger, K.; Sharma, S.; Erasmus, J.H.; Reed, S.G.; Davie, J.W.; Avril, M.; Khandhar, A.P. Preclinical development of lyophilized self-replicating RNA vaccines for COVID-19 and malaria with improved long-term thermostability. J. Control. Release 2025, 377, 81–92. [Google Scholar] [CrossRef]
- Falconer, R.J. Advances in liquid formulations of parenteral therapeutic proteins. Biotechnol. Adv. 2019, 37, 107412. [Google Scholar] [CrossRef]
- Cheng, F.; Wang, Y.; Bai, Y.; Liang, Z.; Mao, Q.; Liu, D.; Wu, X.; Xu, M. Research Advances on the Stability of mRNA Vaccines. Viruses 2023, 15, 668. [Google Scholar] [CrossRef]
- Lewis, L.M.; Badkar, A.V.; Cirelli, D.; Combs, R.; Lerch, T.F. The Race to Develop the Pfizer-BioNTech COVID-19 Vaccine: From the Pharmaceutical Scientists Perspective. J. Pharm. Sci. 2023, 112, 640–647. [Google Scholar] [CrossRef]
- Wayment-Steele, H.K.; Kim, D.S.; A Choe, C.; Nicol, J.J.; Wellington-Oguri, R.; Watkins, A.M.; Sperberg, R.A.P.; Huang, P.-S.; Participants, E.; Das, R. Theoretical basis for stabilizing messenger RNA through secondary structure design. bioRxiv 2021, 49, 10604–10617. [Google Scholar] [CrossRef]
- Fan, Y.; Rigas, D.; Kim, L.J.; Chang, F.-P.; Zang, N.; McKee, K.; Kemball, C.C.; Yu, Z.; Winkler, P.; Su, W.-C.; et al. Physicochemical and structural insights into lyophilized mRNA-LNP from lyoprotectant and buffer screenings. J. Control. Release 2024, 373, 727–737. [Google Scholar] [CrossRef]
- Garidel, P.; Pevestorf, B.; Bahrenburg, S. Stability of buffer-free freeze-dried formulations: A feasibility study of a monoclonal antibody at high protein concentrations. Eur. J. Pharm. Biopharm. 2015, 97, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.H.; Wagner, C.E.; Kamen, A.A. Development of Long-Term Stability of Enveloped rVSV Viral Vector Expressing SARS-CoV-2 Antigen Using a DOE-Guided Approach. Vaccines 2024, 12, 1204. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.H.; Youssef, M.; Nesdoly, S.; Kamen, A.A. Development of Robust Freeze-Drying Process for Long-Term Stability of rVSV-SARS-CoV-2 Vaccine. Viruses 2024, 16, 942. [Google Scholar] [CrossRef] [PubMed]
- Ghaemmaghamian, Z.; Zarghami, R.; Walker, G.; OReilly, E.; Ziaee, A. Stabilizing vaccines via drying: Quality by design considerations. Adv. Drug Deliv. Rev. 2022, 187, 114313. [Google Scholar] [CrossRef]
- Lamoot, A.; Lammens, J.; De Lombaerde, E.; Zhong, Z.; Gontsarik, M.; Chen, Y.; De Beer, T.R.M.; De Geest, B.G. Successful batch and continuous lyophilization of mRNA LNP formulations depend on cryoprotectants and ionizable lipids. Biomater. Sci. 2023, 11, 4327–4334. [Google Scholar] [CrossRef] [PubMed]
- Stitz, L.; Vogel, A.; Schnee, M.; Voss, D.; Rauch, S.; Mutzke, T.; Ketterer, T.; Kramps, T.; Petsch, B.; Rupprecht, C.E. A thermostable messenger RNA based vaccine against rabies. PLoS Negl. Trop. Dis. 2017, 11, 0006108. [Google Scholar] [CrossRef]
- Guimarães, D.; Noro, J.; Silva, C.; Cavaco-Paulo, A.; Nogueira, E. Protective Effect of Saccharides on Freeze-Dried Liposomes Encapsulating Drugs. Front. Bioeng. Biotechnol. 2019, 7, 424. [Google Scholar] [CrossRef]
- Anindita, J.; Tanaka, H.; Oyama, R.; Hagiwara, S.; Shirane, D.; Taneichi, S.; Nakai, Y.; Tange, K.; Hatakeyama, H.; Sakurai, Y.; et al. Development of a Ready-to-Use-Type RNA Vaccine Carrier Based on an Intracellular Environment-Responsive Lipid-like Material with Immune-Activating Vitamin E Scaffolds. Pharmaceutics 2023, 15, 2702. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.M.; Nail, S.L.; Pikal, M.J.; Geidobler, R.; Winter, G.; Hawe, A.; Davagnino, J.; Gupta, S.R. Lyophilized Drug Product Cake Appearance: What Is Acceptable? J. Pharm. Sci. 2017, 106, 1706–1721. [Google Scholar] [CrossRef]
