Antibody Kinetics of Immunological Memory in SARS-CoV-2-Vaccinated Healthcare Workers—The ORCHESTRA Project
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
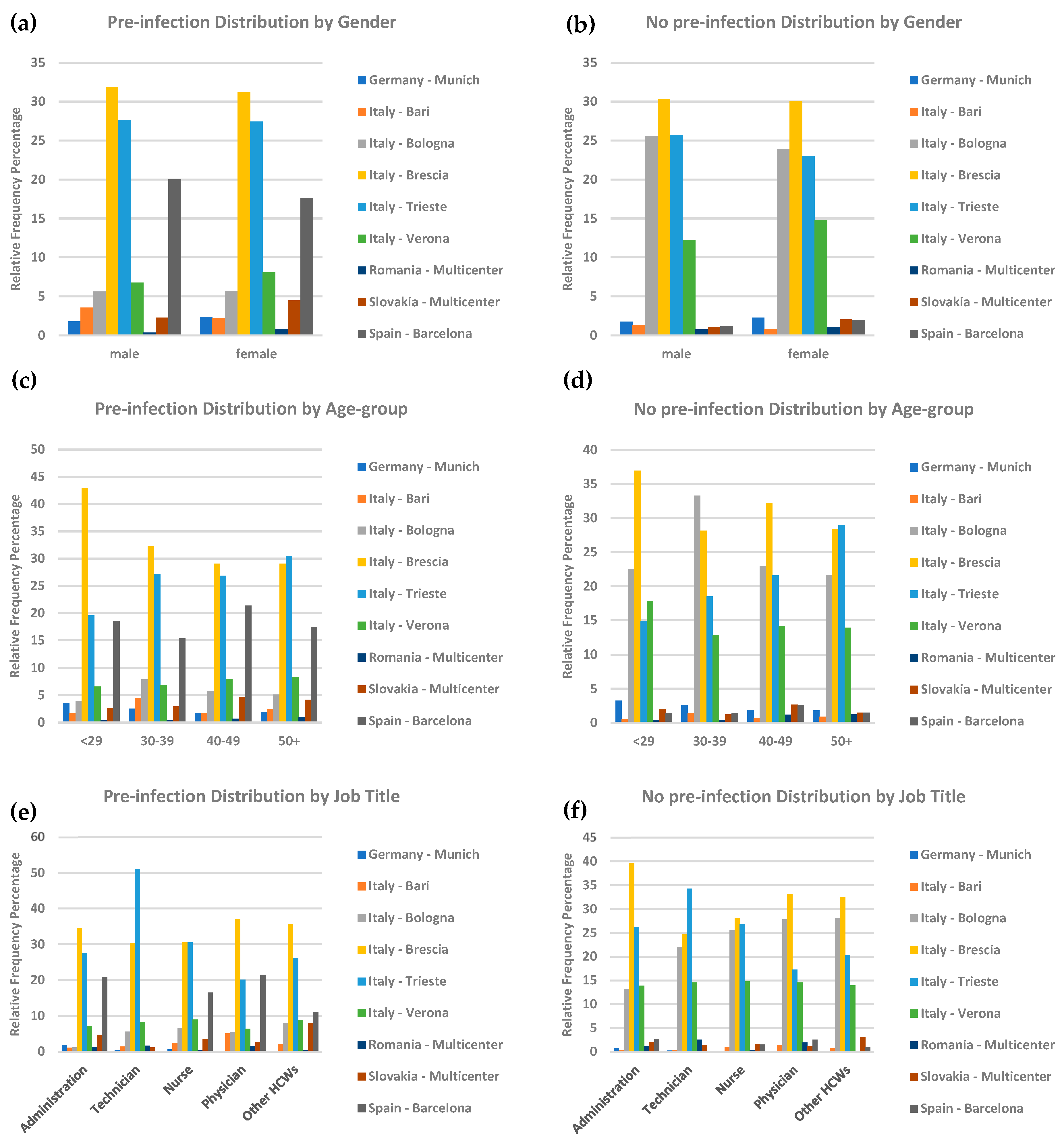
3.1. Participants Characteristics
3.2. Exploring the Serological Response to Vaccination
3.2.1. Unadjusted Response Assessment
3.2.2. Adjusted Response Analysis
3.3. Vaccine-Associated COVID-19 Occurrence
3.3.1. Unadjusted COVID-19 Occurrence
3.3.2. Adjusted COVID-19 Occurrence by Demographics
4. Discussion
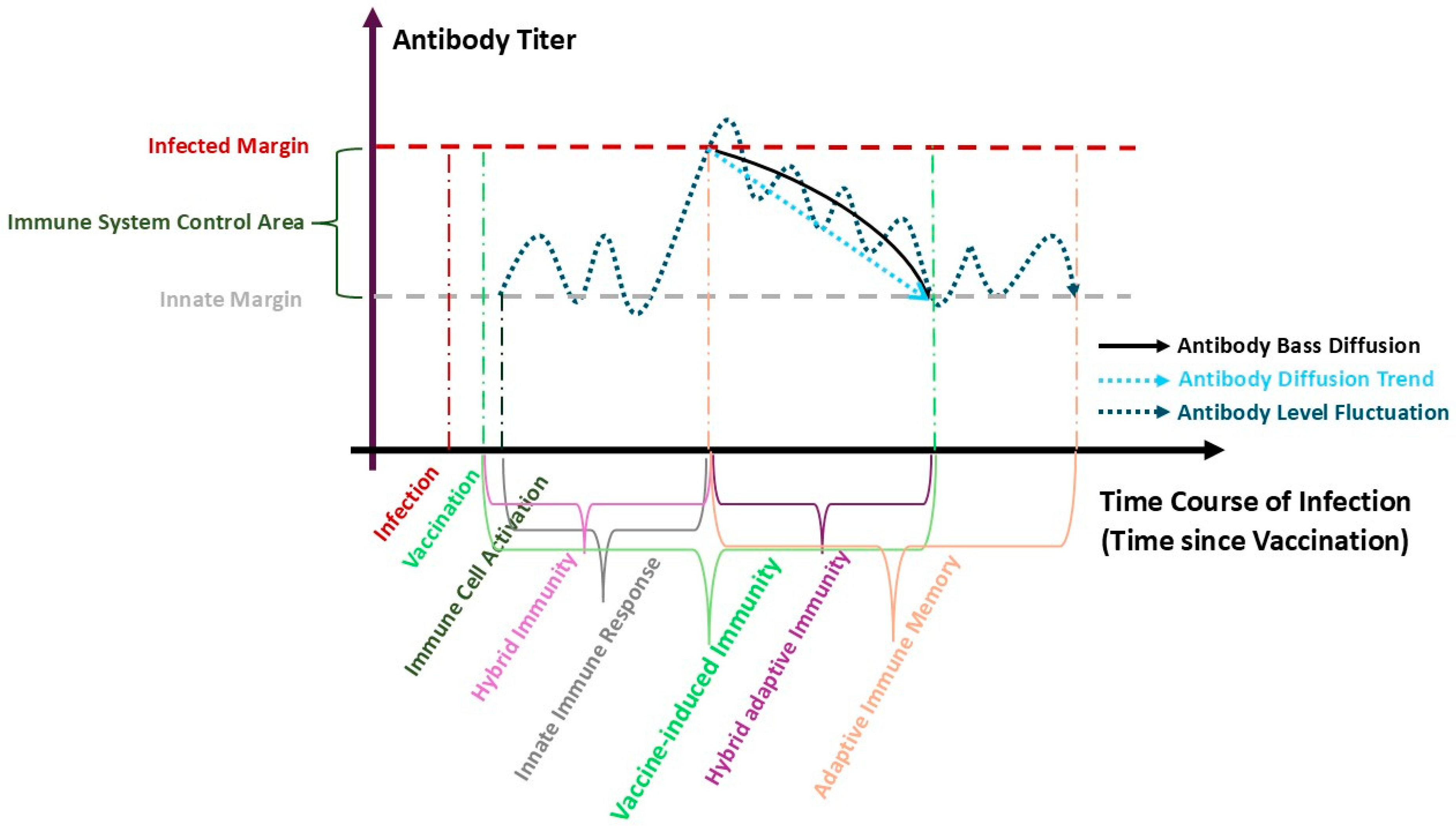
4.1. Antibody Kinetics and Immune Response
4.2. Study Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wietschel, K.A.; Fechtner, K.; Antileo, E.; Abdurrahman, G.; Drechsler, C.A.; Makuvise, M.K.; Rose, R.; Voß, M.; Krumbholz, A.; Michalik, S.; et al. Non-cross-reactive epitopes dominate the humoral immune response to COVID-19 vaccination–kinetics of plasma antibodies, plasmablasts and memory B cells. Front. Immunol. 2024, 15, 1382911. [Google Scholar] [CrossRef]
- Srivastava, K.; Carreño, J.M.; Gleason, C.; Monahan, B.; Singh, G.; Abbad, A.; Tcheou, J.; Raskin, A.; Kleiner, G.; van Bakel, H.; et al. SARS-CoV-2-infection-and vaccine-induced antibody responses are long lasting with an initial waning phase followed by a stabilization phase. Immunity 2024, 57, 587–599. [Google Scholar] [CrossRef]
- Van Elslande, J.; Oyaert, M.; Ailliet, S.; Van Ranst, M.; Lorent, N.; Weygaerde, Y.V.; André, E.; Lagrou, K.; Vandendriessche, S.; Vermeersch, P. Longitudinal follow-up of IgG anti-nucleocapsid antibodies in SARS-CoV-2 infected patients up to eight months after infection. J. Clin. Virol. 2021, 136, 104765. [Google Scholar] [CrossRef]
- Krammer, F.; Simon, V. Serology assays to manage COVID-19. Science 2020, 368, 1060–1061. [Google Scholar] [CrossRef] [PubMed]
- Campo, F.; Venuti, A.; Pimpinelli, F.; Abril, E.; Blandino, G.; Conti, L.; De Virgilio, A.; De Marco, F.; Di Noia, V.; Di Domenico, E.G.; et al. Antibody persistence 6 months post-vaccination with BNT162b2 among health care workers. Vaccines 2021, 9, 1125. [Google Scholar] [CrossRef] [PubMed]
- Violán, C.; Torán-Monserrat, P.; Quirant, B.; Lamonja-Vicente, N.; Carrasco-Ribelles, L.A.; Chacón, C.; Manresa-Dominguez, J.; Josep, M.; Ramos-Roure, F.; Dacosta-Aguayo, R.; et al. Kinetics of humoral immune response over 17 months of COVID-19 pandemic in a large cohort of healthcare workers in Spain: The ProHEpiC-19 study. BMC Infect. Dis. 2022, 22, 721. [Google Scholar] [CrossRef] [PubMed]
