Bioengineering Outer-Membrane Vesicles for Vaccine Development: Strategies, Advances, and Perspectives
Abstract
1. Introduction
2. The Formation, Structure, and Composition of OMVs
3. Vaccine Potential of OMVs
3.1. Distinctive Features of OMVs for Vaccine Development
3.2. Adjuvant Activity of OMVs
3.3. Therapeutic Potential of OMVs for Cancer/Tumour Vaccines
3.4. OMVs and Trained Immunity
3.5. Immune Responses to OMVs
4. OMV-Based Vaccines
4.1. Licenced OMV-Based Vaccines
4.2. OMV-Based Vaccines in Clinical Development
4.3. Trends Emerging in Preclinical Studies: Bioengineered/Modified OMV-Based Vaccines
5. Current Strategies and Latest Updates on OMV Bioengineering
5.1. Selection of OMV Backbone for Vaccine Development
5.2. Reduction in Endotoxin Level
5.3. Strategies to Enhance OMV Production
5.4. Lumen Expression of Heterologous Antigens
5.4.1. Endogenous Loading
5.4.2. Exogenous Loading
5.5. Modifications of OMVs in Terms of Surface Display of Heterologous Antigens
5.5.1. ClyA Fusion
5.5.2. Ice Nucleation Protein (INP) Fusion
5.5.3. OMV Decoration Using Lipoprotein (Lpp) Transport Machinery
5.5.4. The Hemoglobin Protease (Hbp) Display System
5.5.5. Spy Tag (SpT)/Spy Catcher (SpC) System
5.5.6. SnoopTag (SnT)/SnoopCatcher (SnC) System
5.5.7. Display of Biotinylated Antigens on OMV Surfaces via the AvidVax Platform
5.5.8. OMV Surface Decoration by Molecular Painting (MP)
5.5.9. LPS-Binding Peptides for the Display of Antigens on OMV Surfaces
5.5.10. Chemical Conjugation of Antigens to OMVs
5.5.11. Engineering Display of mRNA Antigens on OMV Surfaces
5.6. Strategies to Improve OMV Vaccines’ Cross Protection
5.7. Glycoconjugated and Glycoengineered OMVs
5.8. Nanoparticle-Facilitated OMV Vaccines
5.9. Hybrid OMV-Based Vesicles for Vaccine Development
5.9.1. OMV Fusion with Tumour Cell Membrane
5.9.2. Bacteria–Plant Hybrid Vesicles (BPNs)
5.9.3. Lipid Hybrid OMVs
5.10. In Situ Production of OMVs by Genetically Engineered Bacteria
6. OMVs for Multi-Antigen and Multi-Pathogen Vaccine Development
7. Breakthrough of Artificial Systems Mimicking OMV Features
7.1. Cellular Nanodiscs Based on OMVs for Vaccine Development
7.2. OMV-Based Nanorobots
8. Outlook on OMV Bioengineering Using Synthetic Biology Approaches
8.1. Synthetic OMVs
8.2. A Step Towards Scalable OMV Vaccine Technology: Emerging OMV Isolation and Production Methods
9. Regulatory, Manufacturing, and Translational Challenges in OMV-Based Vaccine Development
10. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, L.; Toyofuku, M.; Hynen, A.L.; Kurosawa, M.; Pessi, G.; Petty, N.K.; Osvath, S.R.; Cárcamo-Oyarce, G.; Gloag, E.S.; Shimoni, R. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat. Commun. 2016, 7, 11220. [Google Scholar] [CrossRef] [PubMed]
- Bishop, D.; Work, E. An extracellular glycolipid produced by Escherichia coli grown under lysine-limiting conditions. Biochem. J. 1965, 96, 567. [Google Scholar] [CrossRef] [PubMed]
- Knox, K.; Vesk, M.; Work, E. Relation between excreted lipopolysaccharide complexes and surface structures of a lysine-limited culture of Escherichia coli. J. Bacteriol. 1966, 92, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Rothfield, L.; Pearlman-Kothencz, M. Synthesis and assembly of bacterial membrane components: A lipopolysaccharide-phospholipid-protein complex excreted by living bacteria. J. Mol. Biol. 1969, 44, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Mergenhagen, S.E.; Bladen, H.A.; Hsu, K.C. Electron microscopic localization of endotoxic lipopolysaccharide in Gram-negagive organisms. Ann. N. Y. Acad. Sci. 1966, 133, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Das, J. Electron microscopic observations on the excretion of cell-wall material by Vibrio cholerae. Microbiology 1967, 49, 1–11. [Google Scholar] [CrossRef] [PubMed]
- DeVoe, I.; Gilchrist, J. Pili on meningococci from primary cultures of nasopharyngeal carriers and cerebrospinal fluid of patients with acute disease. J. Exp. Med. 1975, 141, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Orench-Rivera, N.; Kuehn, M.J. Environmentally controlled bacterial vesicle-mediated export. Cell. Microbiol. 2016, 18, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Cecil, J.D.; Sirisaengtaksin, N.; O’Brien-Simpson, N.M.; Krachler, A.M. Outer membrane vesicle-host cell interactions. Microbiol. Spectr. 2019, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Bauwens, A.; Kunsmann, L.; Marejková, M.; Zhang, W.; Karch, H.; Bielaszewska, M.; Mellmann, A. Intrahost milieu modulates production of outer membrane vesicles, vesicle-associated Shiga toxin 2a and cytotoxicity in Escherichia coli O157: H7 and O104: H4. Environ. Microbiol. Rep. 2017, 9, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Fulsundar, S.; Kulkarni, H.M.; Jagannadham, M.V.; Nair, R.; Keerthi, S.; Sant, P.; Pardesi, K.; Bellare, J.; Chopade, B.A. Molecular characterization of outer membrane vesicles released from Acinetobacter radioresistens and their potential roles in pathogenesis. Microb. Pathog. 2015, 83, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Kothary, M.H.; Gopinath, G.R.; Gangiredla, J.; Rallabhandi, P.V.; Harrison, L.M.; Yan, Q.Q.; Chase, H.R.; Lee, B.; Park, E.; Yoo, Y. Analysis and characterization of proteins associated with outer membrane vesicles secreted by Cronobacter spp. Front. Microbiol. 2017, 8, 134. [Google Scholar]
- Gasperini, G.; Biagini, M.; Arato, V.; Gianfaldoni, C.; Vadi, A.; Norais, N.; Bensi, G.; Delany, I.; Pizza, M.; Arico, B. Outer membrane vesicles (OMV)-based and proteomics-driven antigen selection identifies novel factors contributing to Bordetella pertussis adhesion to epithelial cells. Mol. Cell. Proteom. 2018, 17, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-W.; Kim, S.-C.; Hong, S.-H.; Lee, H.-J. Secretable small RNAs via outer membrane vesicles in periodontal pathogens. J. Dent. Res. 2017, 96, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Mondal, A.; Mitra, S.; Basu, S. Acinetobacter baumannii transfers the bla NDM-1 gene via outer membrane vesicles. J. Antimicrob. Chemother. 2017, 72, 2201–2207. [Google Scholar] [CrossRef] [PubMed]
- Vanhove, A.S.; Duperthuy, M.; Charrière, G.M.; Le Roux, F.; Goudenège, D.; Gourbal, B.; Kieffer-Jaquinod, S.; Couté, Y.; Wai, S.N.; Destoumieux-Garzón, D. Outer membrane vesicles are vehicles for the delivery of Vibrio tasmaniensis virulence factors to oyster immune cells. Environ. Microbiol. 2015, 17, 1152–1165. [Google Scholar] [CrossRef] [PubMed]
- Stentz, R.; Horn, N.; Cross, K.; Salt, L.; Brearley, C.; Livermore, D.M.; Carding, S.R. Cephalosporinases associated with outer membrane vesicles released by Bacteroides spp. protect gut pathogens and commensals against β-lactam antibiotics. J. Antimicrob. Chemother. 2015, 70, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; MacLennan, C.A. Outer membrane vesicle vaccines. Semin. Immunol. 2020, 50, 101433. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Mandujano, A.; Hernández-Cortez, C.; Ibarra, J.A.; Castro-Escarpulli, G. The outer membrane vesicles: Secretion system type zero. Traffic 2017, 18, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Robles, T.; Dillard, R.S.; Cairns, L.S.; Silva-Valenzuela, C.A.; Housman, M.; Ali, A.; Wright, E.R.; Camilli, A. Vibrio cholerae outer membrane vesicles inhibit bacteriophage infection. J. Bacteriol. 2018, 200, 9. [Google Scholar] [CrossRef] [PubMed]
- Urashima, A.; Sanou, A.; Yen, H.; Tobe, T. Enterohaemorrhagic Escherichia coli produces outer membrane vesicles as an active defence system against antimicrobial peptide LL-37. Cell. Microbiol. 2017, 19, e12758. [Google Scholar] [CrossRef] [PubMed]
- Waller, T.; Kesper, L.; Hirschfeld, J.; Dommisch, H.; Kölpin, J.; Oldenburg, J.; Uebele, J.; Hoerauf, A.; Deschner, J.; Jepsen, S. Porphyromonas gingivalis outer membrane vesicles induce selective tumor necrosis factor tolerance in a toll-like receptor 4-and mTOR-dependent manner. Infect. Immun. 2016, 84, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Schertzer, J.W.; Whiteley, M. A bilayer-couple model of bacterial outer membrane vesicle biogenesis. mBio 2012, 3, e00297-11. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, H.M.; Jagannadham, M.V. Biogenesis and multifaceted roles of outer membrane vesicles from Gram-negative bacteria. Microbiology 2014, 160, 2109–2121. [Google Scholar] [CrossRef] [PubMed]
- Pathirana, R.D.; Kaparakis-Liaskos, M. Bacterial membrane vesicles: Biogenesis, immune regulation and pathogenesis. Cell. Microbiol. 2016, 18, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Kaparakis-Liaskos, M.; Ferrero, R.L. Immune modulation by bacterial outer membrane vesicles. Nat. Rev. Immunol. 2015, 15, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Kulp, A.; Kuehn, M.J. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, M.J.; Kesty, N.C. Bacterial outer membrane vesicles and the host–pathogen interaction. Genes. Dev. 2005, 19, 2645–2655. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Ma, Y.; Pang, B.; Zhang, J.; Li, Y.; Rui, Y.; Xu, T.; Zhao, Y.; Qian, Z.; Gu, Y. A cascade targeting strategy based on modified bacterial vesicles for enhancing cancer immunotherapy. J. Nanobiotechnol. 2021, 19, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, D.; van der Laan, J.W.; de Leij, L.; Witholt, B. Release of outer membrane fragments from normally growing Escherichia coli. Biochim. Biophys. Acta Biomembr. 1976, 455, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, A.; Vallström, A.; Petzold, K.; Tegtmeyer, N.; Schleucher, J.; Carlsson, S.; Haas, R.; Backert, S.; Wai, S.N.; Gröbner, G. Biochemical and functional characterization of Helicobacter pylori vesicles. Mol. Microbiol. 2010, 77, 1539–1555. [Google Scholar] [CrossRef] [PubMed]
- Blenkiron, C.; Simonov, D.; Muthukaruppan, A.; Tsai, P.; Dauros, P.; Green, S.; Hong, J.; Print, C.G.; Swift, S.; Phillips, A.R. Uropathogenic Escherichia coli releases extracellular vesicles that are associated with RNA. PLoS ONE 2016, 11, e0160440. [Google Scholar] [CrossRef] [PubMed]
- Dauros-Singorenko, P.; Blenkiron, C.; Phillips, A.; Swift, S. The functional RNA cargo of bacterial membrane vesicles. FEMS Microbiol. Lett. 2018, 365, fny023. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, L.; Toloza, L.; Gimenez, R.; Odena, A.; Oliveira, E.; Aguilar, J.; Badia, J.; Baldomà, L. Proteomic analysis of outer membrane vesicles from the probiotic strain Escherichia coli Nissle 1917. Proteomics 2014, 14, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Irving, A.T.; Mimuro, H.; Kufer, T.A.; Lo, C.; Wheeler, R.; Turner, L.J.; Thomas, B.J.; Malosse, C.; Gantier, M.P.; Casillas, L.N. The immune receptor NOD1 and kinase RIP2 interact with bacterial peptidoglycan on early endosomes to promote autophagy and inflammatory signaling. Cell Host Microbe 2014, 15, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Roier, S.; Zingl, F.G.; Cakar, F.; Durakovic, S.; Kohl, P.; Eichmann, T.O.; Klug, L.; Gadermaier, B.; Weinzerl, K.; Prassl, R. A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria. Nat. Commun. 2016, 7, 10515. [Google Scholar] [CrossRef] [PubMed]
- Lappann, M.; Otto, A.; Becher, D.; Vogel, U. Comparative proteome analysis of spontaneous outer membrane vesicles and purified outer membranes of Neisseria meningitidis. J. Bacteriol. 2013, 195, 4425–4435. [Google Scholar] [CrossRef] [PubMed]
- Scorza, F.B.; Doro, F.; Rodríguez-Ortega, M.J.; Stella, M.; Liberatori, S.; Taddei, A.R.; Serino, L.; Moriel, D.G.; Nesta, B.; Fontana, M.R. Proteomics characterization of outer membrane vesicles from the extraintestinal pathogenic Escherichia coli ΔtolR IHE3034 mutant. Mol. Cell. Proteom. 2008, 7, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.; Garaguso, I.; Adu-Bobie, J.; Doro, F.; Taddei, A.R.; Biolchi, A.; Brunelli, B.; Giuliani, M.M.; Pizza, M.; Norais, N. Outer membrane vesicles from group B Neisseria meningitidis Δgna33 mutant: Proteomic and immunological comparison with detergent-derived outer membrane vesicles. Proteomics 2006, 6, 1856–1866. [Google Scholar] [CrossRef] [PubMed]
- Van de Waterbeemd, B.; Mommen, G.P.; Pennings, J.L.; Eppink, M.H.; Wijffels, R.H.; van der Pol, L.A.; de Jong, A.P. Quantitative proteomics reveals distinct differences in the protein content of outer membrane vesicle vaccines. J. Proteome Res. 2013, 12, 1898–1908. [Google Scholar] [CrossRef] [PubMed]
- Bonnington, K.; Kuehn, M. Protein selection and export via outer membrane vesicles. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2014, 1843, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Manabe, T.; Kato, M.; Ueno, T.; Kawasaki, K. Flagella proteins contribute to the production of outer membrane vesicles from Escherichia coli W3110. Biochem. Biophys. Res. Commun. 2013, 441, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Chaudhuri, K. Association of cholera toxin with Vibrio cholerae outer membrane vesicles which are internalized by human intestinal epithelial cells. FEBS Lett. 2011, 585, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Horstman, A.L.; Kuehn, M.J. Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. J. Biol. Chem. 2000, 275, 12489–12496. [Google Scholar] [CrossRef] [PubMed]
- Wai, S.N.; Lindmark, B.; Söderblom, T.; Takade, A.; Westermark, M.; Oscarsson, J.; Jass, J.; Richter-Dahlfors, A.; Mizunoe, Y.; Uhlin, B.E. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell 2003, 115, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Lindmark, B.; Rompikuntal, P.K.; Vaitkevicius, K.; Song, T.; Mizunoe, Y.; Uhlin, B.E.; Guerry, P.; Wai, S.N. Outer membrane vesicle-mediated release of cytolethal distending toxin (CDT) from Campylobacter jejuni. BMC Microbiol. 2009, 9, 220. [Google Scholar] [CrossRef] [PubMed]
- Fiocca, R.; Necchi, V.; Sommi, P.; Ricci, V.; Telford, J.; Cover, T.L.; Solcia, E. Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. J. Pathol. 1999, 188, 220–226. [Google Scholar] [CrossRef]
- Van Der Pol, L.; Stork, M.; van der Ley, P. Outer membrane vesicles as platform vaccine technology. Biotechnol. J. 2015, 10, 1689–1706. [Google Scholar] [CrossRef] [PubMed]
- Veith, P.D.; Chen, Y.-Y.; Gorasia, D.G.; Chen, D.; Glew, M.D.; O’Brien-Simpson, N.M.; Cecil, J.D.; Holden, J.A.; Reynolds, E.C. Porphyromonas gingivalis outer membrane vesicles exclusively contain outer membrane and periplasmic proteins and carry a cargo enriched with virulence factors. J. Proteome Res. 2014, 13, 2420–2432. [Google Scholar] [CrossRef] [PubMed]
- Diallo, I.; Provost, P. RNA-sequencing analyses of small bacterial RNAs and their emergence as virulence factors in host-pathogen interactions. Int. J. Mol. Sci. 2020, 21, 1627. [Google Scholar] [CrossRef] [PubMed]
- Stanton, B.A. Extracellular vesicles and host–pathogen interactions: A review of inter-kingdom signaling by small noncoding RNA. Genes 2021, 12, 1010. [Google Scholar] [CrossRef] [PubMed]
- Zavan, L.; Bitto, N.J.; Johnston, E.L.; Greening, D.W.; Kaparakis-Liaskos, M. Helicobacter pylori growth stage determines the size, protein composition, and preferential cargo packaging of outer membrane vesicles. Proteomics 2019, 19, 1800209. [Google Scholar] [CrossRef] [PubMed]
- Adriani, R.; Gargari, S.L.M.; Nazarian, S.; Sarvary, S.; Noroozi, N. Immunogenicity of Vibrio cholerae outer membrane vesicles secreted at various environmental conditions. Vaccine 2018, 36, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Devos, S.; Van Putte, W.; Vitse, J.; Van Driessche, G.; Stremersch, S.; Van Den Broek, W.; Raemdonck, K.; Braeckmans, K.; Stahlberg, H.; Kudryashev, M. Membrane vesicle secretion and prophage induction in multidrug-resistant Stenotrophomonas maltophilia in response to ciprofloxacin stress. Environ. Microbiol. 2017, 19, 3930–3937. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Dauros-Singorenko, P.; Whitcombe, A.; Payne, L.; Blenkiron, C.; Phillips, A.; Swift, S. Analysis of the Escherichia coli extracellular vesicle proteome identifies markers of purity and culture conditions. J. Extracell. Vesicles. 2019, 8, 1632099. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, M.; Schild, S.; Kaparakis-Liaskos, M.; Eberl, L. Composition and functions of bacterial membrane vesicles. Nat. Rev. Microbiol. 2023, 21, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Van der Ley, P.; van den Dobbelsteen, G. Next-generation outer membrane vesicle vaccines against Neisseria meningitidis based on nontoxic LPS mutants. Hum. Vaccines 2011, 7, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, R.; Fernández, S.; Zayas, C.; Acosta, A.; Sarmiento, M.E.; Ferro, V.A.; Rosenqvist, E.; Campa, C.; Cardoso, D.; Garcia, L. Bacterial outer membrane vesicles and vaccine applications. Front. Immunol. 2014, 5, 121. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.N.; Leiman, S.A.; Kuehn, M.J. Naturally produced outer membrane vesicles from Pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components. Infect. Immun. 2010, 78, 3822–3831. [Google Scholar] [CrossRef] [PubMed]
