Immunization with Complete Freund’s Adjuvant Reveals Trained Immunity-like Features in A/J Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. BCG Propagation and Enumeration
2.3. Study Design
2.4. Bone Marrow Cells Isolation and Ex Vivo Stimulation
2.5. Cytokine Bead Array Analysis
2.6. RT-qPCR
2.7. Targeted Metabolomics
2.8. Metabolomics Data Analysis
2.9. Chromatin Immunoprecipitation (ChIP)-qPCR Assay
2.10. Statistical Analysis
3. Results and Discussion
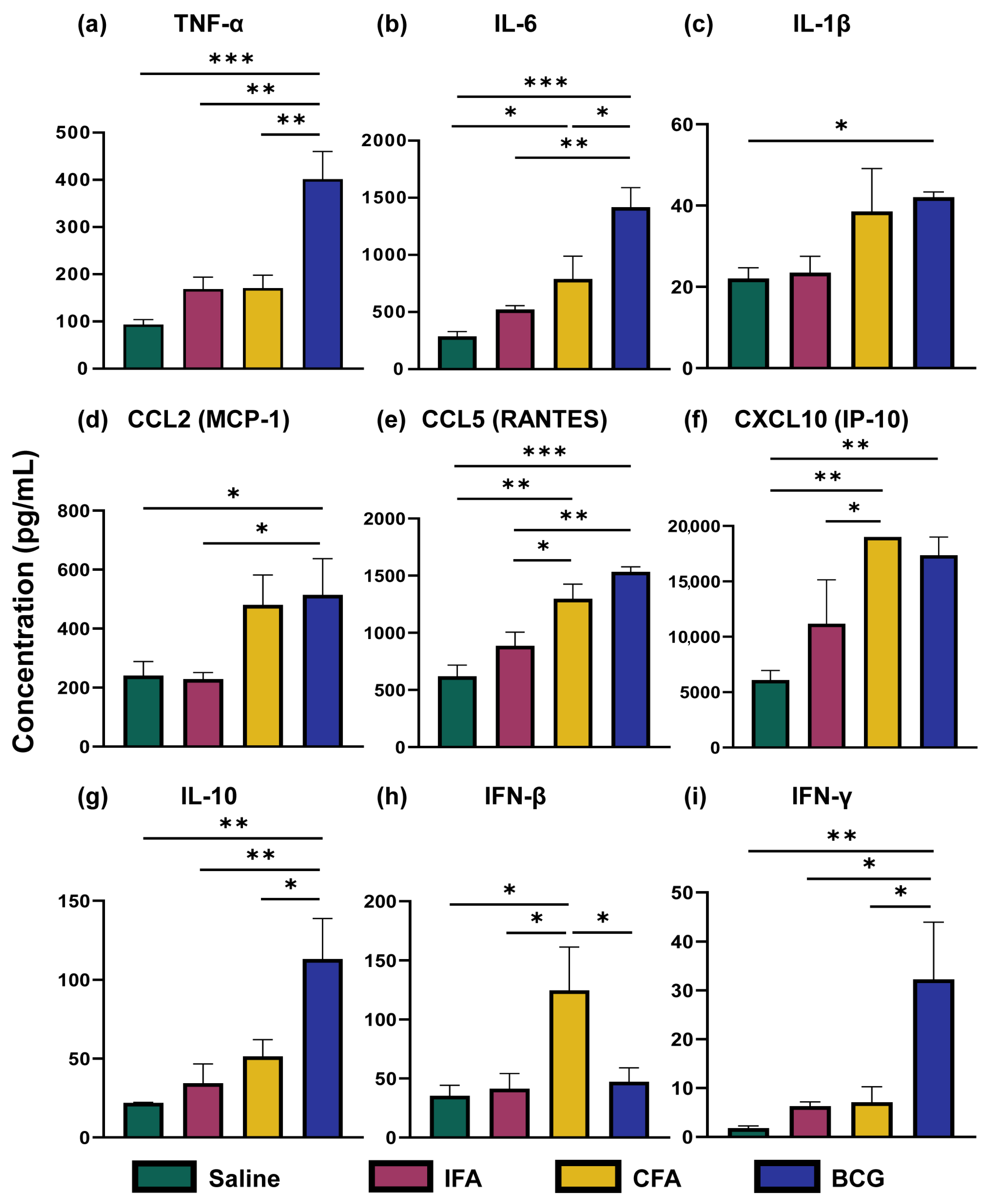
3.1. CFA and BCG Immunizations Were Associated with Distinct Cytokine and Chemokine Profiles Compared to IFA
3.2. Immunizations with Freund’s Adjuvants Did Not Reveal Differences in the Expression of M1 and M2 Markers in BMCs
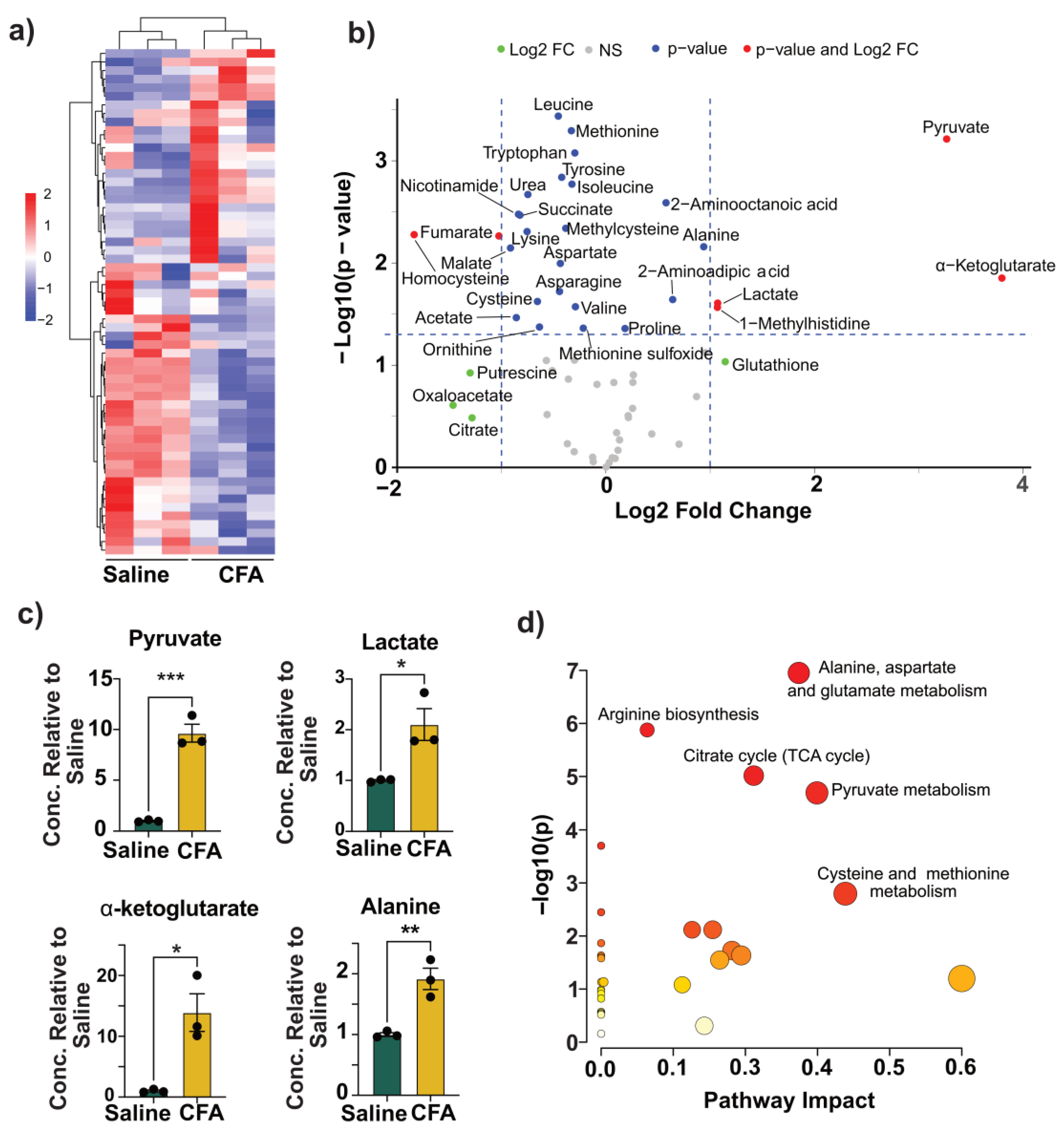
3.3. CFA Immunization Was Associated with Metabolic Reprogramming in the BMCs
3.4. Immunization with BCG Reveals Metabolic Changes That Overlap with CFA
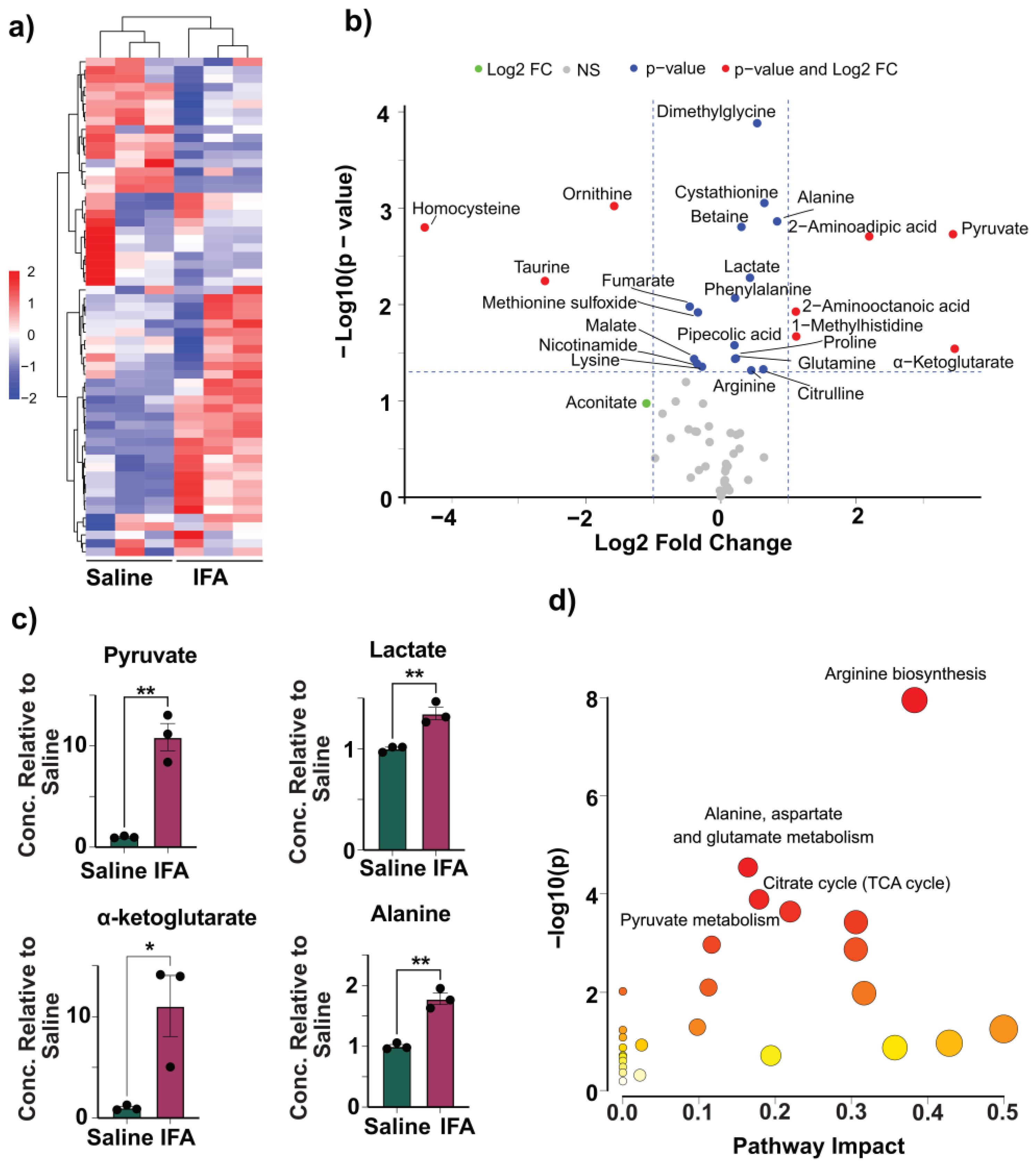
3.5. IFA Induces Modest Changes to Glycolysis
3.6. CFA Immunization Reveals Minor Epigenetic Changes
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| BCG | Bacillus Calmette-Guérin |
| BMC | Bone marrow cells |
| CCL | C-C motif chemokine ligand |
| CFA | Complete Freund’s adjuvant |
| CXCL | C-X-C motif chemokine ligand |
| ChIP | Chromatin Immunoprecipitation |
| FC | Fold change |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| HILIC | Hydrophilic interaction liquid chromatography |
| H3K4me3 | Histone 3 trimethylation of lysine 4 |
| HPLC | High-performance liquid chromatography |
| IFA | Incomplete Freund’s adjuvant |
| IFN | Interferon |
| Ig | Immunoglobulin |
| IL | Interleukin |
| IP | Immunoprecipitation |
| LC-MS | Liquid chromatography coupled with high-resolution mass spectrometry |
| LPS | Lipopolysaccharide |
| M. tb | Mycobacterium tuberculosis |
| MRM | Multiple reaction monitoring |
| NOS | Nitric oxide synthase |
| OD | Optical density |
| PBS | Phosphate-buffered saline |
| PPAR-γ | Peroxisome proliferator-activated receptor-gamma |
| RPM | Revolutions per minute |
| RPMI | Roswell Park Memorial Institute |
| RT-qPCR | Real-time quantitative polymerase chain reaction |
| TCA | Tricarboxylic acid |
| Th | T helper |
| TI | Trained immunity |
| TNF | Tumor necrosis factor |
| TE | Tris-ethylenediaminetetraacetic acid |
References
- Facciola, A.; Visalli, G.; Lagana, A.; Di Pietro, A. An Overview of Vaccine Adjuvants: Current Evidence and Future Perspectives. Vaccines 2022, 10, 819. [Google Scholar] [CrossRef] [PubMed]