- Gilboa, M.; Regev-Yochay, G.; Mandelboim, M.; Indenbaum, V.; Asraf, K.; Fluss, R.; Amit, S.; Mendelson, E.; Doolman, R.; Afek, A.; et al. Durability of immune response after COVID-19 booster vaccination and association with COVID-19 omicron infection. JAMA Netw. Open 2022, 5, e2231778. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Lai, L.; Samaha, H.; Feng, Y.; Hu, M.; Hui, H.S.-Y.; Wali, B.; Ellis, M.; Davis-Gardner, M.E.; Huerta, C.; et al. Durability of immune responses to mRNA booster vaccination against COVID-19. J. Clin. Investig. 2023, 133, e167955. [Google Scholar] [CrossRef]
- Stringhini, S.; Wisniak, A.; Piumatti, G.; Azman, A.S.; Lauer, S.A.; Baysson, H.; De Ridder, D.; Petrovic, D.; Schrempft, S.; Marcus, K.; et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): A population-based study. Lancet 2020, 396, 313–319. [Google Scholar] [CrossRef]
- Richard, A.; Wisniak, A.; Perez-Saez, J.; Garrison-Desany, H.; Petrovic, D.; Piumatti, G.; Baysson, H.; Picazio, A.; Pennacchio, F.; De Ridder, D.; et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies, risk factors for infection and associated symptoms in Geneva, Switzerland: A population-based study. Scand. J. Public Health 2022, 50, 124–135. [Google Scholar] [CrossRef]
- Bass, F.M. A new product growth for model consumer durables. Manag. Sci. 1969, 15, 215–227. [Google Scholar] [CrossRef]
- Sansone, E.; Collatuzzo, G.; Renzetti, S.; Ditano, G.; Bonfanti, C.; Sala, E.; Terlenghi, L.; Matteelli, A.; Abedini, M.; Asafo, S.S.; et al. The effect of the immunization schedule and antibody levels (Anti-S) on the risk of SARS-CoV-2 infection in a large cohort of healthcare workers in Northern Italy. Vaccines 2023, 11, 746. [Google Scholar] [CrossRef] [PubMed]
- Violán, C.; Carrasco-Ribelles, L.A.; Collatuzzo, G.; Ditano, G.; Abedini, M.; Janke, C.; Reinkemeyer, C.; Giang, L.T.T.; Liviero, F.; Scapellato, M.L.; et al. Multimorbidity and serological response to SARS-CoV-2 nine months after 1st vaccine dose: European cohort of healthcare workers—Orchestra project. Vaccines 2023, 11, 1340. [Google Scholar] [CrossRef]
- Collatuzzo, G.; De Palma, G.; Violante, F.S.; Porru, S.; Filon, F.L.; Fabianova, E.; Violán, C.; Vimercati, L.; Leustean, M.; Rodriguez-Suarez, M.M.; et al. Temporal trends of COVID-19 antibodies in vaccinated healthcare workers undergoing repeated serological sampling: An individual-level analysis within 13 months in the ORCHESTRA cohort. Front. Immunol. 2023, 13, 1079884. [Google Scholar] [CrossRef]
- Collatuzzo, G.; Lodi, V.; Feola, D.; De Palma, G.; Sansone, E.; Sala, E.; Janke, C.; Castelletti, N.; Porru, S.; Spiteri, G.; et al. Determinants of Anti-S Immune Response at 9 Months after COVID-19 Vaccination in a Multicentric European Cohort of Healthcare Workers—ORCHESTRA Project. Viruses 2022, 14, 2657. [Google Scholar] [CrossRef]
