Therapeutic Colorectal Cancer Vaccines: Emerging Modalities and Translational Opportunities
Abstract
1. Introduction
2. Colorectal Cancer Development, Genetics and Classification—The Silent Pandemic
2.1. Development
2.2. Genetics
- Sporadic CRC (~60–80%): Occurs in individuals without a hereditary predisposition.
- Familial CRC (~20–40%): Associated with a family history but lacking identifiable high-penetrance mutations.
- Hereditary CRC: Includes familial adenomatous polyposis (FAP) and Lynch syndrome (hereditary nonpolyposis colorectal cancer, HNPCC).
2.3. Classification
3. CRC and Gut Microbes—The Microbiome Factor
4. CRC Screening and Surveillance—Evolving Innovations
5. Tumor Antigens Used in Colorectal Cancer Vaccine Development—The Source
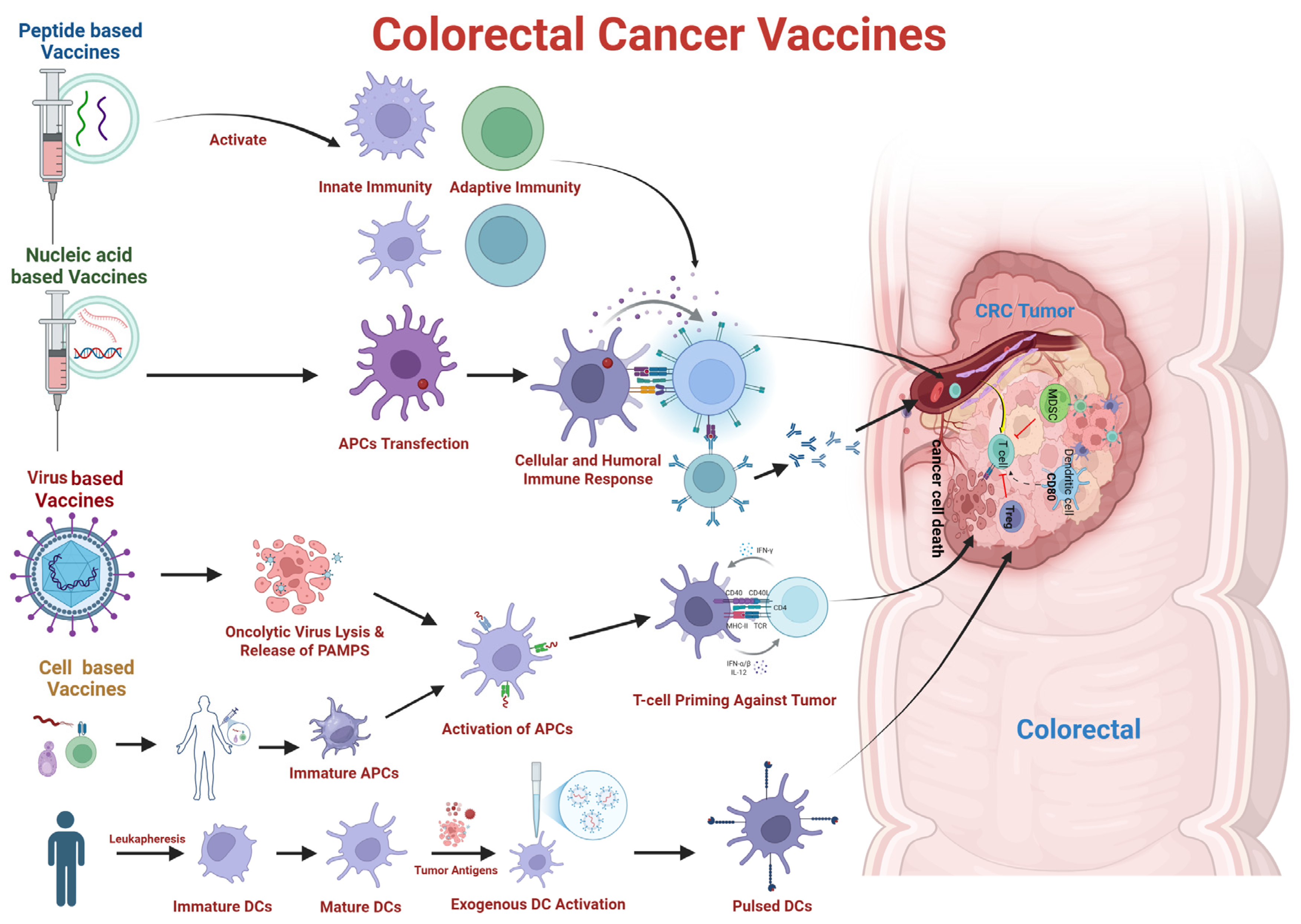
6. Therapeutic Vaccines for CRC—The Savior
6.1. Peptide/Protein Based Vaccines
6.2. Nucleic Acid Based Vaccines
6.3. Cancer Cell Based Vaccines
6.4. Vector-Based Vaccine
6.5. Live-Attenuated Bacteria and Yeast Vaccines
6.6. Neoantigen Vaccines
6.7. Nanovaccines in Colorectal Cancer
6.8. Other Vaccines in Development for Colorectal Cancer
7. Colorectal Cancer Vaccines in Clinical Trials
8. Other Treatment Strategies Against CRC—The Alternatives
8.1. Chemotherapy
8.2. Immunotherapy
8.3. Targeted Therapy
8.4. Adoptive Cell Therapy
8.5. CAR-T Cell Therapy
8.6. Oncolytic Virus Therapy
9. Adjuvants, Dosage and Route of Administration—Value Addition
9.1. Adjuvants
9.2. Dosage and Route of Administration
10. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharma, S.; Singh, N.; Turk, A.A.; Wan, I.; Guttikonda, A.; Dong, J.L.; Zhang, X.; Opyrchal, M. Molecular insights into clinical trials for immune checkpoint inhibitors in colorectal cancer: Unravelling challenges and future directions. World J. Gastroenterol. 2024, 30, 1815–1835. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Greten, F.R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 2021, 21, 653–667. [Google Scholar] [CrossRef]
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Alzahrani, S.M.; Al Doghaither, H.A.; Al-Ghafari, A.B. General insight into cancer: An overview of colorectal cancer (Review). Mol. Clin. Oncol. 2021, 15, 271. [Google Scholar] [CrossRef]
- Roshandel, G.; Ghasemi-Kebria, F.; Malekzadeh, R. Colorectal Cancer: Epidemiology, Risk Factors, and Prevention. Cancers 2024, 16, 1530. [Google Scholar] [CrossRef]
- Jun, S.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Association of the inflammatory balance of diet and lifestyle with colorectal cancer among Korean adults: A case-control study. Epidemiol. Health 2022, 44, e2022084. [Google Scholar] [CrossRef]
- Hossain, M.S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, D.J.; John, A.; Lim, Y.C.; Kibria, K.M.K.; Mohiuddin, A.K.M.; Ming, L.C.; et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef]
- Gupta, S. Screening for Colorectal Cancer. Hematol. Oncol. Clin. N. Am. 2022, 36, 393–414. [Google Scholar] [CrossRef]
- Jiang, S.; Good, D.; Wei, M.Q. Vaccinations for Colorectal Cancer: Progress, Strategies, and Novel Adjuvants. Int. J. Mol. Sci. 2019, 20, 3403. [Google Scholar] [CrossRef]
- Johdi, N.A.; Sukor, N.F. Colorectal Cancer Immunotherapy: Options and Strategies. Front. Immunol. 2020, 11, 1624. [Google Scholar] [CrossRef]
- Cornista, A.M.; Giolito, M.V.; Baker, K.; Hazime, H.; Dufait, I.; Datta, J.; Khumukcham, S.S.; De Ridder, M.; Roper, J.; Abreu, M.T.; et al. Colorectal Cancer Immunotherapy: State of the Art and Future Directions. Gastro Hep Adv. 2023, 2, 1103–1119. [Google Scholar] [CrossRef] [PubMed]
- Hammond, W.A.; Swaika, A.; Mody, K. Pharmacologic resistance in colorectal cancer: A review. Ther. Adv. Med. Oncol. 2016, 8, 57–84. [Google Scholar] [CrossRef] [PubMed]
- Han, C.J.; Ning, X.; Burd, C.E.; Spakowicz, D.J.; Tounkara, F.; Kalady, M.F.; Noonan, A.M.; McCabe, S.; Von Ah, D. Chemotoxicity and Associated Risk Factors in Colorectal Cancer: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 2597. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Shen, X.; Guo, Z.; Cheng, X.; Zhao, R. The future of cancer vaccines against colorectal cancer. Expert. Opin. Biol. Ther. 2024, 24, 269–284. [Google Scholar] [CrossRef]
- Kciuk, M.; Yahya, E.B.; Mohamed Ibrahim Mohamed, M.; Rashid, S.; Iqbal, M.O.; Kontek, R.; Abdulsamad, M.A.; Allaq, A.A. Recent Advances in Molecular Mechanisms of Cancer Immunotherapy. Cancers 2023, 15, 2721. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Z.; Li, S.; Zhang, J. Immunotherapy in colorectal cancer: Statuses and strategies. Heliyon 2025, 11, e41354. [Google Scholar] [CrossRef]
- Shahnazari, M.; Samadi, P.; Pourjafar, M.; Jalali, A. Therapeutic vaccines for colorectal cancer: The progress and future prospect. Int. Immunopharmacol. 2020, 88, 106944. [Google Scholar] [CrossRef]
- Abakushina, E.V.; Gelm, Y.V.; Pasova, I.A.; Bazhin, A.V. Immunotherapeutic Approaches for the Treatment of Colorectal Cancer. Biochemistry 2019, 84, 720–728. [Google Scholar] [CrossRef]
- Fan, T.; Zhang, M.; Yang, J.; Zhu, Z.; Cao, W.; Dong, C. Therapeutic cancer vaccines: Advancements, challenges and prospects. Signal Transduct. Target. Ther. 2023, 8, 450. [Google Scholar] [CrossRef]
- Arvelo, F.; Sojo, F.; Cotte, C. Biology of colorectal cancer. Ecancermedicalscience 2015, 9, 520. [Google Scholar] [CrossRef]
- Pandurangan, A.K.; Divya, T.; Kumar, K.; Dineshbabu, V.; Velavan, B.; Sudhandiran, G. Colorectal carcinogenesis: Insights into the cell death and signal transduction pathways: A review. World J. Gastrointest. Oncol. 2018, 10, 244–259. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, N.; Shahril, M.R.; Shahar, S.; Fenech, M.; Sharif, R. Association between Diet-related Behaviour and Risk of Colorectal Cancer: A Scoping Review. J. Cancer Prev. 2022, 27, 208–220. [Google Scholar] [CrossRef]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef]
- Li, J.; Ma, X.; Chakravarti, D.; Shalapour, S.; DePinho, R.A. Genetic and biological hallmarks of colorectal cancer. Genes. Dev. 2021, 35, 787–820. [Google Scholar] [CrossRef]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.H.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Goel, A.; Chung, D.C. Pathways of Colorectal Carcinogenesis. Gastroenterology 2020, 158, 291–302. [Google Scholar] [CrossRef]
- Bailey, C.E.; Hu, C.Y.; You, Y.N.; Bednarski, B.K.; Rodriguez-Bigas, M.A.; Skibber, J.M.; Cantor, S.B.; Chang, G.J. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. 2015, 150, 17–22. [Google Scholar] [CrossRef]
- Galloway, D.J.; Burns, H.J.; Bear, H.; Jarrett, F.; Boyle, P.; George, W.D. Colorectal cancer in young adults. Clin. Oncol. 1984, 10, 205–211. [Google Scholar]
- Sung, H.; Siegel, R.L.; Laversanne, M.; Jiang, C.; Morgan, E.; Zahwe, M.; Cao, Y.; Bray, F.; Jemal, A. Colorectal cancer incidence trends in younger versus older adults: An analysis of population-based cancer registry data. Lancet Oncol. 2025, 26, 51–63. [Google Scholar] [CrossRef]
- Schreuders, E.H.; Ruco, A.; Rabeneck, L.; Schoen, R.E.; Sung, J.J.; Young, G.P.; Kuipers, E.J. Colorectal cancer screening: A global overview of existing programmes. Gut 2015, 64, 1637–1649. [Google Scholar] [CrossRef]
- Siegel, R.L.; Torre, L.A.; Soerjomataram, I.; Hayes, R.B.; Bray, F.; Weber, T.K.; Jemal, A. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019, 68, 2179–2185. [Google Scholar] [CrossRef] [PubMed]
- Krasteva, N.; Georgieva, M. Promising Therapeutic Strategies for Colorectal Cancer Treatment Based on Nanomaterials. Pharmaceutics 2022, 14, 1213. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, N.; Ugai, T.; Zhong, R.; Hamada, T.; Fujiyoshi, K.; Giannakis, M.; Wu, K.; Cao, Y.; Ng, K.; Ogino, S. Rising incidence of early-onset colorectal cancer—A call to action. Nat. Rev. Clin. Oncol. 2021, 18, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, V.A.; Gheorghe, G.; Bacalbasa, N.; Chiotoroiu, A.L.; Diaconu, C. Colorectal Cancer: From Risk Factors to Oncogenesis. Medicina 2023, 59, 1646. [Google Scholar] [CrossRef]
- Brenner, H.; Kloor, M.; Pox, C.P. Colorectal cancer. Lancet 2014, 383, 1490–1502. [Google Scholar] [CrossRef]
- Li, Q.; Geng, S.; Luo, H.; Wang, W.; Mo, Y.-Q.; Luo, Q.; Wang, L.; Song, G.-B.; Sheng, J.-P.; Xu, B. Signaling pathways involved in colorectal cancer: Pathogenesis and targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 266. [Google Scholar] [CrossRef]
- Singh, M.P.; Rai, S.; Pandey, A.; Singh, N.K.; Srivastava, S. Molecular subtypes of colorectal cancer: An emerging therapeutic opportunity for personalized medicine. Genes. Dis. 2021, 8, 133–145. [Google Scholar] [CrossRef]
- Half, E.; Bercovich, D.; Rozen, P. Familial adenomatous polyposis. Orphanet J. Rare Dis. 2009, 4, 22. [Google Scholar] [CrossRef]
- Katabathina, V.S.; Menias, C.O.; Khanna, L.; Murphy, L.; Dasyam, A.K.; Lubner, M.G.; Prasad, S.R. Hereditary Gastrointestinal Cancer Syndromes: Role of Imaging in Screening, Diagnosis, and Management. RadioGraphics 2019, 39, 1280–1301. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Liu, Y.; Wang, Y.; Hu, H.; Wang, G. Molecular subtyping in colorectal cancer: A bridge to personalized therapy (Review). Oncol. Lett. 2023, 25, 230. [Google Scholar] [CrossRef]