- Billiau, A.; Matthys, P. Modes of action of Freund’s adjuvants in experimental models of autoimmune diseases. J. Leukoc. Biol. 2001, 70, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Jensen, F.C.; Savary, J.R.; Diveley, J.P.; Chang, J.C. Adjuvant activity of incomplete Freund’s adjuvant. Adv. Drug Deliv. Rev. 1998, 32, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Shibaki, A.; Katz, S.I. Induction of skewed Th1/Th2 T-cell differentiation via subcutaneous immunization with Freund’s adjuvant. Exp. Dermatol. 2002, 11, 126–134. [Google Scholar] [CrossRef] [PubMed]
- McGeachy, M.J.; Cua, D.J. Th17 cell differentiation: The long and winding road. Immunity 2008, 28, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Ribechini, E.; Eckert, I.; Beilhack, A.; Du Plessis, N.; Walzl, G.; Schleicher, U.; Ritter, U.; Lutz, M.B. Heat-killed Mycobacterium tuberculosis prime-boost vaccination induces myeloid-derived suppressor cells with spleen dendritic cell-killing capability. JCI Insight 2019, 5, 128664. [Google Scholar] [CrossRef] [PubMed]
- Shenderov, K.; Barber, D.L.; Mayer-Barber, K.D.; Gurcha, S.S.; Jankovic, D.; Feng, C.G.; Oland, S.; Hieny, S.; Caspar, P.; Yamasaki, S.; et al. Cord factor and peptidoglycan recapitulate the Th17-promoting adjuvant activity of mycobacteria through mincle/CARD9 signaling and the inflammasome. J. Immunol. 2013, 190, 5722–5730. [Google Scholar] [CrossRef] [PubMed]
- Damsker, J.M.; Hansen, A.M.; Caspi, R.R. Th1 and Th17 cells: Adversaries and collaborators. Ann. N. Y. Acad. Sci. 2010, 1183, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wakeham, J.; Harkness, R.; Xing, Z. Macrophages are a significant source of type 1 cytokines during mycobacterial infection. J. Clin. Investig. 1999, 103, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Dominguez-Andres, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Jacobs, C.; Xavier, R.J.; van der Meer, J.W.; van Crevel, R.; Netea, M.G. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin. Immunol. 2014, 155, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gao, L.; Wu, X.; Fan, Y.; Liu, M.; Peng, L.; Song, J.; Li, B.; Liu, A.; Bao, F. BCG-induced trained immunity: History, mechanisms and potential applications. J. Transl. Med. 2023, 21, 106. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.J.W.; Moorlag, S.; Novakovic, B.; Li, Y.; Wang, S.Y.; Oosting, M.; Kumar, V.; Xavier, R.J.; Wijmenga, C.; Joosten, L.A.B.; et al. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe 2018, 23, 89–100.e105. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ramon, S.; Conejero, L.; Netea, M.G.; Sancho, D.; Palomares, O.; Subiza, J.L. Trained Immunity-Based Vaccines: A New Paradigm for the Development of Broad-Spectrum Anti-infectious Formulations. Front. Immunol. 2018, 9, 2936. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef] [PubMed]
- Baydemir, I.; Dulfer, E.A.; Netea, M.G.; Dominguez-Andres, J. Trained immunity-inducing vaccines: Harnessing innate memory for vaccine design and delivery. Clin. Immunol. 2024, 261, 109930. [Google Scholar] [CrossRef] [PubMed]
- Quintin, J.; Saeed, S.; Martens, J.H.A.; Giamarellos-Bourboulis, E.J.; Ifrim, D.C.; Logie, C.; Jacobs, L.; Jansen, T.; Kullberg, B.J.; Wijmenga, C.; et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 2012, 12, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Gangaplara, A.; Massilamany, C.; Lasrado, N.; Steffen, D.; Reddy, J. Evidence for Anti-Viral Effects of Complete Freund’s Adjuvant in the Mouse Model of Enterovirus Infection. Vaccines 2020, 8, 364. [Google Scholar] [CrossRef]
- Mone, K.; Garcia, E.J.T.; Abdullatif, F.; Rasquinha, M.T.; Sur, M.; Hanafy, M.; Zinniel, D.K.; Singh, S.; Thomas, R.; Barletta, R.G.; et al. Metabolic Reprogramming in Response to Freund’s Adjuvants: Insights from Serum Metabolomics. Microorganisms 2025, 13, 492. [Google Scholar] [CrossRef] [PubMed]
- Sur, M.; Rasquinha, M.T.; Mone, K.; Massilamany, C.; Lasrado, N.; Gurumurthy, C.; Sobel, R.A.; Reddy, J. Investigation into Cardiac Myhc-alpha 334-352-Specific TCR Transgenic Mice Reveals a Role for Cytotoxic CD4 T Cells in the Development of Cardiac Autoimmunity. Cells 2024, 13, 234. [Google Scholar] [CrossRef] [PubMed]
- Massilamany, C.; Gangaplara, A.; Steffen, D.; Reddy, J. Identification of novel mimicry epitopes for cardiac myosin heavy chain-alpha that induce autoimmune myocarditis in A/J mice. Cell. Immunol. 2011, 271, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Massilamany, C.; Gangaplara, A.; Chapman, N.; Rose, N.; Reddy, J. Detection of cardiac myosin heavy chain-alpha-specific CD4 cells by using MHC class II/IA(k) tetramers in A/J mice. J. Immunol. Methods 2011, 372, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Massilamany, C.; Gangaplara, A.; Basavalingappa, R.H.; Rajasekaran, R.A.; Khalilzad-Sharghi, V.; Han, Z.; Othman, S.; Steffen, D.; Reddy, J. Localization of CD8 T cell epitope within cardiac myosin heavy chain-alpha334-352 that induces autoimmune myocarditis in A/J mice. Int. J. Cardiol. 