- Collatuzzo, G.; Visci, G.; Violante, F.S.; Porru, S.; Spiteri, G.; Monaco, M.G.L.; Fillon, F.L.; Negro, C.; Janke, C.; Castelletti, N.; et al. Determinants of anti-S immune response at 6 months after COVID-19 vaccination in a multicentric European cohort of healthcare workers–ORCHESTRA project. Front. Immunol. 2022, 13, 986085. [Google Scholar] [CrossRef]
- Leomanni, L.; Collatuzzo, G.; Sansone, E.; Sala, E.; De Palma, G.; Porru, S.; Spiteri, G.; Monaco, M.G.L.; Basso, D.; Pavanello, S.; et al. Determinants of Anti-S Immune Response at 12 Months after SARS-CoV-2 Vaccination in a Multicentric European Cohort of Healthcare Workers—ORCHESTRA Project. Vaccines 2023, 11, 1527. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lei, Z. Review of Advances in Active Impulsive Noise Control with Focus on Adaptive Algorithms. Appl. Sci. 2024, 14, 1218. [Google Scholar] [CrossRef]
- Reinkemeyer, C.; Khazaei, Y.; Weigert, M.; Hannes, M.; Le Gleut, R.; Plank, M.; Winter, S.; Noreña, I.; Meier, T.; Xu, L.; et al. The Prospective COVID-19 Post-Immunization Serological Cohort in Munich (KoCo-Impf): Risk Factors and Determinants of Immune Response in Healthcare Workers. Viruses 2023, 15, 1574. [Google Scholar] [CrossRef]
- Rahmandad, H.; Sterman, J. Heterogeneity and network structure in the dynamics of diffusion: Comparing agent-based and differential equation models. Manag. Sci. 2008, 54, 998–1014. [Google Scholar] [CrossRef]
- Norton, J.A.; Bass, F.M. A diffusion theory model of adoption and substitution for successive generations of high-technology products. Manag. Sci. 1987, 33, 1069–1086. [Google Scholar] [CrossRef]
- Dunn, A.G.; Braithwaite, J.; Gallego, B.; Day, R.O.; Runciman, W.; Coiera, E. Nation-scale adoption of new medicines by doctors: An application of the Bass diffusion model. BMC Health Serv. Res. 2012, 12, 248. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.H.; Hashim, M.A. Can the Bass innovation diffusion model describe adsorption breakthrough curves of pharmaceutical contaminants? Green Chem. Eng. 2024, 5, 145–149. [Google Scholar] [CrossRef]
- Duggan, J. Implementing a Metapopulation Bass Diffusion Model using the R Package deSolve. R J. 2017, 9, 153. [Google Scholar] [CrossRef]
- Vynnycky, E.; White, R. An Introduction to Infectious Disease Modelling; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Eryarsoy, E.; Delen, D.; Davazdahemami, B.; Topuz, K. A novel diffusion-based model for estimating cases, and fatalities in epidemics: The case of COVID-19. J. Bus. Res. 2021, 124, 163–178. [Google Scholar] [CrossRef]
