Efficacy, Immunogenicity, and Safety of Pertussis Vaccine During Pregnancy: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Quality Assessment
2.4. Data Extraction
2.5. Statistical Analysis
3. Results
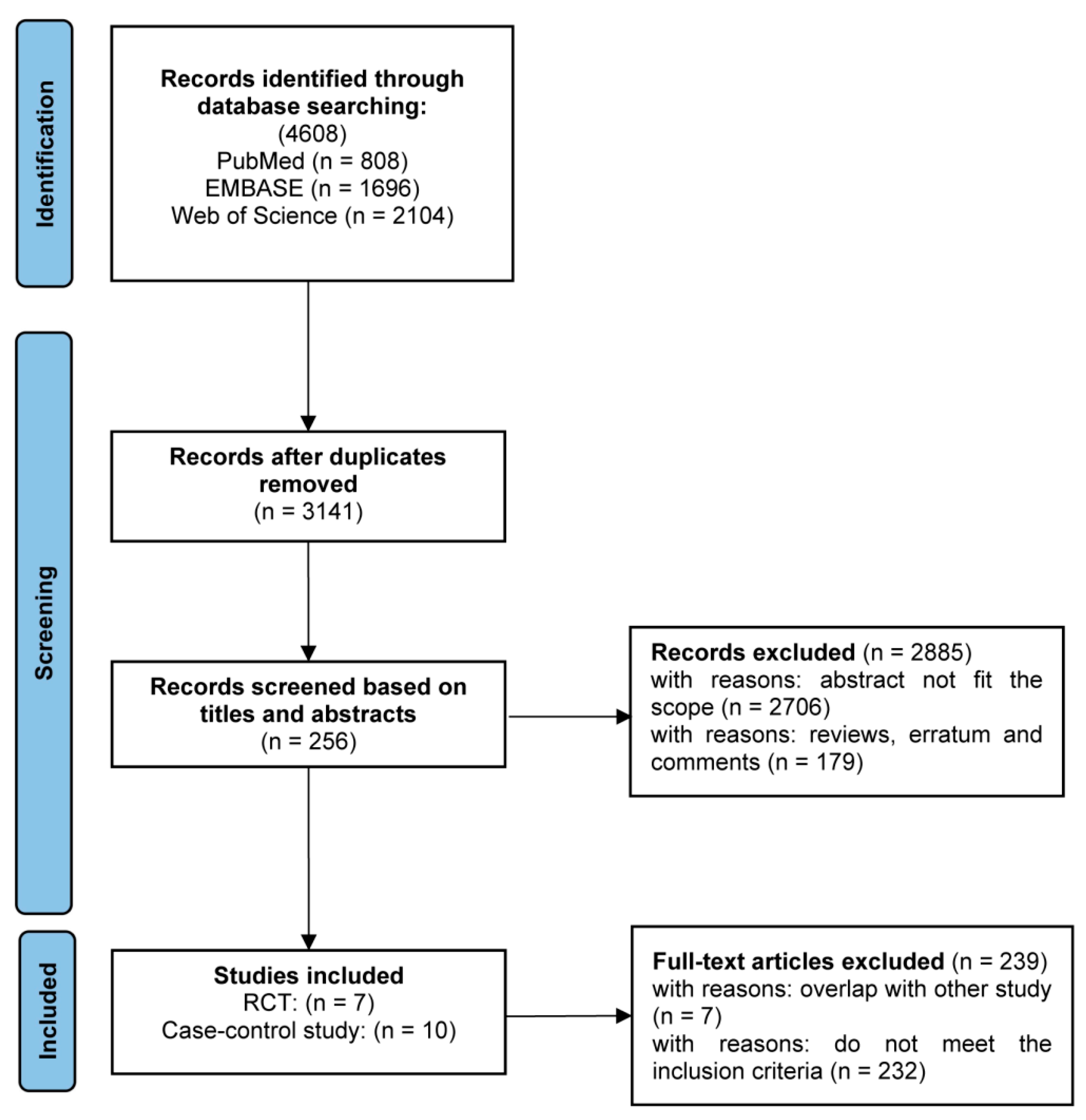
3.1. Study Selection and Quality of the Studies
3.2. Efficacy of Vaccines for Infants Under 3 Months of Age
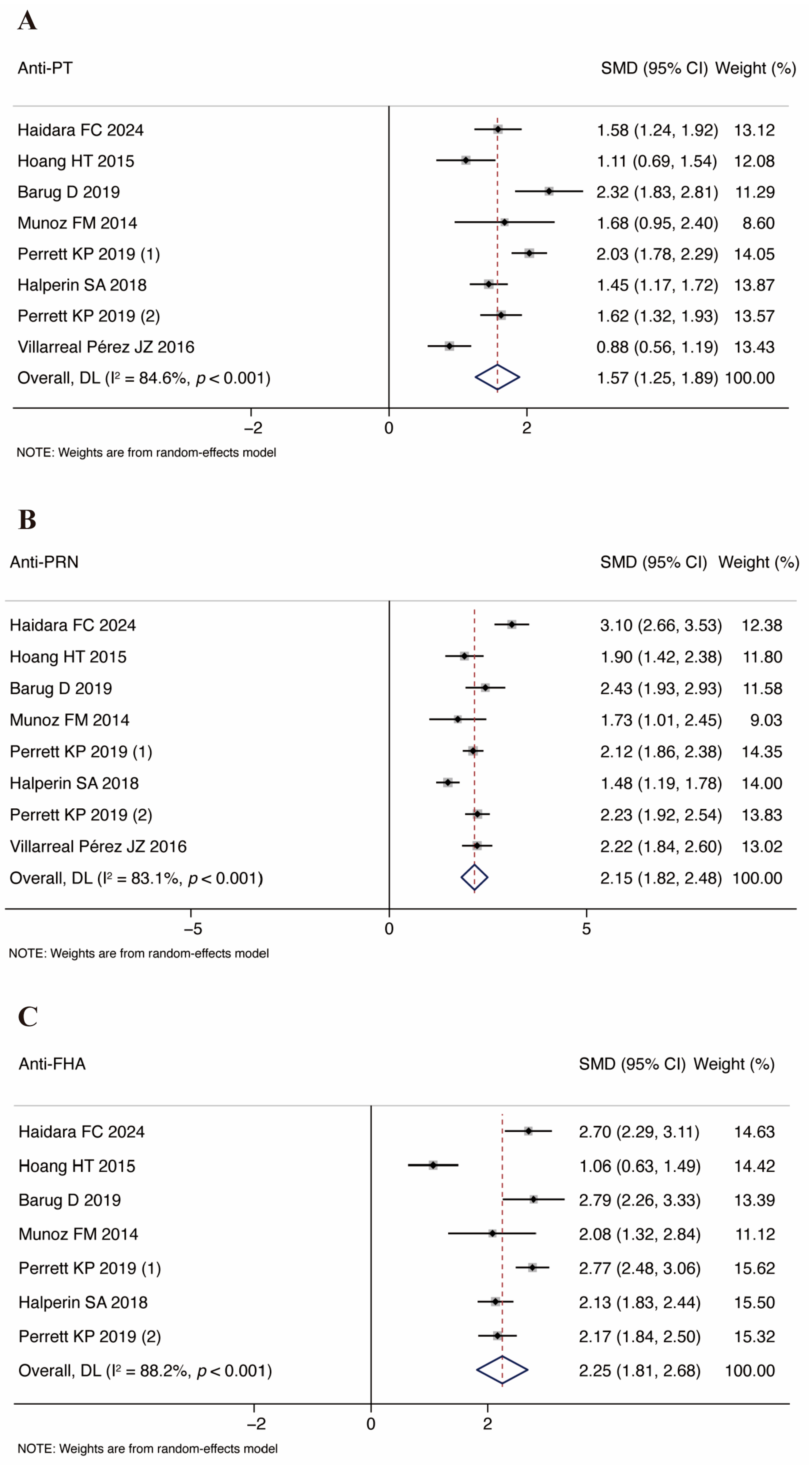
3.3. Vaccine Immunogenicity
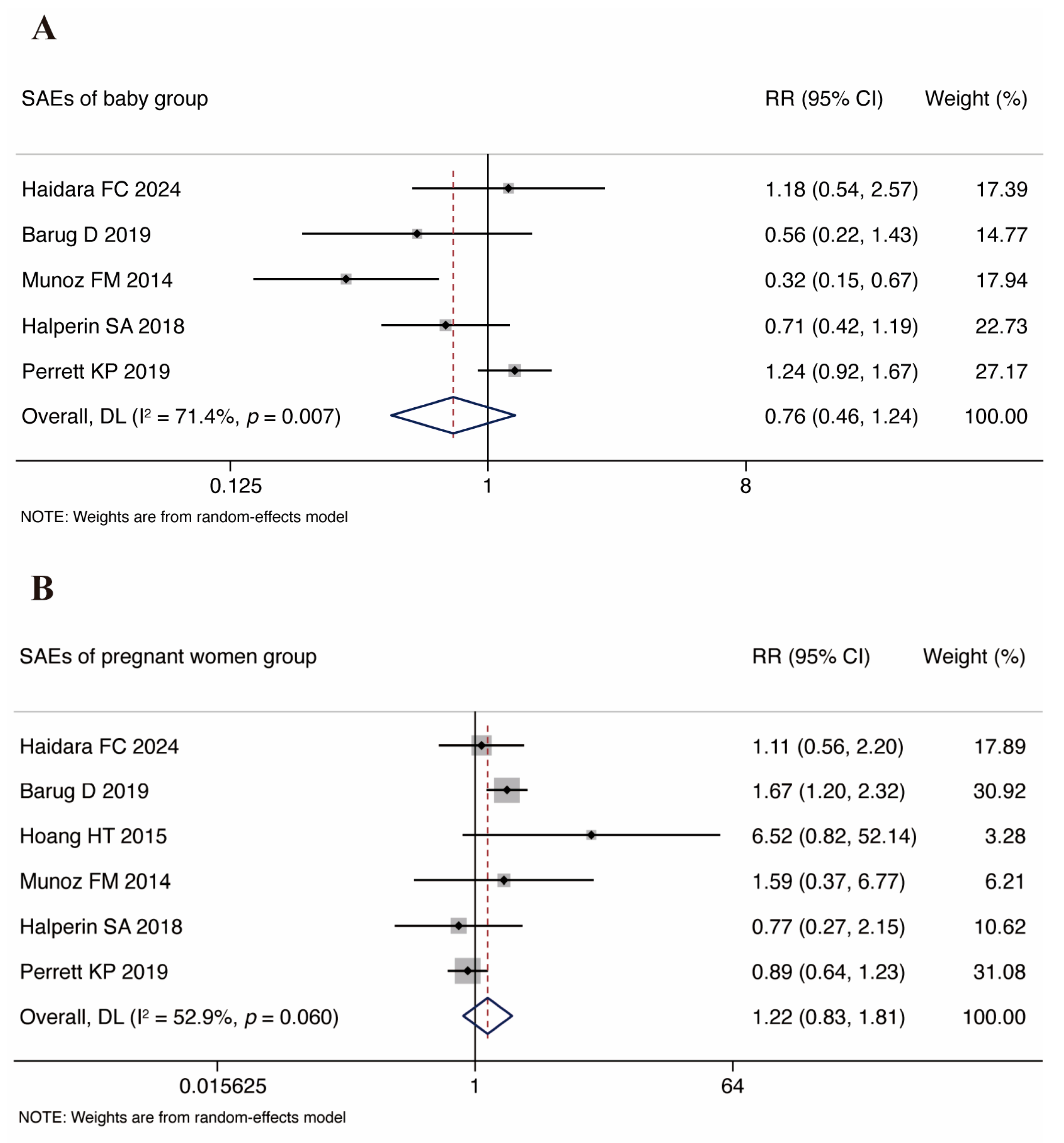
3.4. Vaccine Safety
3.4.1. Infant SAE Analysis
3.4.2. Pregnant Women SAE Analysis
3.5. Sensitivity Analysis and Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- De Weerdt, L.; Herzog, S.A.; Van Damme, P.; Maertens, K. Timing of pertussis vaccination during pregnancy: Evidence and implementation—A systematic review. Vaccine 2024, 42, 126152. [Google Scholar] [CrossRef]
- Masseria, C.; Martin, C.K.; Krishnarajah, G.; Becker, L.K.; Buikema, A.; Tan, T.Q. Incidence and Burden of Pertussis Among Infants Less Than 1 Year of Age. Pediatr. Infect. Dis. J. 2017, 36, e54–e61. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.H.T.; Duclos, P.; Nelson, E.A.S.; Hutubessy, R.C.W. An update of the global burden of pertussis in children younger than 5 years: A modelling study. Lancet Infect. Dis. 2017, 17, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Maertens, K.; Caboré, R.N.; Huygen, K.; Hens, N.; Van Damme, P.; Leuridan, E. Pertussis vaccination during pregnancy in Belgium: Results of a prospective controlled cohort study. Vaccine 2016, 34, 142–150. [Google Scholar] [CrossRef]
- Laverty, M.; Crowcroft, N.; Bolotin, S.; Hawken, S.; Wilson, K.; Amirthalingam, G.; Biringer, A.; Cook, J.; Dubey, V.; Fakhraei, R.; et al. Health Outcomes in Young Children Following Pertussis Vaccination During Pregnancy. Pediatrics 2021, 147, e2020042507. [Google Scholar] [CrossRef]
- Kandeil, W.; van den Ende, C.; Bunge, E.M.; Jenkins, V.A.; Ceregido, M.A.; Guignard, A. A systematic review of the burden of pertussis disease in infants and the effectiveness of maternal immunization against pertussis. Expert Rev. Vaccines 2020, 19, 621–638. [Google Scholar] [CrossRef]