- Ahmed, F.; Alim, A.; Alam, F.; Islam, T.; Talukder, A.A. Bio-Geo-Chemical Characterization of Bangladeshi Textile Effluents. Adv. Microbiol. 2015, 5, 317–324. [Google Scholar] [CrossRef][Green Version]
- Li, B.; Manan, R.S.; Liang, S.-Q.; Gordon, A.; Jiang, A.; Varley, A.; Gao, G.; Langer, R.; Xue, W.; Anderson, D. Combinatorial design of nanoparticles for pulmonary mRNA delivery and genome editing. Nat. Biotechnol. 2023, 41, 1410–1415. [Google Scholar] [CrossRef]
- Sato, S.; Sano, S.; Muto, H.; Kubara, K.; Kondo, K.; Miyazaki, T.; Suzuki, Y.; Uemoto, Y.; Ukai, K. Understanding the Manufacturing Process of Lipid Nanoparticles for mRNA Delivery Using Machine Learning. Chem. Pharm. Bull. 2024, 72, 529–539. [Google Scholar] [CrossRef]
- Identifying Critical Quality Attributes for mRNA/LNP. 2023. Available online: https://www.biophorum.com/news/an-industry-standard-for-mrna-lnp-analytics/ (accessed on 25 May 2025).
- A guide to RNA-LNP Formulation Screening—Inside Therapeutics. Available online: https://insidetx.com/review/a-guide-to-rna-lnp-formulation-screening/ (accessed on 25 May 2025).
- Koganti, V.; Luthra, S.; Pikal, M.J. The Freeze-Drying Process: The Use of Mathematical Modeling in Process Design, Understanding, and Scale-Up. In Chemical Engineering in the Pharmaceutical Industry: R&D to Manufacturing; John Wiley and Sons: Hoboken, NJ, USA, 2010; pp. 801–817. [Google Scholar] [CrossRef]
- Defining CQAs for mRNA/LNP development – BioPhorum. 2023. Available online: https://www.biophorum.com/download/defining-the-required-critical-quality-attributes-cqas-and-phase-requirements-for-mrna-lnp-product-development-and-manufacture/ (accessed on 14 June 2025).
- Haque, M.A.; Shrestha, A.; Mikelis, C.M.; Mattheolabakis, G. Comprehensive analysis of lipid nanoparticle formulation and preparation for RNA delivery. Int. J. Pharm. X 2024, 8, 100283. [Google Scholar] [CrossRef]
- Wang, T.; Yu, T.; Li, W.; Chen, J.; Cheng, S.; Tian, Z.; Sung, T.-C.; Higuchi, A. Development of lyophilized mRNA-LNPs with high stability and transfection efficiency in specific cells and tissues. Regen. Biomater. 2025, 12, rbaf023. [Google Scholar] [CrossRef]
- Schmidt, A.; Helgers, H.; Vetter, F.L.; Juckers, A.; Strube, J. Digital Twin of mRNA-Based SARS-COVID-19 Vaccine Manufacturing towards Autonomous Operation for Improvements in Speed, Scale, Robustness, Flexibility and Real-Time Release Testing. Processes 2021, 9, 748. [Google Scholar] [CrossRef]

| Formulations | Protecting Mechanism | Positive Impacts on mRNA-LNPs | References | |
|---|---|---|---|---|
| Sugars | Sucrose | Protective coating prevents mechanical damage, vitrification (formation of a glassy matrix), water replacement (hydrogen bonds), and cryoprotection | Prevents LNPs aggregation, preserves particle size, maintains mRNA integrity, and reduces freeze and dehydration stresses | [11,32,51,54,55] |
| Trehalose | Increase formulations’ viscosity, high glass transition temperature (Tg), low crystallization risk, vitrification, water replacement, cryoprotection | Higher Tg’ than sucrose, maintains structural integrity, enhances LNPs resistance to drying | [21,54,56,57,58,59] | |
| Maltose | Glass matrix formation | Often combined with sucrose, helps prevent structural collapse | [43,60] | |
| Sugar alcohol | Mannitol | Bulking agent, prevents cake shrinkage | Prevents cake collapse, may reduce aggregation, but can crystallize unfavorably | [42,50,61] |
| Buffer | Tris | Scavenges hydroxyl radicals, stabilizes pH during freezing | Reduces pH shift, improves encapsulation and transfection efficiency, and reduces zeta potential shift | [45,47,62,63,64] |