- Škraba, A.; Vavtar, B.; Stanovov, V.; Semenkin, E.; Stojanović, R. Parametrization of Bass diffusion model on COVID-19 first wave data. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1047, 012084. [Google Scholar] [CrossRef]
- Stanovov, V.; Grabljevec, S.; Akhmedova, S.; Semenkin, E.; Stojanović, R.; Rozman, Č.; Škraba, A. Identification of COVID-19 spread mechanisms based on first-wave data, simulation models, and evolutionary algorithms. PLoS ONE 2022, 17, e0279427. [Google Scholar] [CrossRef]
- Sultan, F.; Farley, J.U.; Lehmann, D.R. A meta-analysis of applications of diffusion models. J. Mark. Res. 1990, 27, 70–77. [Google Scholar] [CrossRef]
- Shmueli, G. To explain or to predict? Stat. Sci. 2010, 25, 289–310. [Google Scholar] [CrossRef]
- Kutner, M.H.; Nachtsheim, C.J.; Neter, J.; Li, W. Applied Linear Statistical Models; McGraw-Hill: New York, NY, USA, 2005. [Google Scholar]
- Meade, N.; Islam, T. Modelling and forecasting the diffusion of innovation–A 25-year review. Int. J. Forecast. 2006, 22, 519–545. [Google Scholar] [CrossRef]
- Chen, X.; Bailleux, F.; Desai, K.; Qin, L.; Dunning, A.J. A threshold method for immunological correlates of protection. BMC Med. Res. Methodol. 2013, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, P.; Vaibhav, K.; Ahluwalia, M.; Mondal, A.K.; Sahajpal, N.; Rojiani, A.M.; Kolhe, R. Infection and immune memory: Variables in robust protection by vaccines against SARS-CoV-2. Front. Immunol. 2021, 12, 660019. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, T.; Takano, T.; Takahashi, Y. Immune responses related to the immunogenicity and reactogenicity of COVID-19 mRNA vaccines. Int. Immunol. 2023, 35, 213–220. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Pooley, N.; Karim, S.S.A.; Combadière, B.; Ooi, E.E.; Harris, R.C.; Seblain, C.E.G.; Kisomi, M.; Shaikh, N. Durability of vaccine-induced and natural immunity against COVID-19: A narrative review. Infect. Dis. Ther. 2023, 12, 367–387. [Google Scholar] [CrossRef]
- Tu, M.K.; Chiang, S.H.; Bender, R.A.; Wong, D.T.; Strom, C.M. The kinetics of COVID-19 vaccine response in a community-vaccinated population. J. Immunol. 2022, 208, 819–826. [Google Scholar] [CrossRef]