- Mbayei, S.A.; Faulkner, A.; Miner, C.; Edge, K.; Cruz, V.; Peña, S.A.; Kudish, K.; Coleman, J.; Pradhan, E.; Thomas, S.; et al. Severe Pertussis Infections in the United States, 2011-2015. Clin. Infect. Dis. 2019, 69, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Quinn, H.E.; Comeau, J.L.; Marshall, H.S.; Elliott, E.J.; Crawford, N.W.; Blyth, C.C.; Kynaston, J.A.; Snelling, T.L.; Richmond, P.C.; Francis, J.R.; et al. Pertussis Disease and Antenatal Vaccine Effectiveness in Australian Children. Pediatr. Infect. Dis. J. 2022, 41, 180–185. [Google Scholar] [CrossRef]
- Skoff, T.H.; Hadler, S.; Hariri, S. The Epidemiology of Nationally Reported Pertussis in the United States, 2000–2016. Clin. Infect. Dis. 2019, 68, 1634–1640. [Google Scholar] [CrossRef]
- McRae, J.E.; Quinn, H.E.; Saravanos, G.L.; Carlson, S.J.; Britton, P.N.; Crawford, N.W.; Wood, N.J.; Marshall, H.S.; Macartney, K.K. Paediatric Active Enhanced Disease Surveillance (PAEDS) 2017 and 2018: Prospective hospital-based surveillance for serious paediatric conditions. Commun. Dis. Intell. (2018) 2020, 44. [Google Scholar] [CrossRef]
- WHO. Pertussis vaccines: WHO position paper, August 2015--Recommendations. Vaccine 2016, 34, 1423–1425. [Google Scholar] [CrossRef]
- Chu, H.Y.; Englund, J.A. Maternal immunization. Birth Defects Res. 2017, 109, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Vilajeliu, A.; Goncé, A.; López, M.; Costa, J.; Rocamora, L.; Ríos, J.; Teixidó, I.; Bayas, J.M. Combined tetanus-diphtheria and pertussis vaccine during pregnancy: Transfer of maternal pertussis antibodies to the newborn. Vaccine 2015, 33, 1056–1062. [Google Scholar] [CrossRef]
- Hardy-Fairbanks, A.J.; Pan, S.J.; Decker, M.D.; Johnson, D.R.; Greenberg, D.P.; Kirkland, K.B.; Talbot, E.A.; Bernstein, H.H. Immune responses in infants whose mothers received Tdap vaccine during pregnancy. Pediatr. Infect. Dis. J. 2013, 32, 1257–1260. [Google Scholar] [CrossRef]