- Lu, J.; Kornmann, M.; Traub, B. Role of Epithelial to Mesenchymal Transition in Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 14815. [Google Scholar] [CrossRef] [PubMed]
- Liaghat, M.; Ferdousmakan, S.; Mortazavi, S.H.; Yahyazadeh, S.; Irani, A.; Banihashemi, S.; Seyedi Asl, F.S.; Akbari, A.; Farzam, F.; Aziziyan, F.; et al. The impact of epithelial-mesenchymal transition (EMT) induced by metabolic processes and intracellular signaling pathways on chemo-resistance, metastasis, and recurrence in solid tumors. Cell Commun. Signal. 2024, 22, 575. [Google Scholar] [CrossRef] [PubMed]
- Greco, L.; Rubbino, F.; Morelli, A.; Gaiani, F.; Grizzi, F.; de’Angelis, G.L.; Malesci, A.; Laghi, L. Epithelial to Mesenchymal Transition: A Challenging Playground for Translational Research. Current Models and Focus on TWIST1 Relevance and Gastrointestinal Cancers. Int. J. Mol. Sci. 2021, 22, 11469. [Google Scholar] [CrossRef] [PubMed]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Valenzuela, G.; Canepa, J.; Simonetti, C.; Solo de Zaldívar, L.; Marcelain, K.; González-Montero, J. Consensus molecular subtypes of colorectal cancer in clinical practice: A translational approach. World J. Clin. Oncol. 2021, 12, 1000–1008. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Sánchez-Alcoholado, L.; Ramos-Molina, B.; Otero, A.; Laborda-Illanes, A.; Ordóñez, R.; Medina, J.A.; Gómez-Millán, J.; Queipo-Ortuño, M.I. The Role of the Gut Microbiome in Colorectal Cancer Development and Therapy Response. Cancers 2020, 12, 1406. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Pandey, H.; Tang, D.W.T.; Wong, S.H.; Lal, D. Gut Microbiota in Colorectal Cancer: Biological Role and Therapeutic Opportunities. Cancers 2023, 15, 866. [Google Scholar] [CrossRef]
- Yoon, K.; Kim, N. The Effect of Microbiota on Colon Carcinogenesis. J. Cancer Prev. 2018, 23, 117–125. [Google Scholar] [CrossRef]
- Patel, M.; McAllister, M.; Nagaraju, R.; Badran, S.S.F.A.; Edwards, J.; McBain, A.J.; Barriuso, J.; Aziz, O. The intestinal microbiota in colorectal cancer metastasis—Passive observer or key player? Crit. Rev. Oncol. Hematol. 2022, 180, 103856. [Google Scholar] [CrossRef] [PubMed]
- Ivleva, E.A.; Grivennikov, S.I. Microbiota-driven mechanisms at different stages of cancer development. Neoplasia 2022, 32, 100829. [Google Scholar] [CrossRef] [PubMed]
- Rebersek, M. Gut microbiome and its role in colorectal cancer. BMC Cancer 2021, 21, 1325. [Google Scholar] [CrossRef] [PubMed]
- Al Moustafa, A.-E.; Al-Antary, N.; Yasmeen, A. High-Risk Human Papillomavirus and Colorectal Carcinogenesis. In Human Papillomavirus—Research in a Global Perspective; Rajkumar, R., Ed.; IntechOpen: Rijeka, Croatia, 2016. [Google Scholar]
- Chen, H.; Chen, X.-Z.; Waterboer, T.; Castro, F.A.; Brenner, H. Viral infections and colorectal cancer: A systematic review of epidemiological studies. Int. J. Cancer 2015, 137, 12–24. [Google Scholar] [CrossRef]
- Dubois, R.N. Role of inflammation and inflammatory mediators in colorectal cancer. Trans. Am. Clin. Clim. Assoc. 2014, 125, 358–372. [Google Scholar]
- Zou, S.; Fang, L.; Lee, M.H. Dysbiosis of gut microbiota in promoting the development of colorectal cancer. Gastroenterol. Rep. 2018, 6, 1–12. [Google Scholar] [CrossRef]
- Li, Q.; von Ehrlich-Treuenstätt, V.; Schardey, J.; Wirth, U.; Zimmermann, P.; Andrassy, J.; Bazhin, A.V.; Werner, J.; Kühn, F. Gut Barrier Dysfunction and Bacterial Lipopolysaccharides in Colorectal Cancer. J. Gastrointest. Surg. 2023, 27, 1466–1472. [Google Scholar] [CrossRef]
- Al-Qadami, G.H.; Secombe, K.R.; Subramaniam, C.B.; Wardill, H.R.; Bowen, J.M. Gut Microbiota-Derived Short-Chain Fatty Acids: Impact on Cancer Treatment Response and Toxicities. Microorganisms 2022, 10, 2048. [Google Scholar] [CrossRef]
- Holbert, C.E.; Casero, R.A., Jr.; Stewart, T.M. Polyamines: The pivotal amines in influencing the tumor microenvironment. Discov. Oncol. 2024, 15, 173. [Google Scholar] [CrossRef]
- Zhang, E.; Ding, C.; Li, S.; Aikemu, B.; Zhou, X.; Fan, X.; Sun, J.; Yang, X.; Zheng, M. Polyamine metabolism patterns characterized tumor microenvironment, prognosis, and response to immunotherapy in colorectal cancer. Cancer Cell Int. 2023, 23, 96. [Google Scholar] [CrossRef]
- Novita Sari, I.; Setiawan, T.; Seock Kim, K.; Toni Wijaya, Y.; Won Cho, K.; Young Kwon, H. Metabolism and function of polyamines in cancer progression. Cancer Lett. 2021, 519, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Thulasinathan, B.; Suvilesh, K.N.; Maram, S.; Grossmann, E.; Ghouri, Y.; Teixeiro, E.P.; Chan, J.; Kaif, J.T.; Rachagani, S. The impact of gut microbial short-chain fatty acids on colorectal cancer development and prevention. Gut Microbes 2025, 17, 2483780. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tian, Y.; Xu, C.; Chen, M.; Xiang, Z.; Gu, L.; Xue, H.; Xu, Q. Crosstalk between gut microbiotas and fatty acid metabolism in colorectal cancer. Cell Death Discov. 2025, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, V.A.; Gheorghe, G.; Georgescu, T.F.; Buica, V.; Catanescu, M.-S.; Cercel, I.-A.; Budeanu, B.; Budan, M.; Bacalbasa, N.; Diaconu, C. Exploring the Role of the Gut Microbiota in Colorectal Cancer Development. Gastrointest. Disord. 2024, 6, 526–537. [Google Scholar] [CrossRef]
- Matsuda, T.; Fujimoto, A.; Igarashi, Y. Colorectal Cancer: Epidemiology, Risk Factors, and Public Health Strategies. Digestion 2025, 106, 91–99. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Olfatifar, M.; Rafiei, F.; Sadeghi, A.; Ataei, E.; Habibi, M.A.; Pezeshgi Modarres, M.; Ghalavand, Z.; Houri, H. Assessing the Colorectal Cancer Landscape: A Comprehensive Exploration of Future Trends in 216 Countries and Territories from 2021 to 2040. J. Epidemiol. Glob. Health 2025, 15, 5. [Google Scholar] [CrossRef]
- Toma, M.; Beluşică, L.; Stavarachi, M.; Apostol, P.; Spandole, S.; Radu, I.; Cimponeriu, D. Rating the environmental and genetic risk factors for colorectal cancer. J. Med. Life 2012, 5, 152–159. [Google Scholar]
- Zhang, Y.; Wang, Y.; Zhang, B.; Li, P.; Zhao, Y. Methods and biomarkers for early detection, prediction, and diagnosis of colorectal cancer. Biomed. Pharmacother. 2023, 163, 114786. [Google Scholar] [CrossRef]
- Sato, Y.; Tsujinaka, S.; Miura, T.; Kitamura, Y.; Suzuki, H.; Shibata, C. Inflammatory Bowel Disease and Colorectal Cancer: Epidemiology, Etiology, Surveillance, and Management. Cancers 2023, 15, 4154. [Google Scholar] [CrossRef]
- Harmon, B.E.; Wirth, M.D.; Boushey, C.J.; Wilkens, L.R.; Draluck, E.; Shivappa, N.; Steck, S.E.; Hofseth, L.; Haiman, C.A.; Le Marchand, L.; et al. The Dietary Inflammatory Index Is Associated with Colorectal Cancer Risk in the Multiethnic Cohort. J. Nutr. 2017, 147, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Maida, M.; Dahiya, D.S.; Shah, Y.R.; Tiwari, A.; Gopakumar, H.; Vohra, I.; Khan, A.; Jaber, F.; Ramai, D.; Facciorusso, A. Screening and Surveillance of Colorectal Cancer: A Review of the Literature. Cancers 2024, 16, 2746. [Google Scholar] [CrossRef]
- Marcellinaro, R.; Spoletini, D.; Grieco, M.; Avella, P.; Cappuccio, M.; Troiano, R.; Lisi, G.; Garbarino, G.M.; Carlini, M. Colorectal Cancer: Current Updates and Future Perspectives. J. Clin. Med. 2023, 13, 40. [Google Scholar] [CrossRef]
- Lopes, S.R.; Martins, C.; Santos, I.C.; Teixeira, M.; Gamito, É.; Alves, A.L. Colorectal cancer screening: A review of current knowledge and progress in research. World J. Gastrointest. Oncol. 2024, 16, 1119–1133. [Google Scholar] [CrossRef]
- Lee, J.K.; Jensen, C.D.; Levin, T.R.; Doubeni, C.A.; Zauber, A.G.; Chubak, J.; Kamineni, A.S.; Schottinger, J.E.; Ghai, N.R.; Udaltsova, N.; et al. Long-term Risk of Colorectal Cancer and Related Death After Adenoma Removal in a Large, Community-based Population. Gastroenterology 2020, 158, 884–894.e885. [Google Scholar] [CrossRef]
- Mishra, N.; Hall, J. Identification of patients at risk for hereditary colorectal cancer. Clin. Colon Rectal Surg. 2012, 25, 67–82. [Google Scholar] [CrossRef][Green Version]
- Kim, H.M.; Kim, T.I. Screening and surveillance for hereditary colorectal cancer. Intest. Res. 2024, 22, 119–130. [Google Scholar] [CrossRef]
- Bevan, R.; Rutter, M.D. Colorectal Cancer Screening—Who, How, and When? Clin. Endosc. 2018, 51, 37–49. [Google Scholar] [CrossRef]
- Johnson, C.D.; Chen, M.-H.; Toledano, A.Y.; Heiken, J.P.; Dachman, A.; Kuo, M.D.; Menias, C.O.; Siewert, B.; Cheema, J.I.; Obregon, R.G. Accuracy of CT colonography for detection of large adenomas and cancers. N. Engl. J. Med. 2008, 359, 1207–1217. [Google Scholar] [CrossRef]
- Ricci, Z.; Kobi, M.; Yee, J. CT Colonography for Colorectal Cancer Screening. J. Radiol. Nurs. 2020, 39, 185–193. [Google Scholar] [CrossRef]