- Jay, C.; Adland, E.; Csala, A.; Lim, N.; Longet, S.; Ogbe, A.; Ratcliff, J.; Sampson, O.; Thompson, C.P.; Turtle, L.; et al. Age-and sex-specific differences in immune responses to BNT162b2 COVID-19 and live-attenuated influenza vaccines in UK adolescents. Front. Immunol. 2023, 14, 1248630. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.A.C.; Jepson, B.; Bhorat, Q.E.; Ahmad, A.; Akhund, T.; Aley, P.K.; Parvinder, K.; Bansal, H.; Bibi, S.; Kelly, E.I.; et al. Immunogenicity and safety of beta variant COVID-19 vaccine AZD2816 and AZD1222 (ChAdOx1 nCoV-19) as primary-series vaccination for previously unvaccinated adults in Brazil, South Africa, Poland, and the UK: A randomised, partly double-blinded, phase 2/3 non-inferiority immunobridging study. Lancet Microbe 2024, 5, 100863. [Google Scholar]
- Falsey, A.R.; Frenck Jr, R.W.; Walsh, E.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Bailey, R.; Swanson, K.A.; Xu, X.; et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N. Engl. J. Med. 2021, 385, 1627–1629. [Google Scholar] [CrossRef]
- Xia, H.; Zou, J.; Kurhade, C.; Cai, H.; Yang, Q.; Cutler, M.; Cooper, D.; Muik, A.; Jansen, K.U.; Xie, X.; et al. Neutralization and durability of 2 or 3 doses of the BNT162b2 vaccine against Omicron SARS-CoV-2. Cell Host Microbe 2022, 30, 485–488. [Google Scholar] [CrossRef]
- Regev-Yochay, G.; Gonen, T.; Gilboa, M.; Mandelboim, M.; Indenbaum, V.; Amit, S.; Meltzer, L.; Asraf, K.; Cohen, C.; Fluss, R.; et al. Efficacy of a fourth dose of COVID-19 mRNA vaccine against Omicron. N. Engl. J. Med. 2022, 386, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
| Between Dose 1–Dose 2 (N = 2138) | Between Dose 2–Dose 3 (N = 28,426) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-Pre-Infected | Pre-Infected | Non-Pre-Infected | Pre-Infected | ||||||
| Effects Predominant | Immunity Type | Effects Predominant | Immunity Type | Effects Predominant | Immunity Type | Effects Predominant | Immunity Type | ||
| Unadjusted | Internal Factors | Adaptive (N = 54) | External Factors | Hybrid (N = 1011) | External Factors | Vaccine Induced (N = 8214) | Internal Factors | Hybrid Adaptive (N = 5332) | |
| Gender | male | Internal Factors | Adaptive (N = 15) | External Factors | Hybrid (N = 260) | External Factors | Vaccine Induced (N = 2132) | External Factors | Hybrid (N = 1411) |
| female | Internal Factors | Adaptive (N = 39) | External Factors | Hybrid (N = 751) | Internal Factors | Adaptive (N = 6082) | External Factors | Hybrid (N = 3921) | |
| Age-group | age ≤ 29 | Internal Factors | Adaptive (N = 6) | External Factors | Hybrid (N = 113) | External Factors | Vaccine Induced (N = 1061) | External Factors | Hybrid (N = 807) |
| 30 ≤ age ≤ 39 | Internal Factors | Adaptive (N = 9) | External Factors | Hybrid (N = 161) | Internal Factors | Adaptive (N = 1586) | External Factors | Hybrid (N = 1025) | |