- Lindsey, B.; Kampmann, B.; Jones, C. Maternal immunization as a strategy to decrease susceptibility to infection in newborn infants. Curr. Opin. Infect. Dis. 2013, 26, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Campbell, H.; Amirthalingam, G.; Andrews, N.; Fry, N.K.; George, R.C.; Harrison, T.G.; Miller, E. Accelerating control of pertussis in England and Wales. Emerg. Infect. Dis. 2012, 18, 38–47. [Google Scholar] [CrossRef]
- Kerr, S.; Van Bennekom, C.M.; Liang, J.L.; Mitchell, A.A. Tdap Vaccination Coverage During Pregnancy—Selected Sites, United States, 2006–2015. MMWR Morb. Mortal. Wkly Rep. 2017, 66, 1105–1108. [Google Scholar] [CrossRef] [PubMed]
- Briga, M.; Goult, E.; Brett, T.S.; Rohani, P.; Domenech de Cellès, M. Maternal pertussis immunization and the blunting of routine vaccine effectiveness: A meta-analysis and modeling study. Nat. Commun. 2024, 15, 921. [Google Scholar] [CrossRef]
- Munoz, F.M.; Bond, N.H.; Maccato, M.; Pinell, P.; Hammill, H.A.; Swamy, G.K.; Walter, E.B.; Jackson, L.A.; Englund, J.A.; Edwards, M.S.; et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: A randomized clinical trial. JAMA 2014, 311, 1760–1769. [Google Scholar] [CrossRef]
- Simayi, A.; Zhu, L.; Jin, H. Safety and Immunogenicity of Pertussis Vaccine Immunization during Pregnancy: A Meta-Analysis of Randomized Clinical Trials. J. Trop Med. 2022, 2022, 4857872. [Google Scholar] [CrossRef]
- Nguyen, H.S.; Vo, N.P.; Chen, S.Y.; Tam, K.W. The optimal strategy for pertussis vaccination: A systematic review and meta-analysis of randomized control trials and real-world data. Am. J. Obstet. Gynecol. 2022, 226, 52–67.e10. [Google Scholar] [CrossRef] [PubMed]
- Calvert, A.; Amirthalingam, G.; Andrews, N.; Basude, S.; Coleman, M.; Cuthbertson, H.; England, A.; Greening, V.; Hallis, B.; Johnstone, E.; et al. Optimising the timing of whooping cough immunisation in mums (OpTIMUM) through investigating pertussis vaccination in pregnancy: An open-label, equivalence, randomised controlled trial. Lancet Microbe 2023, 4, e300–e308. [Google Scholar] [CrossRef] [PubMed]
- Knuutila, A.; Barkoff, A.M.; Ivaska, L.; Tenhu, E.; Teräsjärvi, J.; van Gageldonk, P.; Buisman, A.; Mertsola, J.; He, Q. Effect of immunization during pregnancy and pre-existing immunity on diphtheria-tetanus-acellular pertussis vaccine responses in infants. Emerg. Microbes Infect. 2023, 12, 2204146. [Google Scholar] [CrossRef]