- Palimaka, S.; Blackhouse, G.; Goeree, R. Colon capsule endoscopy for the detection of colorectal polyps: An economic analysis. Ont. Health Technol. Assess. Ser. 2015, 15, 1–43. [Google Scholar] [PubMed]
- Cash, B.D.; Fleisher, M.R.; Fern, S.; Rajan, E.; Haithcock, R.; Kastenberg, D.M.; Pound, D.; Papageorgiou, N.P.; Fernandez-Urien, I.; Schmelkin, I.J. Multicentre, prospective, randomised study comparing the diagnostic yield of colon capsule endoscopy versus CT colonography in a screening population (the TOPAZ study). Gut 2021, 70, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Liles, E.G.; Bent, S.; Levin, T.R.; Corley, D.A. Accuracy of fecal immunochemical tests for colorectal cancer: Systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Song, L.L.; Li, Y.M. Current noninvasive tests for colorectal cancer screening: An overview of colorectal cancer screening tests. World J. Gastrointest. Oncol. 2016, 8, 793–800. [Google Scholar] [CrossRef]
- Gómez-Molina, R.; Suárez, M.; Martínez, R.; Chilet, M.; Bauça, J.M.; Mateo, J. Utility of Stool-Based Tests for Colorectal Cancer Detection: A Comprehensive Review. Healthcare 2024, 12, 1645. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, L.; Song, J.; Wang, G.; Li, P.; Li, W.; Luo, P.; Sun, X.; Wu, J.; Liu, Y. Liquid biopsy at the frontier of detection, prognosis and progression monitoring in colorectal cancer. Mol. Cancer 2022, 21, 86. [Google Scholar] [CrossRef]
- Lofton-Day, C.; Model, F.; DeVos, T.; Tetzner, R.; Distler, J.; Schuster, M.; Song, X.; Lesche, R.; Liebenberg, V.; Ebert, M. DNA methylation biomarkers for blood-based colorectal cancer screening. Clin. Chem. 2008, 54, 414–423. [Google Scholar] [CrossRef]
- Warren, J.D.; Xiong, W.; Bunker, A.M.; Vaughn, C.P.; Furtado, L.V.; Roberts, W.L.; Fang, J.C.; Samowitz, W.S.; Heichman, K.A. Septin 9 methylated DNA is a sensitive and specific blood test for colorectal cancer. BMC Med. 2011, 9, 133. [Google Scholar] [CrossRef]
- Lin, J.S.; Perdue, L.A.; Henrikson, N.B.; Bean, S.I.; Blasi, P.R. Screening for colorectal cancer: Updated evidence report and systematic review for the US Preventive Services Task Force. Jama 2021, 325, 1978–1998. [Google Scholar] [CrossRef]
- Kim, H.J.; Parsa, N.; Byrne, M.F. The role of artificial intelligence in colonoscopy. Semin. Colon Rectal Surg. 2024, 35, 101007. [Google Scholar] [CrossRef]
- Seeliger, B.; Marescaux, J. Endoluminal and next generation robotics in colorectal surgery. Semin. Colon Rectal Surg. 2024, 35, 101006. [Google Scholar] [CrossRef]
- Mota, J.; Almeida, M.J.; Martins, M.; Mendes, F.; Cardoso, P.; Afonso, J.; Ribeiro, T.; Ferreira, J.; Fonseca, F.; Limbert, M.; et al. Artificial Intelligence in Coloproctology: A Review of Emerging Technologies and Clinical Applications. J. Clin. Med. 2024, 13, 5842. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Mullins, C.S.; Linnebacher, M. Colorectal cancer vaccines: Tumor-associated antigens vs neoantigens. World J. Gastroenterol. 2018, 24, 5418–5432. [Google Scholar] [CrossRef] [PubMed]
- de Vries, N.L.; Swets, M.; Vahrmeijer, A.L.; Hokland, M.; Kuppen, P.J. The Immunogenicity of Colorectal Cancer in Relation to Tumor Development and Treatment. Int. J. Mol. Sci. 2016, 17, 1030. [Google Scholar] [CrossRef]
- Hu, Z.; Ott, P.A.; Wu, C.J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol. 2018, 18, 168–182. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, J.A.; Zhang, H.X.; Jiang, Y.N.; Luo, W.H. Cancer vaccines: Targeting KRAS-driven cancers. Expert. Rev. Vaccines 2020, 19, 163–173. [Google Scholar] [CrossRef]
- Linnebacher, M.; Gebert, J.; Rudy, W.; Woerner, S.; Yuan, Y.P.; Bork, P.; von Knebel Doeberitz, M. Frameshift peptide-derived T-cell epitopes: A source of novel tumor-specific antigens. Int. J. Cancer 2001, 93, 6–11. [Google Scholar] [CrossRef]
- Kloor, M.; Reuschenbach, M.; Pauligk, C.; Karbach, J.; Rafiyan, M.R.; Al-Batran, S.E.; Tariverdian, M.; Jäger, E.; von Knebel Doeberitz, M. A Frameshift Peptide Neoantigen-Based Vaccine for Mismatch Repair-Deficient Cancers: A Phase I/IIa Clinical Trial. Clin. Cancer Res. 2020, 26, 4503–4510. [Google Scholar] [CrossRef]
- Fritah, H.; Rovelli, R.; Chiang, C.L.-L.; Kandalaft, L.E. The current clinical landscape of personalized cancer vaccines. Cancer Treat. Rev. 2022, 106, 102383. [Google Scholar] [CrossRef]
- Jou, J.; Harrington, K.J.; Zocca, M.-B.; Ehrnrooth, E.; Cohen, E.E.W. The Changing Landscape of Therapeutic Cancer Vaccines—Novel Platforms and Neoantigen Identification. Clin. Cancer Res. 2021, 27, 689–703. [Google Scholar] [CrossRef]
- Hu, L.F.; Lan, H.R.; Huang, D.; Li, X.M.; Jin, K.T. Personalized Immunotherapy in Colorectal Cancers: Where Do We Stand? Front. Oncol. 2021, 11, 769305. [Google Scholar] [CrossRef] [PubMed]
- Verma, C.; Pawar, V.A.; Srivastava, S.; Tyagi, A.; Kaushik, G.; Shukla, S.K.; Kumar, V. Cancer Vaccines in the Immunotherapy Era: Promise and Potential. Vaccines 2023, 11, 1783. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Che, X.; Wang, X.; Ma, C.; Wu, G. Tumor Vaccines: Unleashing the Power of the Immune System to Fight Cancer. Pharmaceuticals 2023, 16, 1384. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.A.; Holden, J.A.; Lenzo, J.C.; Hadjigol, S.; O’Brien-Simpson, N.M. Multi-disciplinary approaches paving the way for clinically effective peptide vaccines for cancer. NPJ Vaccines 2025, 10, 68. [Google Scholar] [CrossRef]
- Rus Bakarurraini, N.A.A.; Ab Mutalib, N.S.; Jamal, R.; Abu, N. The Landscape of Tumor-Specific Antigens in Colorectal Cancer. Vaccines 2020, 8, 371. [Google Scholar] [CrossRef]
- Hubbard, J.M.; Tőke, E.R.; Moretto, R.; Graham, R.P.; Youssoufian, H.; Lőrincz, O.; Molnár, L.; Csiszovszki, Z.; Mitchell, J.L.; Wessling, J.; et al. Safety and Activity of PolyPEPI1018 Combined with Maintenance Therapy in Metastatic Colorectal Cancer: An Open-Label, Multicenter, Phase Ib Study. Clin. Cancer Res. 2022, 28, 2818–2829. [Google Scholar] [CrossRef]
- Kopetz, S.; Prenen, H.; Sharma, S.; Van Cutsem, E.; Mayol, J.; Trapani, F.; Bogenrieder, T.; Lenz, H. SO-11 KISIMA-01 trial: Safety, tolerability and immunogenicity of ATP128 with or without ezabenlimab (BI 754091) in patients with stage IV colorectal cancer—preliminary results from a phase 1b study. Ann. Oncol. 2021, 32, S206–S207. [Google Scholar] [CrossRef]
- Abd-Aziz, N.; Poh, C.L. Development of Peptide-Based Vaccines for Cancer. J. Oncol. 2022, 2022, 9749363. [Google Scholar] [CrossRef]
- Buonaguro, L.; Tagliamonte, M. Peptide-based vaccine for cancer therapies. Front. Immunol. 2023, 14, 1210044. [Google Scholar] [CrossRef]
- Zahedipour, F.; Jamialahmadi, K.; Zamani, P.; Reza Jaafari, M. Improving the efficacy of peptide vaccines in cancer immunotherapy. Int. Immunopharmacol. 2023, 123, 110721. [Google Scholar] [CrossRef]
- Hao, Q.; Long, Y.; Yang, Y.; Deng, Y.; Ding, Z.; Yang, L.; Shu, Y.; Xu, H. Development and Clinical Applications of Therapeutic Cancer Vaccines with Individualized and Shared Neoantigens. Vaccines 2024, 12, 717. [Google Scholar] [CrossRef] [PubMed]
- Shariati, A.; Khezrpour, A.; Shariati, F.; Afkhami, H.; Yarahmadi, A.; Alavimanesh, S.; Kamrani, S.; Modarressi, M.H.; Khani, P. DNA vaccines as promising immuno-therapeutics against cancer: A new insight. Front. Immunol. 2025, 15, 1498431. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, R.G.; Gonda, T.J. MYB function in normal and cancer cells. Nat. Rev. Cancer 2008, 8, 523–534. [Google Scholar] [CrossRef]
- Cross, R.S.; Malaterre, J.; Davenport, A.J.; Carpinteri, S.; Anderson, R.L.; Darcy, P.K.; Ramsay, R.G. Therapeutic DNA vaccination against colorectal cancer by targeting the MYB oncoprotein. Clin. Transl. Immunol. 2015, 4, e30. [Google Scholar] [CrossRef]
- Williams, B.B.; Wall, M.; Miao, R.Y.; Williams, B.; Bertoncello, I.; Kershaw, M.H.; Mantamadiotis, T.; Haber, M.; Norris, M.D.; Gautam, A. Induction of T cell-mediated immunity using a c-Myb DNA vaccine in a mouse model of colon cancer. Cancer Immunol. Immunother. 2008, 57, 1635–1645. [Google Scholar] [CrossRef]
- Pandya, A.; Shah, Y.; Kothari, N.; Postwala, H.; Shah, A.; Parekh, P.; Chorawala, M.R. The future of cancer immunotherapy: DNA vaccines leading the way. Med. Oncol. 2023, 40, 200. [Google Scholar] [CrossRef]
- Lu, B.; Lim, J.M.; Yu, B.; Song, S.; Neeli, P.; Sobhani, N.; K, P.; Bonam, S.R.; Kurapati, R.; Zheng, J.; et al. The next-generation DNA vaccine platforms and delivery systems: Advances, challenges and prospects. Front. Immunol. 2024, 15, 1332939. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines — a new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Guasp, P.; Reiche, C.; Sethna, Z.; Balachandran, V.P. RNA vaccines for cancer: Principles to practice. Cancer Cell 2024, 42, 1163–1184. [Google Scholar] [CrossRef]
- Katopodi, T.; Petanidis, S.; Grigoriadou, E.; Anestakis, D.; Charalampidis, C.; Chatziprodromidou, I.; Floros, G.; Eskitzis, P.; Zarogoulidis, P.; Koulouris, C.; et al. Immune Specific and Tumor-Dependent mRNA Vaccines for Cancer Immunotherapy: Reprogramming Clinical Translation into Tumor Editing Therapy. Pharmaceutics 2024, 16, 455. [Google Scholar] [CrossRef]