- Arigita, C.; Jiskoot, W.; Westdijk, J.; van Ingen, C.; Hennink, W.E.; Crommelin, D.J.; Kersten, G.F. Stability of mono-and trivalent meningococcal outer membrane vesicle vaccines. Vaccine 2004, 22, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Gerritzen, M.J. Production of Bioengineered Outer Membrane Vesicles as a Vaccine Platform. Ph.D. Thesis, Wageningen University and Research, Wageningen, The Netherlands, 2019. [Google Scholar]
- Li, R.; Liu, Q. Engineered bacterial outer membrane vesicles as multifunctional delivery platforms. Front. Mater. 2020, 7, 202. [Google Scholar] [CrossRef]
- Rappuoli, R.; Black, S.; Bloom, D.E. Vaccines and global health: In search of a sustainable model for vaccine development and delivery. Sci. Transl. Med. 2019, 11, eaaw2888. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ji, H.; Kong, X.; Lei, P.; Yang, Q.; Wu, W.; Jin, L.; Sun, D. Bacterial ghosts-based vaccine and drug delivery systems. Pharmaceutics 2021, 13, 1892. [Google Scholar] [CrossRef] [PubMed]
- Pagliari, S.; Dema, B.; Sanchez-Martinez, A.; Zurbia-Flores, G.M.; Rollier, C.S. DNA vaccines: History, molecular mechanisms and future perspectives. J. Mol. Biol. 2023, 435, 168297. [Google Scholar] [CrossRef] [PubMed]
- Andey, T.; Soni, S.; Modi, S. Conventional vaccination methods: Inactivated and live attenuated vaccines. In Advanced Vaccination Technologies for Infectious and Chronic Diseases; Elsevier: Amsterdam, The Netherlands, 2024; pp. 37–50. [Google Scholar]
- Aminnezhad, S.; Alavi, M.; Azarakhsh, Y. Advancements in liposome-based vaccines: A comprehensive review of mechanisms and applications. Micro. Nano. Bio. Asp. 2024, 3, 19–28. [Google Scholar]
- Minor, P.D. Live attenuated vaccines: Historical successes and current challenges. Virology 2015, 479, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Gote, V.; Bolla, P.K.; Kommineni, N.; Butreddy, A.; Nukala, P.K.; Palakurthi, S.S.; Khan, W. A comprehensive review of mRNA vaccines. Int. J. Mol. Sci. 2023, 24, 2700. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Pellett, S. Recent developments in vaccine design: From live vaccines to recombinant toxin vaccines. Toxins 2023, 15, 563. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.O.; Zha, L.; Cabral-Miranda, G.; Bachmann, M.F. Major findings and recent advances in virus–like particle (VLP)-based vaccines. Semin. Immunol. 2017, 34, 123–132. [Google Scholar] [CrossRef] [PubMed]
- McCann, N.; O’Connor, D.; Lambe, T.; Pollard, A.J. Viral vector vaccines. Curr. Opin. Immunol. 2022, 77, 102210. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liang, B.; Wang, W.; Li, L.; Feng, N.; Zhao, Y.; Wang, T.; Yan, F.; Yang, S.; Xia, X. Viral vectored vaccines: Design, development, preventive and therapeutic applications in human diseases. Signal Transduct. Target. Ther. 2023, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Lua, L.H.; Connors, N.K.; Sainsbury, F.; Chuan, Y.P.; Wibowo, N.; Middelberg, A.P. Bioengineering virus like particles as vaccines. Biotechnol. Bioeng. 2014, 111, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.; Machado, D.; Theizen, T.H.; Guarnieri, J.P.O.; Bernardes, B.G.; Gomide, G.P.; Corat, M.A.F.; Abbehausen, C.; Módena, J.L.P.; Melo, C.F.O.R. Outer membrane vesicles from Neisseria Meningitidis (Proteossome) used for nanostructured Zika virus vaccine production. Sci. Rep. 2018, 8, 8290. [Google Scholar] [CrossRef] [PubMed]
- Muralinath, M.; Kuehn, M.J.; Roland, K.L.; Curtiss III, R. Immunization with Salmonella enterica serovar Typhimurium-derived outer membrane vesicles delivering the pneumococcal protein PspA confers protection against challenge with Streptococcus pneumoniae. Infect. Immun. 2011, 79, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Schild, S.; Nelson, E.J.; Bishop, A.L.; Camilli, A. Characterization of Vibrio cholerae outer membrane vesicles as a candidate vaccine for cholera. Infect. Immun. 2009, 77, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Tavano, R.; Franzoso, S.; Cecchini, P.; Cartocci, E.; Capecchi, B.; Arico, B.; Papini, E. Self-adjuvant and immune-stimulating activity of the anti-meningococcus B vaccine candidate Neisseria meningitidis adhesin A as a soluble recombinant antigen (NadA (Delta) 351-405) or as part of bacterial outer membrane vesicles. New Biotechnol. 2009, 25, S6. [Google Scholar] [CrossRef]
- Minguet, S.; Dopfer, E.P.; Pollmer, C.; Freudenberg, M.A.; Galanos, C.; Reth, M.; Huber, M.; Schamel, W.W. Enhanced B cell activation mediated by TLR4 and BCR crosstalk. Eur. J. Immunol. 2008, 38, 2475–2487. [Google Scholar] [CrossRef] [PubMed]
- Tiku, V.; Tan, M.-W. Host immunity and cellular responses to bacterial outer membrane vesicles. Trends Immunol. 2021, 42, 1024–1036. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Li, R.; Huang, X.; Liu, Q. Outer membrane vesicles: Current status and future direction of these novel vaccine adjuvants. Front. Microbiol. 2018, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Reza Aghasadeghi, M.; Sharifat Salmani, A.; Mehdi Sadat, S.; Javadi, F.; Memarnejadian, A.; Vahabpour, R.; Zabihollahi, R.; Moshiri, A.; Davar Siadat, S. Application of outer membrane vesicle of Neisseria meningitidis serogroup B as a new adjuvant to induce strongly Th1-oriented responses against HIV-1. Curr. HIV Res. 2011, 9, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Kim, S.-H.; Kang, W.; Choi, Y.S.; Lee, S.-H.; Lee, S.-R.; You, S.; Lee, H.K.; Chang, K.-T.; Shin, E.-C. Adjuvant effect of bacterial outer membrane vesicles with penta-acylated lipopolysaccharide on antigen-specific T cell priming. Vaccine 2011, 29, 8293–8301. [Google Scholar] [CrossRef] [PubMed]
- Sardiñas, G.; Reddin, K.; Pajon, R.; Gorringe, A. Outer membrane vesicles of Neisseria lactamica as a potential mucosal adjuvant. Vaccine 2006, 24, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Zhang, M.; Yang, J.; Zhu, Z.; Cao, W.; Dong, C. Therapeutic cancer vaccines: Advancements, challenges and prospects. Signal Transduct. Target. Ther. 2023, 8, 450. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Del. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Gujrati, V.; Prakash, J.; Malekzadeh-Najafabadi, J.; Stiel, A.; Klemm, U.; Mettenleiter, G.; Aichler, M.; Walch, A.; Ntziachristos, V. Bioengineered bacterial vesicles as biological nano-heaters for optoacoustic imaging. Nat. Commun. 2019, 10, 1114. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Wolfram, J. Extracellular vesicles versus synthetic nanoparticles for drug delivery. Nat. Rev. Mater. 2021, 6, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Godet, I.; Shin, Y.J.; Ju, J.A.; Ye, I.C.; Wang, G.; Gilkes, D.M. Fate-mapping post-hypoxic tumor cells reveals a ROS-resistant phenotype that promotes metastasis. Nat. Commun. 2019, 10, 4862. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Zheng, J.H.; Nguyen, V.H.; Jiang, S.-N.; Kim, D.-Y.; Szardenings, M.; Min, J.H.; Hong, Y.; Choy, H.E.; Min, J.-J. RGD peptide cell-surface display enhances the targeting and therapeutic efficacy of attenuated Salmonella-mediated cancer therapy. Theranostics 2016, 6, 1672. [Google Scholar] [CrossRef] [PubMed]
- Gujrati, V.; Kim, S.; Kim, S.-H.; Min, J.J.; Choy, H.E.; Kim, S.C.; Jon, S. Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy. ACS Nano 2014, 8, 1525–1537. [Google Scholar] [CrossRef] [PubMed]
- Pérez Jorge, G.; Gontijo, M.T.P.; Brocchi, M. Salmonella enterica and outer membrane vesicles are current and future options for cancer treatment. Front. Cell. Infect. Microbiol. 2023, 13, 1293351. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ma, N.; Cheng, K.; Feng, Q.; Ma, X.; Yue, Y.; Li, Y.; Zhang, T.; Gao, X.; Liang, J. Bacteria-derived nanovesicles enhance tumour vaccination by trained immunity. Nat. Nanotechnol. 2024, 19, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhu, F.; Cheng, K.; Ma, N.; Ma, X.; Feng, Q.; Xu, C.; Gao, X.; Wang, X.; Shi, J. Outer membrane vesicle based nanohybrids target tumor associated macrophages to enhance trained immunity related vaccine-generated antitumor activity. Adv. Mater. 2023, 35, 2306158. [Google Scholar] [CrossRef] [PubMed]
- Turner, L.; Bitto, N.J.; Steer, D.L.; Lo, C.; D’Costa, K.; Ramm, G.; Shambrook, M.; Hill, A.F.; Ferrero, R.L.; Kaparakis-Liaskos, M. Helicobacter pylori outer membrane vesicle size determines their mechanisms of host cell entry and protein content. Front. Immunol. 2018, 9, 1466. [Google Scholar] [CrossRef] [PubMed]
- Cañas, M.-A.; Giménez, R.; Fábrega, M.-J.; Toloza, L.; Baldomà, L.; Badia, J. Outer membrane vesicles from the probiotic Escherichia coli Nissle 1917 and the commensal ECOR12 enter intestinal epithelial cells via clathrin-dependent endocytosis and elicit differential effects on DNA damage. PLoS ONE 2016, 11, e0160374. [Google Scholar] [CrossRef] [PubMed]
- Kunsmann, L.; Rüter, C.; Bauwens, A.; Greune, L.; Glüder, M.; Kemper, B.; Fruth, A.; Wai, S.N.; He, X.; Lloubes, R. Virulence from vesicles: Novel mechanisms of host cell injury by Escherichia coli O104: H4 outbreak strain. Sci. Rep. 2015, 5, 13252. [Google Scholar] [CrossRef] [PubMed]
- O'donoghue, E.J.; Krachler, A.M. Mechanisms of outer membrane vesicle entry into host cells. Cell. Microbiol. 2016, 18, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Vanaja, S.K.; Russo, A.J.; Behl, B.; Banerjee, I.; Yankova, M.; Deshmukh, S.D.; Rathinam, V.A. Bacterial outer membrane vesicles mediate cytosolic localization of LPS and caspase-11 activation. Cell 2016, 165, 1106–1119. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, E.J.; Sirisaengtaksin, N.; Browning, D.F.; Bielska, E.; Hadis, M.; Fernandez-Trillo, F.; Alderwick, L.; Jabbari, S.; Krachler, A.M. Lipopolysaccharide structure impacts the entry kinetics of bacterial outer membrane vesicles into host cells. PLoS Pathog. 2017, 13, e1006760. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Meng, R.; Tang, Y.; Zhao, K.; Liang, F.; Zhang, R.; Xue, Q.; Chen, F.; Xiao, X.; Wang, H. Toll-like receptor 4 signaling licenses the cytosolic transport of lipopolysaccharide from bacterial outer membrane vesicles. Shock 2019, 51, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Jäger, J.; Keese, S.; Roessle, M.; Steinert, M.; Schromm, A.B. Fusion of Legionella pneumophila outer membrane vesicles with eukaryotic membrane systems is a mechanism to deliver pathogen factors to host cell membranes. Cell. Microbiol. 2015, 17, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Kuzmich, N.N.; Sivak, K.V.; Chubarev, V.N.; Porozov, Y.B.; Savateeva-Lyubimova, T.N.; Peri, F. TLR4 signaling pathway modulators as potential therapeutics in inflammation and sepsis. Vaccines 2017, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Nascimento, L.; Massari, P.; Wetzler, L.M. The role of TLR2 in infection and immunity. Front. Immunol. 2012, 3, 79. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, F.; Smith, K.D.; Ozinsky, A.; Hawn, T.R.; Yi, E.C.; Goodlett, D.R.; Eng, J.K.; Akira, S.; Underhill, D.M.; Aderem, A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001, 410, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K. A Toll-like receptor recognizes bacterial DNA. Nature 2000, 408, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.J.; Behl, B.; Banerjee, I.; Rathinam, V.A. Emerging insights into noncanonical inflammasome recognition of microbes. J. Mol. Biol. 2018, 430, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Gnopo, Y.M.; Watkins, H.C.; Stevenson, T.C.; DeLisa, M.P.; Putnam, D. Designer outer membrane vesicles as immunomodulatory systems–reprogramming bacteria for vaccine delivery. Adv. Drug Del. Rev. 2017, 114, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Alfini, R.; Di Benedetto, R.; Necchi, F.; Schiavo, F.; Mancini, F.; Carducci, M.; Palmieri, E.; Balocchi, C.; Gasperini, G. GMMA is a versatile platform to design effective multivalent combination vaccines. Vaccines 2020, 8, 540. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Palmieri, E.; Samnuan, K.; Ricchetti, B.; Oldrini, D.; McKay, P.F.; Wu, G.; Thorne, L.; Fooks, A.R.; McElhinney, L.M. Generalized Modules for Membrane Antigens (GMMA), an outer membrane vesicle-based vaccine platform, for efficient viral antigen delivery. J. Extracell. Vesicles. 2022, 11, e12247. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Alfini, R.; Di Benedetto, R.; Necchi, F.; Schiavo, F.; Mancini, F.; Carducci, M.; Oldrini, D.; Pitirollo, O.; Gasperini, G. Generalized modules for membrane antigens as carrier for polysaccharides: Impact of sugar length, density, and attachment site on the immune response elicited in animal models. Front. Immunol. 2021, 12, 719315. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.; Micoli, F.; Necchi, F.; Pizza, M.; Berlanda Scorza, F.; Rossi, O. GMMA-based vaccines: The known and the unknown. Front. Immunol. 2021, 12, 715393. [Google Scholar] [CrossRef] [PubMed]
- Piccioli, D.; Bartolini, E.; Micoli, F. GMMA as a ‘plug and play’technology to tackle infectious disease to improve global health: Context and perspectives for the future. Expert Rev. Vaccines 2022, 21, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Van de Waterbeemd, B.; Zomer, G.; Kaaijk, P.; Ruiterkamp, N.; Wijffels, R.H.; van den Dobbelsteen, G.P.; van der Pol, L.A. Improved production process for native outer membrane vesicle vaccine against Neisseria meningitidis. PLoS ONE 2013, 8, e65157. [Google Scholar] [CrossRef] [PubMed]
- Gerritzen, M.J.; Stangowez, L.; van de Waterbeemd, B.; Martens, D.E.; Wijffels, R.H.; Stork, M. Continuous production of Neisseria meningitidis outer membrane vesicles. Appl. Microbiol. Biotechnol. 2019, 103, 9401–9410. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.; Rossi, O.; Necchi, F.; Micoli, F. OMV vaccines and the role of TLR agonists in immune response. Int. J. Mol. Sci. 2020, 21, 4416. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, H.; Yang, C.; Wu, Y.; Zhou, X.; Liu, H.; Wang, Y. Bacterial outer membrane vesicles as a platform for biomedical applications: An update. J. Control. Release 2020, 323, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Koeberling, O.; Ispasanie, E.; Hauser, J.; Rossi, O.; Pluschke, G.; Caugant, D.A.; Saul, A.; MacLennan, C.A. A broadly-protective vaccine against meningococcal disease in sub-Saharan Africa based on generalized modules for membrane antigens (GMMA). Vaccine 2014, 32, 2688–2695. [Google Scholar] [CrossRef] [PubMed]
- Bexsero Summary of Product Characteristics. Available online: https://www.medicines.org.uk/emc/product/5168/smpc (accessed on 23 May 2025).
- Ladhani, S.N.; Andrews, N.; Parikh, S.R.; Campbell, H.; White, J.; Edelstein, M.; Bai, X.; Lucidarme, J.; Borrow, R.; Ramsay, M.E. Vaccination of infants with meningococcal group B vaccine (4CMenB) in England. N. Engl. J. Med. 2020, 382, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Muzzi, A.; Brozzi, A.; Serino, L.; Bodini, M.; Abad, R.; Caugant, D.; Comanducci, M.; Lemos, A.P.; Gorla, M.C.; Křížová, P. Genetic meningococcal antigen typing system (gMATS): A genotyping tool that predicts 4CMenB strain coverage worldwide. Vaccine 2019, 37, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.; Costantino, C.; Martinon-Torres, F.; Westerholt, S.; Lambeth, C.; Chen, Z.; Lumley, J.; Marcek, T.; Johnson, D.; Wilck, M. A phase 4, open-label study to evaluate the safety and immunogenicity of DTaP5-HBV-IPV-Hib in children previously vaccinated with DTaP2-HBV-IPV-Hib or DTaP5-HBV-IPV-Hib (V419-016). Hum. Vaccines Immunother. 2024, 20, 2310900. [Google Scholar] [CrossRef] [PubMed]
- EMA. Procomvax. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/procomvax (accessed on 11 June 2025).
