Real-World Impact of Pneumococcal Conjugate Vaccines on Vaccine Serotypes and Potential Cross-Reacting Non-Vaccine Serotypes
Abstract
1. Introduction
2. Methods
2.1. IPD Data
2.2. Data Analysis
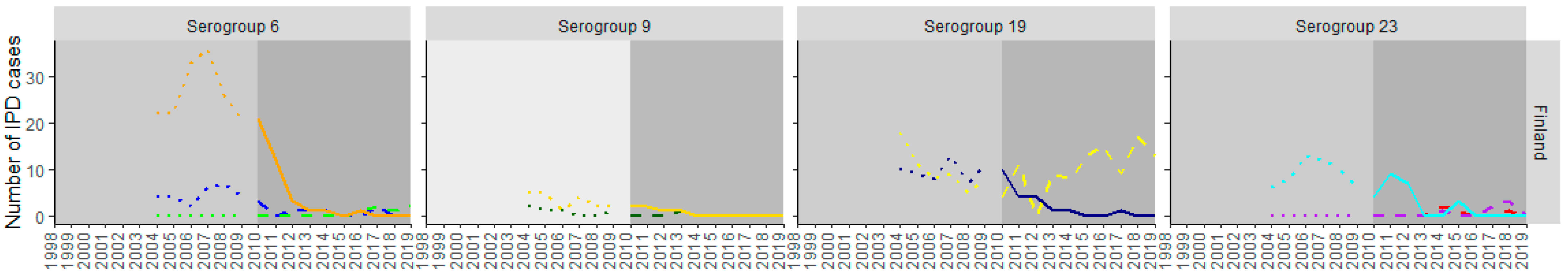
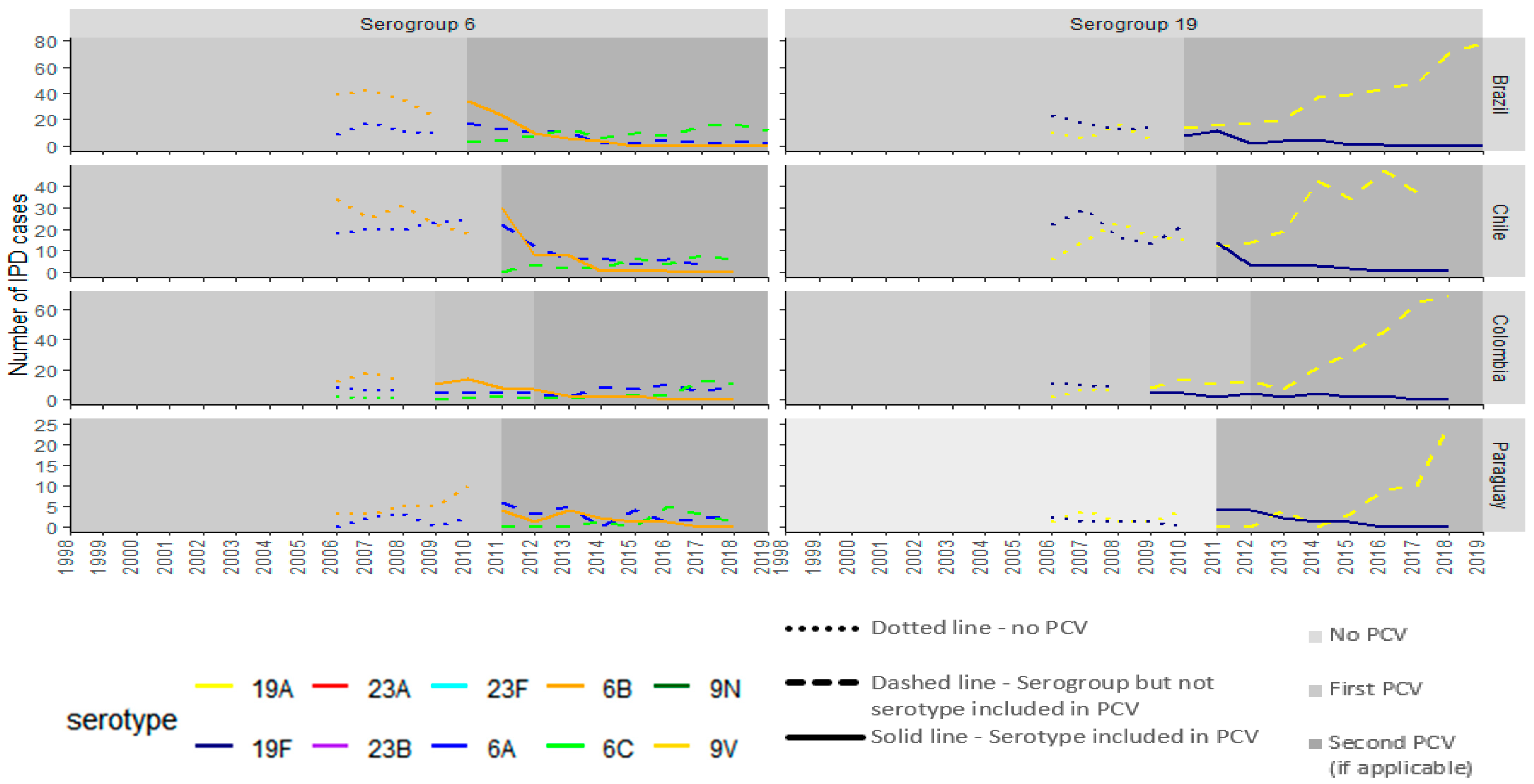
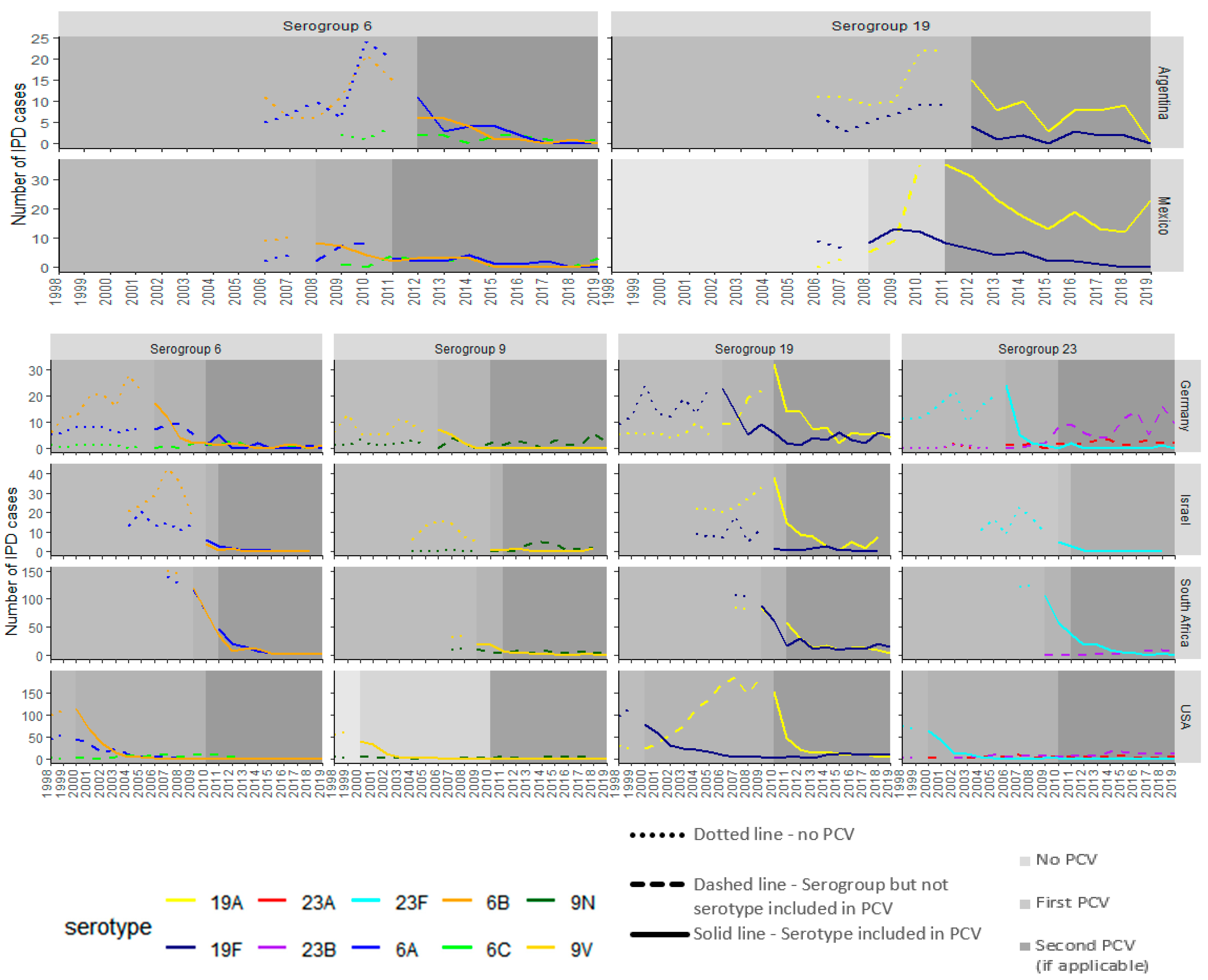
3. Results
3.1. PCV10 Countries
3.2. PCV13 Countries
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2021 Lower Respiratory Infections and Antimicrobial Resistance Collaborators. Global, regional, and national incidence and mortality burden of non-COVID-19 lower respiratory infections and aetiologies, 1990–2021: A systematic analysis from the Global Burden of Disease Study 2021. Lancet Infect. Dis. 2024, 24, 974–1002. [Google Scholar] [CrossRef] [PubMed]
- Vojtek, I.; Buchy, P.; Doherty, T.M.; Hoet, B. Would immunization be the same without cross-reactivity? Vaccine 2019, 37, 539–549. [Google Scholar] [CrossRef]
- Hausdorff, W.P.; Hoet, B.; Schuerman, L. Do pneumococcal conjugate vaccines provide any cross-protection against serotype 19A? BMC Pediatr. 2010, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Poolman, J.; Frasch, C.; Nurkka, A.; Käyhty, H.; Biemans, R.; Schuerman, L. Impact of the conjugation method on the immunogenicity of Streptococcus pneumoniae serotype 19F polysaccharide in conjugate vaccines. Clin. Vaccine Immunol. 2011, 18, 327–336. [Google Scholar] [CrossRef]
- Whitney, C.G.; Pilishvili, T.; Farley, M.M.; Schaffner, W.; Craig, A.S.; Lynfield, R.; Nyquist, A.C.; Gershman, K.A.; Vazquez, M.; Bennett, N.M.; et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: A matched case-control study. Lancet 2006, 368, 1495–1502. [Google Scholar] [CrossRef]
