Bivalent Oral Vaccine Using Attenuated Salmonella Gallinarum Delivering HA and NA-M2e Confers Dual Protection Against H9N2 Avian Influenza and Fowl Typhoid in Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, And Primers
2.2. Vaccine Design
2.3. Confirmation of Clones And Antigen mRNA Transcription In Vitro
2.4. Animals And Ethics
2.5. Chicken Inoculation And Confirmation of Antigen Expression In Vivo
2.6. Immunization And Challenge
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Hemagglutination Inhibition (HI) Assay
2.9. Fluorescence-Activated Cell Sorting (FACS) Analysis
2.10. Assessment of Cytokine Expression by Quantitative Real-Time PCR (qRT-PCR)
2.11. Egg Infectious Dose 50 (EID50)
2.12. Hematoxylin and Eosin (H&E) Staining
2.13. Statistical Analysis
3. Results
3.1. Confirmation of In Vivo Expression of H9N2 Antigens
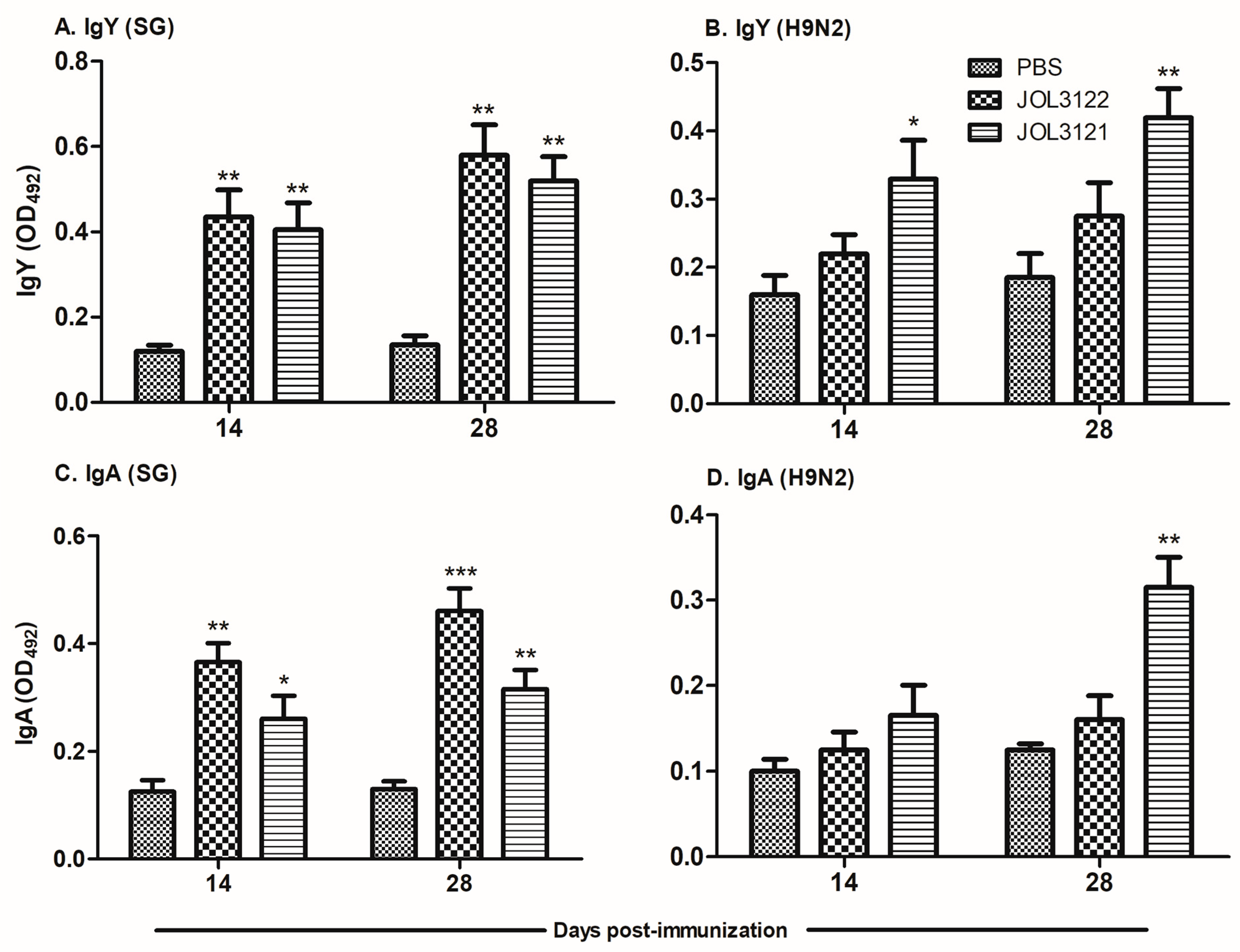
3.2. SG- and H9N2-Specific Antibody Responses
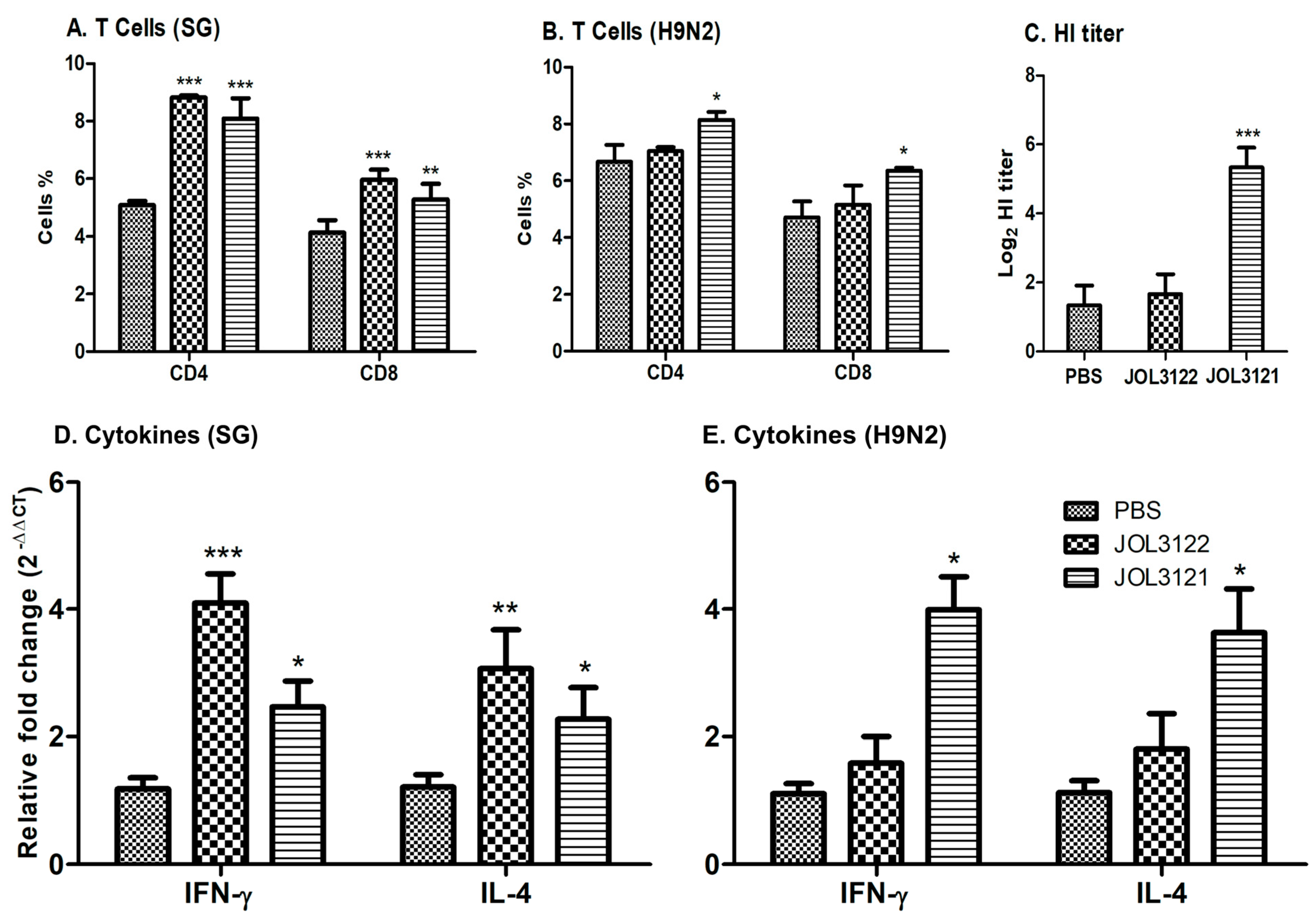
3.3. Cell-Mediated Immune Responses
3.4. HI Titration
3.5. Cytokine Response
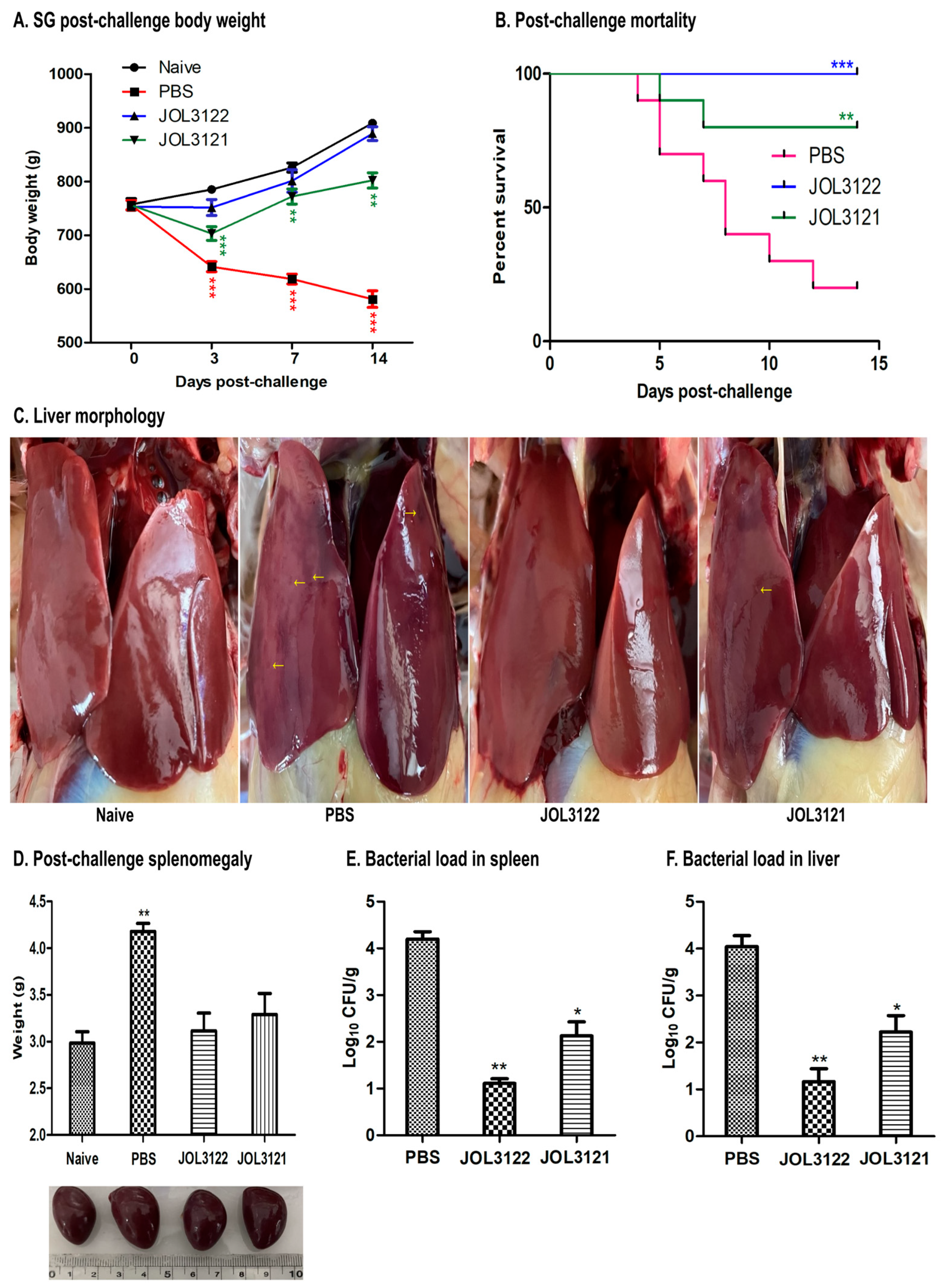
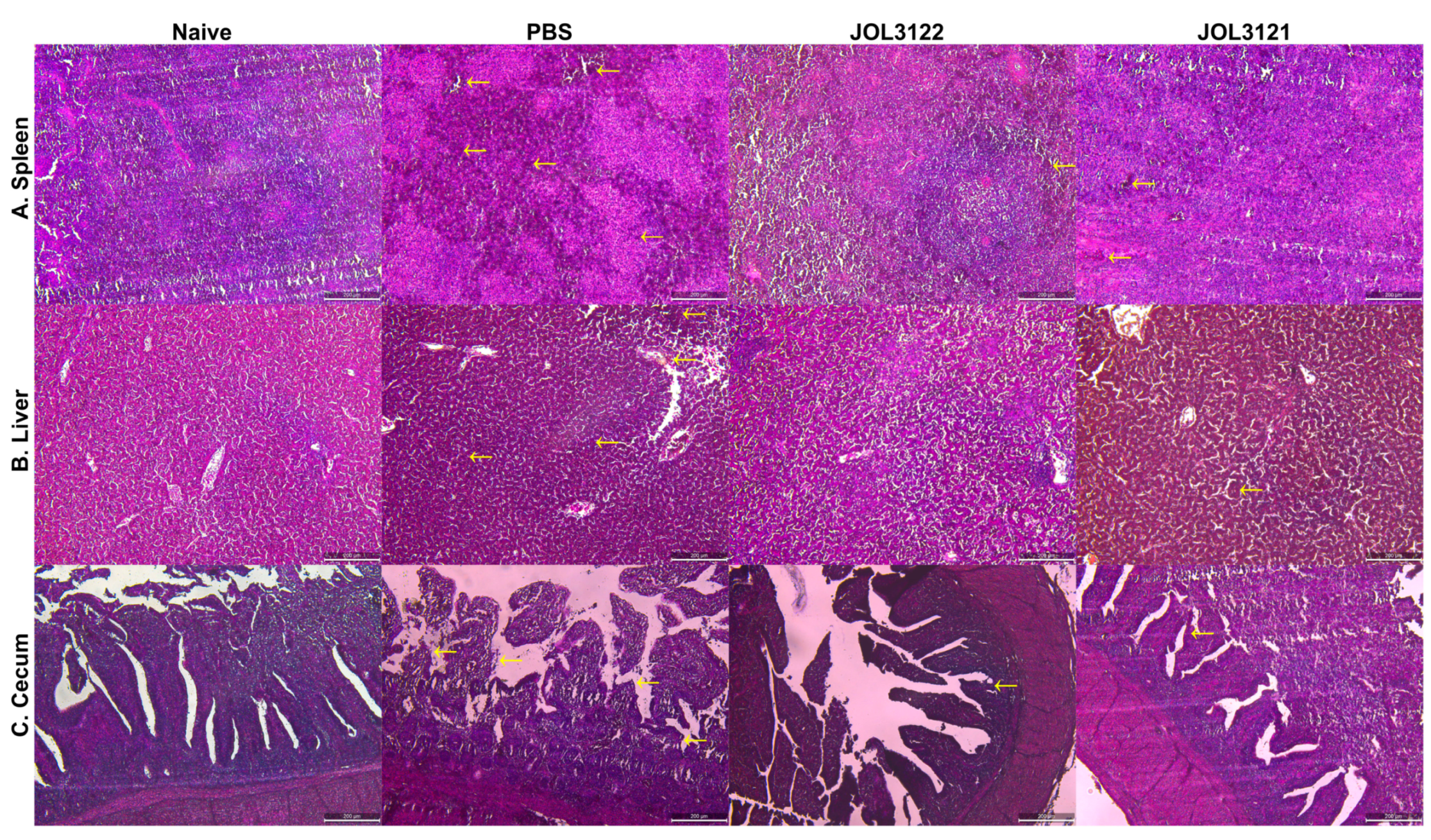
3.6. Protection Against Wild-Type SG
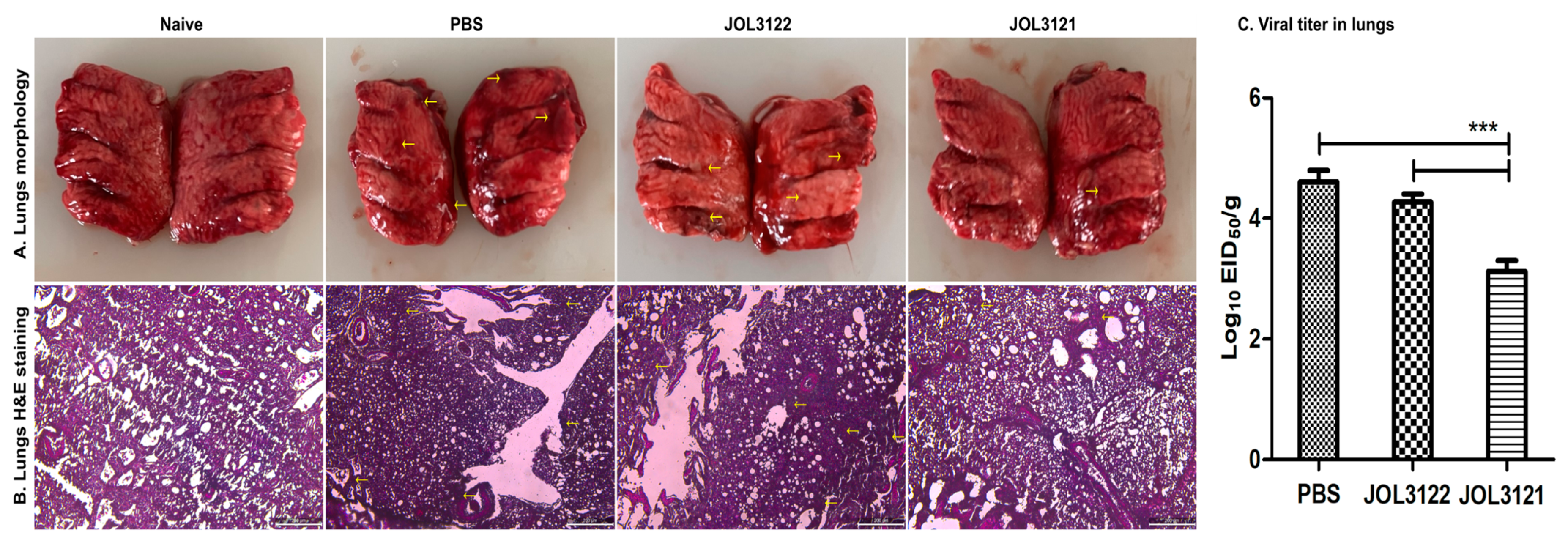
3.7. Protection Against H9N2 Challenge
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peacock, T.H.P.; James, J.; Sealy, J.E.; Iqbal, M.A. Global Perspective on H9N2 Avian Influenza Virus. Viruses 2019, 11, 620. [Google Scholar] [CrossRef]
- Hu, Z.; Ai, H.; Wang, Z.; Huang, S.; Sun, H.; Xuan, X.; Chen, M.; Wang, J.; Yan, W.; Sun, J.; et al. Impact of inactivated vaccine on transmission and evolution of H9N2 avian influenza virus in chickens. NPJ Vaccines 2025, 10, 67. [Google Scholar] [CrossRef]
- Aganja, R.P.; Bakhsh, M.; Senevirathne, A.; Kwon, J.; Lee, J.H. Lipopolysaccharide structural modification in Salmonella Gallinarum targeting lipid a deacylation and O-antigen reduces virulence and endotoxicity with competitive protection against a wild-type challenge in chickens. Dev. Comp. Immunol. 2025, 165, 105343. [Google Scholar] [CrossRef]
- Bin Aslam, H.; Hasler, B.; Iqbal, M.; Yaqub, T.; Alarcon, P. Financial impact of low pathogenic avian influenza virus subtype H9N2 on commercial broiler chicken and egg layer production systems in Pakistan. Prev. Vet. Med. 2024, 233, 106346. [Google Scholar] [CrossRef]