- Haidara, F.C.; Tapia, M.D.; Diallo, F.; Portillo, S.; Williams, M.; Traoré, A.; Rotrosen, E.; Hensel, E.; Makowski, M.; Selamawi, S.; et al. Safety and immunogenicity of a single dose of Tdap compared to Td in pregnant women in Mali and 3 its effect on infant immune responses: A single-centre, randomised, double-blind, active-controlled phase 2 study. eClinicalMedicine 2024, 71, 102556. [Google Scholar] [CrossRef]
- Juscamayta-López, E.; Valdivia, F.; Soto, M.P.; Horna, H.; Pajuelo, M. Case-Control Study to Estimate the Association Between Tdap Vaccination During Pregnancy and Reduced Risk of Pertussis in Newborn Infants in Peru, 2019-2021. Open Forum Infect. Dis. 2023, 10, ofad325. [Google Scholar] [CrossRef] [PubMed]
- Cheuvart, B.; Callegaro, A.; Rosillon, D.; Meyer, N.; Guignard, A. Effectiveness of maternal immunisation with a three-component acellular pertussis vaccine at preventing pertussis in infants in the United States: Post-hoc analysis of a case-control study using Bayesian dynamic borrowing. Vaccine 2023, 41, 5805–5812. [Google Scholar] [CrossRef]
- Regan, A.K.; Moore, H.C.; Binks, M.J.; McHugh, L.; Blyth, C.C.; Pereira, G.; Lust, K.; Sarna, M.; Andrews, R.; Foo, D.; et al. Maternal Pertussis Vaccination, Infant Immunization, and Risk of Pertussis. Pediatrics 2023, 152. [Google Scholar] [CrossRef]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2009, 366, l4898. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Higgins, J.P.; White, I.R.; Anzures-Cabrera, J. Meta-analysis of skewed data: Combining results reported on log-transformed or raw scales. Stat. Med. 2008, 27, 6072–6092. [Google Scholar] [CrossRef]
- Barili, F.; Parolari, A.; Kappetein, P.A.; Freemantle, N. Statistical Primer: Heterogeneity, random- or fixed-effects model analyses? Interact. Cardiovasc. Thorac. Surg. 2018, 27, 317–321. [Google Scholar] [CrossRef]
- Hu, Q.; Xie, Y.; Ji, F.; Zhao, F.; Song, X.; Lu, S.; Li, Z.; Geng, J.; Yang, H.; Long, J.; et al. Effectiveness of EV-A71 Vaccine and Its Impact on the Incidence of Hand, Foot and Mouth Disease: A Systematic Review. Vaccines 2024, 12, 1028. [Google Scholar] [CrossRef] [PubMed]