- Santosham, M.; Wolff, M.; Reid, R.; Hohenboken, M.; Bateman, M.; Goepp, J.; Cortese, M.; Sack, D.; Hill, J.; Newcomer, W. The efficacy in Navajo infants of a conjugate vaccine consisting of Haemophilus influenzae type b polysaccharide and Neisseria meningitidis outer-membrane protein complex. N. Engl. J. Med. 1991, 324, 1767–1772. [Google Scholar] [CrossRef] [PubMed]
- Ahonkhai, V.; Lukacs, L.; Jonas, L.; Calandra, G. Clinical experience with PedvaxHIB, a conjugate vaccine of Haemophilus influenzae type b polysaccharide-Neisseria meningitidis outer membrane protein. Vaccine 1991, 9, S38–S41. [Google Scholar] [CrossRef] [PubMed]
- Gorringe, A.R.; Pajón, R. Bexsero: A multicomponent vaccine for prevention of meningococcal disease. Hum. Vaccines Immunother. 2012, 8, 174–183. [Google Scholar] [CrossRef] [PubMed]
- O’Ryan, M.; Stoddard, J.; Toneatto, D.; Wassil, J.; Dull, P.M. A multi-component meningococcal serogroup B vaccine (4CMenB): The clinical development program. Drugs 2014, 74, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Arnold, R.; Galloway, Y.; McNicholas, A.; O’Hallahan, J. Effectiveness of a vaccination programme for an epidemic of meningococcal B in New Zealand. Vaccine 2011, 29, 7100–7106. [Google Scholar] [CrossRef] [PubMed]
- Sierra, G.; Campa, H.; Varcacel, N.; Garcia, I.; Izquierdo, P.; Sotolongo, P.; Casanueva, G.; Rico, C.; Rodriguez, C.; Terry, M. Vaccine against group B Neisseria meningitidis: Protection trial and mass vaccination results in Cuba. NIPH Ann. 1991, 14, 195–207. [Google Scholar] [PubMed]
- Sierra-González, V.G. Cuban meningococcal vaccine VA-MENGOC-BC: 30 years of use and future potential. MEDICC Rev. 2019, 21, 19–27. [Google Scholar] [PubMed]
- Johnson, B. GSK’s gonorrhea vaccine receives fast-track designation to expedite clinical trials. Nat. Med. 2023, 29, 2146–2147. [Google Scholar] [CrossRef] [PubMed]
- Obiero, C.W.; Ndiaye, A.G.; Sciré, A.S.; Kaunyangi, B.M.; Marchetti, E.; Gone, A.M.; Schütte, L.D.; Riccucci, D.; Auerbach, J.; Saul, A. A phase 2a randomized study to evaluate the safety and immunogenicity of the 1790GAHB generalized modules for membrane antigen vaccine against Shigella sonnei administered intramuscularly to adults from a shigellosis-endemic country. Front. Immunol. 2017, 8, 1884. [Google Scholar] [CrossRef] [PubMed]
- Rossi, O.; Citiulo, F.; Giannelli, C.; Cappelletti, E.; Gasperini, G.; Mancini, F.; Acquaviva, A.; Raso, M.M.; Sollai, L.; Alfini, R. A next-generation GMMA-based vaccine candidate to fight shigellosis. NPJ Vaccines 2023, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Leroux-Roels, I.; Maes, C.; Mancini, F.; Jacobs, B.; Sarakinou, E.; Alhatemi, A.; Joye, J.; Grappi, S.; Cilio, G.L.; Serry-Bangura, A. Safety and Immunogenicity of a 4-Component Generalized Modules for Membrane Antigens Shigella Vaccine in Healthy European Adults: Randomized, Phase 1/2 Study. J. Infect. Dis. 2024, 230, e971–e984. [Google Scholar] [CrossRef] [PubMed]
- Hanumunthadu, B.; Kanji, N.; Owino, N.; Da Silva, C.F.; Robinson, H.; White, R.; Ferruzzi, P.; Nakakana, U.; Canals, R.; Pollard, A.J. Salmonella Vaccine Study in Oxford (SALVO) trial: Protocol for an observer-participant blind randomised placebo-controlled trial of the iNTS-GMMA vaccine within a European cohort. BMJ Open 2023, 13, e072938. [Google Scholar] [CrossRef] [PubMed]
- Skidmore, P.D.; Canals, R.; Ramasamy, M.N. The iNTS-GMMA vaccine: A promising step in non-typhoidal Salmonella vaccine development. Expert. Rev. Vaccines 2023, 22, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Marsay, L.; Dold, C.; Green, C.; Rollier, C.; Norheim, G.; Sadarangani, M.; Shanyinde, M.; Brehony, C.; Thompson, A.; Sanders, H. A novel meningococcal outer membrane vesicle vaccine with constitutive expression of FetA: A phase I clinical trial. J. Infect. 2015, 71, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Keiser, P.; Biggs-Cicatelli, S.; Moran, E.; Schmiel, D.; Pinto, V.; Burden, R.; Miller, L.; Moon, J.; Bowden, R.; Cummings, J. A phase 1 study of a meningococcal native outer membrane vesicle vaccine made from a group B strain with deleted lpxL1 and synX, over-expressed factor H binding protein, two PorAs and stabilized OpcA expression. Vaccine 2011, 29, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Roier, S.; Leitner, D.R.; Iwashkiw, J.; Schild-Prüfert, K.; Feldman, M.F.; Krohne, G.; Reidl, J.; Schild, S. Intranasal immunization with nontypeable Haemophilus influenzae outer membrane vesicles induces cross-protective immunity in mice. PLoS ONE 2012, 7, e42664. [Google Scholar] [CrossRef]
- Rivera, J.; Cordero, R.J.; Nakouzi, A.S.; Frases, S.; Nicola, A.; Casadevall, A. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proc. Natl. Acad. Sci. USA 2010, 107, 19002–19007. [Google Scholar] [CrossRef] [PubMed]
- Avila-Calderón, E.D.; Lopez-Merino, A.; Jain, N.; Peralta, H.; Lopez-Villegas, E.O.; Sriranganathan, N.; Boyle, S.M.; Witonsky, S.; Contreras-Rodríguez, A. Characterization of outer membrane vesicles from Brucella melitensis and protection induced in mice. J. Immunol. Res. 2012, 2012, 352493. [Google Scholar]
- Bartolini, E.; Ianni, E.; Frigimelica, E.; Petracca, R.; Galli, G.; Berlanda Scorza, F.; Norais, N.; Laera, D.; Giusti, F.; Pierleoni, A. Recombinant outer membrane vesicles carrying Chlamydia muridarum HtrA induce antibodies that neutralize chlamydial infection in vitro. J. Extracell. Vesicles. 2013, 2, 20181. [Google Scholar] [CrossRef] [PubMed]
- Bishop, A.L.; Schild, S.; Patimalla, B.; Klein, B.; Camilli, A. Mucosal immunization with Vibrio cholerae outer membrane vesicles provides maternal protection mediated by antilipopolysaccharide antibodies that inhibit bacterial motility. Infect. Immun. 2010, 78, 4402–4420. [Google Scholar] [CrossRef] [PubMed]
- Schild, S.; Nelson, E.J.; Camilli, A. Immunization with Vibrio cholerae outer membrane vesicles induces protective immunity in mice. Infect. Immun. 2008, 76, 4554–4563. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, M.; Siadat, S.D.; Mirabzadeh, E.; Keramati, M.; Vaziri, F.; Shafiei, M.; Shahcheraghi, F. Evaluation of antibody responses to outer membrane vesicles (OMVs) and killed whole cell of Vibrio cholerae O1 El Tor in immunized mice. Iran. J. Microbiol. 2019, 11, 212. [Google Scholar] [CrossRef]
- Kesavalu, L.; Ebersole, J.; Machen, R.; Holt, S. Porphyromonas gingivalis virulence in mice: Induction of immunity to bacterial components. Infect. Immun. 1992, 60, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, E.B.; Prudencio, C.R.; De Gaspari, E. Experimental studies using OMV in a new platform of SARS-CoV-2 vaccines. Hum. Vaccines Immunother. 2021, 17, 2965–2968. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hammer, L.A.; Daamen, J.; Stork, M.; Egilmez, N.K.; Russell, M.W. Microencapsulated IL-12 drives genital tract immune responses to intranasal gonococcal outer membrane vesicle vaccine and induces resistance to vaginal infection with diverse strains of Neisseria gonorrhoeae. Msphere 2023, 8, e00388-22. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hammer, L.A.; Liu, W.; Hobbs, M.M.; Zielke, R.A.; Sikora, A.E.; Jerse, A.E.; Egilmez, N.K.; Russell, M.W. Experimental vaccine induces Th1-driven immune responses and resistance to Neisseria gonorrhoeae infection in a murine model. Mucosal Immunol. 2017, 10, 1594–1608. [Google Scholar] [CrossRef] [PubMed]
- Piliou, S.; Farman, T.A.; Marini, A.; Manoharan, S.; Mastroeni, P. Commensal Neisseria cinerea outer membrane vesicles as a platform for the delivery of meningococcal and gonococcal antigens to the immune system. Vaccine 2023, 41, 7671–7681. [Google Scholar] [CrossRef] [PubMed]
- Rappazzo, C.G.; Watkins, H.C.; Guarino, C.M.; Chau, A.; Lopez, J.L.; DeLisa, M.P.; Leifer, C.A.; Whittaker, G.R.; Putnam, D. Recombinant M2e outer membrane vesicle vaccines protect against lethal influenza A challenge in BALB/c mice. Vaccine 2016, 34, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Ji, H.; Guo, X.; Li, Y.; Ren, T.; Dong, H.; Liu, J.; Liu, Y.; Shi, X.; He, B. Nanoparticle reinforced bacterial outer-membrane vesicles effectively prevent fatal infection of carbapenem-resistant Klebsiella pneumoniae. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102148. [Google Scholar] [CrossRef] [PubMed]
- Martora, F.; Pinto, F.; Folliero, V.; Cammarota, M.; Dell’Annunziata, F.; Squillaci, G.; Galdiero, M.; Morana, A.; Schiraldi, C.; Giovane, A. Isolation, characterization and analysis of pro-inflammatory potential of Klebsiella pneumoniae outer membrane vesicles. Microb. Pathog. 2019, 136, 103719. [Google Scholar] [CrossRef] [PubMed]
- Salverda, M.L.; Meinderts, S.M.; Hamstra, H.-J.; Wagemakers, A.; Hovius, J.W.; van der Ark, A.; Stork, M.; van der Ley, P. Surface display of a borrelial lipoprotein on meningococcal outer membrane vesicles. Vaccine 2016, 34, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Nieves, W.; Petersen, H.; Judy, B.M.; Blumentritt, C.A.; Russell-Lodrigue, K.; Roy, C.J.; Torres, A.G.; Morici, L.A. A Burkholderia pseudomallei outer membrane vesicle vaccine provides protection against lethal sepsis. Clin. Vaccine Immunol. 2014, 21, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Beernink, P.T.; Ispasanie, E.; Lewis, L.A.; Ram, S.; Moe, G.R.; Granoff, D.M. A meningococcal native outer membrane vesicle vaccine with attenuated endotoxin and overexpressed factor H binding protein elicits gonococcal bactericidal antibodies. J. Infect. Dis. 2019, 219, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Gorringe, A.R.; Taylor, S.; Brookes, C.; Matheson, M.; Finney, M.; Kerr, M.; Hudson, M.; Findlow, J.; Borrow, R.; Andrews, N. Phase I safety and immunogenicity study of a candidate meningococcal disease vaccine based on Neisseria lactamica outer membrane vesicles. Clin. Vaccine Immunol. 2009, 16, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Daleke-Schermerhorn, M.H.; Felix, T.; Soprova, Z.; ten Hagen-Jongman, C.M.; Vikström, D.; Majlessi, L.; Beskers, J.; Follmann, F.; De Punder, K.; van Der Wel, N.N. Decoration of outer membrane vesicles with multiple antigens by using an autotransporter approach. Appl. Environ. Microbiol. 2014, 80, 5854–5865. [Google Scholar] [CrossRef] [PubMed]
- Asensio, C.J.; Gaillard, M.E.; Moreno, G.; Bottero, D.; Zurita, E.; Rumbo, M.; Van der Ley, P.; Van der Ark, A.; Hozbor, D. Outer membrane vesicles obtained from Bordetella pertussis Tohama expressing the lipid A deacylase PagL as a novel acellular vaccine candidate. Vaccine 2011, 29, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Pierson, T.; Matrakas, D.; Taylor, Y.U.; Manyam, G.; Morozov, V.N.; Zhou, W.; van Hoek, M.L. Proteomic characterization and functional analysis of outer membrane vesicles of Francisella novicida suggests possible role in virulence and use as a vaccine. J. Proteome Res. 2011, 10, 954–967. [Google Scholar] [CrossRef] [PubMed]
- Alaniz, R.C.; Deatherage, B.L.; Lara, J.C.; Cookson, B.T. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J. Immunol. 2007, 179, 7692–7701. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.; Moreno, G.; Bottero, D.; Gaillard, M.E.; Fingermann, M.; Graieb, A.; Rumbo, M.; Hozbor, D. Outer membrane vesicles as acellular vaccine against pertussis. Vaccine 2008, 26, 4639–4646. [Google Scholar] [CrossRef] [PubMed]
- Raeven, R.H.; van der Maas, L.; Tilstra, W.; Uittenbogaard, J.P.; Bindels, T.H.; Kuipers, B.; van der Ark, A.; Pennings, J.L.; van Riet, E.; Jiskoot, W. Immunoproteomic profiling of Bordetella pertussis outer membrane vesicle vaccine reveals broad and balanced humoral immunogenicity. J. Proteome Res. 2015, 14, 2929–2942. [Google Scholar] [CrossRef] [PubMed]
- Burton, C.; Best, E.; Broom, M.; Heffernan, H.; Briggs, S.; Webb, R. Pediatric invasive meningococcal disease, Auckland, New Zealand (Aotearoa), 2004–2020. Emerg. Infect. Dis. 2023, 29, 686. [Google Scholar] [CrossRef] [PubMed]
- Bjune, G.; Høiby, E.; Grønnesby, J.; Arnesen, Ø.; Fredriksen, J.H.; Lindbak, A.; Nøkleby, H.; Rosenqvist, E.; Solberg, L.; Closs, O. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 1991, 338, 1093–1096. [Google Scholar] [CrossRef] [PubMed]
- Caron, F.; Du Chatelet, I.P.; Leroy, J.-P.; Ruckly, C.; Blanchard, M.; Bohic, N.; Massy, N.; Morer, I.; Floret, D.; Delbos, V. From tailor-made to ready-to-wear meningococcal B vaccines: Longitudinal study of a clonal meningococcal B outbreak. Lancet Infect. Dis. 2011, 11, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Granoff, D.; Anderson, E.; Osterholm, M.; Holmes, S.; McHugh, J.; Belshe, R.; Medley, F.; Murphy, T. Differences in the immunogenicity of three Haemophilus influenzae type b conjugate vaccines in infants. J. Pediatr. 1992, 121, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Procomvax Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/procomvax-epar-product-information_en.pdf (accessed on 28 May 2025).
- Comvax Summary of Product Characteristics. Available online: https://www.drugs.com/pro/comvax-vaccine.html (accessed on 28 May 2025).
- Vaxelis Summary of Product Characteristics. Available online: https://www.medicines.org.uk/emc/product/12264/smpc (accessed on 28 May 2025).
- Gilsdorf, J.R. Hib vaccines: Their impact on Haemophilus influenzae type b disease. J. Infect. Dis. 2021, 224, S321–S330. [Google Scholar] [CrossRef] [PubMed]
- Gerke, C.; Colucci, A.M.; Giannelli, C.; Sanzone, S.; Vitali, C.G.; Sollai, L.; Rossi, O.; Martin, L.B.; Auerbach, J.; Di Cioccio, V. Production of a Shigella sonnei vaccine based on generalized modules for membrane antigens (GMMA), 1790GAHB. PLoS ONE 2015, 10, e0134478. [Google Scholar] [CrossRef] [PubMed]
- Schager, A.E.; Dominguez-Medina, C.C.; Necchi, F.; Micoli, F.; Goh, Y.S.; Goodall, M.; Flores-Langarica, A.; Bobat, S.; Cook, C.N.; Arcuri, M. IgG responses to porins and lipopolysaccharide within an outer membrane-based vaccine against nontyphoidal Salmonella develop at discordant rates. mBio 2018, 9, e02379-17. [Google Scholar] [CrossRef] [PubMed]
- De Benedetto, G.; Alfini, R.; Cescutti, P.; Caboni, M.; Lanzilao, L.; Necchi, F.; Saul, A.; MacLennan, C.; Rondini, S.; Micoli, F. Characterization of O-antigen delivered by Generalized Modules for Membrane Antigens (GMMA) vaccine candidates against nontyphoidal Salmonella. Vaccine 2017, 35, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Nakakana, U.N.; Berlanda Scorza, F. Towards a four-component GMMA-based vaccine against Shigella. Vaccines 2022, 10, 328. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Rondini, S.; Alfini, R.; Lanzilao, L.; Necchi, F.; Negrea, A.; Rossi, O.; Brandt, C.; Clare, S.; Mastroeni, P. Comparative immunogenicity and efficacy of equivalent outer membrane vesicle and glycoconjugate vaccines against nontyphoidal Salmonella. Proc. Natl. Acad. Sci. USA 2018, 115, 10428–10433. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Rossi, O.; Conti, V.; Launay, O.; Sciré, A.S.; Aruta, M.G.; Nakakana, U.N.; Marchetti, E.; Rappuoli, R.; Saul, A. Antibodies elicited by the Shigella sonnei GMMA vaccine in adults trigger complement-mediated serum bactericidal activity: Results from a phase 1 dose escalation trial followed by a booster extension. Front. Immunol. 2021, 12, 671325. [Google Scholar] [CrossRef] [PubMed]
- Launay, O.; Lewis, D.J.; Anemona, A.; Loulergue, P.; Leahy, J.; Scire, A.S.; Maugard, A.; Marchetti, E.; Zancan, S.; Huo, Z. Safety profile and immunologic responses of a novel vaccine against Shigella sonnei administered intramuscularly, intradermally and intranasally: Results from two parallel randomized phase 1 clinical studies in healthy adult volunteers in Europe. eBioMedicine 2017, 22, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Kapulu, M.C.; Nakakana, U.; Scire, A.S.; Sarakinou, E.; Conti, V.; Rossi, O.; Acquaviva, A.; Necchi, F.; Obiero, C.W.; Martin, L.B. Complement-mediated serum bactericidal activity of antibodies elicited by the Shigella sonnei GMMA vaccine in adults from a shigellosis-endemic country: Exploratory analysis of a Phase 2a randomized study. Front. Immunol. 2022, 13, 971866. [Google Scholar] [CrossRef] [PubMed]
- Launay, O.; Ndiaye, A.G.; Conti, V.; Loulergue, P.; Scire, A.S.; Landre, A.M.; Ferruzzi, P.; Nedjaai, N.; Schütte, L.D.; Auerbach, J. Booster vaccination with GVGH Shigella sonnei 1790GAHB GMMA vaccine compared to single vaccination in unvaccinated healthy European adults: Results from a phase 1 clinical trial. Front. Immunol. 2019, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, G.; Fei, X.; Tian, Y.; Wang, S.; Shi, H. Engineered bacterial outer membrane vesicles with lipidated heterologous antigen as an adjuvant-free vaccine platform for Streptococcus suis. Appl. Environ. Microbiol. 2023, 89, e02047-22. [Google Scholar] [CrossRef] [PubMed]
- Gerritzen, M.J.; Martens, D.E.; Wijffels, R.H.; van der Pol, L.; Stork, M. Bioengineering bacterial outer membrane vesicles as vaccine platform. Biotechnol. Adv. 2017, 35, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Vader, P.; Fuhrmann, G. Approaches to surface engineering of extracellular vesicles. Adv. Drug Del. Rev. 2021, 173, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Rossi, O.; Pesce, I.; Giannelli, C.; Aprea, S.; Caboni, M.; Citiulo, F.; Valentini, S.; Ferlenghi, I.; MacLennan, C.A.; D'Oro, U. Modulation of endotoxicity of Shigella generalized modules for membrane antigens (GMMA) by genetic lipid A modifications: Relative activation of TLR4 and TLR2 pathways in different mutants. J. Biol. Chem. 2014, 289, 24922–24935. [Google Scholar] [CrossRef] [PubMed]
- Rossi, O.; Caboni, M.; Negrea, A.; Necchi, F.; Alfini, R.; Micoli, F.; Saul, A.; MacLennan, C.A.; Rondini, S.; Gerke, C. Toll-like receptor activation by generalized modules for membrane antigens from lipid A mutants of Salmonella enterica serovars Typhimurium and Enteritidis. Clin. Vaccine Immunol. 2016, 23, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Tondi, S.; Clemente, B.; Esposito, C.; Sammicheli, C.; Tavarini, S.; Martin, L.B.; Rossi, O.; Micoli, F.; Bartolini, E.; Brazzoli, M. Dissecting in vitro the activation of human immune response induced by Shigella sonnei GMMA. Front. Cell. Infect. Microbiol. 2022, 12, 767153. [Google Scholar] [CrossRef] [PubMed]
- Klimentová, J.; Stulík, J. Methods of isolation and purification of outer membrane vesicles from gram-negative bacteria. Microbiol. Res. 2015, 170, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Zhang, X.; Guo, X.; Wu, G.; Cao, S.; Zhang, Y.; Meng, X.; Wang, T.; Wang, Y.; Song, Y. Delivery of Yersinia pestis antigens via Escherichia coli outer membrane vesicles offered improved protection against plague. Msphere 2024, 9, e00330-24. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg van Saparoea, H.B.; Houben, D.; Kuijl, C.; Luirink, J.; Jong, W.S. Combining protein ligation systems to expand the functionality of semi-synthetic outer membrane vesicle nanoparticles. Front. Microbiol. 2020, 11, 890. [Google Scholar] [CrossRef] [PubMed]
