Abstract
Background: Poliomyelitis is a vaccine-preventable disease, with oral poliomyelitis vaccines (OPVs) and injectable poliomyelitis vaccines. In the Democratic Republic of the Congo (DRC), circulating vaccine-derived polioviruses (VDPVs) persist due to intrinsic and extrinsic factors, including the quality of the cold chain, which may make the vaccines less effective. This study’s objective was to evaluate the cold chain’s quality of OPVs and its effect on the vaccine’s viability and potency at different levels in health systems in Kinshasa. Methods: A cross-sectional study was conducted in Kinshasa, collecting OPVs at different levels of the health pyramid. Vaccine viability was assessed by cell culture using a modified World Health Organization (WHO) protocol, and the viral titer was determined using the Karber formula. The vaccine titer was classified as “very good”, “good”, or “poor” according to the WHO standard’s viral titer. Results: A total of 53 vaccines were collected and analyzed, compressing 38 bivalent oral poliomyelitis (bOPV) vaccines and 15 novel oral poliomyelitis vaccines, type 2 (nOPV2). The viral titer ranged from log105.8 to log 107.3 and from log105.4 to log108.9 for the nOPV2 and the bOPV, respectively. Of these 53 vaccine samples, 10% of the bOPVs showed viral titers below the recommended WHO threshold (>106 CCID50/dose), 100% of the nOPV2 had viral titers within the WHO standards (>105 CCID50/dose), and a significant decline in the viral titer was observed for both types of vaccines (nOPV2 and bOPV) as the distribution progressed along the level of the health pyramid. Conclusions: This study demonstrated that the viral titer of OPV declined from central to peripheral areas in routine and campaign strategies in Kinshasa.
1. Introduction
Poliomyelitis is an endemic–epidemic viral disease caused by the poliovirus. It is a highly contagious and debilitating disease characterized by acute flaccid paralysis, usually unilateral and affecting mainly the lower limbs, most commonly in children under 5 years of age. It can cause death in 10% of cases due to paralysis of the respiratory muscles. The wild poliovirus (WPV) serotypes WPV1, WPV2, and WPV3, which belong to the family Picornaviridae and the genus Enterovirus, have been identified as responsible for the epidemics [1,2,3].
Poliomyelitis remains a preventable disease by vaccination, thanks to the administration of oral poliovirus vaccines (OPVs) and injectable poliovirus vaccines (IPVs), which are included in the national immunization program of several countries, and the vaccine is highly immunogenic [4,5,6,7]. These vaccines have significantly reduced the number of poliomyelitis cases and led to the eradication of wild poliovirus serotype 2 (WPV2) by 2015 and serotype 3 (WPV3) by 2019 through the efforts of the global eradication program led by the World Health Organization (WHO) as part of the Global Polio Eradication Initiative (GPEI) [3,8,9,10,11,12,13].
The Democratic Republic of the Congo (DRC) was classified as an endemic zone in 1988, with 2.13 cases per million population per year, and was reclassified as a country certified free of wild poliovirus in 2015, after the WPV was eradicated in 2011. This achievement was made possible by the efforts of the Expanded Program on Immunization (EPI) in the Democratic Republic of the Congo (EPI-DRC), which was put in place in 1980. Since then, the immunization against poliomyelitis has remained one of the main EPI pillars in managing vaccine-preventable diseases in the DRC using routine and mass campaign immunization strategies [14,15,16,17].
Despite these significant advances in poliomyelitis eradication, the disease remains a global health problem due to persistent circulation of the wild poliovirus strain in some countries and reversion of the vaccine strain to vaccine-derived polioviruses (VDPVs) due to the mutations and genetic recombination of the attenuated poliovirus strains contained in OPVs with other enteroviruses, causing epidemics in regions free of wild poliovirus. These epidemics occur mainly in the context of inadequate immunization coverage in certain communities, particularly in Africa, including the DRC, which reported 263 cases of VDPV in 2023 [7,9,10,15,16,17].
In the DRC, efforts have been made to improve immunization coverage through routine immunization and mass immunization campaigns, which have resulted in over 85% coverage according to post-campaign surveys conducted by EPI-DRC [9,14,17]. Despite efforts to improve coverage, VDPV’s outbreaks, like other vaccine-preventable diseases, continue to occur in certain provinces in the DRC, including Kinshasa, where vaccination is carried out, leading to the resurgence of epidemics [10,12,16,18]. Several factors contribute to this resurgence of epidemics, including intrinsic factors related to the vaccinated individuals (such as immunodeficiency, malnutrition, and lack of vaccination compliance); extrinsic factors related to the pathogen (such as the emergence of strains not covered by the vaccine); and vaccine-related factors, in particular the quality and integrity of the cold chain [19,20,21,22,23,24].
The cold chain plays a crucial role in preserving the immunogenicity of the vaccine from production to the vaccination site. Proper storage of vaccines requires a strong and efficient logistics system, as well as qualified personnel, such as pharmacists, who play a central role in maintaining the cold chain, in particular temperature control, traceability of heat-sensitive products, and training of personnel involved in transporting and storing vaccines. Some vaccines, such as OPVs, require a cold chain capable of maintaining the required temperature range to ensure viability and maintain viral potency [25,26,27,28]. Nevertheless, effective maintenance of the cold chain remains a significant challenge in numerous countries, particularly those with low incomes, such as the DRC, where there are several challenges related to poor infrastructure and logistics, such as unreliable electricity, insufficient or poorly maintained cold chain equipment, and limited human resources and capacity.
However, it is essential to assess or monitor the cold chain for the storage of vaccines. This can be done either directly by closely monitoring the temperature, although this approach, despite technological advances, has several limitations, or indirectly by assessing the vaccine itself. The latter involves assessing the viability of the vaccine and the viral titer to determine whether there are still enough viral particles to trigger an immune response.
This study used the cell culture and viral titration approach, with the main objective of assessing the quality and integrity of the cold chain for vaccine storage and its impact on the viability and viral titer of OPV in Kinshasa, the capital of the DRC.
2. Materials and Methods
2.1. Study Type, Period, and Sites
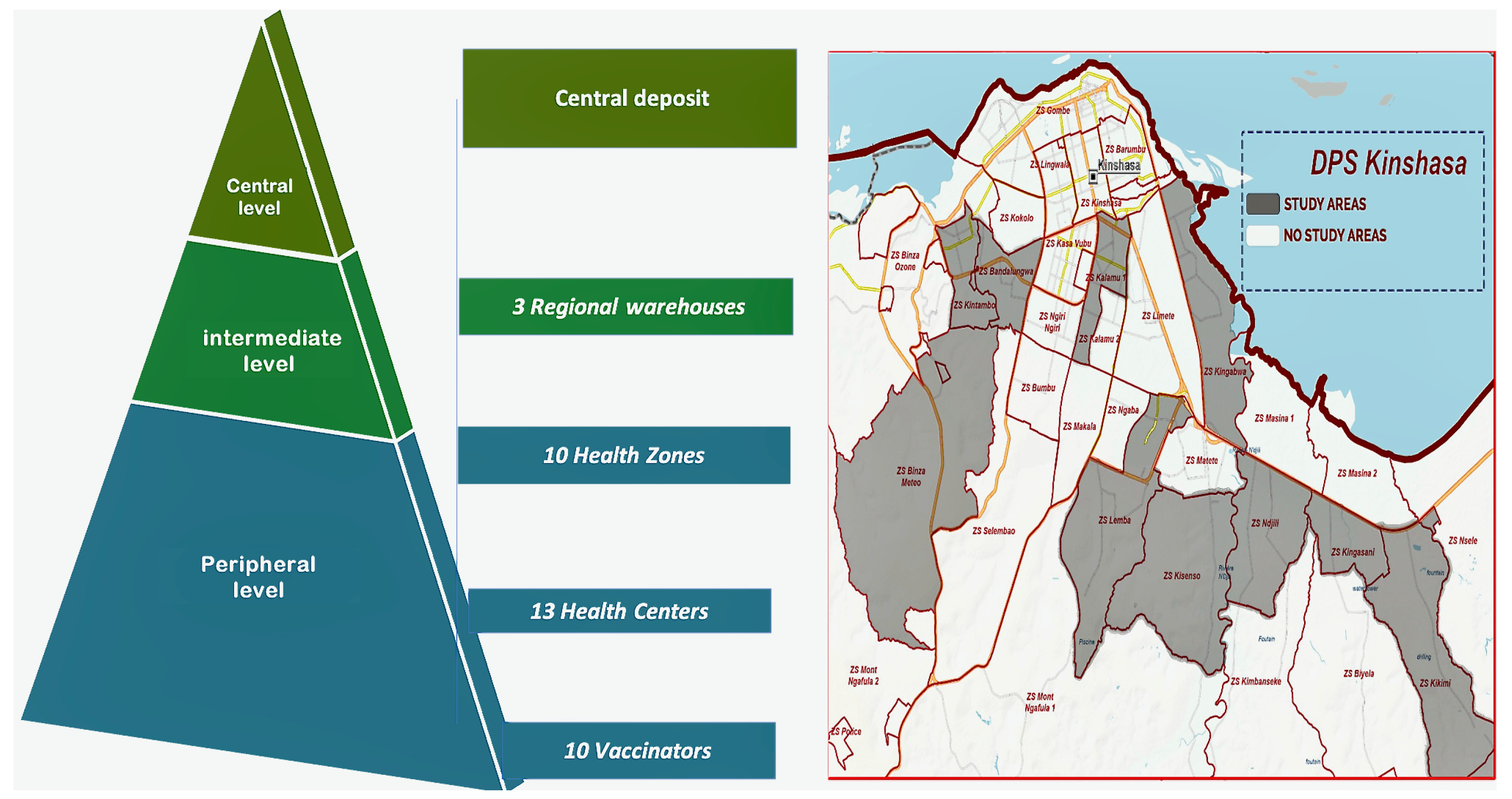
This was a multicenter cross-sectional study conducted from January 2024 to June 2024, as part of an evaluation of vaccination activities. The study was conducted at vaccine storage points of the EPI-DRC. Thirty-seven sites were randomly selected in clusters at a central-level site, intermediate-level sites, and peripheral-level sites (see Figure 1).