- Perrett, K.P.; Halperin, S.A.; Nolan, T.; Martínez Pancorbo, C.; Tapiero, B.; Martinón-Torres, F.; Stranak, Z.; Virta, M.; Vanderkooi, O.G.; Kosina, P.; et al. Immunogenicity, transplacental transfer of pertussis antibodies and safety following pertussis immunization during pregnancy: Evidence from a randomized, placebo-controlled trial. Vaccine 2020, 38, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Barug, D.; Pronk, I.; van Houten, M.A.; Versteegh, F.G.A.; Knol, M.J.; van de Kassteele, J.; Berbers, G.A.M.; Sanders, E.A.M.; Rots, N.Y. Maternal pertussis vaccination and its effects on the immune response of infants aged up to 12 months in the Netherlands: An open-label, parallel, randomised controlled trial. Lancet Infect. Dis. 2019, 19, 392–401. [Google Scholar] [CrossRef]
- Hoang, H.T.; Leuridan, E.; Maertens, K.; Nguyen, T.D.; Hens, N.; Vu, N.H.; Caboré, R.N.; Duong, H.T.; Huygen, K.; Van Damme, P.; et al. Pertussis vaccination during pregnancy in Vietnam: Results of a randomized controlled trial Pertussis vaccination during pregnancy. Vaccine 2016, 34, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Halperin, S.A.; Langley, J.M.; Ye, L.; MacKinnon-Cameron, D.; Elsherif, M.; Allen, V.M.; Smith, B.; Halperin, B.A.; McNeil, S.A.; Vanderkooi, O.G.; et al. A Randomized Controlled Trial of the Safety and Immunogenicity of Tetanus, Diphtheria, and Acellular Pertussis Vaccine Immunization During Pregnancy and Subsequent Infant Immune Response. Clin. Infect. Dis. 2018, 67, 1063–1071. [Google Scholar] [CrossRef]
- Villarreal Pérez, J.Z.; Ramírez Aranda, J.M.; de la, O.C.M.; Zamudio Osuna, M.J.; Perales Dávila, J.; Ballesteros Elizondo, M.R.; Gómez Meza, M.V.; García Elizondo, F.J.; Rodríguez González, A.M. Randomized clinical trial of the safety and immunogenicity of the Tdap vaccine in pregnant Mexican women. Hum. Vaccin. Immunother. 2017, 13, 128–135. [Google Scholar] [CrossRef]
