The Cost-Effectiveness of the Human Papilloma Virus Vaccination in Asia Pacific Countries: What Lessons Can Indonesia Learn?—A Systematic Review
Abstract
1. Introduction
- What types of HPV vaccines are most cost-effective across different economic settings?
- Is gender-neutral vaccination consistently found to be cost-effective across regions?
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
2.3. Data Sources and Search Strategy
2.4. Data Selection
2.5. Data Collection Process
2.6. Quality Assessment
2.7. Data Synthesis
3. Results
4. Discussion
4.1. Perspective for Clinical Practice
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPV | Human Papillomavirus |
| WHO | World Health Organization |
| HIC | High-income country |
| UMIC | Upper-middle-income country |
| LMIC | Low-middle-income country |
| NIP | National Immunization Program |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| CHEERS | Consolidated Health Economic Evaluation Reporting Standards |
| PRIME | Preventable Risk Integrated Model |
| UNIVAC | Universal Vaccine |
| GDP | Gross Domestic Product |
| WTP | Willingness-to-pay |
| QALYs | Quality-adjusted Life Years |
| DALYs | Disability-adjusted Life Years |
| YLS | Years of Life Saved |
| 2vHPV | Bivalent HPV Vaccine |
| 4vHPV | Quadrivalent HPV Vaccine |
| 9vHPV | Nonavalent HPV Vaccine |
| MCSM | Monte Carlo Simulation Models |
| MCMC | Markov Chain Monte Carlo Models |
| CTN | Continuous Time Models |
| HS | Health System |
| Soc | Societal |
| VIA | Visual Inspection with Acetic Acid |
| ICER | Incremental Cost-Effectiveness Ratio |
| DNA | Deoxyribonucleic Acid |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global estimates of incidence and mortality of cervical cancer in 2020: A baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 2023, 11, e197–e206. [Google Scholar] [CrossRef] [PubMed]
- WHO. Regional Implementation Framework for Elimination of Cervical Cancer as a Public Health Problem: 2021–2030; World Health Organization. Regional Office for South-East Asia: New Delhi, India, 2021. [Google Scholar]
- United Nations Department of Economic and Social Affairs. Population Division. World Population Prospects 2024. 2024. Available online: https://population.un.org/wpp/ (accessed on 23 May 2025).
- Bruni, L.A.G.; Serrano, B.; Mena, M.; Collado, J.J.; Gómez, D.; Muñoz, J.; Bosch, F.X.; de Sanjosé, S. Human Papillomavirus and Related Diseases in Asia. 2023. Available online: https://hpvcentre.net/statistics/reports/XSX.pdf (accessed on 15 April 2024).
- Ferlay, J.E.M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. 360 Indonesia Fact Sheet. 2024. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/360-indonesia-fact-sheet.pdf (accessed on 8 April 2024).
- Okunade, K.S. Human papillomavirus and cervical cancer. J. Obs. Gynaecol. 2020, 40, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, M.; Castle, P.E.; Jeronimo, J.; Rodriguez, A.C.; Wacholder, S. Human papillomavirus and cervical cancer. Lancet 2007, 370, 890–907. [Google Scholar] [CrossRef]
- Wild, C.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- WHO. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- WHO. Human papillomavirus vaccines: WHO position paper. Wkly. Epidemiol. Rec. 2022, 50, 645–672. [Google Scholar]
- Brisson, M.; Kim, J.J.; Canfell, K.; Drolet, M.; Gingras, G.; Burger, E.A.; Martin, D.; Simms, K.T.; Bénard, É.; Boily, M.C.; et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: A comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet 2020, 395, 575–590. [Google Scholar] [CrossRef]
- WHO. Introduction of HPV (Human Papillomavirus Vaccine); WHO: Geneva, Switzerland, 2024; Available online: https://www.who.int/teams/immunization-vaccines-and-biologicals/diseases/human-papillomavirus-vaccines-(HPV)/hpv-clearing-house/hpv-dashboard (accessed on 15 April 2024).