| 40 ≤ age ≤ 49 | Internal Factors | Adaptive (N = 15) | External Factors | Hybrid (N = 292) | External Factors | Vaccine Induced (N = 2052) | External Factors | Hybrid (N = 1393) | |
| 50 ≤ age | External Factors | Vaccine Induced (N = 24) | External Factors | Hybrid (N = 445) | External Factors | Vaccine Induced (N = 3514) | External Factors | Hybrid (N = 2107) | |
| Job title | administration | Internal Factors | Adaptive (N = 6) | External Factors | Hybrid (N = 85) | External Factors | Vaccine Induced (N = 1022) | External Factors | Hybrid (N = 452) |
| technician | Not Detected | Not Detected | Internal Factors | Hybrid Adaptive (N = 70) | Not Detected | Not Detected | External Factors | Hybrid (N = 384) | |
| nurse | External Factors | Vaccine Induced (N = 12) | External Factors | Hybrid (N = 434) | External Factors | Vaccine Induced (N = 2698) | External Factors | Hybrid (N = 2253) | |
| physician | Internal Factors | Adaptive (N = 14) | External Factors | Hybrid (N = 166) | External Factors | Vaccine Induced (N = 2002) | External Factors | Hybrid (N = 1101) | |
| other HCWs | Internal Factors | Adaptive (N = 15) | External Factors | Hybrid (N = 195) | Internal Factors | Adaptive (N = 1498) | External Factors | Hybrid (N = 1063) | |
| Between Dose 3–Dose 4 (N = 12,222) | After Dose 4 (N = 128) | ||||||||
| Non-Pre-Infected | Pre-Infected | Non-Pre-Infected | Pre-Infected | ||||||
| Effects Predominant | Immunity Type | Effects Predominant | Immunity Type | Effects Predominant | Immunity Type | Effects Predominant | Immunity Type | ||
| Unadjusted | External Factors | Vaccine Induced (N = 3312) | Internal Factors | Hybrid Adaptive (N = 5232) | Internal Factors | Adaptive (N = 11) | Internal Factors | Hybrid Adaptive (N = 67) | |
| Gender | male | External Factors | Vaccine Induced (N = 822) | External Factors | Hybrid (N = 1278) | Internal Factors | Adaptive (N = 2) | Internal Factors | Hybrid Adaptive (N = 29) |
| female | External Factors | Vaccine Induced (N = 2490) | External Factors | Hybrid (N = 3954) | Internal Factors | Adaptive (N = 9) | Internal Factors | Hybrid Adaptive (N = 38) | |
| Age-group | age ≤ 29 | Internal Factors | Adaptive (N = 403) | External Factors | Hybrid (N = 610) | Not Detected | Not Detected | Internal Factors | Hybrid Adaptive (N = 4) |
| 30 ≤ age ≤ 39 | Internal Factors | Adaptive (N = 541) | External Factors | Hybrid (N = 1000) | Internal Factors | Adaptive (N = 2) | Internal Factors | Hybrid Adaptive (N = 9) | |
| 40 ≤ age ≤ 49 | External Factors | Vaccine Induced (N = 855) | External Factors | Hybrid (N = 1410) | Internal Factors | Adaptive (N = 3) | Internal Factors | Hybrid Adaptive (N = 10) | |