- Weyant, K.B.; Oloyede, A.; DeLisa, M.P. On-Demand Vaccine Production via Dock-and-Display of Biotinylated Antigens on Bacterial Extracellular Vesicles. In Bacterial Extracellular Vesicles: Methods and Protocols; Springer: New York, NY, USA, 2024; pp. 195–216. [Google Scholar]
- Van der Ley, P.A.; Zariri, A.; van Riet, E.; Oosterhoff, D.; Kruiswijk, C.P. An intranasal OMV-based vaccine induces high mucosal and systemic protecting immunity against a SARS-CoV-2 infection. Front. Immunol. 2021, 12, 781280. [Google Scholar] [CrossRef] [PubMed]
- Siadat, S.D.; Vaziri, F.; Eftekhary, M.; Karbasian, M.; Moshiri, A.; Aghasadeghi, M.R.; Ardestani, M.S.; Alitappeh, M.A.; Arsang, A.; Fateh, A. Preparation and evaluation of a new lipopolysaccharide-based conjugate as a vaccine candidate for brucellosis. Osong Public. Health Res. Perspect. 2015, 6, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Gasperini, G.; Alfini, R.; Arato, V.; Mancini, F.; Aruta, M.; Kanvatirth, P.; Pickard, D.; Necchi, F.; Saul, A.; Rossi, O. Salmonella Paratyphi A outer membrane vesicles displaying Vi polysaccharide as a multivalent vaccine against enteric fever. Infect. Immun. 2021, 89, 9. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Valentine, J.L.; Huang, C.-J.; Endicott, C.E.; Moeller, T.D.; Rasmussen, J.A.; Fletcher, J.R.; Boll, J.M.; Rosenthal, J.A.; Dobruchowska, J. Outer membrane vesicles displaying engineered glycotopes elicit protective antibodies. Proc. Natl. Acad. Sci. USA 2016, 113, E3609–E3618. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Fang, R.H.; Thamphiwatana, S.; Luk, B.T.; Li, J.; Angsantikul, P.; Zhang, Q.; Hu, C.-M.J.; Zhang, L. Modulating antibacterial immunity via bacterial membrane-coated nanoparticles. Nano Lett. 2015, 15, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.-Z.; Li, Z.-H.; Bai, X.-F.; Liu, C.-J.; Zhang, X.-Z. Hybrid vesicles based on autologous tumor cell membrane and bacterial outer membrane to enhance innate immune response and personalized tumor immunotherapy. Nano Lett. 2021, 21, 8609–8618. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Zhao, R.; Li, Y.; Qi, Y.; Wang, Y.; Zhang, Y.; Qin, H.; Qin, Y.; Chen, L.; Li, C. Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via Plug-and-Display technology. Nat. Commun. 2021, 12, 2041. [Google Scholar] [CrossRef] [PubMed]
- Fantappiè, L.; de Santis, M.; Chiarot, E.; Carboni, F.; Bensi, G.; Jousson, O.; Margarit, I.; Grandi, G. Antibody-mediated immunity induced by engineered Escherichia coli OMVs carrying heterologous antigens in their lumen. J. Extracell. Vesicles. 2014, 3, 24015. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Goyette-Desjardins, G.; Nothaft, H.; Valguarnera, E.; Szymanski, C.M.; Segura, M.; Feldman, M.F. Glycoengineered outer membrane vesicles: A novel platform for bacterial vaccines. Sci. Rep. 2016, 6, 24931. [Google Scholar] [CrossRef] [PubMed]
- Pritsch, M.; Ben-Khaled, N.; Chaloupka, M.; Kobold, S.; Berens-Riha, N.; Peter, A.; Liegl, G.; Schubert, S.; Hoelscher, M.; Löscher, T. Comparison of intranasal outer membrane vesicles with cholera toxin and injected MF59C. 1 as adjuvants for malaria transmission blocking antigens AnAPN1 and Pfs48/45. J. Immunol. Res. 2016, 2016, 3576028. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.; Aebischer, T. Recombinant outer membrane vesicles to augment antigen-specific live vaccine responses. Vaccine 2009, 27, 6748–6754. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, K.; Wu, Y.; Yue, Y.; Cheng, K.; Feng, Q.; Ma, X.; Liang, J.; Ma, N.; Liu, G. Antigen Capture and Immune Modulation by Bacterial Outer Membrane Vesicles as In Situ Vaccine for Cancer Immunotherapy Post-Photothermal Therapy. Small 2022, 18, 2107461. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Xu, J.; Li, Y.; Cheng, K.; Feng, Q.; Ma, X.; Ma, N.; Zhang, T.; Wang, X.; Zhao, X. Antigen-bearing outer membrane vesicles as tumour vaccines produced in situ by ingested genetically engineered bacteria. Nat. Biomed. Eng. 2022, 6, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Feng, Q.; Wang, J.; Zhao, X. Bacterial outer membrane vesicle-based cancer nanovaccines. Cancer Biol. Med. 2022, 19, 1290. [Google Scholar] [CrossRef] [PubMed]
- Oliver, K.J.; Reddin, K.M.; Bracegirdle, P.; Hudson, M.J.; Borrow, R.; Feavers, I.M.; Robinson, A.; Cartwright, K.; Gorringe, A.R. Neisseria lactamica protects against experimental meningococcal infection. Infect. Immun. 2002, 70, 3621–3626. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.; Troncoso, G.; Ferreiros, C.; Criado, M. Evaluation of cross-reactive antigens as determinants of cross-bactericidal activity in pathogenic and commensal Neisseria. Vaccine 2001, 19, 3390–3398. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.; Oster, P.; Arnold, R.; Tatley, M.; Næss, L.; Aaberge, I.; Galloway, Y.; McNicholas, A.; O'Hallahan, J.; Rosenqvist, E. Vaccines against meningococcal serogroup B disease containing outer membrane vesicles (OMV): Lessons from past programs and implications for the future. Hum. Vaccines Immunother. 2013, 9, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Needham, B.D.; Carroll, S.M.; Giles, D.K.; Georgiou, G.; Whiteley, M.; Trent, M.S. Modulating the innate immune response by combinatorial engineering of endotoxin. Proc. Natl. Acad. Sci. USA 2013, 110, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Van Der Ley, P.; Steeghs, L.; Hamstra, H.J.; ten Hove, J.; Zomer, B.; van Alphen, L. Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: Influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect. Immun. 2001, 69, 5981–5990. [Google Scholar] [CrossRef] [PubMed]
- Fisseha, M.; Chen, P.; Brandt, B.; Kijek, T.; Moran, E.; Zollinger, W. Characterization of native outer membrane vesicles from lpxL mutant strains of Neisseria meningitidis for use in parenteral vaccination. Infect. Immun. 2005, 73, 4070–4080. [Google Scholar] [CrossRef] [PubMed]
- Koeberling, O.; Seubert, A.; Granoff, D.M. Bactericidal antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed factor H–binding protein and genetically attenuated endotoxin. J. Infect. Dis. 2008, 198, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Koeberling, O.; Giuntini, S.; Seubert, A.; Granoff, D.M. Meningococcal outer membrane vesicle vaccines derived from mutant strains engineered to express factor H binding proteins from antigenic variant groups 1 and 2. Clin. Vaccine Immunol. 2009, 16, 156–162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van de Waterbeemd, B.; Streefland, M.; Van der Ley, P.; Zomer, B.; Van Dijken, H.; Martens, D.; Wijffels, R.; Van der Pol, L. Improved OMV vaccine against Neisseria meningitidis using genetically engineered strains and a detergent-free purification process. Vaccine 2010, 28, 4810–4816. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, M.B.; Pinto, V.; Keiser, P.B.; Zollinger, W. Evaluation of a whole-blood cytokine release assay for use in measuring endotoxin activity of group B Neisseria meningitidis vaccines made from lipid A acylation mutants. Clin. Vaccine Immunol. 2010, 17, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Keiser, P.B.; Gibbs, B.T.; Coster, T.S.; Moran, E.E.; Stoddard, M.B.; Labrie III, J.E.; Schmiel, D.H.; Pinto, V.; Chen, P.; Zollinger, W.D. A phase 1 study of a group B meningococcal native outer membrane vesicle vaccine made from a strain with deleted lpxL2 and synX and stable expression of opcA. Vaccine 2010, 28, 6970–6976. [Google Scholar] [CrossRef] [PubMed]
- Zariri, A.; Beskers, J.; van de Waterbeemd, B.; Hamstra, H.J.; Bindels, T.H.; van Riet, E.; van Putten, J.P.; van der Ley, P. Meningococcal outer membrane vesicle composition-dependent activation of the innate immune response. Infect. Immun. 2016, 84, 3024–3033. [Google Scholar] [CrossRef] [PubMed]
- Watkins, H.C.; Rappazzo, C.G.; Higgins, J.S.; Sun, X.; Brock, N.; Chau, A.; Misra, A.; Cannizzo, J.P.; King, M.R.; Maines, T.R. Safe recombinant outer membrane vesicles that display M2e elicit heterologous influenza protection. Mol. Ther. 2017, 25, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Kim, K.-S.; Lee, S.-R.; Kim, E.; Kim, M.-S.; Lee, E.-Y.; Gho, Y.S.; Kim, J.-W.; Bishop, R.E.; Chang, K.-T. Structural modifications of outer membrane vesicles to refine them as vaccine delivery vehicles. Biochim. Biophys. Acta, Biomembr. 2009, 1788, 2150–2159. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.L.; Fonseca, S.; Miquel-Clopés, A.; Cross, K.; Kok, K.-S.; Wegmann, U.; Gil-Cardoso, K.; Bentley, E.G.; Al Katy, S.H.; Coombes, J.L. Bioengineering commensal bacteria-derived outer membrane vesicles for delivery of biologics to the gastrointestinal and respiratory tract. J. Extracell. Vesicles 2019, 8, 1632100. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Howlader, D.R.; Ta, A.; Mitra, S.; Das, S.; Koley, H. Retinoic acid pre-treatment down regulates V. cholerae outer membrane vesicles induced acute inflammation and enhances mucosal immunity. Vaccine 2017, 35, 3534–3547. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Jiang, A.; Ou, X.; Zhou, J.; Li, Z.; Liang, C.; Huang, L.-L.; Wu, G.; Xie, H.-Y. Metal-polyphenol “prison” attenuated bacterial outer membrane vesicle for chemodynamics promoted in situ tumor vaccines. Biomaterials 2024, 304, 122396. [Google Scholar] [CrossRef] [PubMed]
- Rossi, O.; Citiulo, F.; Mancini, F. Outer membrane vesicles: Moving within the intricate labyrinth of assays that can predict risks of reactogenicity in humans. Hum. Vaccines Immunother. 2021, 17, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Molenaar-de Backer, M.W.; Doodeman, P.; Rezai, F.; Verhagen, L.M.; van der Ark, A.; Plagmeijer, E.M.; Metz, B.; van Vlies, N.; Ophorst, O.; Raeven, R.H. In vitro alternative for reactogenicity assessment of outer membrane vesicle based vaccines. Sci. Rep. 2023, 13, 12675. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xue, H.; Du, X.; Nyaruaba, R.; Yang, H.; Wei, H. Outer membrane vesicles generated by an exogenous bacteriophage lysin and protection against Acinetobacter baumannii infection. J. Nanobiotechnol. 2024, 22, 273. [Google Scholar] [CrossRef] [PubMed]
- McCaig, W.D.; Loving, C.L.; Hughes, H.R.; Brockmeier, S.L. Characterization and vaccine potential of outer membrane vesicles produced by Haemophilus parasuis. PLoS ONE 2016, 11, e0149132. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Quan, Y.; Chen, P.; Zhuge, X.; Qin, T.; Chen, S.; Peng, D.; Liu, X. Development of High-Production Bacterial Biomimetic Vesicles for Inducing Mucosal Immunity Against Avian Pathogenic Escherichia coli. Int. J. Mol. Sci. 2024, 25, 12055. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, I.A.; Kuehn, M.J. Stress-induced outer membrane vesicle production by Pseudomonas aeruginosa. J. Bacteriol. 2013, 195, 2971–2981. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Weerapana, E.; Ulanovskaya, O.; Sun, F.; Liang, H.; Ji, Q.; Ye, Y.; Fu, Y.; Zhou, L.; Li, J. Proteome-wide quantification and characterization of oxidation-sensitive cysteines in pathogenic bacteria. Cell Host Microbe 2013, 13, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Van de Waterbeemd, B.; Zomer, G.; van den IJssel, J.; van Keulen, L.; Eppink, M.H.; van der Ley, P.; van der Pol, L.A. Cysteine depletion causes oxidative stress and triggers outer membrane vesicle release by Neisseria meningitidis; implications for vaccine development. PLoS ONE 2013, 8, e54314. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, I.; Schwarz, H.; Hirota, Y.; Henning, U. Cell envelope and shape of Escherichia coli: Multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J. Bacteriol. 1978, 136, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Bernadac, A.; Gavioli, M.; Lazzaroni, J.-C.; Raina, S.; Lloubès, R. Escherichia coli tol-pal mutants form outer membrane vesicles. J. Bacteriol. 1998, 180, 4872–4878. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, J.-i.; Hamada, N.; Kuramitsu, H.K. The autolysin of Porphyromonas gingivalis is involved in outer membrane vesicle release. FEMS Microbiol. Lett. 2002, 216, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Adu-Bobie, J.; Lupetti, P.; Brunelli, B.; Granoff, D.; Norais, N.; Ferrari, G.; Grandi, G.; Rappuoli, R.; Pizza, M. GNA33 of Neisseria meningitidis is a lipoprotein required for cell separation, membrane architecture, and virulence. Infect. Immun. 2004, 72, 1914–1919. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Sinha, R.; Mitobe, J.; Koley, H. Development of a cost-effective vaccine candidate with outer membrane vesicles of a tolA-disrupted Shigella boydii strain. Vaccine 2016, 34, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Turner, L.; Praszkier, J.; Hutton, M.L.; Steer, D.; Ramm, G.; Kaparakis-Liaskos, M.; Ferrero, R.L. Increased outer membrane vesicle formation in a Helicobacter pylori tolB mutant. Helicobacter 2015, 20, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C.; Rodriguez, D.L.; Kuehn, M.J. NlpI-mediated modulation of outer membrane vesicle production through peptidoglycan dynamics in Escherichia coli. Microbiologyopen 2015, 4, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Kulp, A.J.; Sun, B.; Ai, T.; Manning, A.J.; Orench-Rivera, N.; Schmid, A.K.; Kuehn, M.J. Genome-wide assessment of outer membrane vesicle production in Escherichia coli. PLoS ONE 2015, 10, e0139200. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, S.; Saleem, M.; Feavers, I.M.; Wheeler, J.X.; Care, R.; Derrick, J.P. Dissection of the function of the RmpM periplasmic protein from Neisseria meningitidis. Microbiology 2016, 162, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Premjani, V.; Tilley, D.; Gruenheid, S.; Le Moual, H.; Samis, J.A. Enterohemorrhagic Escherichia coli OmpT regulates outer membrane vesicle biogenesis. FEMS Microbiol. Lett. 2014, 355, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Elhenawy, W.; Bording-Jorgensen, M.; Valguarnera, E.; Haurat, M.F.; Wine, E.; Feldman, M.F. LPS remodeling triggers formation of outer membrane vesicles in Salmonella. mBio 2016, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Sidik, S.; Kottwitz, H.; Benjamin, J.; Ryu, J.; Jarrar, A.; Garduno, R.; Rohde, J.R. A Shigella flexneri virulence plasmid encoded factor controls production of outer membrane vesicles. G3 2014, 4, 2493–2503. [Google Scholar] [CrossRef] [PubMed]
- McBroom, A.J.; Johnson, A.P.; Vemulapalli, S.; Kuehn, M.J. Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J. Bacteriol. 2006, 188, 5385–5392. [Google Scholar] [CrossRef] [PubMed]
- Bos, M.P.; Tefsen, B.; Voet, P.; Weynants, V.; Van Putten, J.P.; Tommassen, J. Function of neisserial outer membrane phospholipase A in autolysis and assessment of its vaccine potential. Infect. Immun. 2005, 73, 2222–2231. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berlanda Scorza, F.; Colucci, A.M.; Maggiore, L.; Sanzone, S.; Rossi, O.; Ferlenghi, I.; Pesce, I.; Caboni, M.; Norais, N.; Di Cioccio, V. High yield production process for Shigella outer membrane particles. PLoS ONE 2012, 7, e35616. [Google Scholar] [CrossRef] [PubMed]
- Necchi, F.; Stefanetti, G.; Alfini, R.; Palmieri, E.; Carducci, M.; Di Benedetto, R.; Schiavo, F.; Aruta, M.G.; Giusti, F.; Ferlenghi, I. Neisseria meningitidis factor H binding protein surface exposure on Salmonella typhimurium GMMA is critical to induce an effective immune response against both diseases. Pathogens 2021, 10, 726. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Przysiecki, C.; Flanagan, E.; Bello-Irizarry, S.N.; Ionescu, R.; Muratova, O.; Dobrescu, G.; Lambert, L.; Keister, D.; Rippeon, Y. Sustained high-titer antibody responses induced by conjugating a malarial vaccine candidate to outer-membrane protein complex. Proc. Natl. Acad. Sci. USA 2006, 103, 18243–18248. [Google Scholar] [CrossRef] [PubMed]
- Westermark, M.; Oscarsson, J.; Mizunoe, Y.; Urbonaviciene, J.; Uhlin, B.E. Silencing and activation of ClyA cytotoxin expression in Escherichia coli. J. Bacteriol. 2000, 182, 6347–6357. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Doody, A.M.; Chen, D.J.; Cremona, G.H.; Shuler, M.L.; Putnam, D.; DeLisa, M.P. Engineered bacterial outer membrane vesicles with enhanced functionality. J. Mol. Biol. 2008, 380, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Tzokov, S.B.; Wyborn, N.R.; Stillman, T.J.; Jamieson, S.; Czudnochowski, N.; Artymiuk, P.J.; Green, J.; Bullough, P.A. Structure of the hemolysin E (HlyE, ClyA, and SheA) channel in its membrane-bound form. J. Biol. Chem. 2006, 281, 23042–23049. [Google Scholar] [CrossRef] [PubMed]
- Eifler, N.; Vetsch, M.; Gregorini, M.; Ringler, P.; Chami, M.; Philippsen, A.; Fritz, A.; Müller, S.A.; Glockshuber, R.; Engel, A. Cytotoxin ClyA from Escherichia coli assembles to a 13-meric pore independent of its redox-state. EMBO J. 2006, 25, 2652–2661. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.J.; Osterrieder, N.; Metzger, S.M.; Buckles, E.; Doody, A.M.; DeLisa, M.P.; Putnam, D. Delivery of foreign antigens by engineered outer membrane vesicle vaccines. Proc. Natl. Acad. Sci. USA 2010, 107, 3099–3104. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, Z.; Shi, X.; Shen, M. Engineered cancer cell membranes: An emerging agent for efficient cancer theranostics. Exploration 2022, 2, 20210171. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yan, Q.; Chen, J.; He, Y.; Wang, J.; Zhang, H.; Yu, Z.; Li, L. Molecular characterization of an ice nucleation protein variant (inaQ) from Pseudomonas syringae and the analysis of its transmembrane transport activity in Escherichia coli. Int. J. Biol. Sci. 2012, 8, 1097. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-L.; Oh, J.; Singh, S.; Chen, R.; Chen, W. Functional assembly of minicellulosomes on the Saccharomyces cerevisiae cell surface for cellulose hydrolysis and ethanol production. Appl. Environ. Microbiol. 2009, 75, 6087–6093. [Google Scholar] [CrossRef] [PubMed]
- Amalia, L.; Tsai, S.-L. Functionalization of OMVs for biocatalytic applications. Membranes 2023, 13, 459. [Google Scholar] [CrossRef] [PubMed]
- Cowles, C.E.; Li, Y.; Semmelhack, M.F.; Cristea, I.M.; Silhavy, T.J. The free and bound forms of Lpp occupy distinct subcellular locations in Escherichia coli. Mol. Microbiol. 2011, 79, 1168–1181. [Google Scholar] [CrossRef] [PubMed]
- Basto, A.P.; Leitão, A. Targeting TLR2 for vaccine development. J. Immunol. Res. 2014, 2014, 619410. [Google Scholar] [CrossRef] [PubMed]