- ACPCC. National Strategy for the Elimination of Cervical Cancer in Australia; ACPCC: Carlton, Australia, 2023. [Google Scholar]
- WHO. Human Papillomavirus (HPV) Vaccination Coverage: Indonesia; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- WHO. National Launch of Human Papillomavirus (HPV) Immunization Expansion; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Cangelosi, G.; Sacchini, F.; Mancin, S.; Petrelli, F.; Amendola, A.; Fappani, C.; Sguanci, M.; Morales Palomares, S.; Gravante, F.; Caggianelli, G. Papillomavirus Vaccination Programs and Knowledge Gaps as Barriers to Implementation: A Systematic Review. Vaccines 2025, 13, 460. [Google Scholar] [CrossRef]
- Winarto, H.; Habiburrahman, M.; Dorothea, M.; Wijaya, A.; Nuryanto, K.H.; Kusuma, F.; Utami, T.W.; Anggraeni, T.D. Knowledge, attitudes, and practices among Indonesian urban communities regarding HPV infection, cervical cancer, and HPV vaccination. PLoS ONE 2022, 17, e0266139. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- The World by Income and Region. Available online: https://datatopics.worldbank.org/world-development-indicators/the-world-by-income-and-region.html (accessed on 20 June 2023).
- Husereau, D.; Drummond, M.; Augustovski, F.; de Bekker-Grob, E.; Briggs, A.H.; Carswell, C.; Caulley, L.; Chaiyakunapruk, N.; Greenberg, D.; Loder, E.; et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Statement: Updated Reporting Guidance for Health Economic Evaluations. Value Health 2022, 25, 3–9. [Google Scholar] [CrossRef]
- Jiang, Y.; Ni, W.; Wu, J. Cost-effectiveness and value-based prices of the 9-valent human papillomavirus vaccine for the prevention of cervical cancer in China: An economic modelling analysis. BMJ Open 2019, 9, e031186. [Google Scholar] [CrossRef] [PubMed]
- Levin, C.E.; Sharma, M.; Olson, Z.; Verguet, S.; Shi, J.-F.; Wang, S.-M.; Qiao, Y.-L.; Jamison, D.T.; Kim, J.J. An extended cost-effectiveness analysis of publicly financed HPV vaccination to prevent cervical cancer in China. Vaccine 2015, 33, 2830–2841. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Zhang, Q.; Hu, S.Y.; Zhao, F.H. Effect of vaccination age on cost-effectiveness of human papillomavirus vaccination against cervical cancer in China. BMC Cancer 2016, 16, 164. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; He, H.; Tang, X.; Wang, S.; Zhang, J.; Wu, T.; Chen, Z. Cost-effectiveness of 2-dose human papillomavirus vaccination for 12-year-old girls in Zhejiang Province: Implications for China’s expanded program on immunization. Hum. Vaccines Immunother. 2020, 16, 1623–1629. [Google Scholar] [CrossRef]