- Dabrera, G.; Amirthalingam, G.; Andrews, N.; Campbell, H.; Ribeiro, S.; Kara, E.; Fry, N.K.; Ramsay, M. A case-control study to estimate the effectiveness of maternal pertussis vaccination in protecting newborn infants in England and Wales, 2012–2013. Clin. Infect. Dis. 2015, 60, 333–337. [Google Scholar] [CrossRef]
- Fernandes, E.G.; Sato, A.P.S.; Vaz-de-Lima, L.R.A.; Rodrigues, M.; Leite, D.; de Brito, C.A.; Luna, E.J.A.; Carvalhanas, T.; Ramos, M.; Sato, H.K.; et al. The effectiveness of maternal pertussis vaccination in protecting newborn infants in Brazil: A case-control study. Vaccine 2019, 37, 5481–5484. [Google Scholar] [CrossRef]
- Romanin, V.; Acosta, A.M.; Juarez, M.D.V.; Briere, E.; Sanchez, S.M.; Cordoba, B.L.; Sevilla, M.E.; Lucion, M.F.; Urrutia, A.; Sagradini, S.; et al. Maternal Vaccination in Argentina: Tetanus, Diphtheria, and Acellular Pertussis Vaccine Effectiveness During Pregnancy in Preventing Pertussis in Infants <2 Months of Age. Clin. Infect. Dis. 2020, 70, 380–387. [Google Scholar] [CrossRef]
- Godoy, P.; Garcia-Cenoz, M.; Rius, C.; Munoz-Almagro, C.; Carmona, G.; Alseda, M.; Jane, M.; Vidal, M.-J.; Rodriguez, R.; Alvarez, J.; et al. Effectiveness of maternal pertussis vaccination in protecting newborn: A matched case-control study. J. Infect. 2021, 83, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Merdrignac, L.; Acosta, L.; Habington, A.; Garcìa Cenoz, M.; Pandolfi, E.; Fabiánová, K.; Jordan, I.; O’Sullivan, N.; Navasués, A.; Tozzi, A.E.; et al. Effectiveness of pertussis vaccination in pregnancy to prevent hospitalisation in infants aged <2 months and effectiveness of both primary vaccination and mother’s vaccination in pregnancy in infants aged 2-11 months. Vaccine 2022, 40, 6374–6382. [Google Scholar] [CrossRef] [PubMed]
- Bellido-Blasco, J.; Guiral-Rodrigo, S.; Míguez-Santiyán, A.; Salazar-Cifre, A.; González-Morán, F. A case-control study to assess the effectiveness of pertussis vaccination during pregnancy on newborns, Valencian community, Spain, 1 March 2015 to 29 February 2016. Euro Surveill 2017, 22, 30545. [Google Scholar] [CrossRef] [PubMed]
- Saul, N.; Wang, K.; Bag, S.; Baldwin, H.; Alexander, K.; Chandra, M.; Thomas, J.; Quinn, H.; Sheppeard, V.; Conaty, S. Effectiveness of maternal pertussis vaccination in preventing infection and disease in infants: The NSW Public Health Network case-control study. Vaccine 2018, 36, 1887–1892. [Google Scholar] [CrossRef]