- Hanquet, G.; Lernout, T.; Vergison, A.; Verhaegen, J.; Kissling, E.; Tuerlinckx, D.; Malfroot, A.; Swennen, B.; Sabbe, M. Impact of conjugate 7-valent vaccination in Belgium: Addressing methodological challenges. Vaccine 2011, 29, 2856–2864. [Google Scholar] [CrossRef]
- Harboe, Z.B.; Valentiner-Branth, P.; Benfield, T.L.; Christensen, J.J.; Andersen, P.H.; Howitz, M.; Krogfelt, K.A.; Lambertsen, L.; Konradsen, H.B. Early effectiveness of heptavalent conjugate pneumococcal vaccination on invasive pneumococcal disease after the introduction in the Danish Childhood Immunization Programme. Vaccine 2010, 28, 2642–2647. [Google Scholar] [CrossRef]
- Vestrheim, D.F.; Høiby, E.A.; Aaberge, I.S.; Caugant, D.A. Impact of a pneumococcal conjugate vaccination program on carriage among children in Norway. Clin. Vaccine Immunol. 2010, 17, 325–334. [Google Scholar] [CrossRef]
- Park, I.H.; Moore, M.R.; Treanor, J.J.; Pelton, S.I.; Pilishvili, T.; Beall, B.; Shelly, M.A.; Mahon, B.E.; Nahm, M.H. Differential effects of pneumococcal vaccines against serotypes 6A and 6C. J. Infect. Dis. 2008, 198, 1818–1822. [Google Scholar] [CrossRef]
- Domingues, C.M.; Verani, J.R.; Montenegro Renoiner, E.I.; de Cunto Brandileone, M.C.; Flannery, B.; de Oliveira, L.H.; Santos, J.B.; de Moraes, J.C. Effectiveness of ten-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in Brazil: A matched case-control study. Lancet Respir. Med. 2014, 2, 464–471. [Google Scholar] [CrossRef]