| PBS | Ionic stabilization maintains a stable pH during freezing and drying, but is prone to pH shift in the presence of sodium ions | Common but inferior to Tris, used for its ionic strength, can decrease encapsulation efficiency and stability | [42,45,47,62] | |
| HEPES | PH buffering, stabilizing effect during freeze-thaw | Helps to maintain LNPs integrity during freeze-thaw cycles and long-term storage | [62] | |
| Formulations (w/v) | Buffer/pH | Reconstitution | Stability | References |
|---|---|---|---|---|
| 10% sucrose 10% maltose | 5 mM Tris/ pH 8 | Water | Physicochemical properties do not significantly change for 12 weeks after storage at room temperature and for at least 24 weeks after storage at 4 °C | [43] |
| 8.8% sucrose, 2% trehalose, 0.04% mannitol | - | - | The lyophilized mRNA-LNPs were stable at 2–8 °C, and they did not reduce immunogenicity in vivo or in vitro. | [65] |
| 8.7% sucrose | (PBS) | 90 μL of nuclease-free water | Optimal O9 mRNA-LNPs could be stored at 4 °C for more than 12 weeks and at room temperature for 4 weeks after lyophilization. | [31] |
| 10% sucrose | PBS/pH 7.4 | Deionized water | mRNA vaccines were stably stored in 10% w/v sucrose in PBS at −20 °C for at least 30 days. | [42] |
| 20% maltose | Tris 5 mM/pH 7.4 | 300 μL RNase-free water | Lyophilized LNPs retained their in vivo bioactivity at an almost unaffected level for 1 year when stored at 4 °C. Lyophilized LNPs also presented unaltered thermo-stability at room temperature (25 °C) for 4 weeks. | [45] |
| 12.5% sucrose | 20 mM Tris/ pH 7.4 | 400 μL of Tris-, phosphate- or PBS buffer at pH 7.4 | Lyophilized mRNA-LNPs preserved their functionality when stored at 4 °C, 22 °C and even at 37 °C for 12 weeks. | [47] |
| 5% sucrose/ 5% trehalose | - | - | 5% (w/v) sucrose or trehalose LNPs stored in liquid nitrogen maintained mRNA delivery efficiency for over three months. | [32] |
| 9% trehalose/ 1% PVP | 20 mM Tris/pH 7.4 | 275 μL RNase-free water | The most promising formulations for storage at higher temperatures were identified as 9% (w/v) trehalose + 1% (w/v) PVP, with only a slight increase in size over 6 months at 25 °C, while maintaining PDI and encapsulation efficiency. | [41] |
| 10% sucrose/5% trehalose | 10 mm Tris/pH 7.4 | Water | Lyophilized mRNA-LNPs can be stored at 4 °C for at least 12 months and at least 8 h after reconstitution at ambient temperature without a significant change in product quality. They also preserved the in vitro immunogenicity in mice, comparable to that of freshly prepared mRNA-LNPs. | [66] |
| 10% sucrose/9% mannitol/1% PEG60 | Tris | Water | Dry powder formulation that could maintain the physicochemical properties of mRNA-LNPs after storage at 4 °C for at least two months. | [67] |
| 10% sucrose | - | Nuclease-free water | Lyophilized form of LION/repRNA-CoV-2S with 10% w/v sucrose, maintained in vivo immunogenicity after 1 week at 25 °C and 6 months at 2–8 °C. Lyophilized LION/repRNA-PyCS vaccine with 10% w/v sucrose, stored for 12 months at 2–8 °C, demonstrated no loss in immunogenicity. | [68] |
| Freezing (Temperature/Time) | Primary Drying (Temperature/Pressure/Time) | Secondary Drying (Temperature/Pressure/Time) | References |
|---|---|---|---|
| −45 °C/3 h | −25 °C/2.7 Pa/84 h | 30 °C/2.7 Pa/5 h | [43] |
| −40 °C/2 h | −35 °C/10 Pa/24 h | 25 °C/5 h | [78] |
| −40 °C/2 h | −10 °C/16 Pa/17 h | 2 °C/6.8 Pa/10 h | [79] |
| −80 °C/6 h | −50 °C/6 Pa/24 h | [80] | |
| −30 °C/3 h | −25 °C/5–10 Pa/16–18 h | 22–27 °C/20 Pa/5 h | [31] |
| −80 °C | 12 h | - | [32,54] |
| −40 °C/40 min −40 °C/20 min | −30 °C/1 h −20 °C/1 h −10 °C/1 h 0 °C/1 h | 10 °C/1 h 20 °C/1 h 30 °C/3 h | [46,81] |