2016, 202, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, B.; Massilamany, C.; Basavalingappa, R.H.; Gangaplara, A.; Kang, G.; Li, Q.; Uzal, F.A.; Strande, J.L.; Delhon, G.A.; Riethoven, J.J.; et al. Branched chain alpha-ketoacid dehydrogenase kinase 111-130, a T cell epitope that induces both autoimmune myocarditis and hepatitis in A/J mice. Immun. Inflamm. Dis. 2017, 5, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.C.; Chen, Z.Y.; Huang, X.J.; Wu, J.; Huang, H.; Niu, L.F.; Wang, H.L.; Li, J.H.; Lowrie, D.B.; Hu, Z.; et al. Multi-omics analysis reveals that linoleic acid metabolism is associated with variations of trained immunity induced by distinct BCG strains. Sci. Adv. 2024, 10, eadk8093. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhou, Y.; Zhang, B.; Liang, X.; Feng, J.; Huang, Y.; Weng, S.; Xu, Y.; Su, H. Mucosal recombinant BCG vaccine induces lung-resident memory macrophages and enhances trained immunity via mTORC2/HK1-mediated metabolic rewiring. J. Biol. Chem. 2024, 300, 105518. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.J.W.; Carvalho, A.; La Rocca, C.; Palma, C.; Rodrigues, F.; Silvestre, R.; Kleinnijenhuis, J.; Lachmandas, E.; Goncalves, L.G.; Belinha, A.; et al. Immunometabolic Pathways in BCG-Induced Trained Immunity. Cell Rep. 2016, 17, 2562–2571. [Google Scholar] [CrossRef] [PubMed]
- Lasrado, N.; Gangaplara, A.; Massilamany, C.; Arumugam, R.; Shelbourn, A.; Rasquinha, M.T.; Basavalingappa, R.H.; Delhon, G.; Xiang, S.H.; Pattnaik, A.K.; et al. Attenuated strain of CVB3 with a mutation in the CAR-interacting region protects against both myocarditis and pancreatitis. Sci. Rep. 2021, 11, 12432. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Breitkopf, S.B.; Yang, X.; Asara, J.M. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat. Protoc. 2012, 7, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Team, R.C. R: A Language and Environment for Statistical Computing [Computer Software]; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Kolde, R. Pheatmap: Pretty heatmaps. R Package Version 1.0.12. 2019. Available online: https://rdocumentation.org/packages/pheatmap/versions/1.0.12 (accessed on 14 April 2025).