- Ma, X.; Harripersaud, K.; Smith, K.; Fairley, C.K.; Zou, H.; Zou, Z.; Wang, Y.; Zhuang, G.; Zhang, L. Modeling the epidemiological impact and cost-effectiveness of a combined schoolgirl HPV vaccination and cervical cancer screening program among Chinese women. Hum. Vaccines Immunother. 2021, 17, 1073–1082. [Google Scholar] [CrossRef]
- Mo, X.; Gai Tobe, R.; Wang, L.; Liu, X.; Wu, B.; Luo, H.; Nagata, C.; Mori, R.; Nakayama, T. Cost-effectiveness analysis of different types of human papillomavirus vaccination combined with a cervical cancer screening program in mainland China. BMC Infect. Dis. 2017, 17, 502. [Google Scholar] [CrossRef]
- Phua, L.C.; Choi, H.C.W.; Wu, J.; Jit, M.; Low, J.; Ng, K.; Pearce, F.; Hall, C.; Abdul Aziz, M.I. Cost-effectiveness analysis of the nonavalent human papillomavirus vaccine for the prevention of cervical cancer in Singapore. Vaccine 2021, 39, 2255–2263. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.J.; Hu, S.Y.; Zhao, F.H. Estimating long-term clinical effectiveness and cost-effectiveness of HPV 16/18 vaccine in China. BMC Cancer 2016, 16, 848. [Google Scholar] [CrossRef]
- Zhou, L.; Gu, B.; Wang, J.; Liu, G.; Zhang, X. Human papillomavirus vaccination at the national and provincial levels in China: A cost-effectiveness analysis using the PRIME model. BMC Public Health 2022, 22, 777. [Google Scholar] [CrossRef]
- Zou, Z.; Fairley, C.K.; Ong, J.J.; Hocking, J.; Canfell, K.; Ma, X.; Chow, E.P.F.; Xu, X.; Zhang, L.; Zhuang, G. Domestic HPV vaccine price and economic returns for cervical cancer prevention in China: A cost-effectiveness analysis. Lancet Glob. Health 2020, 8, e1335–e1344. [Google Scholar] [CrossRef]
- Lee, V.J.; Tay, S.K.; Teoh, Y.L.; Tok, M.Y. Cost-effectiveness of different human papillomavirus vaccines in Singapore. BMC Public Health 2011, 11, 203. [Google Scholar] [CrossRef] [PubMed]
- Tay, S.K.; Hsu, T.Y.; Pavelyev, A.; Walia, A.; Kulkarni, A.S. Clinical and economic impact of school-based nonavalent human papillomavirus vaccine on women in Singapore: A transmission dynamic mathematical model analysis. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Tay, S.K.; Hsu, T.Y.; Shcheprov, A.; Walia, A.; Kulkarni, A.S. The clinical and economic benefits of school-based quadrivalent HPV vaccination in Singapore. Int. J. Gynecol. Obstet. 2017, 137, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Tay, S.K.; Lee, B.W.; Sohn, W.Y.; Lee, I.H.; Mathur, G.; Sanicas, M.; Van Kriekinge, G. Cost-effectiveness of two-dose human papillomavirus vaccination in Singapore. Singap. Med. J. 2018, 59, 370–382. [Google Scholar] [CrossRef]