- Jokinen, J.; Rinta-Kokko, H.; Siira, L.; Palmu, A.A.; Virtanen, M.J.; Nohynek, H.; Virolainen-Julkunen, A.; Toropainen, M.; Nuorti, J.P. Impact of ten-valent pneumococcal conjugate vaccination on invasive pneumococcal disease in Finnish children—A population-based study. PLoS ONE 2015, 10, e0120290. [Google Scholar] [CrossRef] [PubMed]
- Deceuninck, G.; De Serres, G.; Boulianne, N.; Lefebvre, B.; De Wals, P. Effectiveness of three pneumococcal conjugate vaccines to prevent invasive pneumococcal disease in Quebec, Canada. Vaccine 2015, 33, 2684–2689. [Google Scholar] [CrossRef]
- Kim, H.W.; Lee, S.; Kim, K.H. Serotype 6B from a pneumococcal polysaccharide vaccine induces cross-functional antibody responses in adults to serotypes 6A, 6C, and 6D. Medicine 2016, 95, e4854. [Google Scholar] [CrossRef]
- Agudelo, C.I.; Castañeda-Orjuela, C.; Brandileone, M.C.C.; Echániz-Aviles, G.; Almeida, S.C.G.; Carnalla-Barajas, M.N.; Regueira, M.; Fossati, S.; Alarcón, P.; Araya, P.; et al. The direct effect of pneumococcal conjugate vaccines on invasive pneumococcal disease in children in the Latin American and Caribbean region (SIREVA 2006-17): A multicentre, retrospective observational study. Lancet Infect. Dis. 2021, 21, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Institutional Repository for Information Sharing (IRIS). Informe Regional de SIREVA II; Organizacion Panamericana de la Salud: Washington, DC, USA, 2018. [Google Scholar]
- Secretaria do Estado da Saúde do Estado de São Paulo. Coordenadoria de Controle de Doenças. Informação da Vigilância das Pneumonias e Meningites Bacterianas; Instituto Adolfo Lutz: Sao Paolo, Brazil, 2019. [Google Scholar]