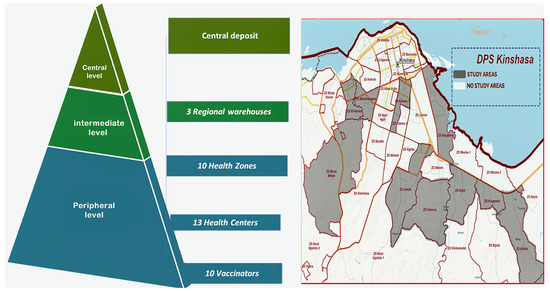
Figure 1.
Mapping of the health pyramid and study sites (DPS: Division Provinciale de la Sante).
2.2. Study Units
For the purposes of this study, the novel oral polio vaccine type 2 (nOPV2) and bivalent oral polio vaccine (bOPV) were selected for indirect evaluation of the cold chain, with the vaccine being stored at EPI vaccine storage sites. At least one representative vaccine from each available vaccine lot number at the site was used, with a minimum of two different lots numbers selected from each level of the storage health pyramid. Sampling was conducted based on both routine and mass campaign immunization strategies depending on availability of the vaccine and limited stock at the sites.
2.3. Data and Vaccines Sample Collection
The following variables were considered:
Vaccine-related data including the location of the vaccine sampling site, the type of vaccine, the manufacturer, the lot number, the expiration date, the receipt date of the vaccine at the vaccination site, the date of sample collection, the date of arrival at the laboratory, the vaccine vial monitor (VVM) stage, the vial condition (opened or closed), the type of storage equipment used for the vaccine, and the storage temperatures at the time of sample collection and upon arrival at the INRB laboratory,
Biological data including the cell culture results and the viral titer results.
Globally, data were collected using both pre-established data collection forms and RedCap software version 5.28.1. The collection of OPV vaccine samples from EPI sites was conducted in accordance with the WHO guidelines [28,29,30]. The vials were meticulously packaged in triple-layer packaging and transported to the laboratory in a cool box at −20 °C. The temperature was monitored using the LogTag® recorders HAXO-8 version 1.0 device from the site to the INRB laboratory [31]. Upon arrival at the virology laboratory, the samples were stored at −20 °C under continuous monitoring until analysis.
2.4. Laboratory Analysis of Vaccine Samples
Prior to the execution of laboratory analyses, a preliminary phase was initiated with the objective of validating the protocol that would subsequently be employed for the testing of vaccine samples. Following the successful validation of the aforementioned protocol, the analyses were conducted using cell culture methods.
- Validation of the Analysis Protocol
In this study, four ranges of analytical protocols were evaluated in accordance with the methodology outlined in the WHO Manual of Laboratory Methods for Vaccines Testing Used by the EPI (we named it WHO2), as well as that described in the Polio Laboratory Manual 4th Edition 2004: Immunization, Vaccines and Biologicals to assess the sensitivity of cell lines (we named it WHO1) [28,29].
Utilizing these established protocols, a control vaccine sample was examined. This sample was retrieved from the central storage facility and had been maintained under optimal conditions at a temperature of −20 °C. The sample was then subjected to testing across a range of vaccine dilution factors (see Table 1 and Table 2). This process was undertaken until a dilution factor that satisfied the validation criteria for a cell culture plate was identified. The validated protocols were designated as WHO1 for nOPV2 and WHO2 for bivalent OPV (bOPV).

Table 1.
Validation of Protocols for nOPV2.

Table 2.
Validation of protocols for bOPV (type 1 and type 3).
Of all the protocols tested, the WHO protocol for the analysis of nOPV2 samples was adapted as follow: the first dilution of the vaccine was carried out at a concentration of 1/100 (instead of a first dilution of 1/10) the dilution factor was 100 (instead of 10); the dilution range of the vaccines was established between 10−6 and 10−9 (instead of 10−4 to 10−7) with a logarithmic difference of 1 between the dilution steps, in a total volume of vaccine-diluted mixture, in and L20B cells of 200 µL. This protocol was validated with a control sample (identified cDC, lot 2224523, Vaccine Vial Monitor Inner square is lighter than outer ring, expiration date not reached, taken at −20 °C from the central deposit) grown on the L20B cell (from CDC Atlanta, Lot 70029026 GR-924) at 2 × 105 cells/mL cell concentration. The protocol was named WHO 1—This study.
The WHO protocol, which we adapted and named WHO2, was validated using the control sample (identified rDC152, lot 1803P152, Vaccine Vial Monitor Inner square is lighter than outer ring, expiration date not reached, taken at −20 °C from the central deposit) cultured on the L20B cell line (from CDC Atlanta, Lot 70029026 GR-924). The first validated dilution was 1/10, and the dilution factor was 10, within a vaccine dilution range of 10−3.5 to 10−8 with a logarithmic difference of 0.5 between the dilution steps, in a total volume of 200 µL vaccine diluent and cells (instead of a total volume of 300 µL vaccine diluent and cells). The modified protocol was used for the analysis of all remaining bOPV samples.
The following criteria were used to validate and interpret the cell culture results:
The plate was considered as valid if 50% to 100% of cytopathic effects (CPEs) were observed at the first considered dilution and 0% to 10% of CPEs at the last considered dilution, depending on nOPV2 or bOPV protocols. In contrast, the plate was considered as not valid when less than 50% of CPEs were observed at the first considered dilution and more than 10% of CPEs at the last considered dilution depending on nOPV2 or bOPV protocols.
After the plate was validated, the results were classified as “very good,” “good,” or “poor” based on the percentage of CPEs on the culture plate and the obtained viral titer. These were then compared with the manufacturer’s reference viral titers [nOPV2 (type 2): >105 CCID50; bOPV (type 1): >106 CCID50; bOPV (type 3): >105.8 CCID50] (Table 3).

Table 3.
Interpretation of results.
- b.
- Oral Poliovirus live attenuated vaccine cell culture.
The OPV live attenuated vaccine was performed in five main steps, as follows:
Step 1: Preparation of permissive cells and working suspension (according to WHO protocol) [28]
Vaccine samples were cultured on L20B cells, a genetically modified mouse-derived cell line expressing the human poliovirus receptor, which allowed selective replication of the poliovirus. The cells were prepared in a growth medium composed of Minimal Essential Medium Eagle (HIMEDIA Ref.RNBL4184 Lot.0000984337) supplemented with 10% fetal bovine serum (gibco Ref.10091-14B, Lot.2577131P) to achieve a confluent layer of healthy, elongated, and compact cells. Cell passages below 15 times and 10 mL of the working cell suspension, containing 1 to 2 × 105 cells/mL, were required per microtitration plate. This suspension was prepared in the maintenance medium using Minimal Essential Medium Eagle containing 2% fetal bovine serum, allowing the formation of a confluent monolayer cells within 2–3 days.
Step 2: Vaccine dilution in maintenance medium in accordance with the WHO protocol [28,29].
The maintenance medium (MM) was distributed into 1 to 10 different dilution tubes (15 mL Eppendorf tubes) according to the protocol, depending on whether the sample was a monovalent vaccine (nOPV2) or a bivalent vaccine (bOPV). The initial dilution 0.1 mL vaccine was mixed with a 9.9 mL diluent solution, and 0.2 mL vaccine was mixed with a 1.8 mL diluent solution for nOPV2 and bOPV, respectively; the diluted sample was then serially diluted through each subsequent dilution tube. The considered dilution ranges were 10−6 to 10−9 and 10−3.5 to 10−8 for nOPV2 and bOPV, respectively [Appendix A, Appendix B].
Step 3: Inoculation of diluted vaccines in to L20B cell line (from CDC Atlanta, Lot 70029026 GR-924)
In columns 1 to 10 of the sterile microtitration plate, 100 µL of the diluted nOPV2 vaccine of 10−6 to 10−9 dilution was inoculated into 100 µL of the L20B cell suspension plate [Appendix A]. Then, 50 µL of the diluted bOPV vaccine of 10−3.5 to 10−8 was inoculated into 100 µL of cell L20B suspension in a sterile, round-bottom, 96-well microtitration plate, which already contained 50 µL of maintenance medium [Appendix B]. In columns 11 and 12, 100 µL of L20B cells was mixed with 100 µL of maintenance medium and was considered to be a negative control. The plate was then hermetically sealed using adhesive tape (Scotch tape). Finally, the plate was incubated at 36 °C in 5–10% of CO2 atmosphere for 5 days.