- Irene, C.; Fantappiè, L.; Caproni, E.; Zerbini, F.; Anesi, A.; Tomasi, M.; Zanella, I.; Stupia, S.; Prete, S.; Valensin, S. Bacterial outer membrane vesicles engineered with lipidated antigens as a platform for Staphylococcus aureus vaccine. Proc. Natl. Acad. Sci. USA 2019, 116, 21780–21788. [Google Scholar] [CrossRef] [PubMed]
- Jong, W.S.; Daleke-Schermerhorn, M.H.; Vikström, D.; ten Hagen-Jongman, C.M.; de Punder, K.; van der Wel, N.N.; van de Sandt, C.E.; Rimmelzwaan, G.F.; Follmann, F.; Agger, E.M. An autotransporter display platform for the development of multivalent recombinant bacterial vector vaccines. Microb. Cell Fact. 2014, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, K.; Daleke-Schermerhorn, M.H.; Jong, W.S.; ten Hagen-Jongman, C.M.; van Opzeeland, F.; Simonetti, E.; Luirink, J.; de Jonge, M.I. Salmonella outer membrane vesicles displaying high densities of pneumococcal antigen at the surface offer protection against colonization. Vaccine 2015, 33, 2022–2029. [Google Scholar] [CrossRef] [PubMed]
- Van Bloois, E.; Winter, R.T.; Kolmar, H.; Fraaije, M.W. Decorating microbes: Surface display of proteins on Escherichia coli. Trends Biotechnol. 2011, 29, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Jong, W.S.; Soprova, Z.; de Punder, K.; ten Hagen-Jongman, C.M.; Wagner, S.; Wickström, D.; de Gier, J.-W.; Andersen, P.; van der Wel, N.N.; Luirink, J. A structurally informed autotransporter platform for efficient heterologous protein secretion and display. Microb. Cell Fact. 2012, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; Chen, L.; Rosenthal, J.A.; Putnam, D.; DeLisa, M.P. Microbial biosynthesis of designer outer membrane vesicles. Curr. Opin. Biotechnol. 2014, 29, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, B.; Fierer, J.O.; Celik, E.; Chittock, E.C.; Schwarz-Linek, U.; Moy, V.T.; Howarth, M. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc. Natl. Acad. Sci. USA 2012, 109, E690–E697. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg van Saparoea, H.B.; Houben, D.; de Jonge, M.I.; Jong, W.S.; Luirink, J. Display of recombinant proteins on bacterial outer membrane vesicles by using protein ligation. Appl. Environ. Microbiol. 2018, 84, e02567-17. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Lin, X.; He, Y.; Zhang, B.; Zhou, N.; Huang, J.-d. A bacterial outer membrane vesicle-based click vaccine elicits potent immune response against Staphylococcus aureus in mice. Front. Immunol. 2023, 14, 1088501. [Google Scholar] [CrossRef] [PubMed]
- Veggiani, G.; Nakamura, T.; Brenner, M.D.; Gayet, R.V.; Yan, J.; Robinson, C.V.; Howarth, M. Programmable polyproteams built using twin peptide superglues. Proc. Natl. Acad. Sci. USA 2016, 113, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Fierer, J.O.; Veggiani, G.; Howarth, M. SpyLigase peptide–peptide ligation polymerizes affibodies to enhance magnetic cancer cell capture. Proc. Natl. Acad. Sci. USA 2014, 111, E1176–E1181. [Google Scholar] [CrossRef] [PubMed]
- Antenucci, F.; Ovsepian, A.; Wrobel, A.; Winther-Larsen, H.C.; Bojesen, A.M. Design and characterization of a novel tool for the antigenic enrichment of Actinobacillus pleuropneumoniae outer membrane. Pathogens 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Weyant, K.B.; Oloyede, A.; Pal, S.; Liao, J.; Jesus, M.R.-D.; Jaroentomeechai, T.; Moeller, T.D.; Hoang-Phou, S.; Gilmore, S.F.; Singh, R. A modular vaccine platform enabled by decoration of bacterial outer membrane vesicles with biotinylated antigens. Nat. Commun. 2023, 14, 464. [Google Scholar] [CrossRef] [PubMed]
- Medof, M.E.; Walter, E.I.; Roberts, W.L.; Haas, R.; Rosenberry, T.L. Decay accelerating factor of complement is anchored to cells by a C-terminal glycolipid. Biochemistry 1986, 25, 6740–6747. [Google Scholar] [CrossRef] [PubMed]
- Paulick, M.G.; Bertozzi, C.R. The glycosylphosphatidylinositol anchor: A complex membrane-anchoring structure for proteins. Biochemistry 2008, 47, 6991–7000. [Google Scholar] [CrossRef] [PubMed]
- Zaruba, M.; Roschitz, L.; Sami, H.; Ogris, M.; Gerner, W.; Metzner, C. Surface modification of E. coli outer membrane vesicles with glycosylphosphatidylinositol-anchored proteins: Generating pro/eukaryote chimera constructs. Membranes 2021, 11, 428. [Google Scholar] [CrossRef] [PubMed]
- Xhindoli, D.; Pacor, S.; Benincasa, M.; Scocchi, M.; Gennaro, R.; Tossi, A. The human cathelicidin LL-37—A pore-forming antibacterial peptide and host-cell modulator. Biochim. Biophys. Acta, Biomembr. 2016, 1858, 546–566. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Cheng, X.; Feng, B.; Fan, D.; Liu, X.; Xie, R.; Luo, T.; Wegner, S.V.; Ma, D.; Chen, F. Engineering versatile bacteria-derived outer membrane vesicles: An adaptable platform for advancing cancer immunotherapy. Adv. Sci. 2024, 11, 2400049. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, L.O.; Schenkman, R.P.; Perciani, C.T.; Carneiro, S.M.; Dias, W.O.; Tanizaki, M.M. Optimization of the conjugation method for a serogroup B/C meningococcal vaccine. Biotechnol. Appl. Biochem. 2006, 45, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, R.; Alfini, R.; Carducci, M.; Aruta, M.G.; Lanzilao, L.; Acquaviva, A.; Palmieri, E.; Giannelli, C.; Necchi, F.; Saul, A. Novel simple conjugation chemistries for decoration of GMMA with heterologous antigens. Int. J. Mol. Sci. 2021, 22, 10180. [Google Scholar] [CrossRef] [PubMed]
- Berti, F.; Micoli, F. Improving efficacy of glycoconjugate vaccines: From chemical conjugates to next generation constructs. Curr. Opin. Immunol. 2020, 65, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Li, L.; Huang, Y.; Delcassian, D.; Chahal, J.; Han, J.; Shi, Y.; Sadtler, K.; Gao, W.; Lin, J. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 2019, 37, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. How COVID unlocked the power of RNA vaccines. Nature 2021, 589, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Blass, E.; Ott, P.A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat. Rev. Clin. Oncol. 2021, 18, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.; Zhang, F.; Liu, Y.; Wang, Z.; Yu, G.; Liang, B.; Niu, G.; Su, T.; Zhu, G.; Lu, G. A bi-adjuvant nanovaccine that potentiates immunogenicity of neoantigen for combination immunotherapy of colorectal cancer. Sci. Adv. 2020, 6, eaaw6071. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lilley, D.M. Structure of a rare non-standard sequence k-turn bound by L7Ae protein. Nucleic Acids Res. 2014, 42, 4734–4740. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.; Zhang, Y.; Fenley, M.O.; Li, H. Molecular basis of box C/D RNA-protein interactions: Cocrystal structure of archaeal L7Ae and a box C/D RNA. Structure 2004, 12, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, Y.; Nie, G.; Zhao, X. mRNA delivery platform based on bacterial outer membrane vesicles for tumor vaccine. Bio-protocol 2023, 13, e4774. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, X.; Yue, Y.; Zhang, K.; Cheng, K.; Feng, Q.; Ma, N.; Liang, J.; Zhang, T.; Zhang, L. Rapid surface display of mRNA antigens by bacteria-derived outer membrane vesicles for a personalized tumor vaccine. Adv. Mater. 2022, 34, 2109984. [Google Scholar] [CrossRef] [PubMed]
- Granoff, D.M. Review of meningococcal group B vaccines. Clin. Infect. Dis. 2010, 50, S54–S65. [Google Scholar] [CrossRef] [PubMed]
- Matthias, K.A.; Strader, M.B.; Nawar, H.F.; Gao, Y.S.; Lee, J.; Patel, D.S.; Im, W.; Bash, M.C. Heterogeneity in non-epitope loop sequence and outer membrane protein complexes alters antibody binding to the major porin protein PorB in serogroup B Neisseria meningitidis. Mol. Microbiol. 2017, 105, 934–953. [Google Scholar] [CrossRef] [PubMed]
- Matthias, K.A.; Reveille, A.; Connolly, K.L.; Jerse, A.E.; Gao, Y.S.; Bash, M.C. Deletion of major porins from meningococcal outer membrane vesicle vaccines enhances reactivity against heterologous serogroup B Neisseria meningitidis strains. Vaccine 2020, 38, 2396–2405. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, Y.; Xu, T.; Guo, Z.; Xu, Q.; Li, Y.; Zeng, L.; Huang, X.; Liu, Q. Disruption of sncRNA improves the protective efficacy of outer membrane vesicles against Helicobacter pylori infection in a mouse model. Infect. Immun. 2022, 90, e00267-22. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, Q.; Yi, J.; Liang, K.; Hu, B.; Zhang, X.; Curtiss III, R.; Kong, Q. Outer membrane vesicles from flagellin-deficient Salmonella enterica serovar Typhimurium induce cross-reactive immunity and provide cross-protection against heterologous Salmonella challenge. Sci. Rep. 2016, 6, 34776. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jie, K.; Li, B.; Yu, H.; Ruan, H.; Wu, J.; Huang, X.; Liu, Q. Immunization with outer membrane vesicles derived from major outer membrane protein-deficient Salmonella Typhimurium mutants for cross protection against Salmonella enteritidis and avian pathogenic Escherichia coli O78 infection in chickens. Front. Microbiol. 2020, 11, 588952. [Google Scholar] [CrossRef] [PubMed]
- Leitner, D.R.; Feichter, S.; Schild-Prüfert, K.; Rechberger, G.N.; Reidl, J.; Schild, S. Lipopolysaccharide modifications of a cholera vaccine candidate based on outer membrane vesicles reduce endotoxicity and reveal the major protective antigen. Infect. Immun. 2013, 81, 2379–2393. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, R.; Li, J.; Zhao, Y.; Lin, H.; Liang, L.; Wang, M.; Liu, H.; Min, Y.; Gao, Y.; Yang, M. Exploiting bacterial outer membrane vesicles as a cross-protective vaccine candidate against avian pathogenic Escherichia coli (APEC). Microb. Cell Fact. 2020, 19, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, U.; Halder, P.; Howlader, D.R.; Banerjee, S.; Maiti, S.; Dutta, S.; Koley, H. A tetravalent Shigella outer membrane vesicles based candidate vaccine offered cross-protection against all the serogroups of Shigella in adult mice. Microb. Infect. 2023, 25, 105100. [Google Scholar] [CrossRef] [PubMed]
- Avci, F.Y.; Kasper, D.L. How bacterial carbohydrates influence the adaptive immune system. Annu. Rev. Immunol. 2009, 28, 107–130. [Google Scholar] [CrossRef] [PubMed]
- Valguarnera, E.; Feldman, M.F. Glycoengineered outer membrane vesicles as a platform for vaccine development. Methods Enzymol. 2017, 597, 285–310. [Google Scholar] [PubMed]
- Raso, M.M.; Gasperini, G.; Alfini, R.; Schiavo, F.; Aruta, M.G.; Carducci, M.; Forgione, M.C.; Martini, S.; Cescutti, P.; Necchi, F. GMMA and Glycoconjugate Approaches Compared in Mice for the Development of a Vaccine against Shigella flexneri Serotype 6. Vaccines 2020, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, E.; Kis, Z.; Ozanne, J.; Di Benedetto, R.; Ricchetti, B.; Massai, L.; Carducci, M.; Oldrini, D.; Gasperini, G.; Aruta, M.G. GMMA as an alternative carrier for a glycoconjugate vaccine against group A Streptococcus. Vaccines 2022, 10, 1034. [Google Scholar] [CrossRef] [PubMed]
- Valentine, J.L.; Chen, L.; Perregaux, E.C.; Weyant, K.B.; Rosenthal, J.A.; Heiss, C.; Azadi, P.; Fisher, A.C.; Putnam, D.; Moe, G.R. Immunization with outer membrane vesicles displaying designer glycotopes yields class-switched, glycan-specific antibodies. Cell Chem. Biol. 2016, 23, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, T.C.; Cywes-Bentley, C.; Moeller, T.D.; Weyant, K.B.; Putnam, D.; Chang, Y.-F.; Jones, B.D.; Pier, G.B.; DeLisa, M.P. Immunization with outer membrane vesicles displaying conserved surface polysaccharide antigen elicits broadly antimicrobial antibodies. Proc. Natl. Acad. Sci. USA 2018, 115, E3106–E3115. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Li, B.; Xu, T.; Yu, H.; Chen, J.; Yu, H.; Li, S.; Zeng, L.; Huang, X.; Liu, Q. Outer membrane vesicles derived from Salmonella enterica serotype Typhimurium can deliver Shigella flexneri 2a O-polysaccharide antigen to prevent Shigella flexneri 2a infection in mice. Appl. Environ. Microbiol. 2021, 87, e00968-21. [Google Scholar] [CrossRef] [PubMed]
- McCright, J.; Naiknavare, R.; Yarmovsky, J.; Maisel, K. Targeting lymphatics for nanoparticle drug delivery. Front. Pharmacol. 2022, 13, 887402. [Google Scholar] [CrossRef] [PubMed]
- Benne, N.; van Duijn, J.; Kuiper, J.; Jiskoot, W.; Slütter, B. Orchestrating immune responses: How size, shape and rigidity affect the immunogenicity of particulate vaccines. J. Control. Release 2016, 234, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.-S.; Sweredoski, M.J.; Graham, R.L.; Hess, S.; Clemons Jr, W.M. Comprehensive proteomic profiling of outer membrane vesicles from Campylobacter jejuni. J. Proteom. 2014, 98, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Bong, J.-H.; Dombovski, A.; Birus, R.; Cho, S.; Lee, M.; Pyun, J.-C.; Jose, J. Covalent coupling of functionalized outer membrane vesicles (OMVs) to gold nanoparticles. J. Colloid. Interface Sci. 2024, 663, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Parodi, A.; Quattrocchi, N.; Van De Ven, A.L.; Chiappini, C.; Evangelopoulos, M.; Martinez, J.O.; Brown, B.S.; Khaled, S.Z.; Yazdi, I.K.; Enzo, M.V. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat. Nanotechnol. 2013, 8, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Zhao, Z.; Wang, J.; Zhang, P.; Ding, Y.; Jiang, Y.; Wang, D.; Li, Y. Engineering autologous tumor cell vaccine to locally mobilize antitumor immunity in tumor surgical bed. Sci. Adv. 2020, 6, eaba4024. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Su, J. Organoid extracellular vesicle-based therapeutic strategies for bone therapy. Biomater. Transl. 2023, 4, 199. [Google Scholar]
- Chen, Q.; Huang, G.; Wu, W.; Wang, J.; Hu, J.; Mao, J.; Chu, P.K.; Bai, H.; Tang, G. A hybrid eukaryotic–prokaryotic nanoplatform with photothermal modality for enhanced antitumor vaccination. Adv. Mater. 2020, 32, 1908185. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, H.; Jiang, W.; Yang, C.; Miao, H.; Wang, Y. Nanovaccines integrating endogenous antigens and pathogenic adjuvants elicit potent antitumor immunity. Nano Today 2020, 35, 101007. [Google Scholar] [CrossRef]
- Chen, L.; Qin, H.; Zhao, R.; Zhao, X.; Lin, L.; Chen, Y.; Lin, Y.; Li, Y.; Qin, Y.; Li, Y. Bacterial cytoplasmic membranes synergistically enhance the antitumor activity of autologous cancer vaccines. Sci. Transl. Med. 2021, 13, eabc2816. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, C.; You, S.; Zhang, K.; Li, M.; Cao, Y.; Wang, C.; Dong, H.; Zhang, X. Bacterial vesicle-cancer cell hybrid membrane-coated nanoparticles for tumor specific immune activation and photothermal therapy. ACS Appl. Mater. Interfaces 2020, 12, 41138–41147. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Dong, X.; Chen, Y.; Ye, J.-J.; Sun, Y.-X.; Zhang, X.-Z. A heterogenic membrane-based biomimetic hybrid nanoplatform for combining radiotherapy and immunotherapy against breast cancer. Biomaterials 2022, 289, 121810. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.; Li, K.; Huang, F.; Dai, Y.; Zhang, T.; Muaibati, M.; Abuduyilimu, A.; Huang, X. Extracellular vesicles hybrid plasmid-loaded lipid nanovesicles for synergistic cancer immunotherapy. Mater. Today Bio. 2023, 23, 100845. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.L.; Luo, G.F.; Chen, W.H.; Zhang, X.Z. Recent advances in engineered materials for immunotherapy-involved combination cancer therapy. Adv. Mater. 2021, 33, 2007630. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zheng, R.; Wu, X.; Xu, K.; Song, P.; Wang, Y.; Yan, J.; Chen, R.; Li, X.; Zhang, H. Thylakoid membranes with unique photosystems used to simultaneously produce self-supplying oxygen and singlet oxygen for hypoxic tumor therapy. Adv. Healthc. Mater. 2021, 10, 2001666. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Dai, Y.; Zhao, Y.; Qi, S.; Liu, L.; Lu, L.; Luo, Q.; Zhang, Z. Melittin-lipid nanoparticles target to lymph nodes and elicit a systemic anti-tumor immune response. Nat. Commun. 2020, 11, 1110. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.-R.; Wang, Y.; Lei, Y.; Zuo, L.; Jiang, A.; Wu, G.; Nie, W.; Huang, L.-L.; Xie, H.-Y. Phytochemical engineered bacterial outer membrane vesicles for photodynamic effects promoted immunotherapy. Nano Lett. 2022, 22, 4491–4500. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Jia, M.; Cheddah, S.; Zhang, Z.; Wang, W.; Li, X.; Wang, Y.; Yan, C. Peptide ligand-SiO(2) microspheres with specific affinity for phosphatidylserine as a new strategy to isolate exosomes and application in proteomics to differentiate hepatic cancer. Bioact. Mater. 2022, 15, 343–354. [Google Scholar] [PubMed]
- Krušić Alić, V.; Malenica, M.; Biberić, M.; Zrna, S.; Valenčić, L.; Šuput, A.; Kalagac Fabris, L.; Wechtersbach, K.; Kojc, N.; Kurtjak, M. Extracellular vesicles from human cerebrospinal fluid are effectively separated by sepharose CL-6B—Comparison of four gravity-flow size exclusion chromatography methods. Biomedicines 2022, 10, 785. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fang, F.; Sun, M.; Zhang, Y.; Hu, M.; Zhang, J. Extracellular vesicles as bioactive nanotherapeutics: An emerging paradigm for regenerative medicine. Theranostics 2022, 12, 4879. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Nelson, K.L.; Uchakin, P.N.; Smith, A.L.; Gu, X.-X.; Daines, D.A. Characterization of extended co-culture of non-typeable Haemophilus influenzae with primary human respiratory tissues. Exp. Biol. Med. 2012, 237, 540–547. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, K.; Lu, Y.; Sun, B.; Sun, J.; Liang, W. Monensin enhanced generation of extracellular vesicles as transfersomes for promoting tumor penetration of pyropheophorbide-a from fusogenic liposome. Nano Lett. 2022, 22, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, M.; Caproni, E.; Benedet, M.; Zanella, I.; Giorgetta, S.; Dalsass, M.; König, E.; Gagliardi, A.; Fantappiè, L.; Berti, A. Outer membrane vesicles from the gut microbiome contribute to tumor immunity by eliciting cross-reactive T cells. Front. Oncol. 2022, 12, 912639. [Google Scholar] [CrossRef] [PubMed]
- Van der Ley, P.; Schijns, V.E. Outer membrane vesicle-based intranasal vaccines. Curr. Opin. Immunol. 2023, 84, 102376. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.J.; Turner, K.B.; Medintz, I.L.; Walper, S.A. Protecting enzymatic function through directed packaging into bacterial outer membrane vesicles. Sci. Rep. 2016, 6, 24866. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S. History of vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- WHO. Weekly Epidemiological Record. WHO Position Papers. Available online: https://www.who.int/publications/i/item/who-wer-10022-193-218 (accessed on 11 June 2025).
- WHO; UNICEF; World Bank. State of the World’s Vaccines and Immunization; World Health Organization: Geneva, Switzerland, 2009; Available online: https://apps.who.int/iris/bitstream/handle/10665/44169/9789241563864_eng.pdf (accessed on 11 June 2025).