- Haabeth, O.A.W.; Blake, T.R.; McKinlay, C.J.; Waymouth, R.M.; Wender, P.A.; Levy, R. mRNA vaccination with charge-altering releasable transporters elicits human T cell responses and cures established tumors in mice. Proc. Natl. Acad. Sci. USA 2018, 115, E9153–E9161. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guo, B. RNA-based therapeutics for colorectal cancer: Updates and future directions. Pharmacol. Res. 2020, 152, 104550. [Google Scholar] [CrossRef] [PubMed]
- Cafri, G.; Gartner, J.J.; Hopson, K.; Meehan, R.S.; Zaks, T.Z.; Robbins, P.; Rosenberg, S.A. Immunogenicity and tolerability of personalized mRNA vaccine mRNA-4650 encoding defined neoantigens expressed by the autologous cancer. J. Clin. Oncol. 2019, 37, 2643. [Google Scholar] [CrossRef]
- Burris, H.A.; Patel, M.R.; Cho, D.C.; Clarke, J.M.; Gutierrez, M.; Zaks, T.Z.; Frederick, J.; Hopson, K.; Mody, K.; Binanti-Berube, A.; et al. A phase I multicenter study to assess the safety, tolerability, and immunogenicity of mRNA-4157 alone in patients with resected solid tumors and in combination with pembrolizumab in patients with unresectable solid tumors. J. Clin. Oncol. 2019, 37, 2523. [Google Scholar] [CrossRef]
- Ni, L. Advances in mRNA-Based Cancer Vaccines. Vaccines 2023, 11, 1599. [Google Scholar] [CrossRef]
- Zhang, A.; Ji, Q.; Sheng, X.; Wu, H. mRNA vaccine in gastrointestinal tumors: Immunomodulatory effects and immunotherapy. Biomed. Pharmacother. 2023, 166, 115361. [Google Scholar] [CrossRef]
- Yaremenko, A.V.; Khan, M.M.; Zhen, X.; Tang, Y.; Tao, W. Clinical advances of mRNA vaccines for cancer immunotherapy. Med 2025, 6, 100562. [Google Scholar] [CrossRef]
- Youssef, M.; Hitti, C.; Puppin Chaves Fulber, J.; Kamen, A.A. Enabling mRNA Therapeutics: Current Landscape and Challenges in Manufacturing. Biomolecules 2023, 13, 1497. [Google Scholar] [CrossRef]
- Diao, L.; Liu, M. Rethinking Antigen Source: Cancer Vaccines Based on Whole Tumor Cell/tissue Lysate or Whole Tumor Cell. Adv. Sci. 2023, 10, e2300121. [Google Scholar] [CrossRef]
- Alzeeb, G.; Tortorelli, C.; Taleb, J.; De Luca, F.; Berge, B.; Bardet, C.; Limagne, E.; Brun, M.; Chalus, L.; Pinteur, B.; et al. Efficacy of novel allogeneic cancer cells vaccine to treat colorectal cancer. Front. Oncol. 2024, 14, 1427428. [Google Scholar] [CrossRef]
- Baars, A.; Claessen, A.M.; Wagstaff, J.; Giaccone, G.; Scheper, R.J.; Meijer, S.; Schakel, M.J.; Gall, H.E.; Meijer, C.J.; Vermorken, J.B.; et al. A phase II study of active specific immunotherapy and 5-FU/Leucovorin as adjuvant therapy for stage III colon carcinoma. Br. J. Cancer 2002, 86, 1230–1234. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Huang, C.Y.; Zhu, Q.; Ferguson, A.K.; Durham, J.N.; Anders, R.A.; Thompson, E.D.; Rozich, N.S.; Thomas, D.L.; Nauroth, J.M.; et al. A phase 2 study of GVAX colon vaccine with cyclophosphamide and pembrolizumab in patients with mismatch repair proficient advanced colorectal cancer. Cancer Med. 2020, 9, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Edil, B.H.; Soares, K.C.; El-Shami, K.; Uram, J.N.; Judkins, C.; Zhang, Z.; Onners, B.; Laheru, D.; Pardoll, D.; et al. A safety and feasibility study of an allogeneic colon cancer cell vaccine administered with a granulocyte-macrophage colony stimulating factor-producing bystander cell line in patients with metastatic colorectal cancer. Ann. Surg. Oncol. 2014, 21, 3931–3937. [Google Scholar] [CrossRef] [PubMed]
- Pittet, M.J.; Di Pilato, M.; Garris, C.; Mempel, T.R. Dendritic cells as shepherds of T cell immunity in cancer. Immunity 2023, 56, 2218–2230. [Google Scholar] [CrossRef]
- Lee, K.W.; Yam, J.W.P.; Mao, X. Dendritic Cell Vaccines: A Shift from Conventional Approach to New Generations. Cells 2023, 12, 2147. [Google Scholar] [CrossRef]
- Lesterhuis, W.J.; De Vries, I.J.; Schreibelt, G.; Schuurhuis, D.H.; Aarntzen, E.H.; De Boer, A.; Scharenborg, N.M.; Van De Rakt, M.; Hesselink, E.J.; Figdor, C.G.; et al. Immunogenicity of dendritic cells pulsed with CEA peptide or transfected with CEA mRNA for vaccination of colorectal cancer patients. Anticancer Res. 2010, 30, 5091–5097. [Google Scholar]
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef]
- Fong, L.; Hou, Y.; Rivas, A.; Benike, C.; Yuen, A.; Fisher, G.A.; Davis, M.M.; Engleman, E.G. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc. Natl. Acad. Sci. USA 2001, 98, 8809–8814. [Google Scholar] [CrossRef]
- Maruoka, S.; Ojima, T.; Iwamoto, H.; Kitadani, J.; Tabata, H.; Tominaga, S.; Katsuda, M.; Hayata, K.; Takeuchi, A.; Yamaue, H. Tumor RNA transfected DCs derived from iPS cells elicit cytotoxicity against cancer cells induced from colorectal cancer patients in vitro. Sci. Rep. 2022, 12, 3295. [Google Scholar] [CrossRef]
- Hazama, S.; Nakamura, Y.; Tanaka, H.; Hirakawa, K.; Tahara, K.; Shimizu, R.; Ozasa, H.; Etoh, R.; Sugiura, F.; Okuno, K.; et al. A phase ΙI study of five peptides combination with oxaliplatin-based chemotherapy as a first-line therapy for advanced colorectal cancer (FXV study). J. Transl. Med. 2014, 12, 108. [Google Scholar] [CrossRef]
- Murahashi, M.; Hijikata, Y.; Yamada, K.; Tanaka, Y.; Kishimoto, J.; Inoue, H.; Marumoto, T.; Takahashi, A.; Okazaki, T.; Takeda, K.; et al. Phase I clinical trial of a five-peptide cancer vaccine combined with cyclophosphamide in advanced solid tumors. Clin. Immunol. 2016, 166–167, 48–58. [Google Scholar] [CrossRef] [PubMed]
- De Mey, W.; Esprit, A.; Thielemans, K.; Breckpot, K.; Franceschini, L. RNA in Cancer Immunotherapy: Unlocking the Potential of the Immune System. Clin. Cancer Res. 2022, 28, 3929–3939. [Google Scholar] [CrossRef] [PubMed]
- Snook, A.E.; Baybutt, T.R.; Xiang, B.; Abraham, T.S.; Flickinger, J.C.; Hyslop, T.; Zhan, T.; Kraft, W.K.; Sato, T.; Waldman, S.A. Split tolerance permits safe Ad5-GUCY2C-PADRE vaccine-induced T-cell responses in colon cancer patients. J. Immunother. Cancer 2019, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Harrop, R.; Connolly, N.; Redchenko, I.; Valle, J.; Saunders, M.; Ryan, M.G.; Myers, K.A.; Drury, N.; Kingsman, S.M.; Hawkins, R.E.; et al. Vaccination of colorectal cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) induces immune responses which correlate with disease control: A phase I/II trial. Clin. Cancer Res. 2006, 12, 3416–3424. [Google Scholar] [CrossRef]
- Jani, C.T.; Manoharan, A.; DeMaria, P.J.; Bilusic, M. Harnessing live vectors for cancer vaccines: Advancing therapeutic immunotherapy. Hum. Vaccines Immunother. 2025, 21, 2469416. [Google Scholar] [CrossRef]
- Lundstrom, K. Viral Vectors in Gene Therapy: Where Do We Stand in 2023? Viruses 2023, 15, 698. [Google Scholar] [CrossRef]
- Morse, M.A.; Chaudhry, A.; Gabitzsch, E.S.; Hobeika, A.C.; Osada, T.; Clay, T.M.; Amalfitano, A.; Burnett, B.K.; Devi, G.R.; Hsu, D.S.; et al. Novel adenoviral vector induces T-cell responses despite anti-adenoviral neutralizing antibodies in colorectal cancer patients. Cancer Immunol. Immunother. 2013, 62, 1293–1301. [Google Scholar] [CrossRef]
- Redman, J.M.; Tsai, Y.T.; Weinberg, B.A.; Donahue, R.N.; Gandhy, S.; Gatti-Mays, M.E.; Abdul Sater, H.; Bilusic, M.; Cordes, L.M.; Steinberg, S.M.; et al. A Randomized Phase II Trial of mFOLFOX6 + Bevacizumab Alone or with AdCEA Vaccine + Avelumab Immunotherapy for Untreated Metastatic Colorectal Cancer. Oncologist 2022, 27, 198–209. [Google Scholar] [CrossRef]
- Anderson, T.S.; McCormick, A.L.; Daugherity, E.A.; Oladejo, M.; Okpalanwaka, I.F.; Smith, S.L.; Appiah, D.; Wood, L.M.; Lowe, D.B. Listeria-based vaccination against the pericyte antigen RGS5 elicits anti-vascular effects and colon cancer protection. Oncoimmunology 2023, 12, 2260620. [Google Scholar] [CrossRef]
- Bilusic, M.; Heery, C.R.; Arlen, P.M.; Rauckhorst, M.; Apelian, D.; Tsang, K.Y.; Tucker, J.A.; Jochems, C.; Schlom, J.; Gulley, J.L. Phase I trial of a recombinant yeast-CEA vaccine (GI-6207) in adults with metastatic CEA-expressing carcinoma. Cancer Immunol. Immunother. 2014, 63, 225–234. [Google Scholar] [CrossRef]
- Cohn, A.; Morse, M.A.; O’Neil, B.; Whiting, S.; Coeshott, C.; Ferraro, J.; Bellgrau, D.; Apelian, D.; Rodell, T.C. Whole Recombinant Saccharomyces cerevisiae Yeast Expressing Ras Mutations as Treatment for Patients With Solid Tumors Bearing Ras Mutations: Results From a Phase 1 Trial. J. Immunother. 2018, 41, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Shen, G.; Gao, W.; Huang, Z.; Huang, C.; Fu, L. Neoantigens: Promising targets for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xiao, Z.; Wang, D.; Jia, L.; Nie, S.; Zeng, X.; Hu, W. The screening, identification, design and clinical application of tumor-specific neoantigens for TCR-T cells. Mol. Cancer 2023, 22, 141. [Google Scholar] [CrossRef]
- Zheng, Y.; Fu, Y.; Wang, P.P.; Ding, Z.Y. Neoantigen: A Promising Target for the Immunotherapy of Colorectal Cancer. Dis. Markers 2022, 2022, 8270305. [Google Scholar] [CrossRef]