- Moatasim, Y.; Kandeil, A.; Mostafa, A.; Elghaffar, S.K.A.; El Shesheny, R.; Elwahy, A.H.M.; Ali, M.A. Single gene reassortment of highly pathogenic avian influenza A H5N1 in the low pathogenic H9N2 backbone and its impact on pathogenicity and infectivity of novel reassortant viruses. Arch. Virol. 2017, 162, 2959–2969. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, J. H9N2 influenza virus in China: A cause of concern. Protein Cell 2015, 6, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Robie, E.R.; Saeed, U.; Jaffar, G.; Bailey, E.S.; Marushchak, L.V.; Kreditor, B.E.; Pulscher, L.A.; Rubrum, A.M.; Webby, R.J.; et al. H9N2 influenza A viruses found to be enzootic in Punjab Pakistan’s bird markets with evidence of human H9N2 nasal colonization. Int. J. Infect. Dis. 2024, 146, 107146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, L.; Liao, M.; Qi, W. H9N2 avian influenza viruses: Challenges and the way forward. Lancet Microbe 2023, 4, e70–e71. [Google Scholar] [CrossRef]
- Peiris, M.; Yuen, K.Y.; Leung, C.W.; Chan, K.H.; Ip, P.L.; Lai, R.W.; Orr, W.K.; Shortridge, K.F. Human infection with influenza H9N2. Lancet 1999, 354, 916–917. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Zhao, C.; Gao, X.; Zhang, Y.; Li, J.; Wang, M.; Zhang, H.; Liu, W.; Wang, C.; et al. Molecular characterization, receptor binding property, and replication in chickens and mice of H9N2 avian influenza viruses isolated from chickens, peafowls, and wild birds in eastern China. Emerg. Microbes Infect. 2021, 10, 2098–2112. [Google Scholar] [CrossRef]
- Kilany, W.H.; Bazid, A.H.; Ali, A.; El-Deeb, A.H.; El-Abideen, M.A.; Sayed, M.E.; El-Kady, M.F. Comparative Effectiveness of Two Oil Adjuvant-Inactivated Avian Influenza H9N2 Vaccines. Avian Dis. 2016, 60, 226–231. [Google Scholar] [CrossRef]
- Swayne, D.E.; Kapczynski, D. Strategies and challenges for eliciting immunity against avian influenza virus in birds. Immunol. Rev. 2008, 225, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, S.; Zhou, Y.; Song, W.; Tang, Y.; Pang, Q.; Miao, Z. Phylogenetic Analysis of Hemagglutinin Genes of H9N2 Avian Influenza Viruses Isolated from Chickens in Shandong, China, between 1998 and 2013. Biomed. Res. Int. 2015, 2015267520. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, H.; Xu, H.; Ma, Z.; Zhang, G. Efficacy of an inactivated bivalent vaccine against the prevalent strains of Newcastle disease and H9N2 avian influenza. Virol. J. 2017, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Alves Batista, D.F.; de Freitas Neto, O.C.; Maria de Almeida, A.; Maboni, G.; de Carvalho, T.F.; de Carvalho, T.P.; Barrow, P.A.; Berchieri, A.J. Evaluation of pathogenicity of Salmonella Gallinarum strains harbouring deletions in genes whose orthologues are conserved pseudogenes in S. Pullorum. PLoS ONE 2018, 13, e0200585. [Google Scholar] [CrossRef]