- Efron, A.; Corso, A. Informe Argentina 2019 SIREVA II; Instituto Nacional de Enfermedades Infecciosas-ANLIS: Buenos Aires, Argentina, 2019. [Google Scholar]
- Instituto Nacional de Salud Publica. Datos por Sexo y por Grupos de Edad Sobre las Características de los Aislamientos de Streptococcus pneumoniae y Neisseria meningitidis en Procesos Infecciosos, 2019; GIVEBPVac (Grupo Interinstitucional para la Vigilancia de Enfermedades Bacterianas Prevenibles por Vacunación): Cuernavaca, Mexico, 2019. [Google Scholar]
- Van der Linden, M. Surveillance. Referenzlabor für Streptokokkoen Uniklinik Aachen. 2021. Available online: https://www.ukaachen.de/kliniken-institute/institut-fuer-medizinische-mikrobiologie/forschung/nationales-referenzzentrum-fuer-streptokokken/publikationen/surveillance/ (accessed on 24 November 2024).
- Ben-Shimol, S.; Regev-Yochay, G.; Givon-Lavi, N.; van der Beek, B.A.; Brosh-Nissimov, T.; Peretz, A.; Megged, O.; Dagan, R. Dynamics of Invasive Pneumococcal Disease in Israel in Children and Adults in the 13-Valent Pneumococcal Conjugate Vaccine (PCV13) Era: A Nationwide Prospective Surveillance. Clin. Infect. Dis. 2022, 74, 1639–1649. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Active Bacterial Core Surveillance. In 1998–2022 Serotype Data for Invasive Pneumococcal Disease Cases by Age Group from Active Bacterial Core Surveillance; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2024. [Google Scholar]
- National Institute for Communicable Diseases. GERMS-SA Annual Report 2019; National Institute for Communicable Disease: Johannesburg, South Africa, 2019.