| 50 ≤ age | External Factors | Vaccine Induced (N = 1513) | External Factors | Hybrid (N = 2211) | Internal Factors | Adaptive (N = 6) | External Factors | Hybrid (N = 44) | |
| Job title | administration | External Factors | Vaccine Induced (N = 424) | External Factors | Hybrid (N = 471) | Internal Factors | Adaptive (N = 1) | Internal Factors | Hybrid Adaptive (N = 4) |
| technician | Internal Factors | Adaptive (N = 256) | Internal Factors | Hybrid Adaptive (N = 517) | Not Detected | Not Detected | Internal Factors | Hybrid Adaptive (N = 7) | |
| nurse | External Factors | Vaccine Induced (N = 1043) | External Factors | Hybrid (N = 2202) | Internal Factors | Adaptive (N = 1) | External Factors | Hybrid (N = 22) | |
| physician | Internal Factors | Adaptive (N = 636) | External Factors | Hybrid (N = 937) | Internal Factors | Adaptive (N = 2) | External Factors | Hybrid (N = 22) | |
| other HCWs | Internal Factors | Adaptive (N = 532) | External Factors | Hybrid (N = 950) | Not Detected | Not Detected | Internal Factors | Hybrid Adaptive (N = 7) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seyedi, S.; Sottile, S.; Abedini, M.; Boffetta, P.; Violante, F.S.; Lodi, V.; De Palma, G.; Sala, E.; Mauro, M.; Rui, F.; et al. Antibody Kinetics of Immunological Memory in SARS-CoV-2-Vaccinated Healthcare Workers—The ORCHESTRA Project. Vaccines 2025, 13, 611. https://doi.org/10.3390/vaccines13060611
Seyedi S, Sottile S, Abedini M, Boffetta P, Violante FS, Lodi V, De Palma G, Sala E, Mauro M, Rui F, et al. Antibody Kinetics of Immunological Memory in SARS-CoV-2-Vaccinated Healthcare Workers—The ORCHESTRA Project. Vaccines. 2025; 13(6):611. https://doi.org/10.3390/vaccines13060611
Chicago/Turabian StyleSeyedi, Seyedalireza, Sara Sottile, Mahsa Abedini, Paolo Boffetta, Francesco Saverio Violante, Vittorio Lodi, Giuseppe De Palma, Emma Sala, Marcella Mauro, Francesca Rui, and et al. 2025. "Antibody Kinetics of Immunological Memory in SARS-CoV-2-Vaccinated Healthcare Workers—The ORCHESTRA Project" Vaccines 13, no. 6: 611. https://doi.org/10.3390/vaccines13060611
APA StyleSeyedi, S., Sottile, S., Abedini, M., Boffetta, P., Violante, F. S., Lodi, V., De Palma, G., Sala, E., Mauro, M., Rui, F., Porru, S., Spiteri, G., Vimercati, L., De Maria, L., Toran-Monserrat, P., Violán, C., Fabiánová, E., Oravec Bérešová, J., Calota, V., & Neamtu, A. (2025). Antibody Kinetics of Immunological Memory in SARS-CoV-2-Vaccinated Healthcare Workers—The ORCHESTRA Project. Vaccines, 13(6), 611. https://doi.org/10.3390/vaccines13060611