- Kwon, Y.K.; Kim, A.; Kang, M.S.; Her, M.; Jung, B.Y.; Lee, K.M.; Jeong, W.; An, B.K.; Kwon, J.H. Prevalence and characterization of Salmonella Gallinarum in the chicken in Korea during 2000 to 2008. Poult. Sci. 2010, 89, 236–242. [Google Scholar] [CrossRef]
- Kim, N.H.; Ha, E.J.; Ko, D.S.; Lee, C.Y.; Kim, J.H.; Kwon, H.J. Molecular evolution of Salmonella enterica subsp. enterica serovar Gallinarum biovar Gallinarum in the field. Vet. Microbiol. 2019, 235, 63–70. [Google Scholar] [CrossRef]
- Shivaprasad, H.L. Fowl typhoid and pullorum disease. Rev. Sci. Tech. 2000, 19, 405–424. [Google Scholar] [CrossRef]
- Aganja, R.P.; Kwon, J.; Senevirathne, A.; Lee, J.H. Deletion of pagL and arnT genes involved in LPS structure and charge modulation in the Salmonella genome confer reduced endotoxicity and retained efficient protection against wild-type Salmonella Gallinarum challenge in chicken. Vet. Res. 2025, 56, 2. [Google Scholar] [CrossRef]
- Ntakiyisumba, E.; Tanveer, M.; Won, G. Integrating meta-analysis with a quantitative microbial risk assessment model to investigate Campylobacter contamination of broiler carcasses. Food Res. Int. 2024, 178, 113983. [Google Scholar] [CrossRef]
- Desin, T.S.; Koster, W.; Potter, A.A. Salmonella vaccines in poultry: Past, present and future. Expert. Rev. Vaccines 2013, 12, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Wigley, P.; Hulme, S.; Powers, C.; Beal, R.; Smith, A.; Barrow, P. Oral infection with the Salmonella enterica serovar Gallinarum 9R attenuated live vaccine as a model to characterise immunity to fowl typhoid in the chicken. BMC Vet. Res. 2005, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Mo, I.P.; Kang, M.S. Protective efficacy of live Salmonella gallinarum 9R vaccine in commercial layer flocks. Avian Pathol. 2007, 36, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Van Immerseel, F.; Studholme, D.J.; Eeckhaut, V.; Heyndrickx, M.; Dewulf, J.; Dewaele, I.; Van Hoorebeke, S.; Haesebrouck, F.; Van Meirhaeghe, H.; Ducatelle, R.; et al. Salmonella Gallinarum field isolates from laying hens are related to the vaccine strain SG9R. Vaccine 2013, 31, 4940–4945. [Google Scholar] [CrossRef]