- Ben-Shimol, S.; Greenberg, D.; Givon-Lavi, N.; Schlesinger, Y.; Somekh, E.; Aviner, S.; Miron, D.; Dagan, R. Early impact of sequential introduction of 7-valent and 13-valent pneumococcal conjugate vaccine on IPD in Israeli children <5 years: An active prospective nationwide surveillance. Vaccine 2014, 32, 3452–3459. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Invasive pneumococcal disease in young children before licensure of 13-valent pneumococcal conjugate vaccine-United States, 2007. MMWR Morb. Mortal. Wkly. Rep. 2010, 59, 253–257. [Google Scholar]
- Mališová, L.; Urbášková, P.; Jakubů, V.; Španělová, P.; Kozáková, J.; Musílek, M.; Žemličková, H. Surveillance of antibiotic resistance of Streptococcus pneumoniae in the Czech Republic, respiratory study results, 2010–2017. Epidemiol. Mikrobiol. Imunol. 2019, 68, 75–81. [Google Scholar]
- Del Amo, E.; Esteva, C.; Hernandez-Bou, S.; Galles, C.; Navarro, M.; Sauca, G.; Diaz, A.; Gassiot, P.; Marti, C.; Larrosa, N.; et al. Serotypes and Clonal Diversity of Streptococcus pneumoniae Causing Invasive Disease in the Era of PCV13 in Catalonia, Spain. PLoS ONE 2016, 11, e0151125. [Google Scholar] [CrossRef]
- Jakobsen, H.; Sigurdsson, V.D.; Sigurdardottir, S.; Schulz, D.; Jonsdottir, I. Pneumococcal serotype 19F conjugate vaccine induces cross-protective immunity to serotype 19A in a murine pneumococcal pneumonia model. Infect. Immun. 2003, 71, 2956–2959. [Google Scholar] [CrossRef]
- Knol, M.J.; Wagenvoort, G.H.; Sanders, E.A.; Elberse, K.; Vlaminckx, B.J.; de Melker, H.E.; van der Ende, A. Invasive Pneumococcal Disease 3 Years after Introduction of 10-Valent Pneumococcal Conjugate Vaccine, the Netherlands. Emerg. Infect. Dis. 2015, 21, 2040–2044. [Google Scholar] [CrossRef] [PubMed]
- Rinta-Kokko, H.; Palmu, A.A.; Auranen, K.; Nuorti, J.P.; Toropainen, M.; Siira, L.; Virtanen, M.J.; Nohynek, H.; Jokinen, J. Long-term impact of 10-valent pneumococcal conjugate vaccination on invasive pneumococcal disease among children in Finland. Vaccine 2018, 36, 1934–1940. [Google Scholar] [CrossRef]
- Mott, M.P.; Caierão, J.; Cunha, G.R.; Del Maschi, M.M.; Pizzutti, K.; d’Azevedo, P.; Dias, C.A.G. Emergence of serotype 19A Streptococcus pneumoniae after PCV10 associated with a ST320 in adult population, in Porto Alegre, Brazil. Epidemiol. Infect. 2019, 147, e93. [Google Scholar] [CrossRef] [PubMed]
- Cassiolato, A.; Almeida, S.; Guerra, S. Changes in invasive Streptococcus pneumoniae serotype (Spn) 19A after introduction of 10-valent pneumococcal conjugate vaccine (PCV10) in Brazil. In Proceedings of the 9th World Congress of the World Society for Pediatric Infectious Diseases (WSPID), Rio de Janeiro, Brazil, 18–21 November 2015. [Google Scholar]
- Camacho Moreno, G.; Imbachi, L.F.; Leal, A.L.; Moreno, V.M.; Patiño, J.A.; Gutiérrez, I.F.; Beltrán, S.; Álvarez-Olmos, M.I.; Mariño, C.; Barrero, R.; et al. Emergence of Streptococcus pneumoniae serotype 19A (Spn19A) in the pediatric population in Bogotá, Colombia as the main cause of invasive pneumococcal disease after the introduction of PCV10. Hum. Vaccin. Immunother. 2020, 16, 2300–2306. [Google Scholar] [CrossRef]
- Instituto de Salid Publica de Chile. Vigilancia de Laboratorio de Streptococcus Pneumoniae Procedente de Enfermedad Invasora in Boletin Instituto de Salud Publica de Chile; Instituto de Salud Publica de Chile: Santiago, Chile, 2015. [Google Scholar]