Step 4: Monitoring cytopathic effects
The plates were examined on a daily basis from day 1 to day 5 using an inverted microscope to monitor the cytopathic effects (CPEs). The CPE manifestations included cell rounding, with cytoplasmic inclusions pushing the nucleus to the periphery, and cell destruction, leading to the detachment of the cell monolayer [28,29,30]. These effects are indicative of the viability of a live attenuated vaccine (see Figure 2).
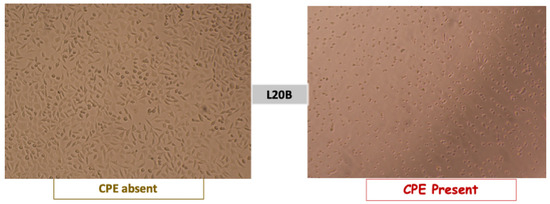
Figure 2.
Poliovirus cytopathic effect observed under an inverted microscope in the L20B cell line. Source: study photo library.
Step 5: Calculation of viral titer [28,29]
On the 5th day of observation, using the lab bench sheet, we calculated the viral titer of the sample using Karber’s formula as follows:
where L is the log of the lowest dilution used in the test, d is the difference between logarithmic dilution steps (0.5 for bOPV and 1 for nOPV2), and S is the sum of the proportion of positive tests (i.e., cultures showing CPEs).
Log CCID50 = L − d (S − 0.5)
The following parameters have been defined:
Cytopathic effects (CPEs) are morphological changes in cells caused by viral growth.
The viral titer (CCID50®) is the smallest amount of virus capable of causing cytopathic effects in 50% of infected cells.
An adequate viral titer is one in which the obtained viral titer is >105 CCID50/0.1 mL for nOPV2 or >105.8 CCID50/0.1 mL for bOPV.
An inadequate viral titer is when the obtained viral titer is ≤105 CCID50/0.1 mL for nOPV2 or ≤105.8 CCID50/0.1 mL for bOPV.
A viable vaccine is any vaccine that, after cell culture, exhibited 50–100% cytopathic effects at the first considered dilution range on the culture plate.
A very good vaccine is a viable vaccine with a 100% positive culture and a viral titer of >105 CCID50/0.1 mL for nOPV2 or >105.8 CCID50/0.1 mL for bOPV.
A good vaccine is a viable vaccine with a 50% positive culture and a viral titer of >105 CCID50/0.1 mL for nOPV2 or >105.8 CCID50/0.1 mL for bOPV.
A poor vaccine does not meet the criteria for a “good” or “very good” vaccine and is a viable vaccine with a 50% positive culture but a viral titer of ≤105 CCID50/0.1 mL for nOPV2 or ≤105.8 CCID50/0.1 mL for bOPV.
cDC is identified from a vaccine sample collected in the central deposit during the immunization campaign.
rDC 152 is an identified vaccine sample collected in the central deposit during the immunization routine.
2.5. Statistical Analysis
Data were collected using RedCap software, version 5.28.1, and exported to Microsoft Excel 2019, version 16.78. Data analysis was carried out using R software, version 4.4.1. Qualitative variables were presented as frequencies and proportions, while quantitative variables were presented as medians, interquartile ranges (IQRs), and the Kruskal–Wallis statistical method for carrying out the analysis of variance. The results are displayed in tables and figures.
2.6. Ethical Approval
This study was conducted as part of the evaluation of EPI related to poliomyelitis vaccination activities in the DRC and was carried out through a partnership between the INRB and the EPI-DRC. The ethical approval was obtained from the committee of Kinshasa University Public Health School (ethical approval number ESP/CE/62/2025).
3. Results
3.1. Protocol Results by Vaccine Type Against Validation Criteria
The WHO1-This study protocol was found to be 100% valid for nOPV2 vaccines and 100% invalid for bOPV. The evaluation of nOPV2 vaccines was facilitated by the aforementioned software. The WHO2 study protocol was found to be 100% valid for bOPV vaccines and 20% invalid for nOPV2. The evaluation of bOPV vaccines was conducted using this methodology (see Table 4).

Table 4.
Result of protocol validation.
3.2. Viability and Titer of OPV Vaccines According to the Level of the Health Pyramid
A total of 53 vaccine samples were collected and analyzed, including 15 nOPV2 and 38 bOPV during the study period. Of the nOPV2, 26.7% (4/15) were collected from vaccinators, while 28.9% (11/38) of the bOPV samples were collected from health zones, health centers, and vaccinators. The central level (A) exhibited a stable titer of 107.2 CCID50/dose for nOPV2 and an average titer of 107.65 CCID50/dose for bOPV; the intermediate level (B) demonstrated the highest average titer at 108.97 CCID50/dose for bOPV; and the peripheral level displayed a progressive decrease in the mean titer for nOPV2 from 106.97 CCID50/dose at the health zone level (Ca) to 106.35 CCID50/dose at the vaccinator level (Cc), with smaller titer variations (Cc: 105.80 to 106.80 CCID50/dose). At the health center level (Cb), the bOPV exhibited the lowest average titer of 106.45 CCID50/dose with a lowest minimum titer of 105.4 CCID50/dose. In contrast, the Cc level had an average titer of 7.30, although it exhibited substantial titer variability from 105.70 to 108.9 CCID50/dose.
At central level A, 100% for both n OPV2 (1/1) and bOPV (2/2) vaccines was interpreted as very good. The same result was obtained at the intermediate level B for the bOPV vaccine. At health zone level Ca, 20% (1/5) of nOPV2 vaccines were classified as good, and 10% (1/11) of bOPV vaccines were classified as poor. At health center level Cb, 60% (3/5) of nOPV2 vaccines were classified as good, and 18% (2/11) of bOPV vaccines were poor. At the vaccinator’s level Cc, 100% (4/4) of nOPV2 vaccines were classified as good, while 10% (1/11) of bOPV vaccines were classified as poor (Table 5 and Figure 3 and Figure 4).

Table 5.
Viability and viral titer of OPV vaccines at different levels of the health pyramid.
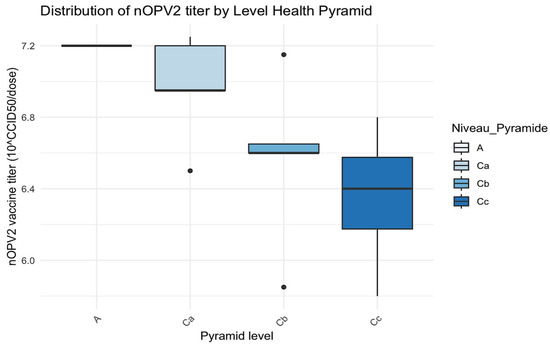
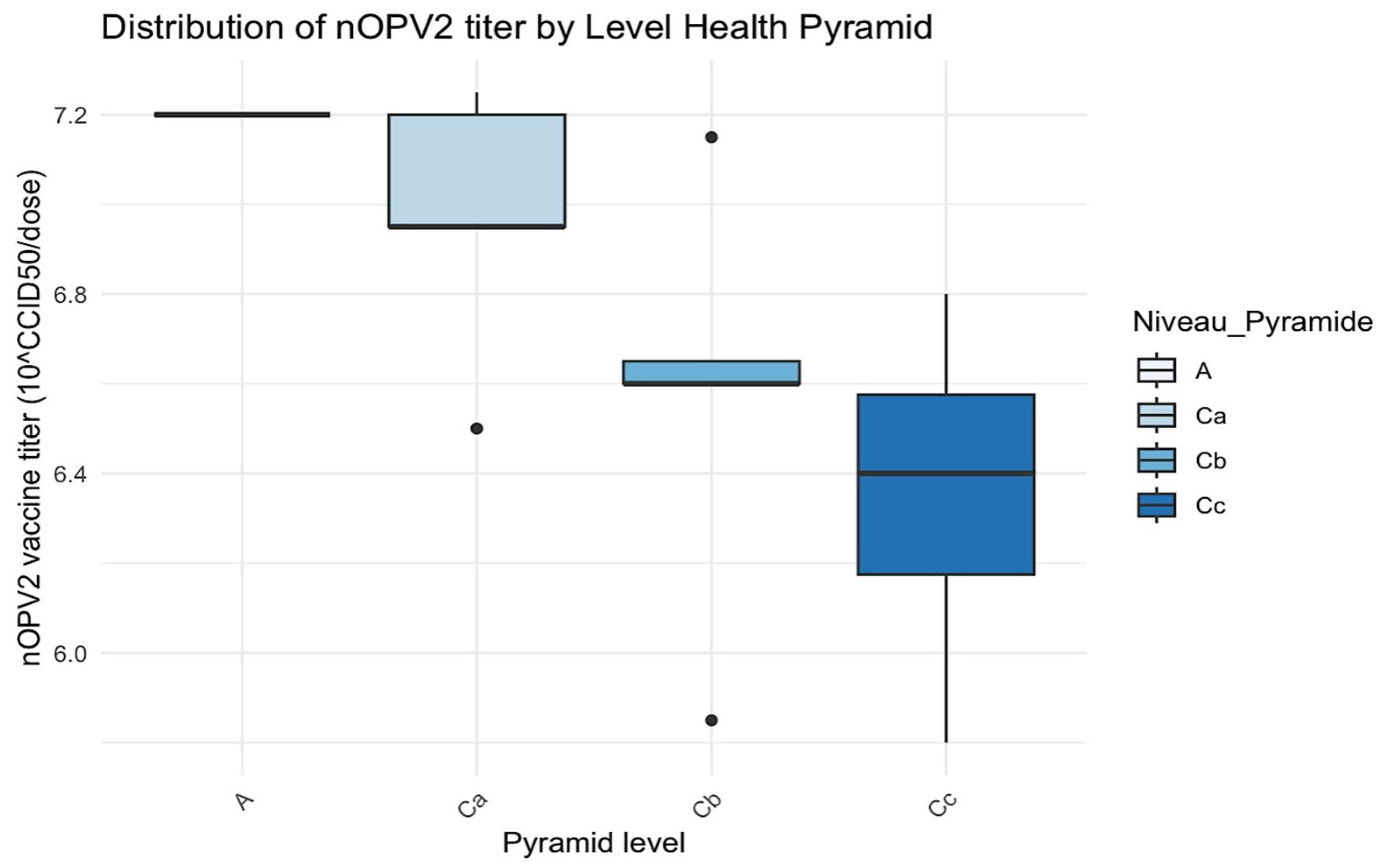
Figure 3.