- Mukhtar, M.; Ghafoor, A.; McClelland, M.; Akhtar, F.; Rasheed, M.A. Construction, molecular characterization, and safety assessment of purB mutant of Salmonella Gallinarum. Front. Microbiol. 2024, 15, 1467230. [Google Scholar] [CrossRef]
- Galen, J.E.; Wahid, R.; Buskirk, A.D. Strategies for Enhancement of Live-Attenuated Salmonella-Based Carrier Vaccine Immunogenicity. Vaccines (Basel) 2021, 9, 162. [Google Scholar] [CrossRef]
- Roberts, M.; Bacon, A.; Li, J.; Chatfield, S. Prior immunity to homologous and heterologous Salmonella serotypes suppresses local and systemic anti-fragment C antibody responses and protection from tetanus toxin in mice immunized with Salmonella strains expressing fragment C. Infect. Immun. 1999, 67, 3810–3815. [Google Scholar] [CrossRef]
- Hajam, I.A.; Kirthika, P.; Hewawaduge, C.; Jawalagatti, V.; Park, S.; Senevirathne, A.; Lee, J.H. Oral immunization with an attenuated Salmonella Gallinarum encoding the H9N2 haemagglutinin and M2 ectodomain induces protective immune responses against H9N2 infection in chickens. Avian Pathol. 2020, 49, 486–495. [Google Scholar] [CrossRef]
- Ntakiyisumba, E.; Tanveer, M.; Won, G. A comprehensive analysis of the current strategy for developing live attenuated vaccines against African swine fever: A systematic review and meta-analysis. Vaccine 2025, 57, 127243. [Google Scholar] [CrossRef]
- Sun, Y.; Cong, Y.; Yu, H.; Ding, Z.; Cong, Y. Assessing the effects of a two-amino acid flexibility in the Hemagglutinin 220-loop receptor-binding domain on the fitness of Influenza A(H9N2) viruses. Emerg. Microbes Infect. 2021, 10, 822–832. [Google Scholar] [CrossRef]
- Russell, C.J. Hemagglutinin Stability and Its Impact on Influenza A Virus Infectivity, Pathogenicity, and Transmissibility in Avians, Mice, Swine, Seals, Ferrets, and Humans. Viruses 2021, 13, 746. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, C.; Zhang, H.; Liu, G.D.; Xue, C.; Cao, Y. Targeting Hemagglutinin: Approaches for Broad Protection against the Influenza A Virus. Viruses 2019, 11, 405. [Google Scholar] [CrossRef] [PubMed]
- Jagadesh, A.; Salam, A.A.; Mudgal, P.P.; Arunkumar, G. Influenza virus neuraminidase (NA): A target for antivirals and vaccines. Arch. Virol. 2016, 161, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Mezhenskaya, D.; Isakova-Sivak, I.; Rudenko, L. M2e-based universal influenza vaccines: A historical overview and new approaches to development. J. Biomed. Sci. 2019, 26, 76. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Cho, K.J.; Fiers, W.; Saelens, X. M2e-Based Universal Influenza A Vaccines. Vaccines (Basel) 2015, 3, 105–136. [Google Scholar] [CrossRef]