- Hendriksen, R.S.; Munk, P.; Njage, P.; Van Bunnik, B.; McNally, L.; Lukjancenko, O.; Röder, T.; Nieuwenhuijse, D.; Pedersen, S.K.; Kjeldgaard, J. Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage. Nat. Commun. 2019, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Bloom, D.E.; Black, S.; Salisbury, D.; Rappuoli, R. Antimicrobial resistance and the role of vaccines. Proc. Natl. Acad. Sci. USA 2018, 115, 12868–12871. [Google Scholar] [CrossRef] [PubMed]
- Orenstein, W.A.; Offit, P.A.; Edwards, K.M.; Plotkin, S.A. Vaccines, 7th ed.; Elsevier: Philadelphia, PA, USA, 2018. [Google Scholar]
- Zahid, A.; Wilson, J.C.; Grice, I.D.; Peak, I.R. Otitis media: Recent advances in otitis media vaccine development and model systems. Front. Microbiol. 2024, 15, 1345027. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Zhao, Q.; Wen, X.; Wu, R.; Wen, Y.; Huang, X.; Huang, Y.; Yan, Q.; Han, X.; Ma, X. A trivalent Apx-fusion protein delivered by E. coli outer membrane vesicles induce protection against Actinobacillus pleuropneumoniae of serotype 1 and 7 challenge in a murine model. PLoS ONE 2018, 13, e0191286. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Shang, Y.; Shen, L.; Yu, X.; Cao, Y.; Zeng, L.; Zhang, H.; Rao, Z.; Li, Y.; Tao, Z. Outer membrane vesicles from genetically engineered Salmonella enterica serovar Typhimurium presenting Helicobacter pylori antigens UreB and CagA induce protection against Helicobacter pylori infection in mice. Virulence 2024, 15, 2367783. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, L.; Bolhassani, A.; Mohit, E.; Baesi, K.; Aghasadeghi, M.R.; Milani, A.; Agi, E. Engineered ClearColi™-derived outer membrane vesicles as functional carriers for development of HIV-1 therapeutic vaccine candidate. Microb. Pathog. 2024, 193, 106749. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Farman, T.A.; Piliou, S.; Mastroeni, P. Characterisation and Immunogenicity of Neisseria cinerea outer membrane vesicles displaying NadA, NHBA and fHbp from Neisseria meningitidis serogroup B. Front. Immunol. 2024, 15, 1473064. [Google Scholar] [CrossRef] [PubMed]
- Wo, J.; Lv, Z.-Y.; Sun, J.-N.; Tang, H.; Qi, N.; Ye, B.-C. Engineering probiotic-derived outer membrane vesicles as functional vaccine carriers to enhance immunity against SARS-CoV-2. Iscience 2023, 26, 105772. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Chen, Y.; Zhang, W.; Liu, X.; Lei, M.; Yuan, H.; Li, M.; Liu, Q.; Kong, Q. Salmonella Typhimurium derived OMV nanoparticle displaying mixed heterologous O-antigens confers immunogenicity and protection against STEC infections in mice. Microb. Cell Fact. 2025, 24, 8. [Google Scholar] [CrossRef] [PubMed]
- König, E.; Gagliardi, A.; Riedmiller, I.; Andretta, C.; Tomasi, M.; Irene, C.; Frattini, L.; Zanella, I.; Berti, F.; Grandi, A. Multi-antigen outer membrane vesicle engineering to develop polyvalent vaccines: The Staphylococcus aureus case. Front. Immunol. 2021, 12, 752168. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Halder, P.; Das, S.; Maiti, S.; Withey, J.H.; Mitobe, J.; Chowdhury, G.; Kitahara, K.; Miyoshi, S.-i.; Mukhopadhyay, A.K. Trivalent outer membrane vesicles-based combination vaccine candidate induces protective immunity against Campylobacter and invasive non-typhoidal Salmonella in adult mice. Med. Microbiol. Immunol. 2024, 213, 21. [Google Scholar] [CrossRef] [PubMed]
- Salvador-Erro, J.; Pastor, Y.; Gamazo, C. A Recombinant Shigella flexneri Strain Expressing ETEC Heat-Labile Enterotoxin B Subunit Shows Promise for Vaccine Development via OMVs. Int. J. Mol. Sci. 2024, 25, 12535. [Google Scholar] [CrossRef] [PubMed]
- Leitner, D.R.; Lichtenegger, S.; Temel, P.; Zingl, F.G.; Ratzberger, D.; Roier, S.; Schild-Prüfert, K.; Feichter, S.; Reidl, J.; Schild, S. A combined vaccine approach against Vibrio cholerae and ETEC based on outer membrane vesicles. Front. Microbiol. 2015, 6, 823. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shehata, M.M.; Mostafa, A.; Teubner, L.; Mahmoud, S.H.; Kandeil, A.; Elshesheny, R.; Boubak, T.A.; Frantz, R.; Pietra, L.L.; Pleschka, S. Bacterial outer membrane vesicles (OMVs)-based dual vaccine for influenza A H1N1 virus and MERS-CoV. Vaccines 2019, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Denisov, I.G.; Grinkova, Y.V.; Lazarides, A.A.; Sligar, S.G. Directed self-assembly of monodisperse phospholipid bilayer Nanodiscs with controlled size. J. Am. Chem. Soc. 2004, 126, 3477–3487. [Google Scholar] [CrossRef] [PubMed]
- Kuai, R.; Ochyl, L.J.; Bahjat, K.S.; Schwendeman, A.; Moon, J.J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 2017, 16, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Noh, I.; Guo, Z.; Zhou, J.; Gao, W.; Fang, R.H.; Zhang, L. Cellular nanodiscs made from bacterial outer membrane as a platform for antibacterial vaccination. ACS Nano 2022, 17, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Tang, D.; Zhou, H.; Li, Y.; Zhou, D.; Peng, X.; Ren, C.; Su, Y.; Zhang, S.; Zheng, H. Bacterial outer membrane vesicle nanorobot. Proc. Natl. Acad. Sci. USA 2024, 121, e2403460121. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Deng, H.; An, X.; Liu, G.; Liu, C. Designing nanodiscs as versatile platforms for on-demand therapy. Nanoscale 2024, 16, 2220–2234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hess, H. Chemically-powered swimming and diffusion in the microscopic world. Nat. Rev. Chem. 2021, 5, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Cai, Y.; Wu, Z.; He, Q. Recent progress on motion control of swimming micro/nanorobots. View 2021, 2, 20200113. [Google Scholar] [CrossRef]
- Kadiri, V.M.; Bussi, C.; Holle, A.W.; Son, K.; Kwon, H.; Schütz, G.; Gutierrez, M.G.; Fischer, P. Biocompatible magnetic micro- and nanodevices: Fabrication of FePt nanopropellers and cell transfection. Adv. Mater. 2020, 32, 2001114. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xing, Y.; Li, X.; Du, X.; Xu, T.; Zhang, X. Cancer cell membrane camouflaged semi-yolk@ spiky-shell nanomotor for enhanced cell adhesion and synergistic therapy. Small 2020, 16, 2003834. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Chen, Q.; Liu, T.; Li, X.; Pei, P.; Zhou, L.; Zhou, S.; Zhang, R.; Liang, K.; Dong, J. Site-selective superassembly of biomimetic nanorobots enabling deep penetration into tumor with stiff stroma. Nat. Commun. 2023, 14, 4628. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Qin, H.; Wang, F.; Liu, L.; Tian, H.; Wang, H.; Wang, S.; Ou, J.; Ye, Y.; Peng, F. Hyperthermia-triggered biomimetic bubble nanomachines. Nat. Commun. 2023, 14, 4867. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Fernández de Ávila, B.; Martín, A.; Soto, F.; Lopez-Ramirez, M.A.; Campuzano, S.; Vasquez-Machado, G.M.; Gao, W.; Zhang, L.; Wang, J. Single cell real-time miRNAs sensing based on nanomotors. ACS Nano 2015, 9, 6756–6764. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pan, R.; Wang, Y.; Guo, P.; Liu, X.; Ji, F.; Hu, J.; Yan, X.; Wang, G.P.; Zhang, L. Carbon Helical Nanorobots Capable of Cell Membrane Penetration for Single Cell Targeted SERS Bio-Sensing and Photothermal Cancer Therapy. Adv. Funct. Mater. 2022, 32, 2200600. [Google Scholar] [CrossRef]
- Hortelao, A.C.; Simó, C.; Guix, M.; Guallar-Garrido, S.; Julián, E.; Vilela, D.; Rejc, L.; Ramos-Cabrer, P.; Cossío, U.; Gómez-Vallejo, V. Swarming behavior and in vivo monitoring of enzymatic nanomotors within the bladder. Sci. Robot. 2021, 6, eabd2823. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Hortelao, A.C.; Miguel-López, A.; Sánchez, S. Bubble-free propulsion of ultrasmall tubular nanojets powered by biocatalytic reactions. J. Am. Chem. Soc. 2016, 138, 13782–13785. [Google Scholar] [CrossRef] [PubMed]
- Terzopoulou, A.; Nicholas, J.D.; Chen, X.-Z.; Nelson, B.J.; Pane, S.; Puigmarti-Luis, J. Metal–organic frameworks in motion. Chem. Rev. 2020, 120, 11175–11193. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Rivera, I.; Mathesh, M.; Wilson, D.A. A supramolecular approach to nanoscale motion: Polymersome-based self-propelled nanomotors. Acc. Chem. Res. 2018, 51, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Chen, H.; Wang, Q.; Niu, Q.; Xu, P.; Yu, Y.; Zhu, T.; Mao, C.; Shen, J. Bio-inspired nitric-oxide-driven nanomotor. Nat. Commun. 2019, 10, 966. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Li, T.; Chen, H.; Mao, C.; Shen, J. Biosafety, functionalities, and applications of biomedical micro/nanomotors. Angew. Chem. Int. Ed. 2021, 60, 13158–13176. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ye, M.; Hu, C.; Qian, H.; Nelson, B.J.; Wang, X. Stimuli-responsive functional micro-/nanorobots: A review. ACS nano 2023, 17, 15254–15276. [Google Scholar] [CrossRef] [PubMed]
- Kelwick, R.; MacDonald, J.T.; Webb, A.J.; Freemont, P. Developments in the tools and methodologies of synthetic biology. Front. Bioeng. Biotechnol. 2014, 2, 60. [Google Scholar] [CrossRef] [PubMed]
- Kelwick, R.J.; Webb, A.J.; Freemont, P.S. Biological materials: The next frontier for cell-free synthetic biology. Front. Bioeng. Biotechnol. 2020, 8, 399. [Google Scholar] [CrossRef] [PubMed]
- Zanella, I.; König, E.; Tomasi, M.; Gagliardi, A.; Frattini, L.; Fantappiè, L.; Irene, C.; Zerbini, F.; Caproni, E.; Isaac, S.J. Proteome-minimized outer membrane vesicles from Escherichia coli as a generalized vaccine platform. J. Extracell. Vesicles. 2021, 10, e12066. [Google Scholar] [CrossRef] [PubMed]
- Porto, E.M.; Komor, A.C.; Slaymaker, I.M.; Yeo, G.W. Base editing: Advances and therapeutic opportunities. Nat. Rev. Drug Discov. 2020, 19, 839–859. [Google Scholar] [CrossRef] [PubMed]
- Ceroni, F.; Boo, A.; Furini, S.; Gorochowski, T.E.; Borkowski, O.; Ladak, Y.N.; Awan, A.R.; Gilbert, C.; Stan, G.-B.; Ellis, T. Burden-driven feedback control of gene expression. Nat. Methods 2018, 15, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Plahar, H.A.; Rich, T.N.; Lane, S.D.; Morrell, W.C.; Springthorpe, L.; Nnadi, O.; Aravina, E.; Dai, T.; Fero, M.J.; Hillson, N.J. BioParts—A biological parts search portal and updates to the ICE parts registry software platform. ACS Synth. Biol. 2021, 10, 2649–2660. [Google Scholar] [CrossRef] [PubMed]
- Casas, A.; Bultelle, M.; Motraghi, C.; Kitney, R. Removing the Bottleneck: Introducing cMatch-A Lightweight Tool for Construct-Matching in Synthetic Biology. Front. Bioeng. Biotechnol. 2022, 9, 785131. [Google Scholar] [CrossRef] [PubMed]
- Haines, M.C.; Carling, B.; Marshall, J.; Shenshin, V.A.; Baldwin, G.S.; Freemont, P.; Storch, M. basicsynbio and the BASIC SEVA collection: Software and vectors for an established DNA assembly method. Synth. Biol. 2022, 7, ysac023. [Google Scholar] [CrossRef] [PubMed]
- Kelwick, R.J.; Webb, A.J.; Heliot, A.; Segura, C.T.; Freemont, P.S. Opportunities to accelerate extracellular vesicle research with cell-free synthetic biology. J. Extracell. Biol. 2023, 2, e90. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Akin, H.; Rao, R.; Hie, B.; Zhu, Z.; Lu, W.; Smetanin, N.; Verkuil, R.; Kabeli, O.; Shmueli, Y. Evolutionary-scale prediction of atomic-level protein structure with a language model. Science 2023, 379, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lisanza, S.; Juergens, D.; Tischer, D.; Watson, J.L.; Castro, K.M.; Ragotte, R.; Saragovi, A.; Milles, L.F.; Baek, M. Scaffolding protein functional sites using deep learning. Science 2022, 377, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Dauparas, J.; Anishchenko, I.; Bennett, N.; Bai, H.; Ragotte, R.J.; Milles, L.F.; Wicky, B.I.; Courbet, A.; de Haas, R.J.; Bethel, N. Robust deep learning–based protein sequence design using ProteinMPNN. Science 2022, 378, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Rezhdo, A.; Islam, M.; Huang, M.; Van Deventer, J.A. Future prospects for noncanonical amino acids in biological therapeutics. Curr. Opin. Biotechnol. 2019, 60, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Duffy, K.; Arangundy-Franklin, S.; Holliger, P. Modified nucleic acids: Replication, evolution, and next-generation therapeutics. BMC Biol. 2020, 18, 112. [Google Scholar] [CrossRef] [PubMed]
- Bista, P.K.; Pillai, D.; Narayanan, S.K. Outer-membrane vesicles of Fusobacterium necrophorum: A proteomic, lipidomic, and functional characterization. Microorganisms 2023, 11, 2082. [Google Scholar] [CrossRef] [PubMed]
- Melo, J.; Cavadas, B.; Pereira, L.; Figueiredo, C.; Leite, M. Transcriptomic remodeling of gastric cells by Helicobacter pylori outer membrane vesicles. Helicobacter 2024, 29, e13031. [Google Scholar] [CrossRef] [PubMed]
- Yadalam, P.K.; Anegundi, R.V.; Saravanan, M.; Meles, H.N.; Heboyan, A. Deep Learning Prediction of Inflammatory Inducing Protein Coding mRNA in P. gingivalis Released Outer Membrane Vesicles. Biomed. Eng. Comput. Biol. 2024, 15, 11795972241277081. [Google Scholar] [CrossRef] [PubMed]
- Kehl, A.; Kuhn, R.; Detzner, J.; Steil, D.; Müthing, J.; Karch, H.; Mellmann, A. Modeling native EHEC outer membrane vesicles by creating synthetic surrogates. Microorganisms 2020, 8, 673. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, J.; Li, M.; Wang, L.; Wang, Z. A facile approach for development of a vaccine made of bacterial double-layered membrane vesicles (DMVs). Biomaterials 2018, 187, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Svennerholm, K.; Crescitelli, R.; Lässer, C.; Gribonika, I.; Lötvall, J. Synthetic bacterial vesicles combined with tumour extracellular vesicles as cancer immunotherapy. J. Extracell. Vesicles. 2021, 10, e12120. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Q.; Wang, S.; Weng, W.; Jing, Y.; Su, J. Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: Advances and perspectives. Bioact. Mater. 2022, 14, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wu, M.; Wang, L.; Tang, M.; Xin, H.; Deng, K. Efficient isolation of outer membrane vesicles (OMVs) secreted by gram-negative bacteria via a novel gradient filtration method. Membranes 2024, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Corso, G.; Mäger, I.; Lee, Y.; Görgens, A.; Bultema, J.; Giebel, B.; Wood, M.J.; Nordin, J.Z.; Andaloussi, S.E. Reproducible and scalable purification of extracellular vesicles using combined bind-elute and size exclusion chromatography. Sci. Rep. 2017, 7, 11561. [Google Scholar] [CrossRef] [PubMed]
- Torres-Vanegas, J.D.; Rincon-Tellez, N.; Guzmán-Sastoque, P.; Valderrama-Rincon, J.D.; Cruz, J.C.; Reyes, L.H. Production and purification of outer membrane vesicles encapsulating green fluorescent protein from Escherichia coli: A step towards scalable OMV technologies. Front. Bioeng. Biotechnol. 2024, 12, 1436352. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Dong, Z.; Ma, C.; Wu, R.; Lv, R.; Liu, S.; Ren, Y.; Liu, Z.; van der Mei, H.C.; Busscher, H.J. High yield, magnetic harvesting of extracellular outer membrane vesicles from Escherichia coli. Small 2022, 18, 2204350. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lu, M.; Peng, T.; Wu, Y.; Zhu, L.; Liu, Y.; Zhang, W.; Xiang, T. An improvised one-step OptiPrep cushion ultracentrifugation method for outer membrane vesicles isolation of Klebsiella pneumoniae. BMC Microbiol. 2024, 24, 548. [Google Scholar] [CrossRef] [PubMed]
- Antenucci, F.; Arak, H.; Gao, J.; Allahgadry, T.; Thøfner, I.; Bojesen, A.M. Hydrostatic filtration enables large-scale production of outer membrane vesicles that effectively protect chickens against Gallibacterium anatis. Vaccines 2020, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Antenucci, F.; Fougeroux, C.; Bossé, J.T.; Magnowska, Z.; Roesch, C.; Langford, P.; Holst, P.J.; Bojesen, A.M. Identification and characterization of serovar-independent immunogens in Actinobacillus pleuropneumoniae. Vet. Res. 2017, 48, 74. [Google Scholar] [CrossRef] [PubMed]
- Pollock, J.; Coffman, J.; Ho, S.V.; Farid, S.S. Integrated continuous bioprocessing: Economic, operational, and environmental feasibility for clinical and commercial antibody manufacture. Biotechnol. Prog. 2017, 33, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L.; O’Connor, T.F.; Yang, X.; Cruz, C.N.; Chatterjee, S.; Madurawe, R.D.; Moore, C.M.; Yu, L.X.; Woodcock, J. Modernizing pharmaceutical manufacturing: From batch to continuous production. J. Pharm. Innov. 2015, 10, 191–199. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, Q.; Molino, N.M.; Wang, S.-W.; Boder, E.T.; Chen, W. Sortase A-mediated multi-functionalization of protein nanoparticles. Chem. Commun. 2015, 51, 12107–12110. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chen, W. A non-chromatographic protein purification strategy using Src 3 homology domains as generalized capture domains. J. Biotechnol. 2016, 234, 27–34. [Google Scholar] [CrossRef] [PubMed]
- FDA. Preclinical Considerations for Products Regulated in OCTGT. Available online: https://www.fda.gov/files/vaccines%2C%20blood%20%26%20biologics/published/Preclinical-Considerations--for-Products-Regulated-in-OCTGT.pdf (accessed on 5 July 2025).