Distribution of nOPV2 titer by level of the health pyramid. The black dots are the outliers.
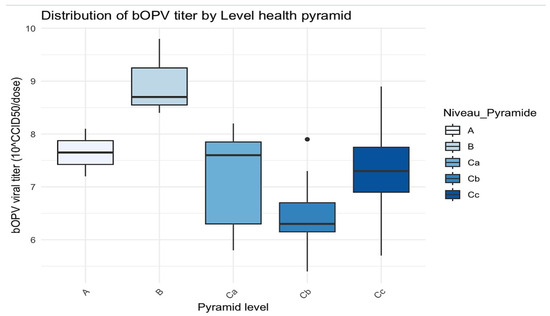
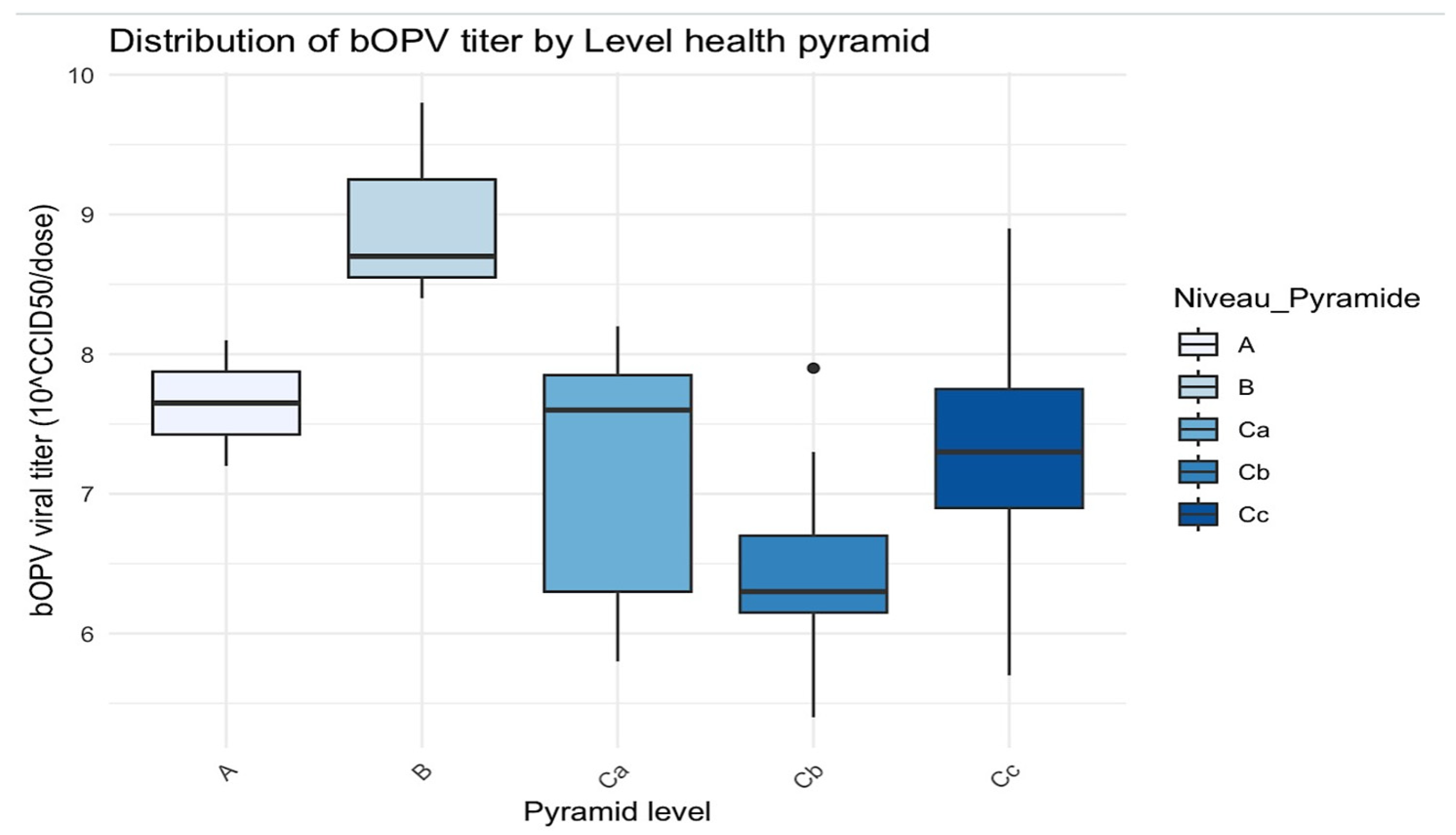
Figure 4.
Distribution of bOPV titer by level of the health pyramid. The black dots are the outliers.
Level A had a single value indicating a lack of variability, with a vaccine titer of 7.2.
In level Ca, the median of viral titer was close to 7.0, and the interquartile range (IQR) was relatively wide, with a low outlier (~6.4).
In level Cb, the variability of viral titers was minimal, with a median of approximately 6.8, accompanied by a lower outlier of around 6.4.
Level Cc had a wide range of vaccine titers (~6.0 to ~7.2), with a median that approached 6.4.
H statistic = 6.40 and p-value = 0.094 (>0.05): no statistically significant difference was found at p < 0.05, although a trend was noted.
In level A, the titers were concentrated between approximately 7.5 and 8, with minimal variability.
Level B demonstrated the highest viral titers, with a median of around 9 and greater variability.
Level C exhibited a wider spread, with a median viral titer of 7.8 but values ranging from 6 to 8.
Level D had a median close to 6.5 and a few outliers.
Level E had a median around 7.5 but with greater variability.
H statistic = 13.7 and p-value = 0.008 (≤0.05): there was a statistically significant difference in bOPV viral titers between the different levels of the health pyramid.
3.3. Viability and Titer of OPV Vaccines According to Immunization Strategies
The viral titers of the nOPV2 vaccines utilized during the vaccination campaign ranged from 105.8 to 107.3 CCID50/dose. Based on culture and viral titer, 60% (9/15) of the nOPV2 vaccines were classified as good, and none were deemed to be poor.
However, the viral titers of the bOPV vaccines utilized during routine immunization ranged from 105.4 to 108.9 CCID50/dose. A total of 10.5% of bOPV vaccines (4/38) were categorized as poor, while 44.7% of bOPV vaccines (17/38) were classified as good or very good, as illustrated in Table 6.

Table 6.
Viability and titer of OPV vaccines by immunization strategies.