- Guo, Y.; He, L.; Song, N.; Li, P.; Sun, S.; Zhao, G.; Tai, W.; Jiang, S.; Du, L.; Zhou, Y. Highly conserved M2e and hemagglutinin epitope-based recombinant proteins induce protection against influenza virus infection. Microbes Infect. 2017, 19, 641–647. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (OIE). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; 2008. Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/A_summry.htm (accessed on 15 June 2025).
- Killian, M.L. Hemagglutination assay for influenza virus. Methods Mol. Biol. 2014, 1161, 3–9. [Google Scholar] [CrossRef]
- Nuwarda, R.F.; Alharbi, A.A.; Kayser, V. An Overview of Influenza Viruses and Vaccines. Vaccines (Basel) 2021, 9, 1032. [Google Scholar] [CrossRef]
- Yamayoshi, S.; Kawaoka, Y. Current and future influenza vaccines. Nat. Med. 2019, 25, 212–220. [Google Scholar] [CrossRef]
- Wang, J.; Wu, M.; Hong, W.; Fan, X.; Chen, R.; Zheng, Z.; Zeng, Y.; Huang, R.; Zhang, Y.; Lam, T.T.; et al. Infectivity and Transmissibility of Avian H9N2 Influenza Viruses in Pigs. J. Virol. 2016, 90, 3506–3514. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Mettenleiter, T.C.; Abdelwhab, E.M. A brief summary of the epidemiology and genetic relatedness of avian influenza H9N2 virus in birds and mammals in the Middle East and North Africa. Epidemiol. Infect. 2017, 145, 3320–3333. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; James, J.; Sadeyen, J.R.; Mahmood, S.; Everest, H.J.; Chang, P.; Walsh, S.K.; Byrne, A.M.P.; Mollett, B.; Lean, F.; et al. Coinfection of Chickens with H9N2 and H7N9 Avian Influenza Viruses Leads to Emergence of Reassortant H9N9 Virus with Increased Fitness for Poultry and a Zoonotic Potential. J. Virol. 2022, 96, e0185621. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Yaqub, T.; Reddy, K.; McCauley, J.W. Novel genotypes of H9N2 influenza A viruses isolated from poultry in Pakistan containing NS genes similar to highly pathogenic H7N3 and H5N1 viruses. PLoS ONE 2009, 4, e5788. [Google Scholar] [CrossRef]
- Myers, M.L.; Gallagher, J.R.; Kim, A.J.; Payne, W.H.; Maldonado-Puga, S.; Assimakopoulos, H.; Bock, K.W.; Torian, U.; Moore, I.N.; Harris, A.K. Commercial influenza vaccines vary in HA-complex structure and in induction of cross-reactive HA antibodies. Nat. Commun. 2023, 14, 1763. [Google Scholar] [CrossRef]