- KS, D.; KR, G. An Overview on Development, Approval and Post Registration Activities for Pharmaceuticals in European Union. Indian J. Pharm. Educ. Res. 2023, 57, 1208. [Google Scholar] [CrossRef]
- Balhuizen, M.D.; Veldhuizen, E.J.; Haagsman, H.P. Outer membrane vesicle induction and isolation for vaccine development. Front. Microbiol. 2021, 12, 629090. [Google Scholar] [CrossRef] [PubMed]
- Preto, R.M.; Dos Santos, V.C.T.; Lordelo, M.V.S.; Pereira, G.H.F.; Leite, L.C.d.C.; Gonçalves, V.M.; Barazzone, G.C. Optimization of methods for isolation and purification of outer membrane vesicles (OMVs) from Neisseria lactamica. Appl. Microbiol. Biotechnol. 2025, 109, 82. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Factors that influence the immune response to vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [PubMed]
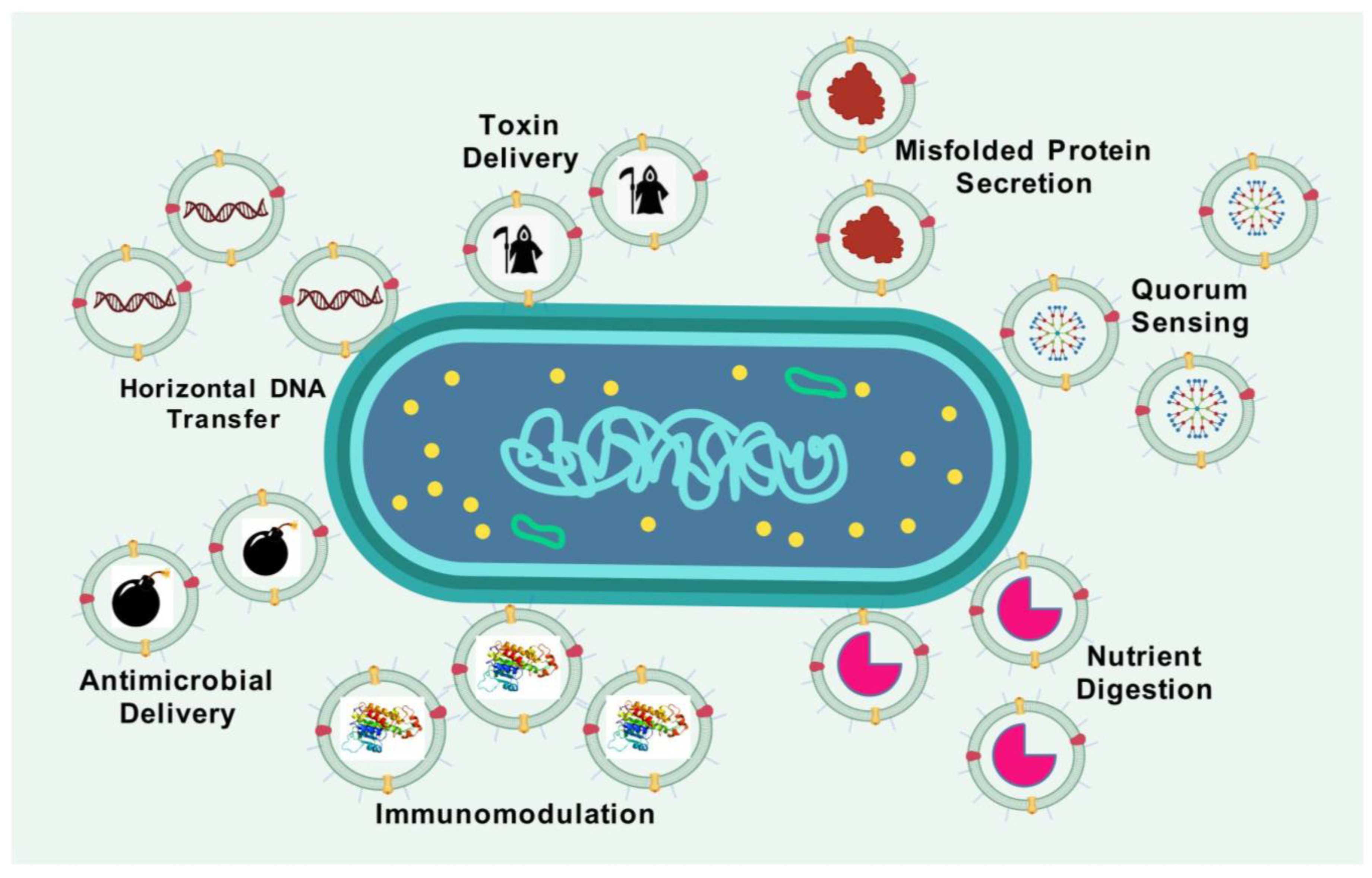
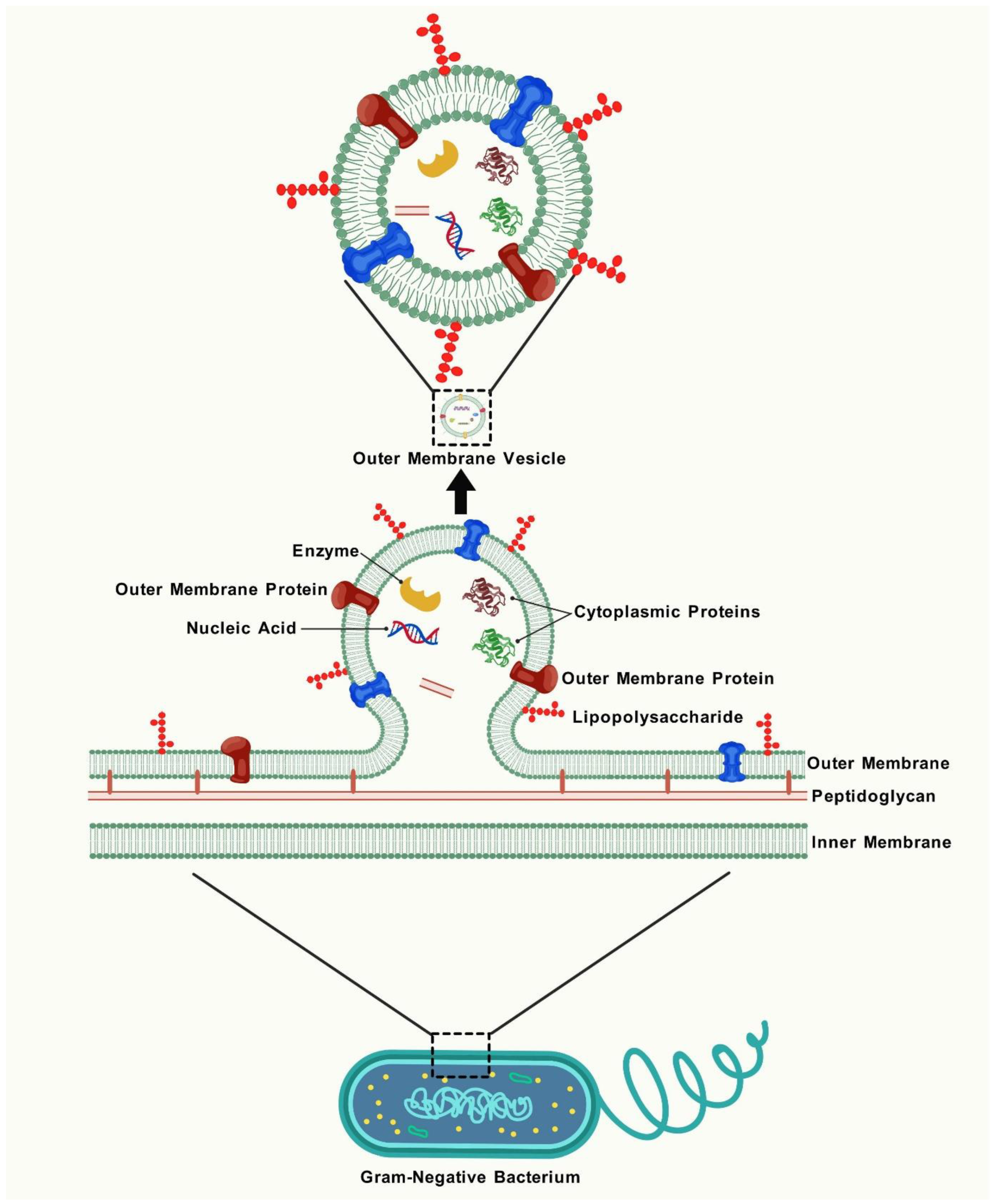
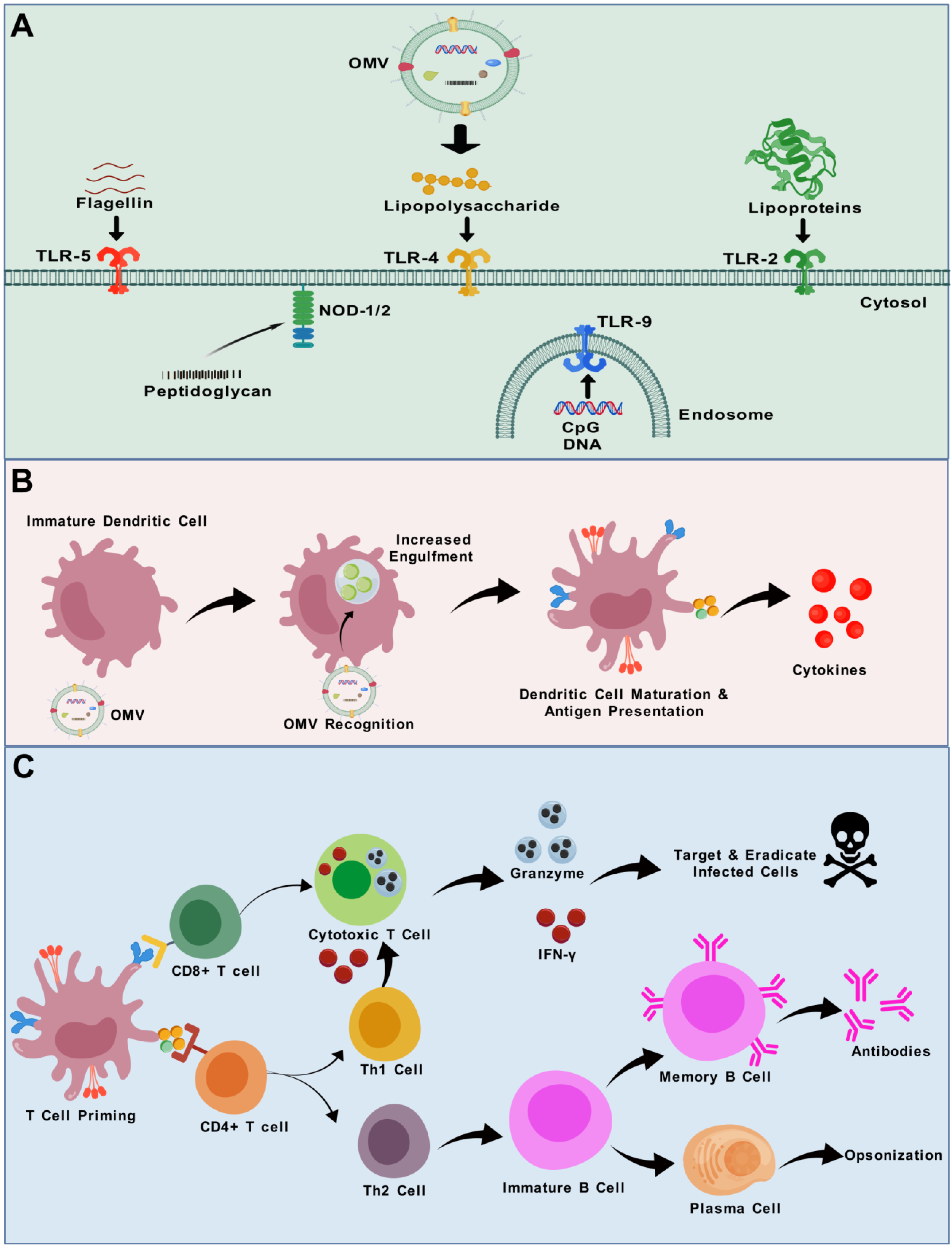
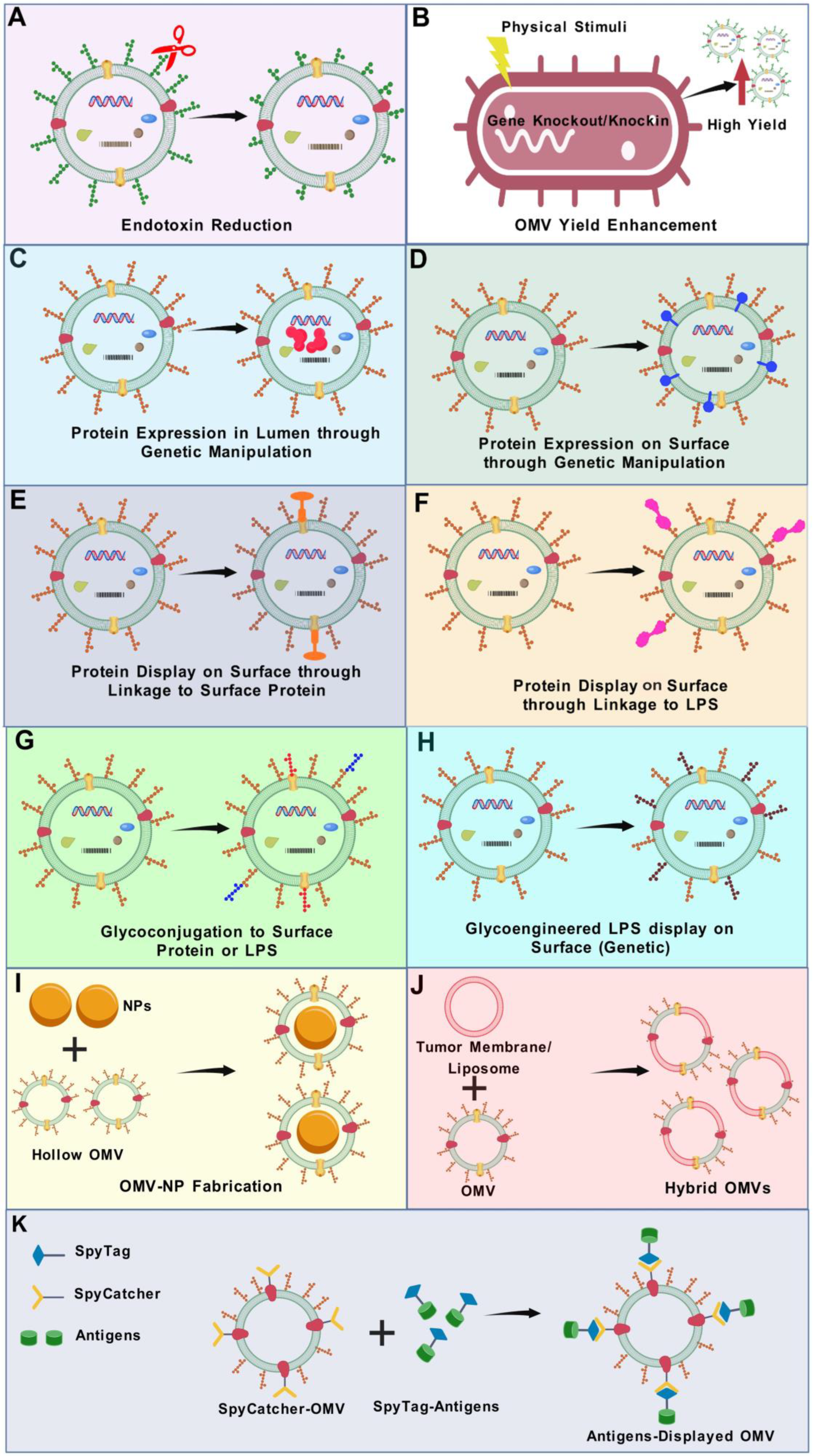
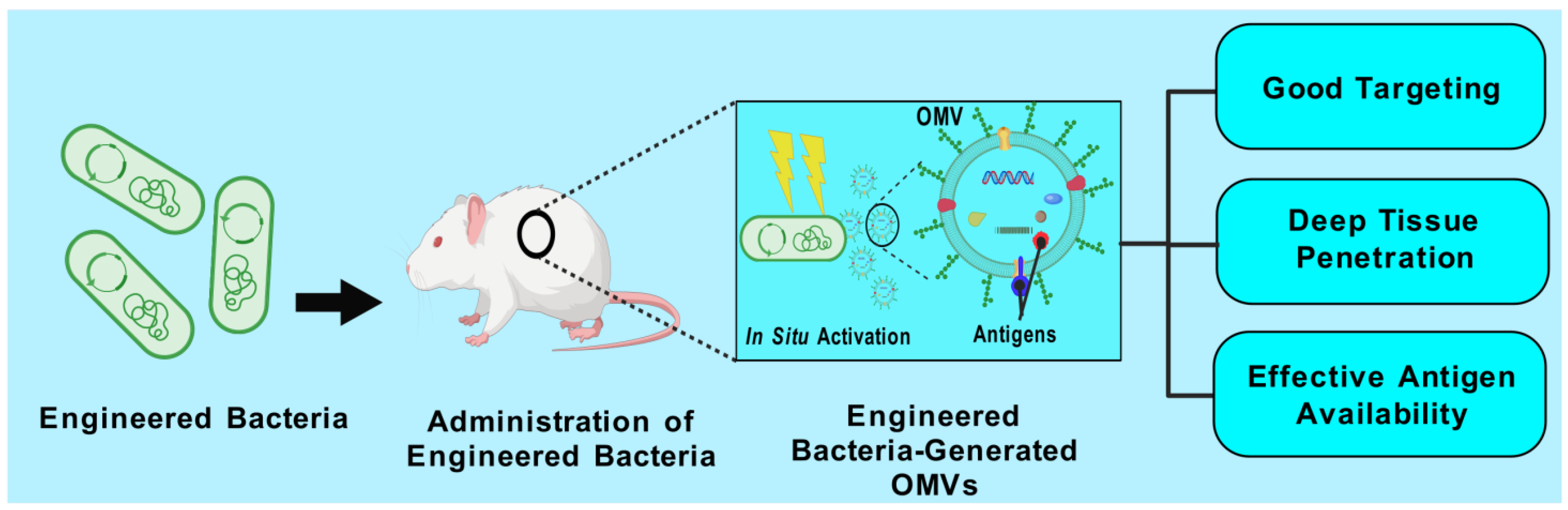
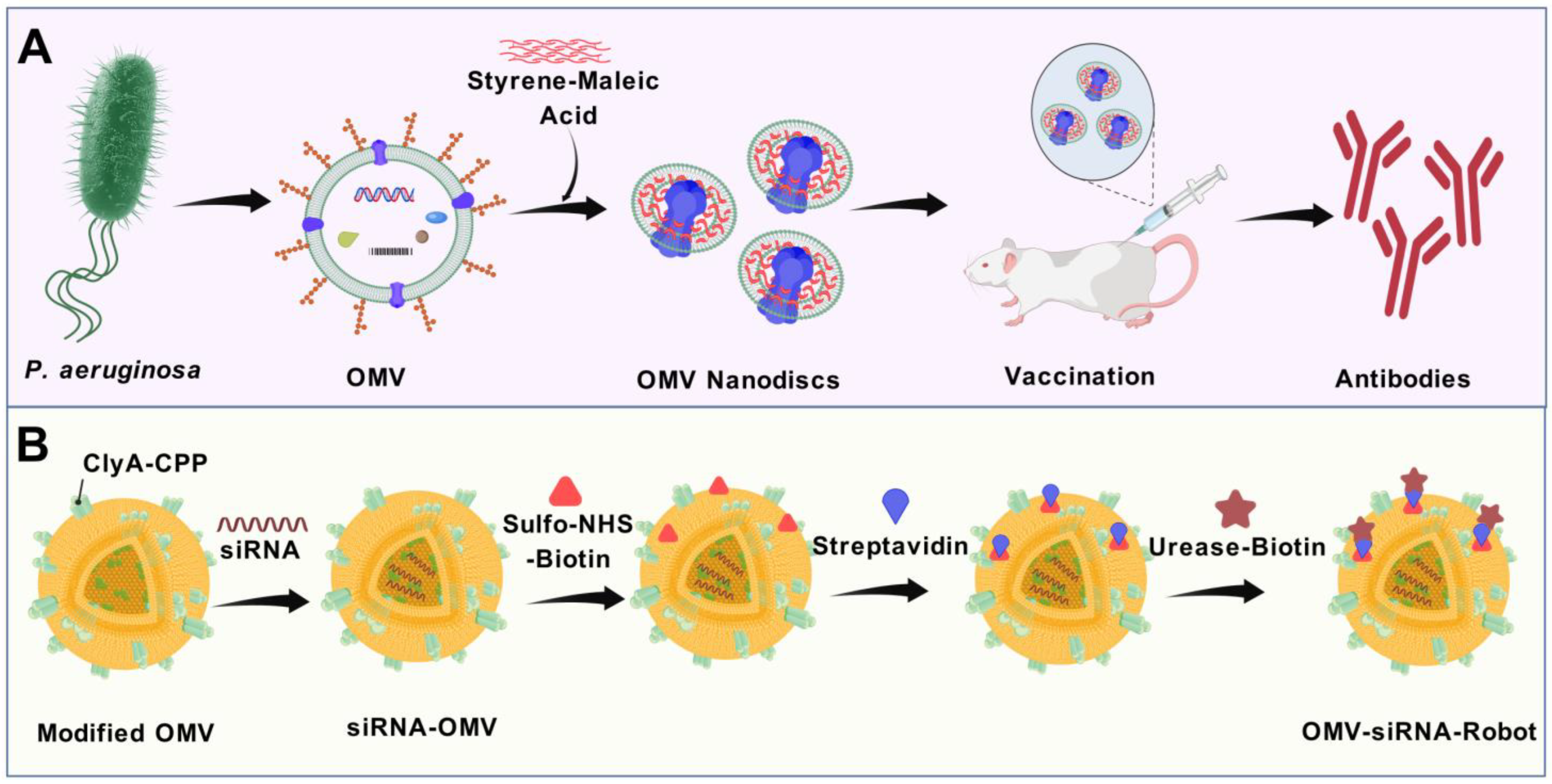
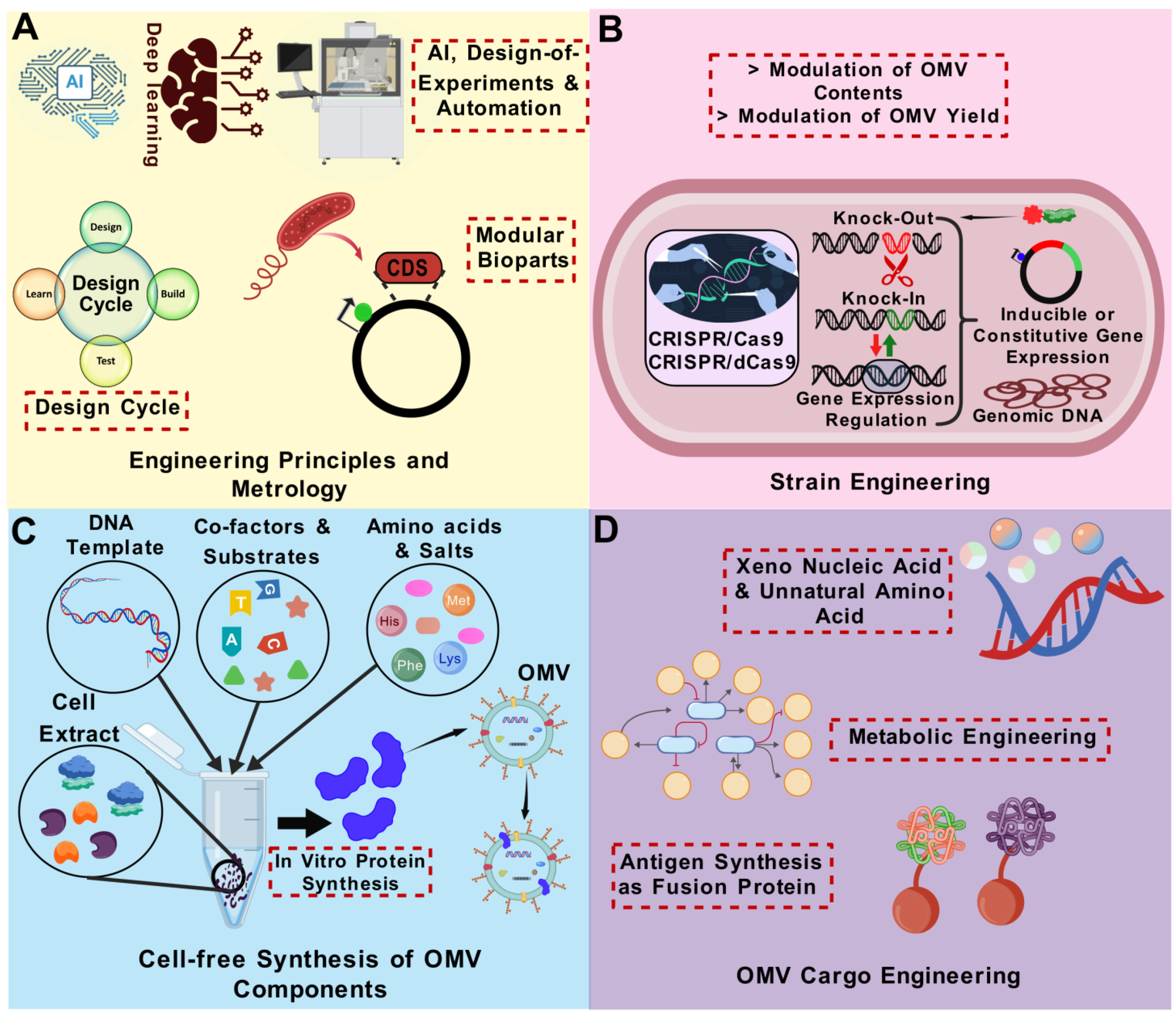
| Vaccine Platform | Particle Size | Material Properties | Advantages | Disadvantages | Examples | Referenes |
|---|---|---|---|---|---|---|
| Bacterial Ghosts | 40–200 nm | Empty envelopes of Gram-negative bacteria | High security; natural immunostimulatory properties | Poor efficiency; complicated production; ambiguous impacts in human application | As of 2025, no human bacterial ghost-based vaccines are fully licenced | [65] |
| DNA Vaccines | Variable | Plasmid DNA-encoding target antigens | Induce both humoral and cellular immunity; stable; easy to produce | Lower immunogenicity in humans; requires specialized delivery devices or methods | ZyCoV-D (COVID-19 vaccine) | [66] |
| Inactivated/Killed Vaccines | 50–200 nm | Chemically or physically inactivated whole pathogens | Safe; cannot revert to virulence; proven efficacy; easier mass production | Weaker immune response; requires boosters | Polio vaccine, Influenza vaccine | [67] |
| Liposome-based Vaccines | 10 nm-hundreds nm | Phospholipid vesicles | Well-studied; excellent compatibility with biological systems; minimal immune response | Leakage of drug content; inefficient loading capacity; complicated quality control process | Epaxal (Hepatitis A vaccine) | [68] |
| Live Attenuated Vaccines | ~30 nm (small viruses) to 5 µm (bacterial vaccines like BCG) | Weakened (attenuated) version of the pathogen | Elicit strong, long-lasting immunity; stimulate both humoral and cellular responses; often require fewer doses; do not require adjuvants | Risk of reversion to virulence; not suitable for immunocompromised individuals; require cold chain storage | Measles, mumps, and rubella (MMR) vaccine, Varicella (chickenpox) vaccine, BCG vaccine (for tuberculosis) | [69] |
| mRNA Vaccines | 50–150 nm | Lipid nanoparticle-encapsulated mRNA | Highly adaptable; rapid production; strong adaptive immune response | Stability issues; cold chain storage required | Pfizer COVID-19 vaccine (Comirnaty), Moderna COVID-19 vaccine (Spikevax) | [70,71] |
| Outer-Membrane Vesicles (OMVs)- based Vaccines | 30–200 nm | Outer-membrane vesicles of Gram-negative bacteria | Naturally immunogenic; good adjuvant properties; comparatively easy production; cost effective; easily modifiable to express various antigens; low risk of evolutionary escape | Inconsistent antigen presentation efficiency; low yields with some strains | Bexsero (meningococcal disease), VA-MENGOC-BC (meningococcal disease) | [49,63] |
| Subunit Vaccines (Polysaccharide vaccines, conjugate vaccines, and protein-based vaccines) | Variable | Contain only specific pieces (subunits) of the pathogen, typically proteins or polysaccharides | Good safety profiles; well-characterized; can be combined with adjuvants | Require adjuvants for strong immunity; costly production | Haemophilus influenzae type B (Hib) vaccine, Shingles vaccine | [72] |
| Toxoid Vaccines | N/A (soluble proteins) | Inactivated bacterial toxins | Good safety profiles; stable; long-lasting immunity | May require boosters; weak innate immune response | Tetanus vaccine, Diphtheria vaccine | [73] |
| Viral Vector Vaccines | Variable | Modified virus to deliver genetic material encoding an antigen | High transduction efficiency; induces robust immune responses; can target specific cells | Pre-existing immunity to vector; potential safety concerns; complex manufacturing | Ebola vaccine, COVID-19 vaccine (AstraZeneca and Johnson & Johnson) | [74,75] |
| Virus-Like Particles (VLPs)-based Vaccines | 20–200 nm | Non-infectious viral protein structures | High immunogenicity; mimic native viruses; strong B- and T-cell responses | Costly and complex production; requires specialized expression systems | Gardasil/ Cervarix (HPV vaccines), Hecolin (Hepatitis E vaccine) | [73,76] |
| Feature | Native OMV | Detergent-Extracted OMV | Modified OMV | References |
|---|---|---|---|---|
| Production Method | Naturally secreted by Gram-negative bacteria | Vesicles extracted from bacterial cells using detergents | Produced through the genetic modification of bacterial strains to enhance vesicle release or introduce foreign antigens; the development of modified strains can be difficult depending upon the type of modification; risk of off-target or secondary mutations during strain development | [114,119] |
| Scalability | Limited production output depending on the bacterial species and strain used | High yield due to enhanced release through detergent action | High yield facilitated by strain engineering | [49,120] |
| Ease of Purification | Streamlined and cost-effective purification process | Purification process can be more complex compared to other OMV types | Simple and cost-effective purification | [49,120] |
| Safety Profile | Contains unaltered pathogen-associated molecular patterns (PAMPs), including LPS, contributing to high reactogenicity | Detergent treatment lowers LPS and lipoprotein levels, reducing endotoxicity | Genetic detoxification of lipid A and deletion of unwanted toxins and antigens enhances safety | [121] |
| Immunostimulatory Capacity | Exhibits self-adjuvanting effects due to native PAMPs | Reduced immunogenic potential due to lower PAMP content | Preserves adjuvanticity depending on the degree of PAMP modification or removal | |
| Antigen Presentation | May exhibit low levels of relevant protective antigens | Detergent use can lead to loss of key antigens such as lipoproteins and LPS | Allows for the overexpression of conserved antigens, enabling broader immune coverage | [40,122,123] |
| Breadth of Immune Protection | Often induces strain-specific immunity with limited cross-protection | Tends to remain strain-specific with minimal species-wide protection | Capable of expressing homologous and heterologous antigens, providing cross-strain and cross-species immunity | [114] |
| Developmental Status | Disease(s) | Pathogen(s) | Vaccine Name | References |
|---|---|---|---|---|
| Licenced | Diphtheria, tetanus, Whooping cough, Hib infections, and Hepatitis B | Diphtheria, Clostridium tetani, Bordetella pertussis, Poliomyelitis, Haemophilus infuenzae type b, and Hepatitis B | Vaxelis | [127] |
| Hib disease and Hepatitis B | H. infuenzae type b and Hepatitis B | Procomvax/Comvax * (PRP-OMPC and hepatitis B) | [128] | |
| Hib disease | H. infuenzae type b | PedvaxHib (PRP-OMPC) | [129,130] | |
| Meningitis | Neisseria meningitidis B | Bexsero (4CMenB) | [131,132] | |
| Meningitis | N. meningitidis B | MenZB (NZ dOMV) | [133] | |
| Meningitis | N. meningitidis B | VA-MENGOC-BC | [134,135] | |
| Phase II Clinical Trials | Gonorrhea | N. gonorrhoea | Neisseria gonorrhoeae GMMA (NgG) | [136] NCT05630859 |
| Shigellosis | Shigella sonnei, Shigella flexneri 1b, 2a, and 3a | altSonfex1-2-3 | [137,138,139] | |
| Phase I Clinical Trials | COVID-19 | SARS-CoV-2 | Avacc 10 | NCT05604690 |
| Invasive non-typhoidal Salmonella disease | Invasive non-typhoidal Salmonella | iNTS-GMMA | [140,141] | |
| Meningitis | N. meningitidis B | MenPF-1 | [142] NCT01640652 | |
| Meningitis | N. meningitidis B | Native outer-membrane vesicle (NOMV) vaccine | [143] | |