3.4. Summary of Evaluations of the Two Immunization Strategies According to the Level of the Health Pyramid with OPV Vaccines and Study Sites
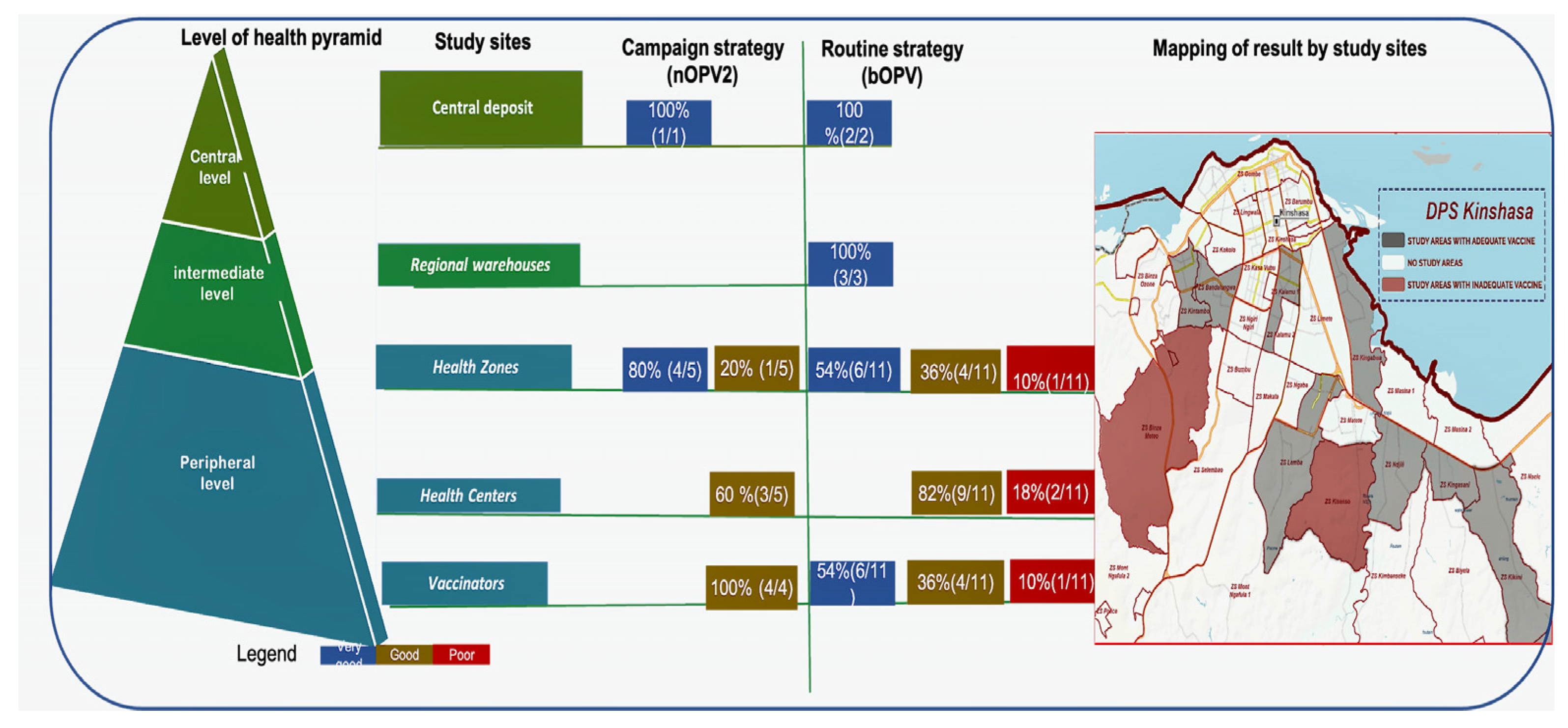
For the campaign strategy (nOPV2 vaccine), at the central level, 100% was very good; at the health zone level, 80% was very good, and 20% good; at the health center level, 40% very good, and 60% was good; and 100% of vaccinators’ nOPV2 was good. For the routine strategy (bOPV vaccine), 100% was very good at the central and intermediate levels; at the health zone level, 10% was bad; at the health center level, 18% were classified as poor, i.e., 2/11; 10% of vaccinators’ bOPV were poor. Furthermore, 8.1% (3/37: Binza Météo health zone, Kasavubu Health Centre and Kasavubu’s vaccinator, Kinsenso Gare Health Centre) of sites had inadequate vaccines (with titers below the WHO reference threshold) (Figure 5).
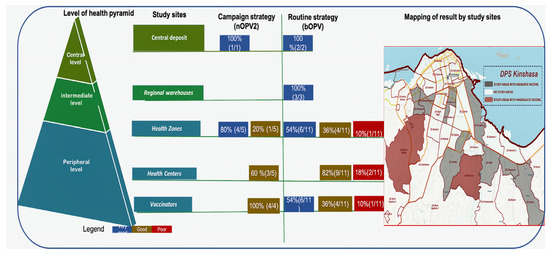
Figure 5.
Schematic summary of OPV vaccine results by immunization strategies and geographical mapping of vaccines.
4. Discussion
This study provides evidence of the quality of the OPV vaccine storage cold chain at different levels of the heath pyramid, particularly in the field, during both campaigns and routine immunization activities in Kinshasa, the capital city of the DRC.
The study collected a total of 53 vials, including 15 vials of nOPV2 obtained during the immunization mass campaign and 38 vials of bOPV collected during routine immunization activities. Logistical constraints, including vaccine stockouts at specific sites and the inability to reach all study sites simultaneously during the immunization, particularly for vaccinators, justified the difference in the size of both nOPV2 and bOPV. However, the sample size used in the present study was sufficient for titer and viability evaluation according to the literature [25,26,27].
At the central level, the viral titer was 107.2 CCID50/dose for n OPV2, while the mean titer of bOPV was 107.65 CCD50/dose for b OPV, indicating that the vaccine was stable, and the initial storage condition (−20 °C) was satisfactory [28,29,30].
This outcome was consistent with those reported by Zipursky et al. in 2011 in Chad [25], who examined the stability of two control vaccines stored under identical conditions. The intermediate level exhibited the highest mean titer of 108.97 CCID50/dose for bOPV, attributable to the more dependable cold chain (a negative cold room at –20 °C) and the stringent temperature control implemented at this level. However, the present study observed a progressive decline in viral titer as the central level was transitioned to the peripheral level, for both nOPV2 and bOPV utilized during the mass campaign and routine immunization, respectively. The p-value of viral titer for nOPV2 was close to the threshold (0.05), suggesting a trend toward a difference. With a larger sample size, a significant difference might emerge. The graphical analysis (Figure 3) showed an apparent decrease in titers from level A to level Cc.
At the peripheral level, this study demonstrated a decline in the mean viral titer for nOPV2 range from 106.97 CCID50/dose at the health zone level to 106.35 CCID50/dose at the vaccinator level (Cc), with smaller titer variations (105.80 to 106.80 CCID50/dose), which suggested a gradual loss of vaccine stability, which might be related to less stringent storage conditions, particularly temperatures above +8 °C. The mean viral titer found was consistent with the mean titer of 106.25CCID50/dose found by Muhammad et al. in Nigeria during exposure to +60 °C in vaccine samples from the local government [26] and the mean titer of 106.2 CCID50/dose found by Zipursky et al. in Chad after the vaccine had been exposed to ambient temperature for 2 days [25]. For bOPV, at the health center level, we found vaccines with the lowest mean titer of 106.45 CCID50/dose and the lowest minimum titer of 105.4 CCID50/dose, while at the vaccinator level we found high titer variability ranging from 105.70 to 108.90 CCID50/dose, suggesting a failure of the cold chain at the peripheral level, where storage conditions were uneven (ranging from refrigerators at the health zone and health center levels to vaccine carriers sometimes without ice packs at the vaccinator level). These findings are of major public health relevance as they highlight critical weaknesses in the last mile of the vaccine supply chain that could compromise immunization efforts. Ensuring potent vaccines reach the population, especially in remote or underserved areas, is crucial for preventing poliovirus transmission and achieving eradication goals in the DRC. They also imply that failure in maintaining recommended cold chain temperatures may contribute to the re-emergence of vaccine-derived polioviruses (VDPVs), thus reinforcing the urgency to strengthen cold chain infrastructure and monitoring. This is consistent with findings from Muhammad et al. in 2010, Zipursky et al. in 2011, and Eswaran et al. in 2003, who found a titer of 106.90 CCID50/dose at +60 °C [26], a titer of 105.6 CCID50/dose below the threshold of 105.80 CCID50/dose after exposure to ambient temperatures [25], and vaccines at the threshold of 105.80 CID50/dose [27], respectively.
In this study, the campaign immunization strategy demonstrated that vaccines met the expectations of the vaccination program, with viral titers exceeding the WHO reference threshold of 105 CCID50/dose [28,29]. These titers exhibited relative homogeneity, with a marginal decline at lower levels. All nOPV2 vaccines were deemed adequate (ranging from very good to good). This outcome was consistent with those reported by Zipursky et al. in Chad, who found similar viral titers of 105.60 to 106.40 CCID50/dose [25]. The decline in viral titer at the peripheral level of the health pyramid might be related to low vigilance in temperature monitoring by vaccinators. These results further demonstrate that mass vaccination campaigns using nOPV2 can achieve better control of cold chain quality compared with routine services, which is an important insight for planning national immunization strategies.
In the context of the routine strategy, 10% of vaccines had titers below the WHO reference threshold of 105.80 CCID50/dose [27], which was already observed at the peripheral level (health zones and vaccinator), with the lowest titer recorded at 105.4 CCID50/dose, categorizing this as inadequate (poor vaccines). This outcome aligned with the findings reported by Zipursky et al. in Chad, where vaccines were exposed to ambient temperatures (105.60 CCID50/dose), and by Muhammad et al. in 2010, where vaccines were exposed to +60 °C in the laboratory in Nigeria [25,26]. However, this result diverges with finding reported by Eswaran et al. in India, who found a range of 105.83 to 106.49 CCID50/dose [27]. All these findings point out the exposure of vaccines to temperatures out of the WHO range [28,29] at the sites due to poor storage conditions and a lack of temperature monitoring in the storage equipment. Continuous monitoring of the quality of the cold chain is essential to ensure that vaccines remain effective until they are administered. This can be done by performing regular temperature checks and assessing the viability of the vaccines.