- Sha, D.; Jin, Z.; Budczies, J.; Kluck, K.; Stenzinger, A.; Sinicrope, F.A. Tumor Mutational Burden as a Predictive Biomarker in Solid Tumors. Cancer Discov. 2020, 10, 1808–1825. [Google Scholar] [CrossRef]
- Chen, G.L.; Kong, D.X.; Lin, Y. Neo-Antigen-Reactive T Cells Immunotherapy for Colorectal Cancer: A More Personalized Cancer Therapy Approach. Glob. Chall. 2023, 7, 2200186. [Google Scholar] [CrossRef]
- Ishikawa, T.; Fujita, T.; Suzuki, Y.; Okabe, S.; Yuasa, Y.; Iwai, T.; Kawakami, Y. Tumor-specific immunological recognition of frameshift-mutated peptides in colon cancer with microsatellite instability. Cancer Res. 2003, 63, 5564–5572. [Google Scholar]
- Toubaji, A.; Achtar, M.; Provenzano, M.; Herrin, V.E.; Behrens, R.; Hamilton, M.; Bernstein, S.; Venzon, D.; Gause, B.; Marincola, F. Pilot study of mutant ras peptide-based vaccine as an adjuvant treatment in pancreatic and colorectal cancers. Cancer Immunol. Immunother. 2008, 57, 1413–1420. [Google Scholar] [CrossRef]
- Kloor, M.; Reuschenbach, M.; Karbach, J.; Rafiyan, M.; Al-Batran, S.-E.; Pauligk, C.; Jaeger, E.; von Knebel Doeberitz, M. Vaccination of MSI-H colorectal cancer patients with frameshift peptide antigens: A phase I/IIa clinical trial. J. Clin. Oncol. 2015, 33, 3020. [Google Scholar] [CrossRef]
- Leoni, G.; D’Alise, A.M.; Cotugno, G.; Langone, F.; Garzia, I.; De Lucia, M.; Fichera, I.; Vitale, R.; Bignone, V.; Tucci, F.G.; et al. A Genetic Vaccine Encoding Shared Cancer Neoantigens to Treat Tumors with Microsatellite Instability. Cancer Res. 2020, 80, 3972–3982. [Google Scholar] [CrossRef]
- Overman, M.J.; Maurel, J.; Oberstein, P.E.; Roselló-Keränen, S.; Le, D.T.; Pedersen, K.S.; Mukherjee, S.; D’Alise, A.M.; Leoni, G.; Siani, L.; et al. Results of phase I-II bridging study for Nous-209, a neoantigen cancer immunotherapy, in combination with pembrolizumab as first line treatment in patients with advanced dMMR/MSI-h colorectal cancer. J. Clin. Oncol. 2023, 41, e14665. [Google Scholar] [CrossRef]
- Al-Hetty, H.; Kadhim, M.S.; Al-Tamimi, J.H.Z.; Ahmed, N.M.; Jalil, A.T.; Saleh, M.M.; Kandeel, M.; Abbas, R.H. Implications of biomimetic nanocarriers in targeted drug delivery. Emergent Mater. 2023, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ghazizadeh, Y.; Hossein, S.S.; Fatemeh, M.; Sedigheh, N.; Adabi, M. Advances in cancer nanovaccines: A focus on colorectal cancer. Nanomedicine 2025, 20, 1029–1041. [Google Scholar] [CrossRef]
- Overman, M.J.; Adam, L.; Raghav, K.; Wang, J.; Kee, B.; Fogelman, D.; Eng, C.; Vilar, E.; Shroff, R.; Dasari, A.; et al. Phase II study of nab-paclitaxel in refractory small bowel adenocarcinoma and CpG island methylator phenotype (CIMP)-high colorectal cancer. Ann. Oncol. 2018, 29, 139–144. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, S.; Liu, X.; Xu, Y.; Zhao, J.; Si, X.; Li, H.; Huang, Z.; Wang, Z.; Tang, Z. Supramolecular assembled programmable nanomedicine as in situ cancer vaccine for cancer immunotherapy. Adv. Mater. 2021, 33, e2007293. [Google Scholar] [CrossRef]
- Ni, Q.; Zhang, F.; Liu, Y.; Wang, Z.; Yu, G.; Liang, B.; Niu, G.; Su, T.; Zhu, G.; Lu, G. A bi-adjuvant nanovaccine that potentiates immunogenicity of neoantigen for combination immunotherapy of colorectal cancer. Sci. Adv. 2020, 6, eaaw6071. [Google Scholar] [CrossRef]
- Schimanski, C.C.; Kasper, S.; Hegewisch-Becker, S.; Schröder, J.; Overkamp, F.; Kullmann, F.; Bechstein, W.O.; Vöhringer, M.; Öllinger, R.; Lordick, F. Adjuvant MUC vaccination with tecemotide after resection of colorectal liver metastases: A randomized, double-blind, placebo-controlled, multicenter AIO phase II trial (LICC). Oncoimmunology 2020, 9, 1806680. [Google Scholar] [CrossRef]
- Moehler, M.; Folprecht, G.; Heinemann, V.; Holch, J.W.; Maderer, A.; Kasper, S.; Hegewisch-Becker, S.; Schröder, J.; Overkamp, F.; Kullmann, F. Survival after secondary liver resection in metastatic colorectal cancer: Comparing data of three prospective randomized European trials (LICC, CELIM, FIRE-3). Int. J. Cancer 2022, 150, 1341–1349. [Google Scholar] [CrossRef]
- Mai, J.; Li, Z.; Xia, X.; Zhang, J.; Li, J.; Liu, H.; Shen, J.; Ramirez, M.; Li, F.; Li, Z. Synergistic activation of antitumor immunity by a particulate therapeutic vaccine. Adv. Sci. 2021, 8, 2100166. [Google Scholar] [CrossRef]
- Li, J.; Huang, D.; Cheng, R.; Figueiredo, P.; Fontana, F.; Correia, A.; Wang, S.; Liu, Z.; Kemell, M.; Torrieri, G. Multifunctional biomimetic nanovaccines based on photothermal and Weak-Immunostimulatory nanoparticulate cores for the immunotherapy of solid tumors. Adv. Mater. 2022, 34, 2108012. [Google Scholar] [CrossRef]
- Chang, M.; Hou, Z.; Jin, D.; Zhou, J.; Wang, M.; Wang, M.; Shu, M.; Ding, B.; Li, C.; Lin, J. Colorectal tumor microenvironment-activated bio-decomposable and metabolizable Cu2O@ CaCO3 nanocomposites for synergistic oncotherapy. Adv. Mater. 2020, 32, 2004647. [Google Scholar] [CrossRef] [PubMed]
- Worth, L.L.; Jia, S.-F.; An, T.; Kleinerman, E.S. ImmTher, a lipophilic disaccharide derivative of muramyl dipeptide, up-regulates specific monocyte cytokine genes and activates monocyte-mediated tumoricidal activity. Cancer Immunol. Immunother. 1999, 48, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Vosika, G.J.; Cornelius, D.A.; Gilbert, C.W.; Sadlik, J.R.; Bennek, J.A.; Doyle, A.; Hertsgaard, D. Phase I trial of ImmTher, a new liposome-incorporated lipophilic disaccharide tripeptide. J. Immunother. 1991, 10, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Neidhart, J.; Allen, K.O.; Barlow, D.L.; Carpenter, M.; Shaw, D.R.; Triozzi, P.L.; Conry, R.M. Immunization of colorectal cancer patients with recombinant baculovirus-derived KSA (Ep-CAM) formulated with monophosphoryl lipid A in liposomal emulsion, with and without granulocyte-macrophage colony-stimulating factor. Vaccine 2004, 22, 773–780. [Google Scholar] [CrossRef]
- Lee, Y.J.; Shin, K.J.; Chae, Y.C. Regulation of cargo selection in exosome biogenesis and its biomedical applications in cancer. Exp. Mol. Med. 2024, 56, 877–889. [Google Scholar] [CrossRef]
- Lahouty, M.; Fadaee, M.; Shanehbandi, D.; Kazemi, T. Exosome-driven nano-immunotherapy: Revolutionizing colorectal cancer treatment. Mol. Biol. Rep. 2024, 52, 83. [Google Scholar] [CrossRef]
- Thomas, S.C.; Kim, J.W.; Pauletti, G.M.; Hassett, D.J.; Kotagiri, N. Exosomes: Biological Pharmaceutical Nanovectors for Theranostics. Front. Bioeng. Biotechnol. 2021, 9, 808614. [Google Scholar] [CrossRef]
- Dai, S.; Wei, D.; Wu, Z.; Zhou, X.; Wei, X.; Huang, H.; Li, G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol. Ther. 2008, 16, 782–790. [Google Scholar] [CrossRef]
- Oxley, K.L.; Hanson, B.M.; Zani, A.N.; Bishop, G.A. Activated B lymphocytes and tumor cell lysate as an effective cellular cancer vaccine. Cancer Immunol. Immunother. 2021, 70, 3093–3103. [Google Scholar] [CrossRef]
- Ren, H.; Zhao, S.; Li, W.; Dong, H.; Zhou, M.; Cao, M.; Hu, H.-M.; Wang, L.-x. Therapeutic antitumor efficacy of B cells loaded with tumor-derived autophagasomes vaccine (DRibbles). J. Immunother. 2014, 37, 383–393. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Speiser, D.E.; Knuth, A.; Bachmann, M.F. Virus-like particles for vaccination against cancer. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1579. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, B.; Al-Barwani, F.; Pelham, S.J.; Young, K.; Ward, V.K.; Young, S.L. Multi-target chimaeric VLP as a therapeutic vaccine in a model of colorectal cancer. J. Immunother. Cancer 2017, 5, 69. [Google Scholar] [CrossRef]
- Morse, M.A.; Nair, S.K.; Mosca, P.J.; Hobeika, A.C.; Clay, T.M.; Deng, Y.; Boczkowski, D.; Proia, A.; Neidzwiecki, D.; Clavien, P.A.; et al. Immunotherapy with autologous, human dendritic cells transfected with carcinoembryonic antigen mRNA. Cancer Investig. 2003, 21, 341–349. [Google Scholar] [CrossRef]
- Gögenur, M.; Balsevicius, L.; Bulut, M.; Colak, N.; Justesen, T.F.; Fiehn, A.K.; Jensen, M.B.; Høst-Rasmussen, K.; Cappelen, B.; Gaggar, S.; et al. Neoadjuvant intratumoral influenza vaccine treatment in patients with proficient mismatch repair colorectal cancer leads to increased tumor infiltration of CD8+ T cells and upregulation of PD-L1: A phase 1/2 clinical trial. J. Immunother. Cancer 2023, 11, e006774. [Google Scholar] [CrossRef]
- Shebbo, S.; Binothman, N.; Darwaish, M.; Niaz, H.A.; Abdulal, R.H.; Borjac, J.; Hashem, A.M.; Mahmoud, A.B. Redefining the battle against colorectal cancer: A comprehensive review of emerging immunotherapies and their clinical efficacy. Front. Immunol. 2024, 15, 1350208. [Google Scholar] [CrossRef]
- Ning, N.; Pan, Q.; Zheng, F.; Teitz-Tennenbaum, S.; Egenti, M.; Yet, J.; Li, M.; Ginestier, C.; Wicha, M.S.; Moyer, J.S. Cancer stem cell vaccination confers significant antitumor immunity. Cancer Res. 2012, 72, 1853–1864. [Google Scholar] [CrossRef]
- ClinicalTrial.gov. Available online: https://clinicaltrials.gov/study/NCT04491955?cond=colorectal%20cancer%20vaccine&aggFilters=status:com&sort=StudyFirstPostDate&rank=9 (accessed on 22 April 2025).