| Preclinical Studies (Selective Examples) | Acute otitis media, Sinusitis, and Bronchitis | Non-typeable H. influenzae | - | [144] |
| Anthrax | Bacillus anthracis | [145] | ||
| Brucellosis | Brucella melitensis | - | [146] | |
| Chlamydia | Chlamydia muridarum | - | [147] | |
| Cholera | Vibrio cholerae | - | [54,148,149,150] | |
| Chronic periodontitis | Porphyromonas gingivalis | - | [151] | |
| COVID-19 | SARS-CoV2 | - | [152] | |
| Diarrhea | Enterotoxigenic Escherichia coli (ETEC) | - | [59] | |
| Gonorrhea | Neisseria gonorrhoea | Avacc11 | [153] | |
| Gonorrhea | N. gonorrhoea | - | [154,155] | |
| Influenza | Influenza A virus | - | [156] | |
| Lung infections | Klebsiella pneumoniae | - | [157,158] | |
| Lyme | Borrelia burgdorferi | [159] | ||
| Melioidosis | Burkholderia pseudomallei | - | [160] | |
| Meningitis | N. meningitidis B | - | [161] | |
| Meningitis | Neisseria lactamica | - | [162] | |
| Tuberculosis | Mycobacterium tuberculosis | - | [163] | |
| Pneumonia, Meningitis, and Sepsis | Acinetobacter baumannii | - | [164] | |
| Tularemia | Francisella (different strains) | - | [165] | |
| Typhoid fever, Gastroenteritis | Salmonella Typhimurium | - | [166] | |
| Whooping cough | B. pertussis | - | [164,167,168] |
| No. | Pathogens | Antigens | OMV Backbone | Developmental Status | Major Study Outcomes | References |
|---|---|---|---|---|---|---|
| 1 | Actinobacillus Pleuropneumoniae | A trivalent Apx-fusion protein | Escherichia coli | Preclinical | Immunization in mice led to elevated antigen-specific IgG levels and a balanced Th1/Th2 cytokine response, resulting in significant protection against challenges with serotypes 1 and 7 | [342] |
| 2 | Chlamydia trachomatis | Multi-epitope polypeptide of C. trachomatis major outer membrane protein (MOMP) | Salmonella enterica serovar Typhimurium | Preclinical | Vaccine induced robust systemic and mucosal immune responses in mice, characterized by high levels of specific IgG and IgA antibodies | [163] |
| 3 | Helicobacter pylori | UreB and CagA of H. pylori | S. enterica serovar Typhimurium | Preclinical | Vaccination elicited strong humoral and cellular immune responses, including elevated levels of specific antibodies and cytokines, leading to reduced bacterial colonization in the gastric mucosa of mice | [343] |
| 4 | HIV-1 | HIV-1 Tat and Nef proteins | ClearColi™ | Preclinical | The engineered OMVs induced potent antigen-specific immune responses in mice, with the significant production of IgG antibodies and activation of CD4+ and CD8+ T-cells | [344] |
| 5 | Mycobacterium tuberculosis | ESAT6, Ag85B, and Rv2660c antigens of M. tuberculosis | E. coli and S. enterica serovar Typhimurium | Preclinical | Immunization resulted in strong Th1-type immune responses, characterized by increased IFN-γ production and enhanced protection against M. tuberculosis challenge in mice | [163] |
| 6 | Neisseria meningitidis | NadA, NHBA and fHbp from N. meningitidis serogroup B | Neisseria cinerea | Preclinical | Vaccine elicited bactericidal antibodies against multiple N. meningitidis serogroup B strains, demonstrating cross-protective potential in murine models | [345] |
| 7 | SARS-CoV-2 | NG06 fragment and the receptor-binding domain (RBD) of the spike protein | Lumen of E. coli Nissle 1917 OMVs | Preclinical | The bivalent OMV vaccine induced strong neutralizing antibody responses and T-cell activation, providing protection against SARS-CoV-2 challenge in mice | [346] |
| 8 | Shiga toxin-producing E. coli (STEC) | O-antigen polysaccharides of E. coli O26, O45, O145, and O103 | S. enterica serovar Typhimurium | Preclinical | Immunization with the multivalent OMVs elicited robust antibody responses and conferred protection against multiple STEC serotypes in murine models | [347] |
| 9 | Staphylococcus aureus | Five protective antigens of S. aureus as fusions to a lipoprotein leader sequence | E. coli | Preclinical | Vaccine induced strong antigen-specific antibody responses and provided significant protection against S. aureus infection in mice | [263] |
| 10 | S. aureus | ClfAY338A, LukE, SpAKKAA, and HlaH35L | E. coli BL21(DE3)Δ60 | Preclinical | Multivalent OMVs elicited functional antibodies capable of neutralizing key virulence factors, leading to enhanced survival rates in infected mice | [348] |
| 11 | Campylobacter jejuni, S. enterica ser. Typhimurium and S. enterica ser. Enteritidis | Isolated OMVs from each pathogen | C. jejuni, S. enterica ser. Typhimurium and S. enterica ser. Enteritidis (OMV pooling) | Preclinical | The trivalent OMV vaccine induced broad-spectrum immune responses and conferred protection against all three pathogens in murine models | [349] |
| 12 | Enterotoxigenic E. coli (ETEC) and Shigella flexneri | ETEC enterotoxin B | S. flexneri | Preclinical | Vaccine elicited strong mucosal and systemic immune responses, providing protection against both ETEC and Shigella infections in mice | [350] |
| 13 | Enterotoxigenic E. coli (ETEC) and Shigella sonnei | E. coli antigens SslE and FdeC | S. sonnei | Preclinical | Immunization resulted in robust antibody responses against both pathogens | [114] |
| 14 | Enterotoxigenic E. coli (ETEC) and Vibrio cholerae | Lipopolysaccharide modified and toxin negative OMVs from both pathogens | V. cholerae and ETEC (OMV pooling) | Preclinical | The dual-OMV vaccine induced cross-protective immune responses, with significant reductions in bacterial colonization observed in vaccinated mice | [351] |
| 15 | Influenza A virus (IAV) and Middle East Respiratory Syndrome (MERS) Virus | H1 hemagglutinin antigen of IAV and receptor binding domain (RBD) of MERS | E. coli | Preclinical | The bivalent OMV vaccine elicited strong neutralizing antibody responses against both viruses, providing protection in murine challenge models | [352] |
| 16 | N. meningitidis and S. enterica ser. Typhimurium | MenA and MenC oligosaccharides | S. enterica ser. Typhimurium | Preclinical | Vaccine-induced robust antibody responses against both pathogens, demonstrating potential for dual protection in mice | [114] |
| 17 | N. meningitidis and Neisseria gonorrhoeae | fHbp and NHBA-2 of N. meningitidis and NHBA-542 of N. gonorrhoeae | Neisseria cinerea | Preclinical | Immunization elicited cross-reactive antibodies capable of targeting both N. meningitidis and N. gonorrhoeae | [155] |
| 18 | S. enterica serovar Paratyphi A and S. enterica serovar Typhi | S. Paratyphi A homologous O:2 antigens and S. Typhi heterologous Vi | S. enterica serovar Paratyphi A | Preclinical | Vaccine induced a strong antibody response against both Vi and O:2, and these antibodies were functional in a serum bactericidal assay | [198] |
| 19 | S. enterica ser. Typhimurium and Influenza A Virus (IAV) | S. enterica ser. Typhimurium OmpA and SseB, and H-stalk protein H5 of IAV | Bacteroides thetaiotaomicron | Preclinical | Vaccine induced antigen-specific immune and antibody responses in both mucosal tissues and systemically against both bacterial and viral pathogens | [224] |
| 20 | S. flexneri 2a, S. flexneri 3a, S. flexneri 6 and S. sonnei I | OMVs from all strains mixed in 1:1:1:1 ratio | Shigella | Preclinical | Vaccine induced broad-spectrum immune responses, providing protection against multiple Shigella serotypes in murine models | [301] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahid, A.; Ismail, H.; Wilson, J.C.; Grice, I.D. Bioengineering Outer-Membrane Vesicles for Vaccine Development: Strategies, Advances, and Perspectives. Vaccines 2025, 13, 767. https://doi.org/10.3390/vaccines13070767
Zahid A, Ismail H, Wilson JC, Grice ID. Bioengineering Outer-Membrane Vesicles for Vaccine Development: Strategies, Advances, and Perspectives. Vaccines. 2025; 13(7):767. https://doi.org/10.3390/vaccines13070767
Chicago/Turabian StyleZahid, Ayesha, Hazrat Ismail, Jennifer C. Wilson, and I. Darren Grice. 2025. "Bioengineering Outer-Membrane Vesicles for Vaccine Development: Strategies, Advances, and Perspectives" Vaccines 13, no. 7: 767. https://doi.org/10.3390/vaccines13070767
APA StyleZahid, A., Ismail, H., Wilson, J. C., & Grice, I. D. (2025). Bioengineering Outer-Membrane Vesicles for Vaccine Development: Strategies, Advances, and Perspectives. Vaccines, 13(7), 767. https://doi.org/10.3390/vaccines13070767