Our results indicated a sequential deterioration of the cold chain across three distinct levels of vaccine supply and storage in Kinshasa. This indicated that the issue could have occurred from the health zone that supplied the health center, which in turn supplied the vaccinator within the same geographical area. These findings support the need to prioritize investment in cold chain infrastructure and monitoring in low-performing areas to ensure the equity and effectiveness of immunization programs across the DRC.
In the present study, the individual serotype was not subjected to titration. Had this been conducted, it would have yielded insights into the potency of the individual vaccine strain for bOPV, i.e., type 1 and type 3. However, given that the total titer was set in OPV, estimating the composite titer was sufficient to evaluate the b OPV. It should be noted that in this study, the sample was limited to the capital region, and only a single manufacturer of vaccine was used.
This study has the merit of being the first pilot study to assess the viability of a vaccine in the DRC in the context of the re-emergence of poliomyelitis caused by the vaccine strain, despite significant vaccination efforts. It also underscores the importance of cold chain management in vaccine storage to ensure the effectiveness of the immunization program in the DRC. Its findings are critical for guiding national strategies to close operational gaps and ultimately interrupt the transmission of vaccine-derived polioviruses in the country.
This study suggests to conduct similar study in other provinces where poliomyelitis is also occurring.
5. Conclusions
The study revealed a significant decline in vaccine viability from the central level to the peripheral level, particularly in routine strategy. While these findings cannot be generalized, they contribute to the understanding of the rise in vaccine-preventable diseases despite high vaccine coverage. The findings are of particular significance for decision-makers as they underscore the necessity for improvements in vaccine storage, especially in low-income countries. The results underscore the need for further studies in DRC provinces where poliomyelitis outbreaks are still recurrent.
Author Contributions
Conceptualization, G.K.-M. and S.A.-M.; methodology, G.K.-M., A.M.-M., F.M.-M., J.B.-P., L.K., E.M.-M., A.M.w.b.C. and S.A.-M.; software, G.K.-M., A.M.-M. and G.W.-T.; validation: G.K.-M., A.M.-M., F.M.-M., M.M.-K., A.N.-N. and S.A.-M.; formal analysis, G.K.-M., A.M.-M., G.W.-T., T.K.-M., F.N., P.N.-M., B.N.-M. and S.A.-M.; investigation, G.K.-M. and A.M.-M.; resources, G.K.-M., L.K., E.M.-M., A.M.w.b.C. and S.A.-M.; data curation, G.K.-M. and S.A.-M.; writing—original draft preparation, G.K.-M., A.M.-M., F.M.-M., M.M.-K., T.K.-M. and S.A.-M.; writing—review and editing, G.K.-M., A.M.-M., F.M.-M., M.M.-K., J.B.-P., T.K.-M., P.N.-M., A.N.-N., L.K., E.M.-M., A.M.w.b.C. and S.A.-M.; visualization, G.K.-M., A.M.-M., F.M.-M., M.M.-K., J.B.-P., A.N.-N. and S.A.-M.; supervision, G.K.-M. and S.A.-M.; funding acquisition, G.K.-M. and S.A.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding and was conducted as part of the evaluation of polio immunization activities in the DRC and was carried out through a partnership between the INRB and the EPI-DRC. However, it benefited from funding from Plateforme de Recherche Internationale en Santé Mondiale (PRISME) for scientific publication.
Institutional Review Board Statement
The School of Public Health’s Ethics Committee at the University of Kinshasa provided consent for the data to be used in scientific publications following the ethical approval number ESP/CE/62/2025.
Data Availability Statement
Lab analysis and culture data are available on request from the corresponding and senior authors.
Acknowledgments
This work was made possible by the contributions of several individuals and organizations. We are deeply indebted to the Ministry of Health and la Division Provinciale de la Santé of the Democratic Republic of the Congo (DRC), the Institut National de Recherche Biomédicale (INRB), and the Expanded Program on Immunization (EPI) in the DRC for their invaluable support. Our sincere thanks are directed toward the virology team and Laboratory of Virus Emergent team at the INRB for their excellent collaboration.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| bOPV | Bivalent oral polio vaccine |
| CCID50 | Cell culture infectious dose 50% |
| CPE | Cytopathic effects |
| DRC | Democratic Republic of the Congo |
| DPS | Division Provinciale de la Santé |
| EPI-DRC | Expanded Program on Immunization in the Democratic Republic of the Congo. |
| FBS | Fetal bovine serum |
| GPEI | Global Polio Eradication Initiative |
| IQRs | Interquartile ranges |
| INRB | Institut National de Recherche Biomédicale |
| nOPV2 | Novel oral polio vaccine type 2 |
| OPV | Oral polio vaccine |
| VV | Vaccine viability |
| VVM | Vaccine vial monitoring |
| WPV1 | Poliovirus serotypes 1 |
| WPV2 | Poliovirus serotypes 1 |
| WPV3 | Poliovirus serotypes 1 |
| WHO | World Health Organization |
Appendix A. Procedure of nOPV2 Titration
Appendix A.1. Equipment
- -
- Indelible ink markers (2 colors);
- -
- Disposable 1 mL and 10 mL plastic pipettes;
- -
- Sterile 15 mL tubes Eppendorf with caps for dilution;
- -
- Vortex mixer;
- -
- Sterile 96-well cell culture microtiter plate, flat-bottomed, with lids;
- -
- Sterile, non-toxic plate sealers (if CO2 incubator is not used);
- -
- Pipettes with aerosol-resistant tips (ART);
- -
- Flask with confluence layer of healthy L20B cells, containing 1 to 2 × 105 cells/mL;
- -
- Vaccine samples nOPV2 to be tested with known titre (105 CCID50/dose);
- -
- Maintenance Medium (Minimal Essential Medium with 2% of SVF.
Appendix A.2. Procedure
- (1)
- 1 Vial of vaccine nOPV2 = 5 mL = 50 doses; 1 dose = 0.1 mL (=105 infectious units)
- (2)
- Label the dilution tubes: 10−2 to 10−9.
- (3)
- Dispense 9 mL of MM into tubes 1 to 8.
- (4)
- Dilute the vaccine in 2% Maintenance Medium This is the 10−1 dilution: 0.1 mL of vaccine in 9.9 of MM.
- (5)
- Draw 1 mL of diluted vaccine from the first tube using a sterile pipette or a pipette with an ART tip.
- (6)
- Cover the tube and vortex gently.
- (7)
- Repeat the dilution steps, transferring 1 mL each time and always changing pipette/pipette tip between dilutions, up to tube 8 (see Figure A1).
- (8)
- Add 100 µL of the Vaccine dilutions (10−6 to 10−9) to 1 to 10 rows A-H, i.e. 20 wells per dilution (Figure A2).
- (9)
- Add 100 µL of maintenance medium to wells A11 to H12 in rows A to H for the cell controls.
- (10)
- Add 100 µL of cells from a cell suspension containing 1 to 2 × 105 cells/mL of L20B to all wells in rows A to H of the plate.
- (11)
- Cover the plate and incubate at 36 °C under CO2.
- (12)
- Examine the development of an EPC using an inverted microscope and record daily measurements for 5 to 7 days. For a valid test, the cell control must contain a complete monolayer of healthy cells.
- (13)
- Calculation of viral titre using Kärber’s formula (Figure A3)log CCID50 = L − d (S − 0.5), where:L = log of the lowest dilution used in the testd = difference between log dilution stepsS = sum of the proportion of ‘positive’ tests (i.e., cultures showing EPC)In this example:L = −6.0; d = 1.0; S = 1 + 0.65 + 0.45 + 0 = 2.10Log CCID50 = −7.60; Virus titre = 107.6 CCID50/0.1 mL
Appendix A.3. Interpretation
- -
- If the small dilution used does not give CPE in 90 to 100% of the wells (the 20), the test is invalid.
- -
- If the largest dilution gives CPE in more than 10% of its wells, the test is invalid.
Appendix B. Procedure of bOPV Titration
The procedure is standard for determining the potency of OPV, both for total virus content (type 1 + type 2 + type 3) and individual serotypes separately using the microneutralization test.
Appendix B.1. Equipment
- -
- Indelible ink markers (2 colors);
- -
- Disposable 1 mL and 10 mL plastic pipettes;
- -
- Sterile 15 mL tubes with caps for dilution;
- -
- Vortex mixer;
- -
- Sterile 96-well cell culture microtiter plate, flat-bottomed, with lids;
- -
- Sterile, non-toxic plate sealers (if CO2 incubator is not used);
- -
- Pipettes with aerosol-resistant tips (ART);
- -
- Flask with confluence layer of healthy L20B cells, containing 1 to 2 × 105 cells/mL;
- -
- Vaccine samples bOPV to be tested with known titre (105.8 CCID50/dose and 106 CCID50/dose);
- -
- 2% MEM maintenance medium
Appendix B.2. Procedure
- -
- Add 0.05 mL of test medium to all wells of the plate for the test vaccine.
- -
- Add 0.05 mL of vaccine dilution to a column of 8 wells. Start by transferring the highest dilution into column 10, and use the same dropper pipette for the whole range.
- -
- Add 0.1 mL of test medium, to equalize the volumes, to each of the cell control wells.
- -
- During this time, wash L20B monolayer flask cultures, trypsinize, count cells and prepare a cell suspension in test medium to contain approximately 1–2 × 105 cells per mL.