- Siegrist, C.A. Blame vaccine interference, not neonatal immunization, for suboptimal responses after neonatal diphtheria, tetanus, and acellular pertussis immunization. J. Pediatr. 2008, 153, 305–307. [Google Scholar] [CrossRef]
- Olin, P.; Rasmussen, F.; Gustafsson, L.; Hallander, H.O.; Heijbel, H. Randomised controlled trial of two-component, three-component, and five-component acellular pertussis vaccines compared with whole-cell pertussis vaccine. Ad Hoc Group for the Study of Pertussis Vaccines. Lancet 1997, 350, 1569–1577. [Google Scholar] [CrossRef]

| Study ID | Study Period | Study Design | Intervention of Women (Exposure/Control) | N of Women (Exposure/Control) | Country | Gestational Age in Weeks of Vaccination | Major Vaccine Manufacturers | Registration No. |
|---|---|---|---|---|---|---|---|---|
| Perrett et al. [33] | 2015–2017 | RCT obsever-blind | Tdap/placebo | 341/346 | Australia, Canada, Czech Republic, Finland, Italy, and Spain | 27–36 weeks | GSK | NCT02377349 |
| Barug et al. [34] | 2014–2016 | RCT open-label | Tdap/-- | 58/60 | Netherlands | 30–32 weeks | GSK | EudraCT 2012-004006-9/NTR4314 |
| Munoz et al. [19] | 2008–2012 | RCT double-blind | Tdap/placebo | 33/15 | The United States | 30–32 weeks | SP | NCT00707148 |
| Villarreal Pérez et al. [37] | 2011–2014 | RCT double-blind | Tdap/placebo | 90/81 | Vietnam | 30–32 weeks | SP | NCT01445743 |
| Halperin et al. [36] | 2007–2011 | RCT obsever-blind | Tdap/Td | 135/138 | Canada | ≥30 weeks | SP | NCT00553228 |
| Haidara et al. [24] | 2019 | RCT double-blind | Tdap/Td | 133/67 | Mali | 14–27 weeks | GSK | NCT03589768 |
| Hoang et al. [35] | 2015 | RCT | Tdap/TT | 52/51 | Vietnam | 18–36 weeks | SP | NA |
| Study ID | Study Period | Vaccine Efficacy (95% Confidence Interval) | N of Women (Exposure/Control) | Country | Gestational Age in Weeks of Vaccination | Major Vaccine Manufacturers |
|---|---|---|---|---|---|---|