- Institute of Environmental Science and Research Ltd. (ESR). Invasive Pneumococcal Disease in New Zealand; Institute of Environmental Science and Research: Wellington, New Zealand, 2014. [Google Scholar]
- Ekinci, E.; Van Heirstraeten, L.; Willen, L.; Desmet, S.; Wouters, I.; Vermeulen, H.; Lammens, C.; Goossens, H.; Van Damme, P.; Verhaegen, J.; et al. Serotype 19A and 6C Account for One-Third of Pneumococcal Carriage Among Belgian Day-Care Children Four Years After a Shift to a Lower-Valent PCV. J. Pediatric Infect. Dis. Soc. 2023, 12, 36–42. [Google Scholar] [CrossRef]
- Desmet, S.; Theeten, H.; Laenen, L.; Cuypers, L.; Maes, P.; Bossuyt, W.; Van Heirstraeten, L.; Peetermans, W.E.; Lagrou, K. Characterization of Emerging Serotype 19A Pneumococcal Strains in Invasive Disease and Carriage, Belgium. Emerg. Infect. Dis. 2022, 28, 1606–1614. [Google Scholar] [CrossRef]
- Liesenborghs, L.; Verhaegen, J.; Peetermans, W.E.; Vandeven, J.; Flamaing, J. Trends in serotype prevalence in invasive pneumococcal disease before and after infant pneumococcal vaccination in Belgium, 2002–2010. Vaccine 2013, 31, 1529–1534. [Google Scholar] [CrossRef]
- Isturiz, R.; Sings, H.L.; Hilton, B.; Arguedas, A.; Reinert, R.R.; Jodar, L. Streptococcus pneumoniae serotype 19A: Worldwide epidemiology. Expert Rev. Vaccines 2017, 16, 1007–1027. [Google Scholar] [CrossRef]
- Vesikari, T.; Wysocki, J.; Chevallier, B.; Karvonen, A.; Czajka, H.; Arsène, J.P.; Lommel, P.; Dieussaert, I.; Schuerman, L. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. Pediatr. Infect. Dis. J. 2009, 28 (Suppl. S4), S66–S76. [Google Scholar] [CrossRef]
- Ladhani, S.N.; Collins, S.; Djennad, A.; Sheppard, C.L.; Borrow, R.; Fry, N.K.; Andrews, N.J.; Miller, E.; Ramsay, M.E. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–2017: A prospective national observational cohort study. Lancet Infect. Dis. 2018, 18, 441–451. [Google Scholar] [CrossRef]
- Lepoutre, A.; Varon, E.; Georges, S.; Dorléans, F.; Janoir, C.; Gutmann, L.; Lévy-Bruhl, D. Impact of the pneumococcal conjugate vaccines on invasive pneumococcal disease in France, 2001–2012. Vaccine 2015, 33, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Naucler, P.; Galanis, I.; Morfeldt, E.; Darenberg, J.; Örtqvist, Å.; Henriques-Normark, B. Comparison of the Impact of Pneumococcal Conjugate Vaccine 10 or Pneumococcal Conjugate Vaccine 13 on Invasive Pneumococcal Disease in Equivalent Populations. Clin. Infect. Dis. 2017, 65, 1780–1789. [Google Scholar] [CrossRef] [PubMed]
- Polkowska, A.; Rinta-Kokko, H.; Toropainen, M.; Palmu, A.A.; Nuorti, J.P. Long-term population effects of infant 10-valent pneumococcal conjugate vaccination on pneumococcal meningitis in Finland. Vaccine 2021, 39, 3216–3224. [Google Scholar] [CrossRef]
- Peckeu, L.; van der Ende, A.; de Melker, H.E.; Sanders, E.A.M.; Knol, M.J. Impact and effectiveness of the 10-valent pneumococcal conjugate vaccine on invasive pneumococcal disease among children under 5 years of age in the Netherlands. Vaccine 2021, 39, 431–437. [Google Scholar] [CrossRef] [PubMed]
- McEllistrem, M.C.; Nahm, M.H. Novel pneumococcal serotypes 6C and 6D: Anomaly or harbinger. Clin. Infect. Dis. 2012, 55, 1379–1386. [Google Scholar] [CrossRef]
- Cooper, D.; Yu, X.; Sidhu, M.; Nahm, M.H.; Fernsten, P.; Jansen, K.U. The 13-valent pneumococcal conjugate vaccine (PCV13) elicits cross-functional opsonophagocytic killing responses in humans to Streptococcus pneumoniae serotypes 6C and 7A. Vaccine 2011, 29, 7207–7211. [Google Scholar] [CrossRef]