- -
- Add 0.1 mL of cell suspension to all wells of the plate. Avoid touching the wells with micropipette tips during this procedure.
- -
- Cover the plates with a sealer.
- -
- Incubate all plates at 36 °C for 5 days.
- -
- During this time, monitor the cell control wells to ensure that the cells are forming a monolayer. Also score the positive wells starting at day 3 and keep good records of the data.
- -
- Calculate the titre in CCID50 per human dose using the Kärber formula.
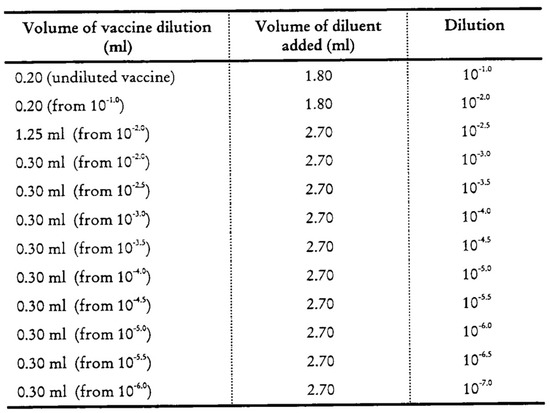
Figure A1.
Arrangement of tube for bOPV dilutions.
Figure A1.
Arrangement of tube for bOPV dilutions.
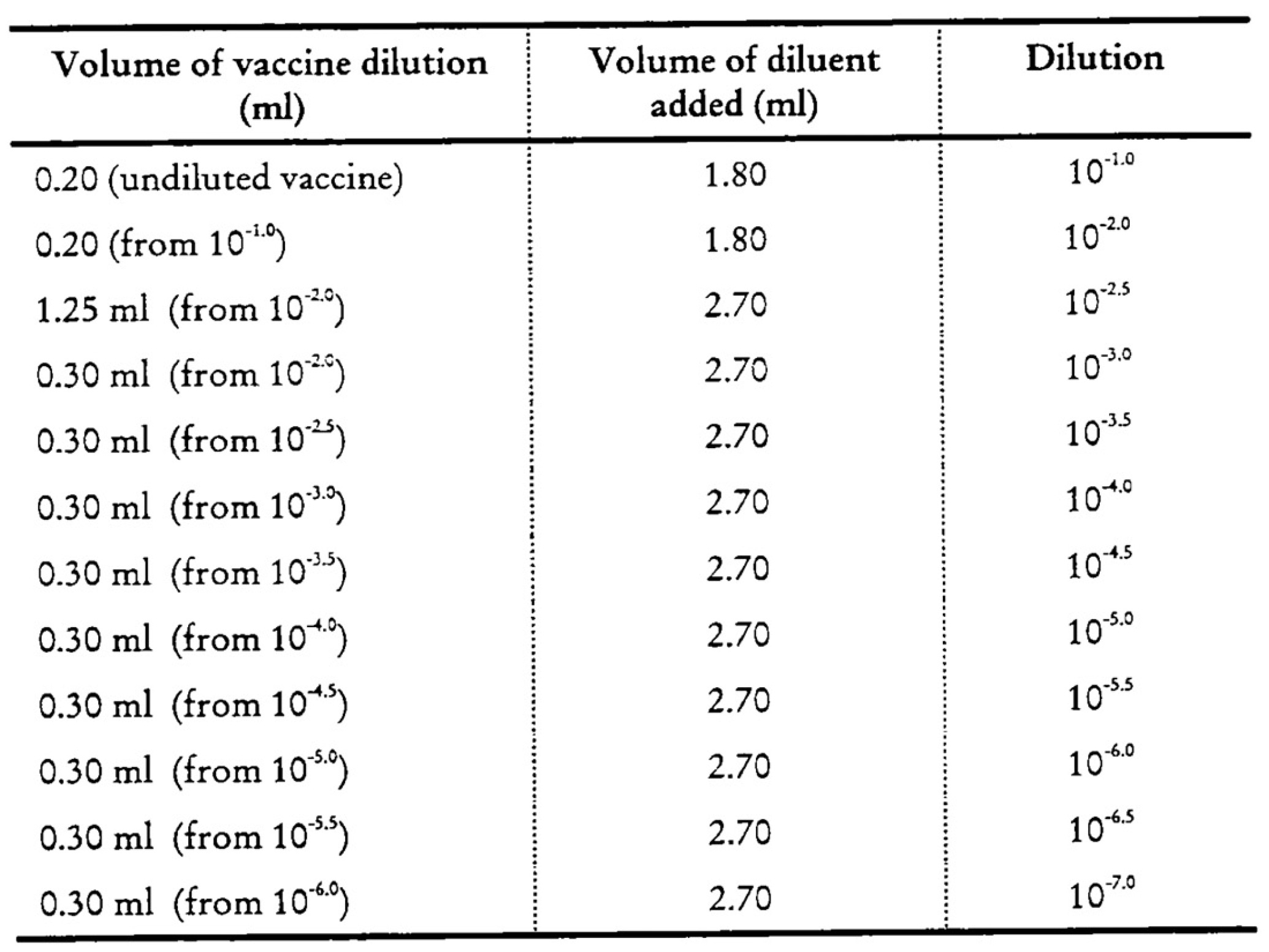
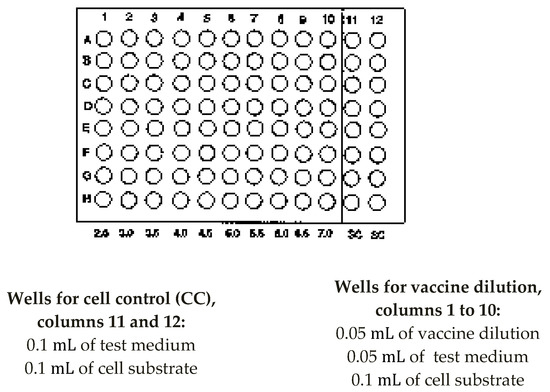
Figure A2.
Arrangement of microtitre plate for OPV potency testing.
Figure A2.
Arrangement of microtitre plate for OPV potency testing.
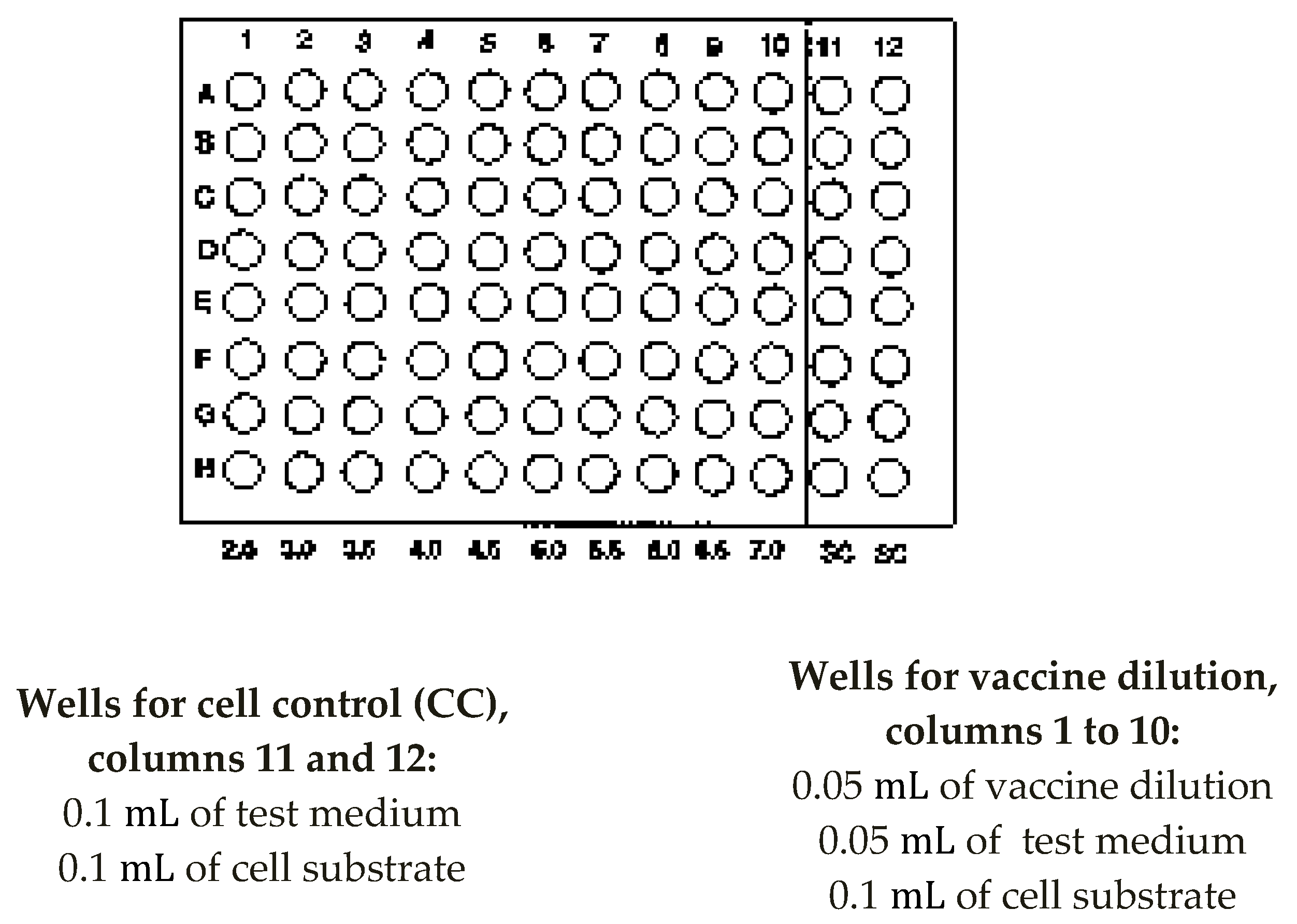
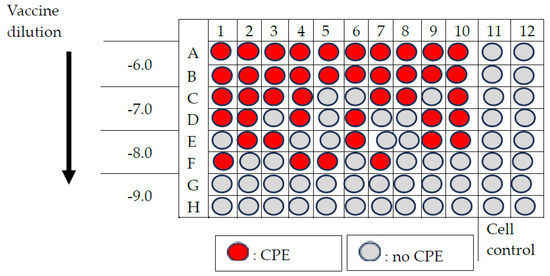
Figure A3.