- ClinicalTrial.gov. Available online: https://clinicaltrials.gov/study/NCT03357276?cond=colorectal%20cancer%20vaccine&aggFilters=status:com&sort=StudyFirstPostDate&rank=21 (accessed on 22 April 2025).
- Available online: https://clinicaltrials.gov/study/NCT02380443?cond=colorectal%20cancer%20vaccine&aggFilters=status:com&sort=StudyFirstPostDate&rank=26#publications (accessed on 22 April 2025).
- Bever, K.M.; Thomas, D.L.; Zhang, J.; Diaz Rivera, E.A.; Rosner, G.L.; Zhu, Q.; Nauroth, J.M.; Christmas, B.; Thompson, E.D.; Anders, R.A. A feasibility study of combined epigenetic and vaccine therapy in advanced colorectal cancer with pharmacodynamic endpoint. Clin. Epigenet. 2021, 13, 25. [Google Scholar] [CrossRef]
- ClinicalTrial.gov. Available online: https://clinicaltrials.gov/study/NCT01671592?cond=colorectal%20cancer%20vaccine&aggFilters=status:com&rank=2&tab=table (accessed on 22 April 2025).
- Español-Rego, M.; Fernández-Martos, C.; Elez, E.; Foguet, C.; Pedrosa, L.; Rodríguez, N.; Ruiz-Casado, A.; Pineda, E.; Cid, J.; Cabezón, R.; et al. A Phase I-II multicenter trial with Avelumab plus autologous dendritic cell vaccine in pre-treated mismatch repair-proficient (MSS) metastatic colorectal cancer patients; GEMCAD 1602 study. Cancer Immunol. Immunother. 2023, 72, 827–840. [Google Scholar] [CrossRef]
- Lesterhuis, W.; De Vries, I.; Schuurhuis, D.; Boullart, A.; Jacobs, J.; De Boer, A.; Scharenborg, N.; Brouwer, H.; Van De Rakt, M.; Figdor, C. Vaccination of colorectal cancer patients with CEA-loaded dendritic cells: Antigen-specific T cell responses in DTH skin tests. Ann. Oncol. 2006, 17, 974–980. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/study/NCT03948763?cond=colorectal%20cancer%20vaccine&aggFilters=status:com&sort=StudyFirstPostDate&rank=11 (accessed on 22 April 2025).
- Rappaport, A.R.; Kyi, C.; Lane, M.; Hart, M.G.; Johnson, M.L.; Henick, B.S.; Liao, C.-Y.; Mahipal, A.; Shergill, A.; Spira, A.I. A shared neoantigen vaccine combined with immune checkpoint blockade for advanced metastatic solid tumors: Phase 1 trial interim results. Nat. Med. 2024, 30, 1013–1022. [Google Scholar] [CrossRef]
- ClinicalTrial.gov. Available online: https://clinicaltrials.gov/study/NCT03827967?cond=colorectal%20cancer%20vaccine&aggFilters=status:com&sort=StudyFirstPostDate&rank=12 (accessed on 22 April 2025).
- Palmer, C.D.; Rappaport, A.R.; Davis, M.J.; Hart, M.G.; Scallan, C.D.; Hong, S.-J.; Gitlin, L.; Kraemer, L.D.; Kounlavouth, S.; Yang, A. Individualized, heterologous chimpanzee adenovirus and self-amplifying mRNA neoantigen vaccine for advanced metastatic solid tumors: Phase 1 trial interim results. Nat. Med. 2022, 28, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Gatti-Mays, M.E.; Redman, J.M.; Donahue, R.N.; Palena, C.; Madan, R.A.; Karzai, F.; Bilusic, M.; Sater, H.A.; Marté, J.L.; Cordes, L.M. A phase I trial using a multitargeted recombinant adenovirus 5 (CEA/MUC1/Brachyury)-based immunotherapy vaccine regimen in patients with advanced cancer. Oncologist 2020, 25, 479–e899. [Google Scholar] [CrossRef] [PubMed]
- Crosby, E.J.; Hobeika, A.C.; Niedzwiecki, D.; Rushing, C.; Hsu, D.; Berglund, P.; Smith, J.; Osada, T.; Gwin III, W.R.; Hartman, Z.C. Long-term survival of patients with stage III colon cancer treated with VRP-CEA (6D), an alphavirus vector that increases the CD8+ effector memory T cell to Treg ratio. J. Immunother. Cancer 2020, 8, e001662. [Google Scholar] [CrossRef] [PubMed]
- Maughan, T.; Adams, R.A.; Mayer-Mokler, A.; Nowara, E.; Torday, L.; Cseh, J.; Hoehler, T.; Hitre, E.; Folprecht, G.; Fisher, D.; et al. Overall survival (OS) of advanced colorectal cancer (aCRC) patients (pts) treated with the multipeptide vaccine IMA910: Results of a matched-pair analysis with arm C pts from COIN. J. Clin. Oncol. 2012, 30, 3530. [Google Scholar] [CrossRef]
- Hubbard, J.M.; Zemla, T.J.; Graham, R.P.; Jin, Z.; Zhu, M.; Mitchell, J.L.; Novo, E.; Vegh, E.; Lorincz, O.; Csiszovszki, Z.; et al. PolyPEPI1018 vaccine in combination with TAS-102 in participants with late-stage microsatellite-stable metastatic colorectal cancer (MSS mCRC): A phase Ib study to evaluate safety, tolerability, immunogenicity and efficacy (OBERTO-201). J. Clin. Oncol. 2023, 41, 3595. [Google Scholar] [CrossRef]
- Schoen, R.E.; Boardman, L.A.; Cruz-Correa, M.; Bansal, A.; Kastenberg, D.; Hur, C.; Dzubinski, L.; Kaufman, S.F.; Rodriguez, L.M.; Richmond, E. Randomized, double-blind, placebo-controlled trial of MUC1 peptide vaccine for prevention of recurrent colorectal adenoma. Clin. Cancer Res. 2023, 29, 1678–1688. [Google Scholar] [CrossRef]
- ClinicalTrial.gov. Available online: https://clinicaltrials.gov/study/NCT00019591 (accessed on 22 April 2025).
- Aurisicchio, L.; Fridman, A.; Mauro, D.; Sheloditna, R.; Chiappori, A.; Bagchi, A.; Ciliberto, G. Safety, tolerability and immunogenicity of V934/V935 hTERT vaccination in cancer patients with selected solid tumors: A phase I study. J. Transl. Med. 2020, 18, 39. [Google Scholar] [CrossRef]
- Filip, S.; Vymetalkova, V.; Petera, J.; Vodickova, L.; Kubecek, O.; John, S.; Cecka, F.; Krupova, M.; Manethova, M.; Cervena, K.; et al. Distant Metastasis in Colorectal Cancer Patients-Do We Have New Predicting Clinicopathological and Molecular Biomarkers? A Comprehensive Review. Int. J. Mol. Sci. 2020, 21, 5255. [Google Scholar] [CrossRef]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef]
- Shin, Y.M.; Han, H.S.; Lim, S.W.; Kim, B.C.; Cheoi, K.S.; Eum, Y.O.; Kim, S.T.; Lee, K.H. Combination chemotherapy of oxaliplatin, 5-fluorouracil and low dose leucovorin in patients with advanced colorectal cancer. Cancer Res. Treat. 2005, 37, 284–289. [Google Scholar] [CrossRef]
- Guglielmi, A.P.; Sobrero, A.F. Second-line therapy for advanced colorectal cancer. Gastrointest. Cancer Res. 2007, 1, 57–63. [Google Scholar] [PubMed]
- Temraz, S.; Mukherji, D.; Alameddine, R.; Shamseddine, A. Methods of overcoming treatment resistance in colorectal cancer. Crit. Rev. Oncol. Hematol. 2014, 89, 217–230. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Ghemrawi, R.; Abuamer, L.; Kremesh, S.; Hussien, G.; Ahmed, R.; Mousa, W.; Khoder, G.; Khair, M. Revolutionizing Cancer Treatment: Recent Advances in Immunotherapy. Biomedicines 2024, 12, 2158. [Google Scholar] [CrossRef]
- Golshani, G.; Zhang, Y. Advances in immunotherapy for colorectal cancer: A review. Ther. Adv. Gastroenterol. 2020, 13, 1756284820917527. [Google Scholar] [CrossRef]
- Granier, C.; De Guillebon, E.; Blanc, C.; Roussel, H.; Badoual, C.; Colin, E.; Saldmann, A.; Gey, A.; Oudard, S.; Tartour, E. Mechanisms of action and rationale for the use of checkpoint inhibitors in cancer. ESMO Open 2017, 2, e000213. [Google Scholar] [CrossRef]
- Makaremi, S.; Asadzadeh, Z.; Hemmat, N.; Baghbanzadeh, A.; Sgambato, A.; Ghorbaninezhad, F.; Safarpour, H.; Argentiero, A.; Brunetti, O.; Bernardini, R.; et al. Immune Checkpoint Inhibitors in Colorectal Cancer: Challenges and Future Prospects. Biomedicines 2021, 9, 1075. [Google Scholar] [CrossRef]
- Song, D.; Hou, S.; Ma, N.; Yan, B.; Gao, J. Efficacy and safety of PD-1/PD-L1 and CTLA-4 immune checkpoint inhibitors in the treatment of advanced colorectal cancer: A systematic review and meta-analysis. Front. Immunol. 2024, 15, 1485303. [Google Scholar] [CrossRef]
- Chen, X.; Chen, L.-J.; Peng, X.-F.; Deng, L.; Wang, Y.; Li, J.-J.; Guo, D.-L.; Niu, X.-H. Anti-PD-1/PD-L1 therapy for colorectal cancer: Clinical implications and future considerations. Transl. Oncol. 2024, 40, 101851. [Google Scholar] [CrossRef]
- Rahimi, A.; Baghernejadan, Z.; Hazrati, A.; Malekpour, K.; Samimi, L.N.; Najafi, A.; Falak, R.; Khorramdelazad, H. Combination therapy with immune checkpoint inhibitors in colorectal cancer: Challenges, resistance mechanisms, and the role of microbiota. Biomed. Pharmacother. 2025, 186, 118014. [Google Scholar] [CrossRef]
- Bou-Assaly, W.; Mukherji, S. Cetuximab (erbitux). AJNR Am. J. Neuroradiol. 2010, 31, 626–627. [Google Scholar] [CrossRef]
- Crutcher, M.M.; Baybutt, T.R.; Kopenhaver, J.S.; Snook, A.E.; Waldman, S.A. Emerging drug targets for colon cancer: A preclinical assessment. Expert. Opin. Ther. Targets 2022, 26, 207–216. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Restifo, N.P.; Yang, J.C.; Morgan, R.A.; Dudley, M.E. Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nat. Rev. Cancer 2008, 8, 299–308. [Google Scholar] [CrossRef]
- Fan, J.; Shang, D.; Han, B.; Song, J.; Chen, H.; Yang, J.-M. Adoptive Cell Transfer: Is it a Promising Immunotherapy for Colorectal Cancer? Theranostics 2018, 8, 5784–5800. [Google Scholar] [CrossRef]
- Ellis, G.I.; Sheppard, N.C.; Riley, J.L. Genetic engineering of T cells for immunotherapy. Nat. Rev. Genet. 2021, 22, 427–447. [Google Scholar] [CrossRef]