- Løchen, A.; Croucher, N.J.; Anderson, R.M. Divergent serotype replacement trends and increasing diversity in pneumococcal disease in high income settings reduce the benefit of expanding vaccine valency. Sci. Rep. 2020, 10, 18977. [Google Scholar] [CrossRef]
- Savulescu, C.; Krizova, P.; Valentiner-Branth, P.; Ladhani, S.; Rinta-Kokko, H.; Levy, C.; Mereckiene, J.; Knol, M.; Winje, B.A.; Ciruela, P.; et al. Effectiveness of 10 and 13-valent pneumococcal conjugate vaccines against invasive pneumococcal disease in European children: SpIDnet observational multicentre study. Vaccine 2022, 40, 3963–3974. [Google Scholar] [CrossRef]
- Grant, L.R.; Apodaca, K.; Deshpande, L.; Kimbrough, J.H.; Hayford, K.; Yan, Q.; Mendes, R.; Cané, A.; Gessner, B.D.; Arguedas, A. Characterization of Streptococcus pneumoniae isolates obtained from the middle ear fluid of US children, 2011–2021. Front. Pediatr. 2024, 12, 1383748. [Google Scholar] [CrossRef]
- Wysocki, J.; Tejedor, J.C.; Grunert, D.; Konior, R.; Garcia-Sicilia, J.; Knuf, M.; Bernard, L.; Dieussaert, I.; Schuerman, L. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) when coadministered with different neisseria meningitidis serogroup C conjugate vaccines. Pediatr. Infect. Dis. J. 2009, 28 (Suppl. S4), S77–S88. [Google Scholar] [CrossRef]
- Bermal, N.; Szenborn, L.; Edison, A.; Hernandez, M.; Pejcz, J.; Majda-Stanislawska, E.; Gatchalian, S.; Fanic, A.; Dieussaert, I.; Schuerman, L. Safety and immunogenicity of a booster dose of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine coadministered with DTPw-HBV/Hib and poliovirus vaccines. Pediatr. Infect. Dis. J. 2011, 30, 69–72. [Google Scholar] [CrossRef] [PubMed]
- van den Bergh, M.R.; Spijkerman, J.; François, N.; Swinnen, K.; Borys, D.; Schuerman, L.; Veenhoven, R.H.; Sanders, E.A. Immunogenicity, safety, and reactogenicity of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine and DTPa-IPV-Hib when coadministered as a 3-dose primary vaccination schedule in The Netherlands: A randomized controlled trial. Pediatr. Infect. Dis. J. 2011, 30, e170–e178. [Google Scholar] [PubMed]
- Kim, C.H.; Kim, J.S.; Cha, S.H.; Kim, K.N.; Kim, J.D.; Lee, K.Y.; Kim, H.M.; Kim, J.H.; Hyuk, S.; Hong, J.Y.; et al. Response to primary and booster vaccination with 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine in Korean infants. Pediatr. Infect. Dis. J. 2011, 30, e235–e243. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.R.; Link-Gelles, R.; Schaffner, W.; Lynfield, R.; Lexau, C.; Bennett, N.M.; Petit, S.; Zansky, S.M.; Harrison, L.H.; Reingold, A.; et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: Analysis of multisite, population-based surveillance. Lancet Infect. Dis. 2015, 15, 301–309. [Google Scholar] [CrossRef]
- Grant, L.R.; O’Brien, S.E.; Burbidge, P.; Haston, M.; Zancolli, M.; Cowell, L.; Johnson, M.; Weatherholtz, R.C.; Reid, R.; Santosham, M.; et al. Comparative immunogenicity of 7 and 13-valent pneumococcal conjugate vaccines and the development of functional antibodies to cross-reactive serotypes. PLoS ONE 2013, 8, e74906. [Google Scholar] [CrossRef]
- Szu, S.C.; Lee, C.J.; Parke, J.C., Jr.; Schiffman, G.; Henrichsen, J.; Austrian, R.; Rastogi, S.C.; Robbins, J.B. Cross-immunogenicity of pneumococcal group 9 capsular polysaccharides in adult volunteers. Infect. Immun. 1982, 35, 777–782. [Google Scholar] [CrossRef]