- Ward, J.H., Jr. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Pohlert, T. dixonTest: Dixon’s Ratio Test for Outlier Detection. R Package Version 1.0.4. 2022. Available online: https://cran.r-project.org/package=dixonTest (accessed on 14 April 2025).
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef] [PubMed]
- Vierboom, M.P.M.; Dijkman, K.; Sombroek, C.C.; Hofman, S.O.; Boot, C.; Vervenne, R.A.W.; Haanstra, K.G.; van der Sande, M.; van Emst, L.; Dominguez-Andres, J.; et al. Stronger induction of trained immunity by mucosal BCG or MTBVAC vaccination compared to standard intradermal vaccination. Cell Rep. Med. 2021, 2, 100185. [Google Scholar] [CrossRef] [PubMed]
- Jeyanathan, M.; Vaseghi-Shanjani, M.; Afkhami, S.; Grondin, J.A.; Kang, A.; D’Agostino, M.R.; Yao, Y.; Jain, S.; Zganiacz, A.; Kroezen, Z.; et al. Parenteral BCG vaccine induces lung-resident memory macrophages and trained immunity via the gut-lung axis. Nat. Immunol. 2022, 23, 1687–1702. [Google Scholar] [CrossRef] [PubMed]
- Joosten, S.A.; van Meijgaarden, K.E.; Arend, S.M.; Prins, C.; Oftung, F.; Korsvold, G.E.; Kik, S.V.; Arts, R.J.; van Crevel, R.; Netea, M.G.; et al. Mycobacterial growth inhibition is associated with trained innate immunity. J. Clin. Investig. 2018, 128, 1837–1851. [Google Scholar] [CrossRef] [PubMed]
- Moorlag, S.; Folkman, L.; Ter Horst, R.; Krausgruber, T.; Barreca, D.; Schuster, L.C.; Fife, V.; Matzaraki, V.; Li, W.; Reichl, S.; et al. Multi-omics analysis of innate and adaptive responses to BCG vaccination reveals epigenetic cell states that predict trained immunity. Immunity 2024, 57, 171–187.e114. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Saeed, A.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Strizova, Z.; Benesova, I.; Bartolini, R.; Novysedlak, R.; Cecrdlova, E.; Foley, L.K.; Striz, I. M1/M2 macrophages and their overlaps—Myth or reality? Clin. Sci. 2023, 137, 1067–1093. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Jeljeli, M.; Riccio, L.G.C.; Doridot, L.; Chene, C.; Nicco, C.; Chouzenoux, S.; Deletang, Q.; Allanore, Y.; Kavian, N.; Batteux, F. Trained immunity modulates inflammation-induced fibrosis. Nat. Commun. 2019, 10, 5670. [Google Scholar] [CrossRef] [PubMed]
- Chi, H. Immunometabolism at the intersection of metabolic signaling, cell fate, and systems immunology. Cell. Mol. Immunol. 2022, 19, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Etchegaray, J.P.; Mostoslavsky, R. Interplay between Metabolism and Epigenetics: A Nuclear Adaptation to Environmental Changes. Mol. Cell 2016, 62, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, D.R.; Bultman, S.J. Metaboloepigenetics: Interrelationships between energy metabolism and epigenetic control of gene expression. J. Cell. Physiol. 2012, 227, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yang, X.; Yuan, Z.; Wang, H. Metabolic Reprogramming in Immune Response and Tissue Inflammation. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1990–2001. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.A.; Rutter, J. Metabolites as signalling molecules. Nat. Rev. Mol. Cell Biol. 2023, 24, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.; Rao, N.A.; Aghajanirefah, A.; et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.V.; Dominguez-Andres, J.; Merlo Pich, L.M.; Joosten, L.A.B.; Netea, M.G. Metabolic Regulation in the Induction of Trained Immunity. Semin. Immunopathol. 2024, 46, 7. [Google Scholar] [CrossRef] [PubMed]
- Seravalli, J.; Portugal, F. Putrescine Detected in Strains of Staphylococcus aureus. Pathogens 2023, 12, 881. [Google Scholar] [CrossRef] [PubMed]
- Basu, U.; Seravalli, J.; Madayiputhiya, N.; Adamec, J.; Case, A.J.; Zimmerman, M.C. Rapid metabolism of exogenous angiotensin II by catecholaminergic neuronal cells in culture media. Physiol. Rep. 2015, 3, e12287. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Wind, F.; Negelein, E. The Metabolism of Tumors in the Body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Mancuso, A.; Daikhin, E.; Nissim, I.; Yudkoff, M.; Wehrli, S.; Thompson, C.B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 19345–19350. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Morris, S.M., Jr. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336 Pt 1, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ming, X.F. Functions of arginase isoforms in macrophage inflammatory responses: Impact on cardiovascular diseases and metabolic disorders. Front. Immunol. 2014, 5, 533. [Google Scholar] [CrossRef] [PubMed]