- Dascalu, S.; Sealy, J.E.; Sadeyen, J.R.; Flammer, P.G.; Fiddaman, S.; Preston, S.G.; Dixon, R.J.; Bonsall, M.B.; Smith, A.L.; Iqbal, M. Immunisation of chickens with inactivated and/or infectious H9N2 avian influenza virus leads to differential immune B-cell repertoire development. Front. Immunol. 2024, 15, 1461678. [Google Scholar] [CrossRef]
- Wei, C.J.; Crank, M.C.; Shiver, J.; Graham, B.S.; Mascola, J.R.; Nabel, G.J. Next-generation influenza vaccines: Opportunities and challenges. Nat. Rev. Drug Discov. 2020, 19, 239–252. [Google Scholar] [CrossRef]
- Chen, S.; Pounraj, S.; Sivakumaran, N.; Kakkanat, A.; Sam, G.; Kabir, M.T.; Rehm, B.H.A. Precision-engineering of subunit vaccine particles for prevention of infectious diseases. Front. Immunol. 2023, 14, 1131057. [Google Scholar] [CrossRef]
- Liu, T.; Liang, Y.; Huang, L. Development and Delivery Systems of mRNA Vaccines. Front. Bioeng. Biotechnol. 2021, 9, 718753. [Google Scholar] [CrossRef]
- Aganja, R.P.; Kim, I.S.; Tae, H.J.; Lee, J.H. Expression and delivery of HA1-M2e antigen using an innovative attenuated Salmonella-mediated delivery system confers promising protection against H9N2 avian influenza challenge. Poult. Sci. 2025, 104, 104602. [Google Scholar] [CrossRef]
- Park, B.S.; Song, D.H.; Kim, H.M.; Choi, B.S.; Lee, H.; Lee, J.O. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 2009, 458, 1191–1195. [Google Scholar] [CrossRef]
- Senevirathne, A.; Hewawaduge, C.; Sivasankar, C.; Lee, J.H. Prospective lipid-A altered live attenuated Salmonella Gallinarum confers protectivity, DIVA capability, safety and low endotoxicity against fowl typhoid. Vet. Microbiol. 2022, 274, 109572. [Google Scholar] [CrossRef]
- Kong, Q.; Six, D.A.; Roland, K.L.; Liu, Q.; Gu, L.; Reynolds, C.M.; Wang, X.; Raetz, C.R.; Curtiss, R. 3rd. Salmonella synthesizing 1-monophosphorylated lipopolysaccharide exhibits low endotoxic activity while retaining its immunogenicity. J. Immunol. 2011, 187, 412–423. [Google Scholar] [CrossRef]
- Ito, R.; Ozaki, Y.A.; Yoshikawa, T.; Hasegawa, H.; Sato, Y.; Suzuki, Y.; Inoue, R.; Morishima, T.; Kondo, N.; Sata, T.; et al. Roles of anti-hemagglutinin IgA and IgG antibodies in different sites of the respiratory tract of vaccinated mice in preventing lethal influenza pneumonia. Vaccine 2003, 21, 2362–2371. [Google Scholar] [CrossRef]