- Wahab, M.T.; Tan, R.K.J.; Cook, A.R.; Prem, K. Impact of including boys in the national school-based human papillomavirus vaccination programme in Singapore: A modelling-based cost-effectiveness analysis. Vaccine 2023, 41, 1934–1942. [Google Scholar] [CrossRef]
- Cody, P.; Tobe, K.; Abe, M.; Elbasha, E.H. Public health impact and cost effectiveness of routine and catch-up vaccination of girls and women with a nine-valent HPV vaccine in Japan: A model-based study. BMC Infect. Dis. 2021, 21, 11. [Google Scholar] [CrossRef]
- Connelly, L.B.; Le, H.N.D. Cost-effectiveness of a bivalent human papillomavirus vaccination program in Japan. Sex. Health 2015, 12, 520–531. [Google Scholar] [CrossRef]
- Konno, R.; Sasagawa, T.; Fukuda, T.; Van Kriekinge, G.; Demarteau, N. Cost-effectiveness analysis of prophylactic cervical cancer vaccination in Japanese women. Int. J. Gynecol. Cancer 2010, 20, 385–392. [Google Scholar] [CrossRef]
- Yamabe, K.; Singhal, P.K.; Abe, M.; Dasbach, E.J.; Elbasha, E.H. The cost-effectiveness analysis of a quadrivalent human papillomavirus vaccine (6/11/16/18) for females in Japan. Value Health Reg. Issues 2013, 2, 92–97. [Google Scholar] [CrossRef][Green Version]
- Yamamoto, N.; Mori, R.; Jacklin, P.; Osuga, Y.; Kawana, K.; Shibuya, K.; Taketani, Y. Introducing HPV vaccine and scaling up screening procedures to prevent deaths from cervical cancer in Japan: A cost-effectiveness analysis. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 177–186. [Google Scholar] [CrossRef]
- Chou, H.H.; Chang, S.C.; Sukarom, I.; Saxena, K.; Pavelyev, A.; Wu, Y.H.; Chang, C.J. The Clinical and Economic Impact of a Nonavalent Versus Bivalent Human Papillomavirus National Vaccination Program in Taiwan. Value Health Reg. Issues 2022, 32, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Dasbach, E.J.; Insinga, R.P.; Yang, Y.C.; Pwu, R.F.; Lac, C.; Elbasha, E.H. The cost-effectiveness of a quadrivalent human papillomavirus vaccine in Taiwan. Asian Pac. J. Cancer Prev. 2008, 9, 459–466. [Google Scholar] [PubMed]