- Sharda, D.R.; Yu, S.; Ray, M.; Squadrito, M.L.; De Palma, M.; Wynn, T.A.; Morris, S.M., Jr.; Hankey, P.A. Regulation of macrophage arginase expression and tumor growth by the Ron receptor tyrosine kinase. J. Immunol. 2011, 187, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef] [PubMed]
- Geiger, R.; Rieckmann, J.C.; Wolf, T.; Basso, C.; Feng, Y.; Fuhrer, T.; Kogadeeva, M.; Picotti, P.; Meissner, F.; Mann, M.; et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell 2016, 167, 829–842.e813. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N. Role of methionine on epigenetic modification of DNA methylation and gene expression in animals. Anim. Nutr. 2018, 4, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Lauinger, L.; Kaiser, P. Sensing and Signaling of Methionine Metabolism. Metabolites 2021, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, K.; Jakob, U. The role of thiols in antioxidant systems. Free Radic. Biol. Med. 2019, 140, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Franchina, D.G.; Kurniawan, H.; Grusdat, M.; Binsfeld, C.; Guerra, L.; Bonetti, L.; Soriano-Baguet, L.; Ewen, A.; Kobayashi, T.; Farinelle, S.; et al. Glutathione-dependent redox balance characterizes the distinct metabolic properties of follicular and marginal zone B cells. Nat. Commun. 2022, 13, 1789. [Google Scholar] [CrossRef] [PubMed]
- Ritz, N.; Hanekom, W.A.; Robins-Browne, R.; Britton, W.J.; Curtis, N. Influence of BCG vaccine strain on the immune response and protection against tuberculosis. FEMS Microbiol. Rev. 2008, 32, 821–841. [Google Scholar] [CrossRef] [PubMed]
- Gillard, J.; Blok, B.A.; Garza, D.R.; Venkatasubramanian, P.B.; Simonetti, E.; Eleveld, M.J.; Berbers, G.A.M.; van Gageldonk, P.G.M.; Joosten, I.; de Groot, R.; et al. BCG-induced trained immunity enhances acellular pertussis vaccination responses in an explorative randomized clinical trial. NPJ Vaccines 2022, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhenyukh, O.; Civantos, E.; Ruiz-Ortega, M.; Sanchez, M.S.; Vazquez, C.; Peiro, C.; Egido, J.; Mas, S. High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free Radic. Biol. Med. 2017, 104, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.; Scaini, G.; Furlanetto, C.B.; Galant, L.S.; Vuolo, F.; Dall’Igna, D.M.; Schuck, P.F.; Ferreira, G.C.; Dal-Pizzol, F.; Streck, E.L. Administration of branched-chain amino acids alters the balance between pro-inflammatory and anti-inflammatory cytokines. Int. J. Dev. Neurosci. 2016, 48, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Koeken, V.; Qi, C.; Mourits, V.P.; de Bree, L.C.J.; Moorlag, S.; Sonawane, V.; Lemmers, H.; Dijkstra, H.; Joosten, L.A.B.; van Laarhoven, A.; et al. Plasma metabolome predicts trained immunity responses after antituberculosis BCG vaccination. PLoS Biol. 2022, 20, e3001765. [Google Scholar] [CrossRef] [PubMed]
- Gokkusu, C.; Tulubas, F.; Unlucerci, Y.; Ozkok, E.; Umman, B.; Aydin, M. Homocysteine and pro-inflammatory cytokine concentrations in acute heart disease. Cytokine 2010, 50, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Bekkering, S.; Arts, R.J.W.; Novakovic, B.; Kourtzelis, I.; van der Heijden, C.; Li, Y.; Popa, C.D.; Ter Horst, R.; van Tuijl, J.; Netea-Maier, R.T.; et al. Metabolic Induction of Trained Immunity through the Mevalonate Pathway. Cell 2018, 172, 135–146.e139. [Google Scholar] [CrossRef] [PubMed]
- Fanucchi, S.; Dominguez-Andres, J.; Joosten, L.A.B.; Netea, M.G.; Mhlanga, M.M. The Intersection of Epigenetics and Metabolism in Trained Immunity. Immunity 2021, 54, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Benayoun, B.A.; Pollina, E.A.; Ucar, D.; Mahmoudi, S.; Karra, K.; Wong, E.D.; Devarajan, K.; Daugherty, A.C.; Kundaje, A.B.; Mancini, E.; et al. H3K4me3 breadth is linked to cell identity and transcriptional consistency. Cell 2014, 158, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Valtanen, P.; Guzman-Genuino, R.M.; Williams, D.L.; Hayball, J.D.; Diener, K.R. Evaluation of trained immunity by beta-1, 3 (d)-glucan on murine monocytes in vitro and duration of response in vivo. Immunol. Cell Biol. 2017, 95, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Owen, A.M.; Fults, J.B.; Patil, N.K.; Hernandez, A.; Bohannon, J.K. TLR Agonists as Mediators of Trained Immunity: Mechanistic Insight and Immunotherapeutic Potential to Combat Infection. Front. Immunol. 2020, 11, 622614. [Google Scholar] [CrossRef] [PubMed]