| Merdrignac et al. [42] | 2015–2019 | 87.0% [CI]: 55.0–96.0 | 75/201 | Czech Republic, Ireland, Italy, Spain | Czech Republic: 28–36 weeks, Ireland: 16–36 weeks, Italy: >27 weeks, Spain: 27–36 weeks | NA |
| Fernandes et al. [39] | 2015–2016 | 80.7% [CI]: 55.9–91.6 | 42/249 | Brazil | Case group: 18–37 weeks Control group: 10–40 weeks | NA |
| Cheuvart et al. [26] | 2011–2014 | 83.4% [CI]: 55.7–92.5 | 108/183 | Australia | During pregnancy | GSK |
| Quinn et al. [8] | 2012–2019 | 84.3% [CI]: 26.1–96.7 | 17/52 | Australia | During pregnancy | NA |
| Godoy et al. [41] | 2016–2018 | 88.0% [CI]: 53.8–96.5 | 47/124 | Spain | During pregnancy | NA |
| Romanin et al. [40] | 2012–2016 | 80.7% [CI]: 52.1–92.2 | 71/300 | Argentina | During pregnancy | NA |
| López et al. [25] | 2019–2021 | 81.0% [CI]: 14.0–96.0 | 50/150 | Peru | 27–36 weeks | NA |
| Dabrera et al. [38] | 2012–2013 | 93.0% [CI]: 81.0–97.0 | 58/55 | Britain | 26–38 weeks | NA |
| Saul et al. [44] | 2015–2016 | 69.0% [CI]: 13.0–89.0 * | 117/117 | Australia | 28–32 weeks | GSK |
| Blasco et al. [43] | 2015–2016 | 90.9% [CI]: 56.6–98.1 * | 22/66 | Spain | 28–36 weeks | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Q.; Li, J.; Hu, Q.; Cheng, C.; Yang, K.; Li, X.; Song, X.; Chen, S.; Duan, G. Efficacy, Immunogenicity, and Safety of Pertussis Vaccine During Pregnancy: A Meta-Analysis. Vaccines 2025, 13, 666. https://doi.org/10.3390/vaccines13070666
Shi Q, Li J, Hu Q, Cheng C, Yang K, Li X, Song X, Chen S, Duan G. Efficacy, Immunogenicity, and Safety of Pertussis Vaccine During Pregnancy: A Meta-Analysis. Vaccines. 2025; 13(7):666. https://doi.org/10.3390/vaccines13070666
Chicago/Turabian StyleShi, Qianqian, Jun Li, Quanman Hu, Cheng Cheng, Kun Yang, Xiaoyu Li, Xiaoru Song, Shuaiyin Chen, and Guangcai Duan. 2025. "Efficacy, Immunogenicity, and Safety of Pertussis Vaccine During Pregnancy: A Meta-Analysis" Vaccines 13, no. 7: 666. https://doi.org/10.3390/vaccines13070666
APA StyleShi, Q., Li, J., Hu, Q., Cheng, C., Yang, K., Li, X., Song, X., Chen, S., & Duan, G. (2025). Efficacy, Immunogenicity, and Safety of Pertussis Vaccine During Pregnancy: A Meta-Analysis. Vaccines, 13(7), 666. https://doi.org/10.3390/vaccines13070666