- Suzuki, T.; Kawaguchi, A.; Ainai, A.; Tamura, S.; Ito, R.; Multihartina, P.; Setiawaty, V.; Pangesti, K.N.; Odagiri, T.; Tashiro, M.; et al. Relationship of the quaternary structure of human secretory IgA to neutralization of influenza virus. Proc. Natl. Acad. Sci. USA 2015, 112, 7809–7814. [Google Scholar] [CrossRef] [PubMed]
- Hemann, E.A.; Kang, S.M.; Legge, K.L. Protective CD8 T cell-mediated immunity against influenza A virus infection following influenza virus-like particle vaccination. J. Immunol. 2013, 191, 2486–2494. [Google Scholar] [CrossRef] [PubMed]
- Whitmire, J.K.; Tan, J.T.; Whitton, J.L. Interferon-gamma acts directly on CD8+ T cells to increase their abundance during virus infection. J. Exp. Med. 2005, 201, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Macaulay, A.E.; DeKruyff, R.H.; Goodnow, C.C.; Umetsu, D.T. Antigen-specific B cells preferentially induce CD4(+) T cells to produce IL-4. J. Immunol. 1997, 158, 4171–4179. [Google Scholar] [CrossRef]
- Duerkop, B.A.; Vaishnava, S.; Hooper, L.V. Immune responses to the microbiota at the intestinal mucosal surface. Immunity 2009, 31, 368–376. [Google Scholar] [CrossRef]
- Endt, K.; Stecher, B.; Chaffron, S.; Slack, E.; Tchitchek, N.; Benecke, A.; Van Maele, L.; Sirard, J.C.; Mueller, A.J.; Heikenwalder, M.; et al. The microbiota mediates pathogen clearance from the gut lumen after non-typhoidal Salmonella diarrhea. PLoS Pathog. 2010, 6, e1001097. [Google Scholar] [CrossRef]
- Ismail, N.; Olano, J.P.; Feng, H.M.; Walker, D.H. Current status of immune mechanisms of killing of intracellular microorganims. Fems Microbiol. Lett. 2002, 207, 111–120. [Google Scholar] [CrossRef]
- Wolfenden, R.E.; Layton, S.L.; Wolfenden, A.D.; Khatiwara, A.; Gaona-Ramirez, G.; Pumford, N.R.; Cole, K.; Kwon, Y.M.; Tellez, G.; Bergman, L.R.; et al. Development and evaluation of candidate recombinant Salmonella-vectored Salmonella vaccines. Poult. Sci. 2010, 89, 2370–2379. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Scarpellini, G.; Kong, W.; Shi, H.; Baek, C.H.; Gunn, B.; Wanda, S.Y.; Roland, K.L.; Zhang, X.; et al. Salmonella vaccine vectors displaying delayed antigen synthesis in vivo to enhance immunogenicity. Infect. Immun. 2010, 78, 3969–3980. [Google Scholar] [CrossRef]
| Bacteria/Primers | Description | References |
|---|---|---|
| S. Gallinarum | ||
| JOL422 JOL2997 | Wild type Δlon ΔpagL | Lab stock Lab stock [3] |
| JOL3062 | Δlon ΔpagL Δasd | Lab stock |
| JOL3121 | pJHL270 (pro HA + eu NA-M2e) in JOL3062 | This study |
| JOL3122 | pJHL270 empty vector in JOL3062 | This study |
| Plasmid | ||