- Covian, C.; Fernandez-Fierro, A.; Retamal-Diaz, A.; Diaz, F.E.; Vasquez, A.E.; Lay, M.K.; Riedel, C.A.; Gonzalez, P.A.; Bueno, S.M.; Kalergis, A.M. BCG-Induced Cross-Protection and Development of Trained Immunity: Implication for Vaccine Design. Front. Immunol. 2019, 10, 2806. [Google Scholar] [CrossRef] [PubMed]
- Whelan, A.O.; Wright, D.C.; Chambers, M.A.; Singh, M.; Hewinson, R.G.; Vordermeier, H.M. Evidence for enhanced central memory priming by live Mycobacterium bovis BCG vaccine in comparison with killed BCG formulations. Vaccine 2008, 26, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Benn, C.S.; Joosten, L.A.; Jacobs, C.; van Loenhout, J.; Xavier, R.J.; Aaby, P.; van der Meer, J.W.; et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 2014, 6, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Moorlag, S.; Lefkovith, A.; Li, B.; Matzaraki, V.; van Emst, L.; Kang, H.A.; Latorre, I.; Jaeger, M.; Joosten, L.A.B.; et al. Single-cell transcriptomic profiles reveal changes associated with BCG-induced trained immunity and protective effects in circulating monocytes. Cell Rep. 2021, 37, 110028. [Google Scholar] [CrossRef] [PubMed]
- Kang, A.; Ye, G.; Afkhami, S.; Aleithan, F.; Singh, K.; Dvorkin-Gheva, A.; Berg, T.; Miller, M.S.; Jeyanathan, M.; Xing, Z. LPS-induced lung tissue-resident trained innate immunity provides differential protection against pneumococci and SARS-CoV-2. Cell Rep. 2024, 43, 114849. [Google Scholar] [CrossRef] [PubMed]
- Angelidou, A.; Diray-Arce, J.; Conti, M.G.; Netea, M.G.; Blok, B.A.; Liu, M.; Sanchez-Schmitz, G.; Ozonoff, A.; van Haren, S.D.; Levy, O. Human Newborn Monocytes Demonstrate Distinct BCG-Induced Primary and Trained Innate Cytokine Production and Metabolic Activation In Vitro. Front. Immunol. 2021, 12, 674334. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, J.; Wu, Z. The Regulatory Role of alpha-Ketoglutarate Metabolism in Macrophages. Mediators Inflamm. 2021, 2021, 5577577. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; He, L.; Yao, K. The Antioxidative Function of Alpha-Ketoglutarate and Its Applications. Biomed. Res. Int. 2018, 2018, 3408467. [Google Scholar] [CrossRef] [PubMed]
- Klysz, D.; Tai, X.; Robert, P.A.; Craveiro, M.; Cretenet, G.; Oburoglu, L.; Mongellaz, C.; Floess, S.; Fritz, V.; Matias, M.I.; et al. Glutamine-dependent alpha-ketoglutarate production regulates the balance between T helper 1 cell and regulatory T cell generation. Sci. Signal 2015, 8, ra97. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Li, H.; Huang, N.; Zhou, X.; Tian, J.; Li, T.; Wu, J.; Tian, Y.; Yin, Y.; Yao, K. Alpha-ketoglutarate suppresses the NF-kappaB-mediated inflammatory pathway and enhances the PXR-regulated detoxification pathway. Oncotarget 2017, 8, 102974–102988. [Google Scholar] [CrossRef] [PubMed]
- Ron-Harel, N.; Ghergurovich, J.M.; Notarangelo, G.; LaFleur, M.W.; Tsubosaka, Y.; Sharpe, A.H.; Rabinowitz, J.D.; Haigis, M.C. T Cell Activation Depends on Extracellular Alanine. Cell Rep. 2019, 28, 3011–3021.e3014. [Google Scholar] [CrossRef] [PubMed]
- Peace, C.G.; O’Carroll, S.M.; O’Neill, L.A.J. Fumarate hydratase as a metabolic regulator of immunity. Trends Cell Biol. 2024, 34, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Hoogendoorn, A.; Avery, T.D.; Li, J.; Bursill, C.; Abell, A.; Grace, P.M. Emerging Therapeutic Applications for Fumarates. Trends Pharmacol. Sci. 2021, 42, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Kern Coquillat, N.; Picq, L.; Hamond, A.; Megy, P.; Benezech, S.; Drouillard, A.; Lager-Lachaud, N.; Cahoreau, E.; Moreau, M.; Fallone, L.; et al. Pivotal role of exogenous pyruvate in human natural killer cell metabolism. Nat. Metab. 2025, 7, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Shi, R.C.; Xiang, Y.C.; Fan, L.; Tang, H.; He, G.; Zhou, M.; Feng, X.Z.; Tan, J.D.; Huang, P.; et al. Malate initiates a proton-sensing pathway essential for pH regulation of inflammation. Signal Transduct. Target. Ther. 2024, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Drummer, C.t.; Saaoud, F.; Shao, Y.; Sun, Y.; Xu, K.; Lu, Y.; Ni, D.; Atar, D.; Jiang, X.; Wang, H.; et al. Trained Immunity and Reactivity of Macrophages and Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Vuscan, P.; Kischkel, B.; Joosten, L.A.B.; Netea, M.G. Trained immunity: General and emerging concepts. Immunol. Rev. 2024, 323, 164–185. [Google Scholar] [CrossRef] [PubMed]