- Demarteau, N.; Tang, C.H.; Chen, H.C.; Chen, C.J.; Van Kriekinge, G. Cost-effectiveness analysis of the bivalent compared with the quadrivalent human papillomavirus vaccines in Taiwan. Value Health 2012, 15, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.H.; Hu, F.C.; Lee, P.I.; Chow, S.N.; Huang, C.W.; Wang, J.D. Cost-effectiveness of human papillomavirus vaccination for prevention of cervical cancer in Taiwan. BMC Health Serv. Res. 2010, 10, 11. [Google Scholar] [CrossRef][Green Version]
- Tang, C.H.; Cheng, W.F.; Jiang, J.H.; You, S.L.; Huang, L.W.; Hsieh, J.Y.; Mukherjee, P.; Van Kriekinge, G.; Lee, C. Cost-Effectiveness Analysis of Human Papillomavirus Vaccination in Adolescent Girls in Taiwan. Asian Pac. J. Cancer Prev. APJCP 2019, 20, 1377–1387. [Google Scholar] [CrossRef]
- Aljunid, S.; Maimaiti, N.; Nur, A.M.; Noor, M.R.; Puteh, S.E. Cost-effectiveness of HPV vaccination regime: Comparing twice versus thrice vaccinations dose regime among adolescent girls in Malaysia. BMC Public Health 2016, 16, 71. [Google Scholar] [CrossRef]
- Ezat, W.P.; Aljunid, S. Cost-effectiveness of HPV vaccination in the prevention of cervical cancer in Malaysia. Asian Pac. J. Cancer Prev. APJCP 2010, 11, 79–90. [Google Scholar]
- Van Kriekinge, G.; Sohn, W.Y.; Aljunid, S.M.; Soon, R.; Yong, C.M.; Chen, J.; Lee, I.H. Comparative Cost-Effectiveness Analysis of Two Different Two-Dose Human Papillomavirus Vaccines in Malaysia. Asian Pac. J. Cancer Prev. 2018, 19, 933–940. [Google Scholar] [CrossRef]
- Ezat, S.W.P.; Aljunid, S. Comparative cost-effectiveness of HPV vaccines in the prevention of cervical cancer in Malaysia. Asian Pac. J. Cancer Prev. 2010, 11, 943–951. [Google Scholar]
- Kulasingam, S.; Connelly, L.; Conway, E.; Hocking, J.S.; Myers, E.; Regan, D.G.; Roder, D.; Ross, J.; Wain, G. A cost-effectiveness analysis of adding a human papillomavirus vaccine to the Australian National Cervical Cancer Screening Program. Sex. Health 2007, 4, 165–175. [Google Scholar] [CrossRef]
- Mahumud, R.A.; Alam, K.; Dunn, J.; Gow, J. The cost-effectiveness of controlling cervical cancer using a new 9-valent human papillomavirus vaccine among school-aged girls in Australia. PLoS ONE 2019, 14, e0223658. [Google Scholar] [CrossRef] [PubMed]
- Simms, K.T.; Laprise, J.F.; Smith, M.A.; Lew, J.B.; Caruana, M.; Brisson, M.; Canfell, K. Cost-effectiveness of the next generation nonavalent human papillomavirus vaccine in the context of primary human papillomavirus screening in Australia: A comparative modelling analysis. Lancet Public Health 2016, 1, e66–e75. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Kobus, K.E.; Diaz, M.; O'Shea, M.; Van Minh, H.; Goldie, S.J. Exploring the cost-effectiveness of HPV vaccination in Vietnam: Insights for evidence-based cervical cancer prevention policy. Vaccine 2008, 26, 4015–4024. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Sy, S.; Kim, J.J. The value of male human papillomavirus vaccination in preventing cervical cancer and genital warts in a low-resource setting. BJOG 2016, 123, 917–926. [Google Scholar] [CrossRef]
- Van Minh, H.; My, N.T.T.; Jit, M. Cervical cancer treatment costs and cost-effectiveness analysis of human papillomavirus vaccination in Vietnam: A PRIME modeling study. BMC Health Serv. Res. 2017, 17, 353. [Google Scholar] [CrossRef]
- Sharma, M.; Ortendahl, J.; Van Der Ham, E.; Sy, S.; Kim, J.J. Cost-effectiveness of human papillomavirus vaccination and cervical cancer screening in Thailand. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 166–176. [Google Scholar] [CrossRef]
- Termrungruanglert, W.; Havanond, P.; Khemapech, N.; Lertmaharit, S.; Pongpanich, S.; Khorprasert, C.; Taneepanichskul, S. Cost and effectiveness evaluation of prophylactic HPV vaccine in developing countries. Value Health 2012, 15, S29–S34. [Google Scholar] [CrossRef][Green Version]
- Termrungruanglert, W.; Khemapech, N.; Vasuratna, A.; Havanond, P.; Deebukkham, P.; Kulkarni, A.S.; Pavelyev, A. The epidemiologic and economic impact of a quadrivalent human papillomavirus vaccine in Thailand. PLoS ONE 2021, 16, e0245894. [Google Scholar] [CrossRef]
- Cheung, T.H.; Cheng, S.S.Y.; Hsu, D.C.; Wong, Q.W.L.; Pavelyev, A.; Walia, A.; Saxena, K.; Prabhu, V.S. The impact and cost-effectiveness of 9-valent human papillomavirus vaccine in adolescent females in Hong Kong. Cost. Eff. Resour. Alloc. 2021, 19, 75. [Google Scholar] [CrossRef]
- Cheung, T.H.; Cheng, S.S.Y.; Hsu, D.; Wing-Lei Wong, Q.; Pavelyev, A.; Sukarom, I.; Saxena, K. Health impact and cost-effectiveness of implementing gender-neutral vaccination with the 9-valent HPV vaccine in Hong Kong. Hum. Vaccines Immunother. 2023, 19, 2184605. [Google Scholar] [CrossRef]
- Germar, M.J.; Purugganan, C.; Bernardino, M.S.; Cuenca, B.; Chen, Y.C.; Li, X.; Van Kriekinge, G.; Lee, I.H. Cost-effectiveness analysis of AS04-adjuvanted human papillomavirus 16/18 vaccine compared with human papillomavirus 6/11/16/18 vaccine in the Philippines, with the new 2-dose schedule. Hum. Vaccines Immunother. 2017, 13, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Llave, C.L.; Uy, M.E.V.; Lam, H.Y.; Aldaba, J.G.; Yacapin, C.C.; Miranda, M.B.; Valverde, H.A.; Silva, W.T.; Nawaz, S.; Slavkovsky, R.C.; et al. The cost-effectiveness of human papillomavirus vaccination in the Philippines. Vaccine 2022, 40, 3802–3811. [Google Scholar] [CrossRef] [PubMed]