| pJHL270 pET28a (+) | asd+, CMV eukaryotic promoter, Ptrc prokaryotic promoter, BlaSS secretory signal sequence, pBR322 ori IPTG-inducible expression vector; kanamycin resistant | Lab stock Novagen, USA |
| Primers | ||
| IFN-γ | Forward: CAAAGCCGCACATCAAACA Reverse: TTTCACCTTCTTCACGCCAT | Lab stock |
| IL-4 | Forward: GGAGAGCATCCGGATAGTGA Reverse: TGACGCATGTTGAGGAAGAG | Lab stock |
| GAPDH HA NA M2e | Forward: AGAACATCATCCCAGCGTCC Reverse: CGGCAGGTCAGGTCAACA Forward: AGAATGGAAACCATTCTGGTGGTG Reverse: TTACGCAATCACCAGGCTGCT Forward: CTTGGGCAGGGAACCA Reverse: AGCCCACCCTTTCACT Forward: ATGAGTCTTCTAACCGAG Reverse: TTACTCCAGCTCTA | Lab stock This study This study This study |
| Group | Route | Dosage (CFU/200 µL) | Booster Dosage (CFU/200 µL) | SG Challenge (CFU/200 µL, Oral) | H9N2 Challenge (EID50/100 µL, Intranasal) |
|---|---|---|---|---|---|
| PBS | Oral | 200 µL | 200 µL | 1 × 106 | 1 × 106 |
| JOL3122 | Oral | 1 × 108 | 1 × 108 | 1 × 106 | 1 × 106 |
| JOL3121 | Oral | 1 × 108 | 1 × 108 | 1 × 106 | 1 × 106 |
| Naïve | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhsh, M.; Senevirathne, A.; Riaz, J.; Kwon, J.; Aganja, R.P.; Cabarles, J.C., Jr.; Oh, S.-I.; Lee, J.H. Bivalent Oral Vaccine Using Attenuated Salmonella Gallinarum Delivering HA and NA-M2e Confers Dual Protection Against H9N2 Avian Influenza and Fowl Typhoid in Chickens. Vaccines 2025, 13, 790. https://doi.org/10.3390/vaccines13080790
Bakhsh M, Senevirathne A, Riaz J, Kwon J, Aganja RP, Cabarles JC Jr., Oh S-I, Lee JH. Bivalent Oral Vaccine Using Attenuated Salmonella Gallinarum Delivering HA and NA-M2e Confers Dual Protection Against H9N2 Avian Influenza and Fowl Typhoid in Chickens. Vaccines. 2025; 13(8):790. https://doi.org/10.3390/vaccines13080790
Chicago/Turabian StyleBakhsh, Muhammad, Amal Senevirathne, Jamal Riaz, Jun Kwon, Ram Prasad Aganja, Jaime C. Cabarles, Jr., Sang-Ik Oh, and John Hwa Lee. 2025. "Bivalent Oral Vaccine Using Attenuated Salmonella Gallinarum Delivering HA and NA-M2e Confers Dual Protection Against H9N2 Avian Influenza and Fowl Typhoid in Chickens" Vaccines 13, no. 8: 790. https://doi.org/10.3390/vaccines13080790
APA StyleBakhsh, M., Senevirathne, A., Riaz, J., Kwon, J., Aganja, R. P., Cabarles, J. C., Jr., Oh, S.-I., & Lee, J. H. (2025). Bivalent Oral Vaccine Using Attenuated Salmonella Gallinarum Delivering HA and NA-M2e Confers Dual Protection Against H9N2 Avian Influenza and Fowl Typhoid in Chickens. Vaccines, 13(8), 790. https://doi.org/10.3390/vaccines13080790