- Rodríguez-Nava, C.; Ortuño-Pineda, C.; Illades-Aguiar, B.; Flores-Alfaro, E.; Leyva-Vázquez, M.A.; Parra-Rojas, I.; Del Moral-Hernández, O.; Vences-Velázquez, A.; Cortés-Sarabia, K.; Alarcón-Romero, L.D.C. Mechanisms of Action and Limitations of Monoclonal Antibodies and Single Chain Fragment Variable (scFv) in the Treatment of Cancer. Biomedicines 2023, 11, 1610. [Google Scholar] [CrossRef]
- Morandi, F.; Yazdanifar, M.; Cocco, C.; Bertaina, A.; Airoldi, I. Engineering the Bridge between Innate and Adaptive Immunity for Cancer Immunotherapy: Focus on γδ T and NK Cells. Cells 2020, 9, 1757. [Google Scholar] [CrossRef]
- Zhen, Y.H.; Liu, X.H.; Yang, Y.; Li, B.; Tang, J.L.; Zeng, Q.X.; Hu, J.; Zeng, X.N.; Zhang, L.; Wang, Z.J.; et al. Phase I/II study of adjuvant immunotherapy with sentinel lymph node T lymphocytes in patients with colorectal cancer. Cancer Immunol. Immunother. 2015, 64, 1083–1093. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, G.; Wan, X. Challenges and new technologies in adoptive cell therapy. J. Hematol. Oncol. 2023, 16, 97. [Google Scholar] [CrossRef]
- Mannan, A.; Kakkar, C.; Dhiman, S.; Singh, T.G. Advancing the frontiers of adaptive cell therapy: A transformative mechanistic journey from preclinical to clinical settings. Int. Immunopharmacol. 2023, 125, 111095. [Google Scholar] [CrossRef]
- Sadelain, M.; Brentjens, R.; Rivière, I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013, 3, 388–398. [Google Scholar] [CrossRef]
- Rus Bakarurraini, N.A.A.; Kamarudin, A.A.; Jamal, R.; Abu, N. Engineered T cells for Colorectal Cancer. Immunotherapy 2024, 16, 987–998. [Google Scholar] [CrossRef]
- Smith, R. Bringing cell therapy to tumors: Considerations for optimal CAR binder design. Antib. Ther. 2023, 6, 225–239. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, D.Y.; Choi, J.U.; Park, J.S.; Lee, S.M.; Kang, C.H.; Park, C.H. Optimized conditions for gene transduction into primary immune cells using viral vectors. Sci. Rep. 2023, 13, 12365. [Google Scholar] [CrossRef]
- Ghazi, B.; El Ghanmi, A.; Kandoussi, S.; Ghouzlani, A.; Badou, A. CAR T-cells for colorectal cancer immunotherapy: Ready to go? Front. Immunol. 2022, 13, 978195. [Google Scholar] [CrossRef]
- Qin, X.; Wu, F.; Chen, C.; Li, Q. Recent advances in CAR-T cells therapy for colorectal cancer. Front. Immunol. 2022, 13, 904137. [Google Scholar] [CrossRef]
- Ouladan, S.; Orouji, E. Chimeric Antigen Receptor-T Cells in Colorectal Cancer: Pioneering New Avenues in Solid Tumor Immunotherapy. J. Clin. Oncol. 2025, 43, 994–1005. [Google Scholar] [CrossRef]
- Li, G.; Wu, X.; Chan, I.H.; Trager, J.B. Abstract 4235: A combination of CAR-NK and CAR-T cells results in rapid and persistent anti-tumor efficacy while reducing CAR-T cell mediated cytokine release and T-cell proliferation. Cancer Res. 2020, 80, 4235. [Google Scholar] [CrossRef]
- Li, H.; Yang, C.; Cheng, H.; Huang, S.; Zheng, Y. CAR-T cells for Colorectal Cancer: Target-selection and strategies for improved activity and safety. J. Cancer 2021, 12, 1804–1814. [Google Scholar] [CrossRef]
- Rahman, M.M.; McFadden, G. Oncolytic Viruses: Newest Frontier for Cancer Immunotherapy. Cancers 2021, 13, 5452. [Google Scholar] [CrossRef]
- Tian, Y.; Xie, D.; Yang, L. Engineering strategies to enhance oncolytic viruses in cancer immunotherapy. Signal Transduct. Target. Ther. 2022, 7, 117. [Google Scholar] [CrossRef] [PubMed]
- Pol, J.; Kroemer, G.; Galluzzi, L. First oncolytic virus approved for melanoma immunotherapy. Oncoimmunology 2016, 5, e1115641. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Lee, W.S.; Kim, C.W.; Lee, S.J.; Yang, H.; Kong, S.J.; Ning, J.; Yang, K.M.; Kang, B.; Kim, W.R.; et al. Oncolytic vaccinia virus reinvigorates peritoneal immunity and cooperates with immune checkpoint inhibitor to suppress peritoneal carcinomatosis in colon cancer. J. Immunother. Cancer 2020, 8, e000857. [Google Scholar] [CrossRef]
- Ren, Y.; Miao, J.-M.; Wang, Y.-Y.; Fan, Z.; Kong, X.-B.; Yang, L.; Cheng, G. Oncolytic viruses combined with immune checkpoint therapy for colorectal cancer is a promising treatment option. Front. Immunol. 2022, 13, 961796. [Google Scholar] [CrossRef]
- Yuan, S.; Wu, Y.; Wang, Y.; Chen, J.; Chu, L. An Oncolytic Adenovirus Expressing SNORD44 and GAS5 Exhibits Antitumor Effect in Colorectal Cancer Cells. Hum. Gene Ther. 2017, 28, 690–700. [Google Scholar] [CrossRef]
- Hwang, J.K.; Hong, J.; Yun, C.O. Oncolytic Viruses and Immune Checkpoint Inhibitors: Preclinical Developments to Clinical Trials. Int. J. Mol. Sci. 2020, 21, 8627. [Google Scholar] [CrossRef]
- Wang, G.; Kang, X.; Chen, K.S.; Jehng, T.; Jones, L.; Chen, J.; Huang, X.F.; Chen, S.Y. An engineered oncolytic virus expressing PD-L1 inhibitors activates tumor neoantigen-specific T cell responses. Nat. Commun. 2020, 11, 1395. [Google Scholar] [CrossRef]
- Wu, C.; Wu, M.; Liang, M.; Xiong, S.; Dong, C. A novel oncolytic virus engineered with PD-L1 scFv effectively inhibits tumor growth in a mouse model. Cell Mol. Immunol. 2019, 16, 780–782. [Google Scholar] [CrossRef]
- Lovatt, C.; Parker, A.L. Oncolytic Viruses and Immune Checkpoint Inhibitors: The “Hot” New Power Couple. Cancers 2023, 15, 4178. [Google Scholar] [CrossRef]
- Temizoz, B.; Kuroda, E.; Ishii, K.J. Vaccine adjuvants as potential cancer immunotherapeutics. Int. Immunol. 2016, 28, 329–338. [Google Scholar] [CrossRef]
- Cox, A.; Cevik, H.; Feldman, H.A.; Canaday, L.M.; Lakes, N.; Waggoner, S.N. Targeting natural killer cells to enhance vaccine responses. Trends Pharmacol. Sci. 2021, 42, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.; Das, A.; Abir, M.H.; Nafiz, I.H.; Mahmud, A.R.; Sarker, M.R.; Emran, T.B.; Hassan, M.M. Cytokines and their role as immunotherapeutics and vaccine Adjuvants: The emerging concepts. Cytokine 2023, 169, 156268. [Google Scholar] [CrossRef] [PubMed]
- Curti, B.D.; Koguchi, Y.; Leidner, R.S.; Rolig, A.S.; Sturgill, E.R.; Sun, Z.; Wu, Y.; Rajamanickam, V.; Bernard, B.; Hilgart-Martiszus, I.; et al. Enhancing clinical and immunological effects of anti-PD-1 with belapectin, a galectin-3 inhibitor. J. Immunother. Cancer 2021, 9, e002371. [Google Scholar] [CrossRef]
- Schlom, J.; Sabzevari, H.; Grosenbach, D.W.; Hodge, J.W. A Triad of Costimulatory Molecules Synergize to Amplify T--Cell Activation in Both Vector--Based and Vector--Infected Dendritic Cell Vaccines. Artif. Cells Blood Substit. Biotechnol. 2003, 31, 193–228. [Google Scholar] [CrossRef]
- Goodwin, T.J.; Huang, L. Investigation of phosphorylated adjuvants co-encapsulated with a model cancer peptide antigen for the treatment of colorectal cancer and liver metastasis. Vaccine 2017, 35, 2550–2557. [Google Scholar] [CrossRef]
- Liu, C.; Xie, Y.; Sun, B.; Geng, F.; Zhang, F.; Guo, Q.; Wu, H.; Yu, B.; Wu, J.; Yu, X.; et al. MUC1- and Survivin-based DNA Vaccine Combining Immunoadjuvants CpG and interleukin-2 in a Bicistronic Expression Plasmid Generates Specific Immune Responses and Antitumour Effects in a Murine Colorectal Carcinoma Model. Scand. J. Immunol. 2018, 87, 63–72. [Google Scholar] [CrossRef]
- Zanetti, B.F.; Ferreira, C.P.; Vasconcelos, J.R.C.; Han, S.W. Adjuvant properties of IFN-γ and GM-CSF in the scFv6. C4 DNA vaccine against CEA-expressing tumors. Gene Ther. 2023, 30, 41–50. [Google Scholar] [CrossRef]
- Le Naour, J.; Galluzzi, L.; Zitvogel, L.; Kroemer, G.; Vacchelli, E. Trial watch: TLR3 agonists in cancer therapy. Oncoimmunology 2020, 9, 1771143. [Google Scholar] [CrossRef]
- Wang, F.; Su, H.; Xu, D.; Dai, W.; Zhang, W.; Wang, Z.; Anderson, C.F.; Zheng, M.; Oh, R.; Wan, F. Tumour sensitization via the extended intratumoural release of a STING agonist and camptothecin from a self-assembled hydrogel. Nat. Biomed. Eng. 2020, 4, 1090–1101. [Google Scholar] [CrossRef]
- Sultan, H.; Kumai, T.; Nagato, T.; Wu, J.; Salazar, A.M.; Celis, E. The route of administration dictates the immunogenicity of peptide-based cancer vaccines in mice. Cancer Immunol. Immunother. 2019, 68, 455–466. [Google Scholar] [CrossRef]
- Bouazzaoui, A.; Abdellatif, A.A.H. Vaccine delivery systems and administration routes: Advanced biotechnological techniques to improve the immunization efficacy. Vaccine X 2024, 19, 100500. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, C.S.; Hsu, J.C.; Hosseini, I.; Shen, B.-Q.; Rotte, A.; Twomey, P.; Girish, S.; Wu, B. Personalized Cancer Vaccines: Clinical Landscape, Challenges, and Opportunities. Mol. Ther. 2021, 29, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Mochida, Y.; Uchida, S. mRNA vaccine designs for optimal adjuvanticity and delivery. RNA Biol. 2024, 21, 422–448. [Google Scholar] [CrossRef]
- Johansen, P.; Storni, T.; Rettig, L.; Qiu, Z.; Der-Sarkissian, A.; Smith, K.A.; Manolova, V.; Lang, K.S.; Senti, G.; Müllhaupt, B.; et al. Antigen kinetics determines immune reactivity. Proc. Natl. Acad. Sci. USA 2008, 105, 5189–5194. [Google Scholar] [CrossRef]