- Minute, L.; Bergon-Gutierrez, M.; Mata-Martinez, P.; Fernandez-Pascual, J.; Terron, V.; Bravo-Robles, L.; Bicakcioglu, G.; Zapata-Fernandez, G.; Aguilo, N.; Lopez-Collazo, E.; et al. Heat-killed Mycobacterium tuberculosis induces trained immunity in vitro and in vivo administered systemically or intranasally. iScience 2024, 27, 108869. [Google Scholar] [CrossRef] [PubMed]
- Krahenbuhl, J.L.; Sharma, S.D.; Ferraresi, R.W.; Remington, J.S. Effects of muramyl dipeptide treatment on resistance to infection with Toxoplasma gondii in mice. Infect. Immun. 1981, 31, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, K.A.; Gaudet, A.D.; Amici, S.A.; Popovich, P.G.; Guerau-de-Arellano, M. Control of the Inflammatory Macrophage Transcriptional Signature by miR-155. PLoS ONE 2016, 11, e0159724. [Google Scholar] [CrossRef] [PubMed]
- Overbergh, L.; Giulietti, A.; Valckx, D.; Decallonne, R.; Bouillon, R.; Mathieu, C. The use of real-time reverse transcriptase PCR for the quantification of cytokine gene expression. J. Biomol. Tech. 2003, 14, 33–43. [Google Scholar] [PubMed]
- Ishida, Y.; Kimura, A.; Kuninaka, Y.; Inui, M.; Matsushima, K.; Mukaida, N.; Kondo, T. Pivotal role of the CCL5/CCR5 interaction for recruitment of endothelial progenitor cells in mouse wound healing. J. Clin. Investig. 2012, 122, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Ozga, A.J.; Chow, M.T.; Lopes, M.E.; Servis, R.L.; Di Pilato, M.; Dehio, P.; Lian, J.; Mempel, T.R.; Luster, A.D. CXCL10 chemokine regulates heterogeneity of the CD8(+) T cell response and viral set point during chronic infection. Immunity 2022, 55, 82–97.e88. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, K.; Kano, F.; Shiota, K.; Murata, M. Expression of the peroxisome proliferator activated receptor gamma gene is repressed by DNA methylation in visceral adipose tissue of mouse models of diabetes. BMC Biol. 2009, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Villalba, A.; Mattiotti, A.; Gunst, Q.D.; Cano-Ballesteros, S.; van den Hoff, M.J.; Ruijter, J.M. Reference genes for gene expression studies in the mouse heart. Sci. Rep. 2017, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, W.; Zhang, X.; van Zomeren, K.C.; Jacobs, S.; Georgieva, P.B.; Wolf, S.A.; Kettenmann, H.; Janova, H.; Saiepour, N.; Hanisch, U.K.; et al. Long-lasting pro-inflammatory suppression of microglia by LPS-preconditioning is mediated by RelB-dependent epigenetic silencing. Brain Behav. Immun. 2015, 48, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Liang, Z.; Weng, S.; Sun, C.; Huang, J.; Zhang, T.; Wang, X.; Wu, S.; Zhang, Z.; Zhang, Y.; et al. miR-9-5p regulates immunometabolic and epigenetic pathways in beta-glucan-trained immunity via IDH3alpha. JCI Insight 2021, 6, 144260. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vazquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mone, K.; Singh, S.; Abdullatif, F.; Sur, M.; Rasquinha, M.T.; Seravalli, J.; Zinniel, D.K.; Mukhopadhyay, I.; Barletta, R.G.; Gebregiworgis, T.; et al. Immunization with Complete Freund’s Adjuvant Reveals Trained Immunity-like Features in A/J Mice. Vaccines 2025, 13, 768. https://doi.org/10.3390/vaccines13070768
Mone K, Singh S, Abdullatif F, Sur M, Rasquinha MT, Seravalli J, Zinniel DK, Mukhopadhyay I, Barletta RG, Gebregiworgis T, et al. Immunization with Complete Freund’s Adjuvant Reveals Trained Immunity-like Features in A/J Mice. Vaccines. 2025; 13(7):768. https://doi.org/10.3390/vaccines13070768
Chicago/Turabian StyleMone, Kiruthiga, Shraddha Singh, Fatema Abdullatif, Meghna Sur, Mahima T. Rasquinha, Javier Seravalli, Denise K. Zinniel, Indranil Mukhopadhyay, Raul G. Barletta, Teklab Gebregiworgis, and et al. 2025. "Immunization with Complete Freund’s Adjuvant Reveals Trained Immunity-like Features in A/J Mice" Vaccines 13, no. 7: 768. https://doi.org/10.3390/vaccines13070768
APA StyleMone, K., Singh, S., Abdullatif, F., Sur, M., Rasquinha, M. T., Seravalli, J., Zinniel, D. K., Mukhopadhyay, I., Barletta, R. G., Gebregiworgis, T., & Reddy, J. (2025). Immunization with Complete Freund’s Adjuvant Reveals Trained Immunity-like Features in A/J Mice. Vaccines, 13(7), 768. https://doi.org/10.3390/vaccines13070768