- Ravenscroft, N.; Omar, A.; Hlozek, J.; Edmonds-Smith, C.; Follador, R.; Serventi, F.; Lipowsky, G.; Kuttel, M.M.; Cescutti, P.; Faridmoayer, A. Genetic and structural elucidation of capsular polysaccharides from Streptococcus pneumoniae serotype 23A and 23B, and comparison to serotype 23F. Carbohydr. Res. 2017, 450, 19–29. [Google Scholar] [CrossRef]
- Robbins, J.B.; Austrian, R.; Lee, C.J.; Rastogi, S.C.; Schiffman, G.; Henrichsen, J.; Mäkelä, P.H.; Broome, C.V.; Facklam, R.R.; Tiesjema, R.H.; et al. Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J. Infect. Dis. 1983, 148, 1136–1159. [Google Scholar] [CrossRef]
| Country | First PCV and Posology Used in NIP | Year of First PCV Introduction | Second PCV and Posology Used in NIP | Second PCV Year of Introduction During Study Period | Years of Data Available | Serotype Data Available |
|---|---|---|---|---|---|---|
| Colombia | PCV7 (2 + 1) | 2009 | PCV10 (2 + 1) | 2012 | 2006–2018 | 6A, 6B, 6C, 19A, 19F |
| Chile * | PCV10 (3 + 1) | 2011 | - | - | 2006–2018 | 6A, 6B, 6C, 19A, 19F |
| Finland | PCV10 (2 + 1) | 2010 | - | - | 2004–2019 | 6A, 6B, 6C, 9N, 9V, 19A, 19F, 23A, 23B, 23F |
| Brazil | PCV10 (3 + 1) | 2010 | - | - | 2006–2018 | 6A, 6B, 6C, 19A, 19F |
| Paraguay | PCV10 (2 + 1) | 2011 | - | - | 2006–2018 | 6A, 6B, 6C, 19A, 19F |
| Germany ** | PCV7 (3 + 1) | 2006 | PCV13 (2 + 1) | 2010 | 1998–2019 | 6A, 6B, 6C, 9N, 9V, 19A, 19F, 23A, 23B, 23F |
| Israel | PCV7 (2 + 1) | 2009 | PCV13 (2 + 1) | 2010 | 2004–2019 | 6A, 6B, 6C, 9N, 9V, 19A, 19F |
| USA | PCV7 (3 + 1) | 2000 | PCV13 (3 + 1) | 2010 | 1998–2019 | 6A, 6B, 6C, 9N, 9V, 19A, 19F, 23A, 23B, 23F |
| Mexico | PCV7 (3 + 0) | 2008 | PCV13 (2 + 1) | 2011 | 2006–2018 | 6A, 6B, 6C, 19A, 19F |
| South Africa | PCV7 (2 + 1) | 2009 | PCV13 (2 + 1) | 2011 | 2009–2019 | 6A, 6B, 6C, 9N, 9V, 19A, 19F, 23B, 23F |
| Argentina | PCV13 (2 + 1) | 2012 | - | - | 2006–2018 | 6A, 6B, 6C, 19A, 19F |
| Serogroup | Serotypes | PCV7 | PCV10 | PCV13 |
|---|---|---|---|---|
| 6 | 6A | VRT | VRT | VT |
| 6B | VT | VT | VT | |
| 6C | VRT | VRT | VRT | |
| 9 | 9N | VRT | VRT | VRT |
| 9V | VT | VT | VT | |
| 19 | 19A | VRT | VRT | VT |
| 19F | VT | VT | VT | |
| 23 | 23A | VRT | VRT | VRT |
| 23B | VRT | VRT | VRT | |
| 23F | VT | VT | VT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apodaca, K.; Grant, L.R.; Perdrizet, J.; Daigle, D.; Mircus, G.; Gessner, B.D. Real-World Impact of Pneumococcal Conjugate Vaccines on Vaccine Serotypes and Potential Cross-Reacting Non-Vaccine Serotypes. Vaccines 2025, 13, 651. https://doi.org/10.3390/vaccines13060651
Apodaca K, Grant LR, Perdrizet J, Daigle D, Mircus G, Gessner BD. Real-World Impact of Pneumococcal Conjugate Vaccines on Vaccine Serotypes and Potential Cross-Reacting Non-Vaccine Serotypes. Vaccines. 2025; 13(6):651. https://doi.org/10.3390/vaccines13060651
Chicago/Turabian StyleApodaca, Kevin, Lindsay R. Grant, Johnna Perdrizet, Derek Daigle, Gabriel Mircus, and Bradford D. Gessner. 2025. "Real-World Impact of Pneumococcal Conjugate Vaccines on Vaccine Serotypes and Potential Cross-Reacting Non-Vaccine Serotypes" Vaccines 13, no. 6: 651. https://doi.org/10.3390/vaccines13060651
APA StyleApodaca, K., Grant, L. R., Perdrizet, J., Daigle, D., Mircus, G., & Gessner, B. D. (2025). Real-World Impact of Pneumococcal Conjugate Vaccines on Vaccine Serotypes and Potential Cross-Reacting Non-Vaccine Serotypes. Vaccines, 13(6), 651. https://doi.org/10.3390/vaccines13060651