Example of result of titration of nOPV2.
Figure A3.
Example of result of titration of nOPV2.
References
- World Health Organization. Polio Laboratory Manual, 4th ed.; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Fomban Leke, R.G.; King, A.; Pallansch, M.A.; Tangermann, R.H.; Mkanda, P.; Chunsuttiwat, S.; Jack, A.; Kaboré, B.J.; Kane, I.; Khomo, N.E.; et al. Certifying the Interruption of Wild Poliovirus Transmission in the WHO African Region on the Turbulent Journey to a Polio-Free World. Lancet Glob. Health 2020, 8, e1345–e1351. [Google Scholar] [CrossRef] [PubMed]
- Platt, L.R.; Estivariz, C.F.; Sutter, R.W. Vaccine-Associated Paralytic Poliomyelitis: A Review of the Epidemiology and Estimation of the Global Burden. J. Infect. Dis. 2014, 210 (Suppl. S1), S380–S389. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A. Vaccines: Past, Present and Future. Nat. Med. 2005, 11, S5–S11. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S. History of Vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef] [PubMed]
- Rappuoli, R.; Black, S.; Lambert, P.H. Vaccine Discovery and Translation of New Vaccine Technology. Lancet 2011, 378, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Global Polio Eradication Initiative. Inactivated Polio Vaccine Now Introduced Worldwide. Available online: https://polioeradication.org/news-post/inactivated-polio-vaccine-now-introduced-worldwide/ (accessed on 4 April 2025).
- Global Polio Eradication Initiative. Independent Experts Advise Move to Next Use Phase for Novel Oral Polio Vaccine Type 2—GPEI. Available online: https://polioeradication.org/news-post/independent-experts-advise-transition-to-next-use-phase-for-novel-oral-polio-vaccine-type-2-nopv2/ (accessed on 4 April 2025).
- Tuma, J.N.; Wilkinson, A.L.; Diop, O.M.; Jorba, J.; Gardner, T.; Snider, C.J.; Anand, A.; Ahmed, J. Surveillance to Track Progress Toward Polio Eradication—Worldwide, 2019–2020. Morb. Mortal. Wkly. Rep. 2021, 70, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.T.; Bujaki, E.; Dolan, P.T.; Smith, M.; Wahid, R.; Konz, J.; Weiner, A.J.; Bandyopadhyay, A.S.; Van Damme, P.; De Coster, I.; et al. Engineering the Live-Attenuated Polio Vaccine to Prevent Reversion to Virulence. Cell Host Microbe 2020, 27, 736–751.e8. [Google Scholar] [CrossRef] [PubMed]
- WHO. Projet de Plan D’action Mondial Pour la Sécurité des Patients 2021–2030: Vers L’élimination des Préjudices Évitables Dans le Cadre des Soins de Santé, 1st ed.; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Delpeyroux, F.; Colbère-Garapin, F.; Razafindratsimandresy, R.; Sadeuh-Mba, S.; Joffret, M.-L.; Rousset, D.; Blondel, B. Éradication de la poliomyélite et émergence de poliovirus pathogènes dérivés du vaccin: De Madagascar au Cameroun. Med. Sci. 2013, 29, 1034–1041. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sáez-Llorens, X.; Bandyopadhyay, A.S.; Gast, C.; Leon, T.D.; DeAntonio, R.; Jimeno, J.; Caballero, M.I.; Aguirre, G.; Oberste, M.S.; Weldon, W.C.; et al. Safety and Immunogenicity of Two Novel Type 2 Oral Poliovirus Vaccine Candidates Compared with a Monovalent Type 2 Oral Poliovirus Vaccine in Children and Infants: Two Clinical Trials. Lancet 2021, 397, 27–38. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Poliomyélite. WHO, Soixante-Seizième Assemblée Mondiale de la Santé, A76/13M. Available online: https://apps.who.int/gb/ebwha/pdf_files/WHA76/A76_13-fr.pdf. (accessed on 4 April 2025).
- World Health Organization. Poliovirus Sauvage de Type 1 (PVS1)—Malawi. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/wild-poliovirus-type-1-(WPV1)-malawi (accessed on 4 April 2025).
- Global Polio Eradication Initiative. Classification and Reporting of Vaccine-Derived Polioviruses (VDPV). 2016. Available online: https://polioeradication.org/wp-content/uploads/2016/09/Reporting-and-Classification-of-VDPVs_Aug2016_EN.pdf (accessed on 4 April 2025).
- Rai, A.; Uwishema, O.; Uweis, L.; El Saleh, R.; Arab, S.; Abbass, M.; Wellington, J.; Musabirema, F.; Adanur, I.; Patrick Onyeaka, C.V. Polio Returns to the USA: An Epidemiological Alert. Ann. Med. Surg. 2022, 82. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Weekly Epidemiological Record, No 22, 31 May 2024. Available online: https://www.who.int/publications/journals/weekly-epidemiological-record (accessed on 5 June 2025).
- World Health Organization African Region. Weekly Monitoring of Measles Epidemics and Polio Eradication in Central Africa. Available online: https://www.afro.who.int/health-topics/disease-outbreaks/outbreaks-and-other-emergencies-updates (accessed on 5 June 2025).
- Savolainen-Kopra, C.; Blomqvist, S. Mechanisms of Genetic Variation in Polioviruses. Rev. Med. Virol. 2010, 20, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, D.; Pons-Salort, M.; Shaw, A.G.; Grassly, N.C. The Role of Genetic Sequencing and Analysis in the Polio Eradication Programme. Virus Evol. 2020, 6, veaa040. [Google Scholar] [CrossRef] [PubMed]
- Burton, A. WHO and UNICEF Estimates of National Infant Immunization Coverage: Methods and Processes. Bull. World Health Organ. 2009, 87, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.M.; Kalkowska, D.A. Potential Future Use, Costs, and Value of Poliovirus Vaccines. Risk Anal. 2021, 41, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Greene, S.A.; Burns, C.C.; Tallis, G.; Wassilak, S.G. Progress towards Polio Eradication—Worldwide, January 2021–March 2023. Wkly. Epidemiol. Rec. 2023, 12, 195–204. [Google Scholar]
- Zipursky, S.; Boualam, L.; Cheikh, D.O.; Fournier-Caruana, J.; Hamid, D.; Janssen, M.; Kartoglu, U.; Waeterloos, G.; Ronveaux, O. Assessing the Potency of Oral Polio Vaccine Kept Outside of the Cold Chain during a National Immunization Campaign in Chad. Vaccine 2011, 29, 5652–5656. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, T.; Baba, S.S.; Zaria, L.T.; Abdul-Dahiru, E.-Y.; Thilza, I.B. Determination of Thermal Stability of Oral Polio Vaccine (Opv) at Different Temperature under Laboratory Conditions. Stem Cell 2010, 1, 69–73. [Google Scholar]
- Eswaran, S.P.; Praharaj, A.; Chander, Y.; Nagendra, A. Potency Titration of Oral Polio Vaccine by Estimation of Live Virus Content Using Tissue Culture Technique. Med. J. Armed Forces India 2003, 59, 105–107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- World Health Organization. Vaccine Supply and Quality Unit. In Manual of Laboratory Methods for Testing of Vaccines Used in the WHO Expanded Programme on Immunization; WHO: Geneva, Switzerland, 1997; No. WHO/VSQ/97.04. [Google Scholar]
- World Health Organization. Polio Laboratory Manual; WHO: Geneva, Switzerland, 2004; No. WHO/IVB/04.10. [Google Scholar]
- WHO Expanded Programme on Immunization; World Health Organization; Division of Communicable Diseases. Manual for the Virological Investigation of Poliomyelitis. In Global Poliomyelitis Eradication by the Year 2000; WHO: Geneva, Switzerland, 1990; No. WHO/EPI/CDS/POLIO/90.1, Unpublished. [Google Scholar]
- LogTag® HAXO-8 Temperature Recorder: Technical Specifications. 2014. Available online: http://www.logtagrecorders.com (accessed on 5 June 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).