| −50 °C/5 h | −15 °C/24 Pa/12 h | 30 °C/13.3 Pa/7 h | [45] |
| −40 °C/3 h | −20 °C/13 Pa/10 h | 25 °C/5 h | [41] |
| −20 °C | −30 °C/3 Pa/30 h | 25 °C/3 Pa/6 h | [66] |
| −50 °C/3 h | −50 °C/1 h/27 Pa −40 °C/1 h/27 Pa −35 °C/12 h/27 Pa | 30 °C/10 h | [67] |
| −50 °C/1.5 h | −30 °C/7 Pa/17.5 h | 25 °C/7 Pa/1.5 h | [68] |
| Critical Process Parameters (CPPs) | Critical Quality Attributes (CQAs) |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Property | Analytical Method | Reference Study | Recommended Standard |
|---|---|---|---|
| Particle size | Dynamic light scattering (DLS) | [31,32,42,43,45,47,65] | Between 80 and 110 nm for optimal cellular uptake and biodistribution |
| Nanoparticle morphology, size, and internal structure | Transmission electron microscopy (TEM) Scanning electron microscopy (SEM) Cryogenic electron microscope Cryo-transmission electron microscopy (Cryo-TEM) | [23,31,41,42,43,45,65,66,67] | Uniform spherical or vesicular structures, depending on the design Between 70 and 90 nm for optimal cellular uptake and biodistribution |
| Polydispersity index (PDI) | Dynamic light scattering (DLS) | [31,43,45,47,65] | ≤0.2 indicates a homogeneous particle population |
| Zeta potential | Electrophoretic light scattering (ELS) Dynamic light scattering (DLS) | [31,32,42,45,47] | ±20 to 30 mV is generally sufficient for colloidal stability and minimal aggregation |
| mRNA encapsulation efficiency | Quant-it Ribogreen fluorescence assay | [31,43,45,47,65] | ≥90–95% is typically targeted for therapeutic efficacy |
| mRNA concentration | Ribogreen fluorescence assay | [43,45] | Consistency across batches is key; the quantitative threshold depends on dose |
| mRNA integrity | Capillary electrophoresis | [31,43,45,65] | Intact single bands, degradation products should be minimal or absent |
| Lipid content | Ultra high-performance liquid chromatography (UHPLC) | [43] | Must match expected lipid: mRNA molar ratios |
| Residual moisture | Karl Fischer titration | [41] | <1% w/w is typically recommended to ensure long-term stability and prevent degradation |
| Visual appearance (cake quality) | Visual inspection (macroscopic evaluation) | [41] | Cake should be uniform, white, intact, without collapse or shrinkage |
| In vitro transfection efficiency | Luciferase report assay, GFP expression assay | [45,91] | Comparable or improved transfection vs. freshly prepared LNPs |
| In vitro cytotoxicity | Cell viability assays (CCK-8, MTT) | [65,91] | Usually, >80% cell viability |
| In vivo immunogenicity | ELISA, HAI assay/titer | [23,43,65] | Robust and comparable immune response to fresh vaccine |
| In vivo biodistribution | IVIS imaging, fluorescence/RNA quantification in organs | [45,91] | Distribution to the target tissue, with low off-target accumulation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.F.H.; Baudin, F.; Sudalaiyadum Perumal, A.; Kamen, A.A. Freeze-Drying of mRNA-LNPs Vaccines: A Review. Vaccines 2025, 13, 853. https://doi.org/10.3390/vaccines13080853
Khan MFH, Baudin F, Sudalaiyadum Perumal A, Kamen AA. Freeze-Drying of mRNA-LNPs Vaccines: A Review. Vaccines. 2025; 13(8):853. https://doi.org/10.3390/vaccines13080853
Chicago/Turabian StyleKhan, MD Faizul Hussain, Floriane Baudin, Ayyappasamy Sudalaiyadum Perumal, and Amine A. Kamen. 2025. "Freeze-Drying of mRNA-LNPs Vaccines: A Review" Vaccines 13, no. 8: 853. https://doi.org/10.3390/vaccines13080853
APA StyleKhan, M. F. H., Baudin, F., Sudalaiyadum Perumal, A., & Kamen, A. A. (2025). Freeze-Drying of mRNA-LNPs Vaccines: A Review. Vaccines, 13(8), 853. https://doi.org/10.3390/vaccines13080853