- Gupta, M.; Wahi, A.; Sharma, P.; Nagpal, R.; Raina, N.; Kaurav, M.; Bhattacharya, J.; Rodrigues Oliveira, S.M.; Dolma, K.G.; Paul, A.K.; et al. Recent Advances in Cancer Vaccines: Challenges, Achievements, and Futuristic Prospects. Vaccines 2022, 10, 2011. [Google Scholar] [CrossRef]
- Stanich, P.P.; Pelstring, K.R.; Hampel, H.; Pearlman, R. A High Percentage of Early-age Onset Colorectal Cancer Is Potentially Preventable. Gastroenterology 2021, 160, 1850–1852. [Google Scholar] [CrossRef]
- Cavestro, G.M.; Mannucci, A.; Balaguer, F.; Hampel, H.; Kupfer, S.S.; Repici, A.; Sartore-Bianchi, A.; Seppälä, T.T.; Valentini, V.; Boland, C.R.; et al. Delphi Initiative for Early-Onset Colorectal Cancer (DIRECt) International Management Guidelines. Clin. Gastroenterol. Hepatol. 2023, 21, 581–603.e533. [Google Scholar] [CrossRef]

| Classification | Prevalence | Features |
|---|---|---|
| CMS 1 (Immune) | 14% | MSI-H CIMP high; hypermutation strong immune activation and JAK-STAT signaling pathway activation |
| CMS 2 (Canonical) | 37% | SCNA high; Wnt/MYC signaling activation |
| CMS 3 (Metabolic) | 13% | Mixed MSI status, SCNA low, CIMP high; Metabolic dysregulation |
| CMS 4 (Mesenchymal) | 23% | SCNA high; Stromal infiltration, TGF-β activation, angiogenesis |
| Vaccine Types | Type of Immunotherapy | Clinical Phase | ROA | Vaccination Strategy | Combination Therapies | Status | NCT Number | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cellular Vaccines | Cancer Stem Cell Vaccine | I/II | i.v | Cancer Stem Cell Vaccine against Specific Antigen in Metastatic Adenocarcinoma of the Colorectal Cancer | NA | Completed | NCT02176746 | [187] |
| Combination Vaccine | Combination Immunotherapy in Subjects with Advanced Small Bowel and Colorectal Cancers | II | s.c, i.v | Combination of vaccines and immunotherapy drugs can reduce the tumor for colorectal cancers | II) CEA/MUC1 Vaccines + M7824 + N-803 + NHSIL12 (Quadruple Therapy) | Completed | NCT04491955 | [188] |
| I) CEA/MUC1 Vaccines + M7824 + N-803 (Triple Therapy) | ||||||||
| Combination Vaccine | Mix Vaccine | I/II | s.c | Mix vaccine to small metastases of colorectal cancer | NA | Completed | NCT03357276 | [189] |
| Combination Vaccine | GVAX Colon Vaccine (With Cyclophosphamide) and Pembrolizumab | II | i.d | For Patients with Mismatch Repair-Proficient (MMR-p) in Advanced Colorectal Cancer | Cyclophosphamide, Pembrolizumab | Completed | NCT02981524 | [133] |
| Combination Vaccine | AlloStim® Immunotherapy Alone and in Combination with Cryoablation as Third Line Therapy | II | i.t, i.v | Bioengineered allogeneic immune cells acts as adjuvant to modulate immune response and kill metastatic tumor cells | Cryoablation | Completed | NCT02380443 | [190] |
| Combination Vaccine | SGI-110 in Combination with an Allogeneic Colon Cancer Cell Vaccine (GVAX) and Cyclophosphamide (CY) | I | i.d, i.v | Against Metastatic Colorectal Cancer (mCRC) as Maintenance Therapy | GM-CSF, Cyclophosphamide | Completed | NCT01966289 | [191] |
| DC Vaccine | RNA-Pulsed DC vaccine | I/II | i.v | CEA RNA Pulsed patients’ dendritic cells are reinfused into the patient’s body | NA | Completed | NCT00003433 | [184] |
| DC Vaccine | Labelled DC Vaccine | I | i.d | MRI-based Tracking of Alpha-type-1 DC Vaccines in Patients with Colorectal Cancer | NA | Completed | NCT01671592 | [192] |
| DC Vaccine | Autologous Dendritic Cell Vaccine plus Avelumab | I/II | i.d | For Pre-treated Mismatch Repair-proficient (MSS-p) Metastatic Colorectal Cancer Patients | Avelumab | Completed | NCT03152565 | [193] |
| DC Vaccine | CEA-loaded dendritic cell vaccine | I/II | i.d, i.v | Induction of Specific T Cell Responses in Colorectal Cancer Patients with Liver Metastases | oxaliplatin/capecitabine | Completed | NCT00228189 | [194] |
| Nucleic acid Vaccine | V941(mRNA-5671/V941) | I | i.v, i.m | Participants with KRAS Mutant Colorectal Cancer | Pembrolizumab | Completed | NCT03948763 | [195] |
| Neoantigen Vaccine | Cancer Vaccine Targeting Shared Neoantigens (GRT-C903 and GRT-R904) | I/II | s.c, i.v, i.m | Neoantigen in combination with checkpoint inhibitors to stimulate immune response against CRC patients | nivolumab | Completed | NCT03953235 | [196] |
| ipilimumab | ||||||||
| Neoantigen Vaccine | Neoadjuvant PalloV-CC | I | i.d | Autologous DC vaccine with silicate-capped yeast cell wall protein by ex vivo priming loaded with tumor lysate for colon cancer | NA | Completed | NCT03827967 | [197] |
| Neoantigen Vaccine | Cancer Vaccine Targeting Shared Neoantigens (GRT-C901 and GRT-R902) | I/II | s.c, i.v, i.m | Vaccine regimen uses two vaccine vectors as a heterologous prime/boost approach (GRT-C901 first followed by GRT-R902) to stimulate an immune response. | nivolumab | Completed | NCT03639714 | [198] |
| ipilimumab | ||||||||
| OV Vaccine | Influenza Vaccine for Early Colorectal Cancer | I/II | i.t | Intratumoral application of an unattenuated influenza vaccine | Curative Surgery | Completed | NCT04591379 | [185] |
| OV Vaccine | Recombinant Ad5 (CEA/MUC1/ Brachyury) Based Immunotherapy Vaccine | I | s.c | Novel adenovirus-based vaccine to elucidate antitumor cytolytic T-cell responses | NA | Completed | NCT03384316 | [199] |
| OV Vaccine | Immunotherapy With CEA(6D) VRP Vaccine (AVX701) | I | i.m | Active Immunotherapy against patients with Stage III Colorectal Cancer | Completed | NCT01890213 | [200] | |
| OV Vaccine | 5T4-MVA (TroVax) | II | i.m | Against patients undergoing surgical resection for colorectal liver metastases | NA | Completed | NCT00259844 | [145] |
| Peptide Vaccine | IMA910 Plus GM-CSF | I/II | i.d | For advanced colorectal carcinoma patients | GM-CSF, Cyclophosphamide | Completed | NCT00785122 | [201] |
| Peptide Vaccine | Colorectal Cancer Peptide Vaccine PolyPEPI1018 | I | s.c | Used against proteins present on the surface of CRC tumor cells | TAS-102 | Completed | NCT05130060 | [202] |
| Peptide Vaccine | PolyPEPI1018 Vaccine and CDx | I/II | s.c | Add-on Immunotherapy to the Standard-of-Care Maintenance Therapy | Fluoropyrimidine/Bevacizumab maintenance therapy | Completed | NCT03391232 | [107] |
| Peptide Vaccine | MUC1 Peptide-Poly-ICLC Adjuvant Vaccine | II | s.c | For Individuals with Advanced Colorectal Adenoma | NA | Completed | NCT00773097 | [203] |
| Peptide Vaccine | Vaccine Therapy with or Without Interleukin-2 | I/II | i.v, s.c | For Treatment of HLA A2.1 Positive Patients with Colorectal Cancer | NA | Completed | NCT00019591 | [204] |
| Vector based Vaccine | V934/V935 hTERT Vaccination | I | i.m, i.d | Vaccination in Cancer Patients with Selected Solid Tumors | NA | Completed | NCT00753415 | [205] |
| Yeast based Vaccine | Vaccine (GI-6207) | I | s.c | Vaccine for Metastatic CEA- Expressing Carcinoma | NA | Completed | NCT00924092 | [151] |
| Name | Commercial Names | Target | Year of Commercialization | Treatment |
|---|---|---|---|---|
| Bevacizumab | Avastin | VEGF | 2004 | Metastatic colorectal cancer |
| Cetuximab | ERBITUX | EGFR | 2004 | Metastatic and Recurrent colorectal cancer |
| Panitumumab | Vectibix | EGFR | 2006 | Metastatic carcinoma of the colorectal cancer |
| Regorafenib | Stivarga | VEGFR2&3; TIE2 | 2012 | Metastatic carcinoma of the colorectal cancer |
| Ziv-aflibercept | Zaltrap | VEGF and PlGF | 2012 | Metastatic carcinoma of the colorectal cancer |
| Ramucirumab | Cyramza | VEGFR2 | 2015 | Metastatic carcinoma of the colorectal cancer |
| Nivolumab | Opdivo | PD-1 | 2017 | Advanced MSI-H/dMMR colorectal cancer |
| Ipilimumab | Yervoy | CTLA-4 | 2018 | MSI-H or dMMR colorectal cancer |
| Pembrolizumab | Keytruda | PD-1 | 2020 | Advanced or unresectable MSI-Hor dMMR CRC cases |
| Dostarlimab | Jemperli | PD-1 | 2021 | Recurrent or advanced dMMR solid tumors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muthukutty, P.; Woo, H.Y.; Yoo, S.Y. Therapeutic Colorectal Cancer Vaccines: Emerging Modalities and Translational Opportunities. Vaccines 2025, 13, 689. https://doi.org/10.3390/vaccines13070689
Muthukutty P, Woo HY, Yoo SY. Therapeutic Colorectal Cancer Vaccines: Emerging Modalities and Translational Opportunities. Vaccines. 2025; 13(7):689. https://doi.org/10.3390/vaccines13070689
Chicago/Turabian StyleMuthukutty, Palaniyandi, Hyun Young Woo, and So Young Yoo. 2025. "Therapeutic Colorectal Cancer Vaccines: Emerging Modalities and Translational Opportunities" Vaccines 13, no. 7: 689. https://doi.org/10.3390/vaccines13070689
APA StyleMuthukutty, P., Woo, H. Y., & Yoo, S. Y. (2025). Therapeutic Colorectal Cancer Vaccines: Emerging Modalities and Translational Opportunities. Vaccines, 13(7), 689. https://doi.org/10.3390/vaccines13070689