- Blakely, T.; Kvizhinadze, G.; Karvonen, T.; Pearson, A.L.; Smith, M.; Wilson, N. Cost-effectiveness and equity impacts of three HPV vaccination programmes for school-aged girls in New Zealand. Vaccine 2014, 32, 2645–2656. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.H.; Wu, T.; Hu, Y.M.; Wei, L.H.; Li, M.Q.; Huang, W.J.; Chen, W.; Huang, S.J.; Pan, Q.J.; Zhang, X.; et al. Efficacy, safety, and immunogenicity of an Escherichia coli-produced Human Papillomavirus (16 and 18) L1 virus-like-particle vaccine: End-of-study analysis of a phase 3, double-blind, randomised, controlled trial. Lancet Infect. Dis. 2022, 22, 1756–1768. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, D.; Dolk, F.C.; Suwantika, A.A.; Westra, T.A.; Wilschut, J.C.; Postma, M.J. Cost-Utility Analysis of Human Papillomavirus Vaccination and Cervical Screening on Cervical Cancer Patient in Indonesia. Value Health Reg. Issues 2016, 9, 84–92. [Google Scholar] [CrossRef]
- Fesenfeld, M.; Hutubessy, R.; Jit, M. Cost-effectiveness of human papillomavirus vaccination in low and middle income countries: A systematic review. Vaccine 2013, 31, 3786–3804. [Google Scholar] [CrossRef]
- Setiawan, D.; Andrijono; Hadinegoro, S.R.; Meyta, H.; Sitohang, R.V.; Tandy, G.; Perwitasari, D.A.; Postma, M.J. Cervical cancer prevention in Indonesia: An updated clinical impact, cost-effectiveness and budget impact analysis. PLoS ONE 2020, 15, e0230359. [Google Scholar] [CrossRef]
- World Health Organization. WHO Cervical Cancer Elimination Initiative: From Call to Action to Global Movement; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Dykens, J.A.; Peterson, C.E.; Holt, H.K.; Harper, D.M. Gender neutral HPV vaccination programs: Reconsidering policies to expand cancer prevention globally. Front. Public Health 2023, 11, 1067299. [Google Scholar] [CrossRef]

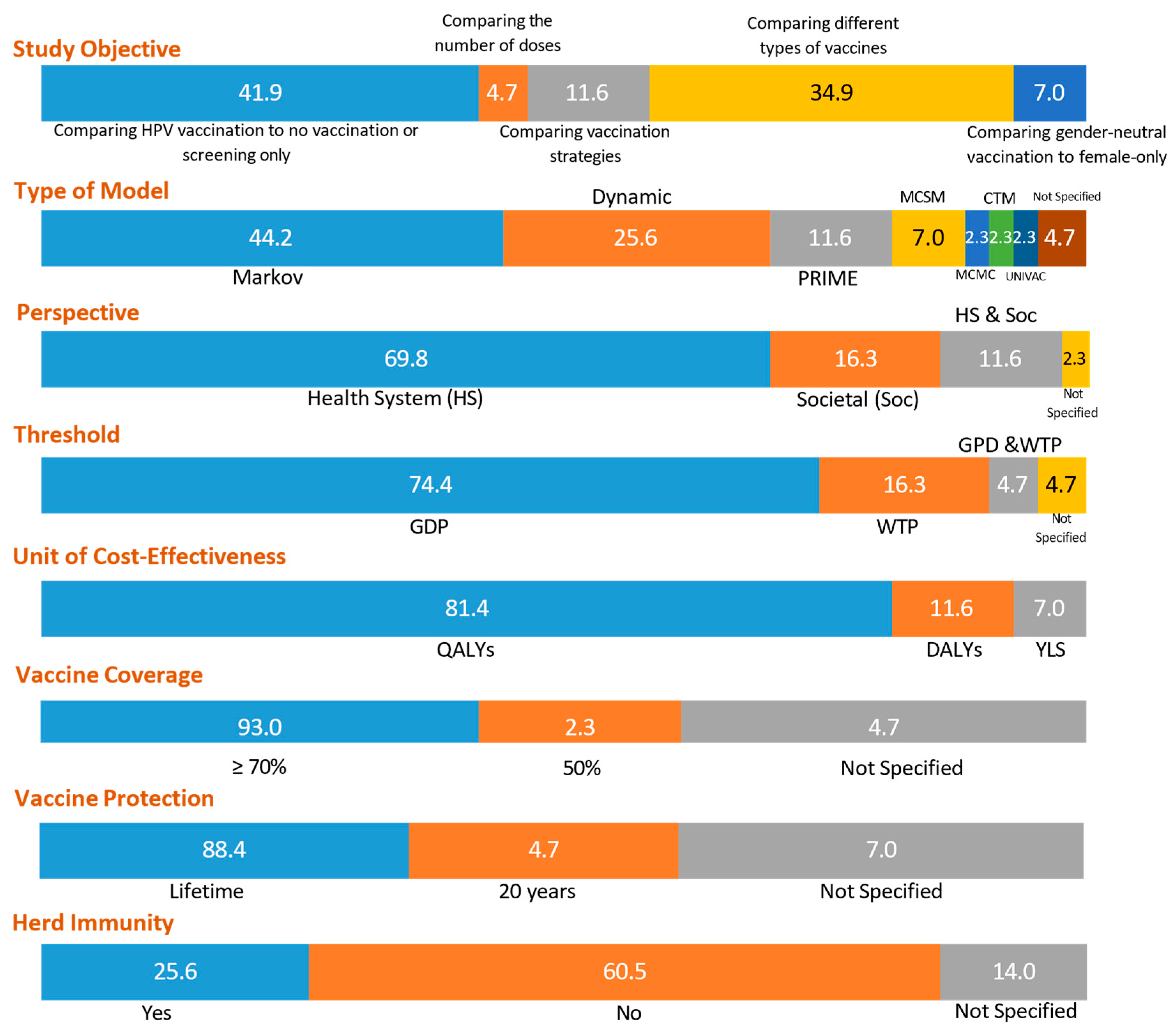
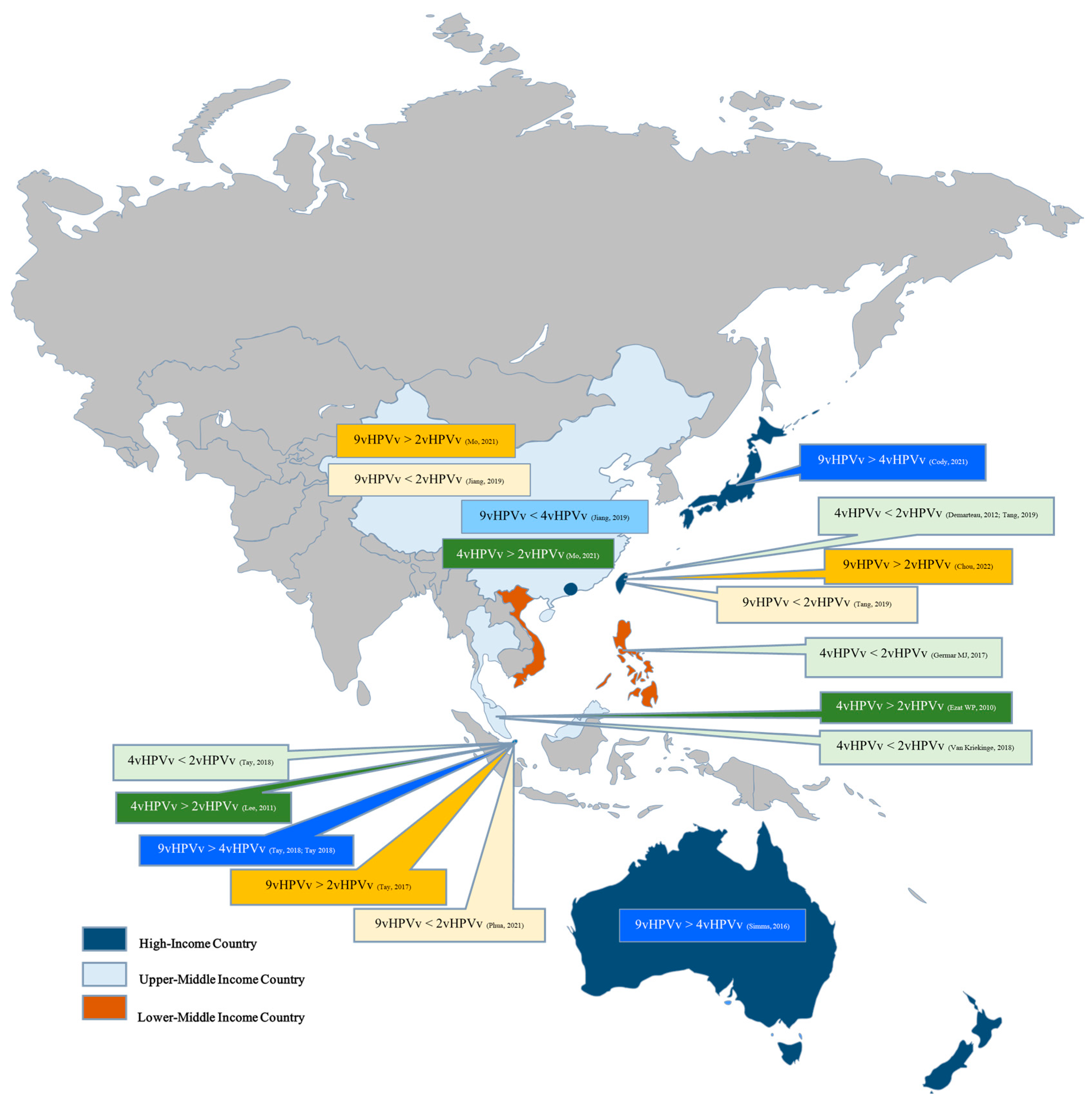
| Characteristics | Number of Studies (n) | Percentage (%) |
|---|---|---|
| Selected articles | 43 | 100 |
| Study setting | ||
| China | 9 | 21 |
| Singapore | 6 | 14 |
| Japan | 5 | 12 |
| Taiwan | 5 | 12 |
| Malaysia | 4 | 9 |
| Australia | 3 | 7 |
| Vietnam | 3 | 7 |
| Thailand | 3 | 7 |
| Hong Kong | 2 | 5 |
| Philippines | 2 | 5 |
| New Zealand | 1 | 2 |
| Economic classification | ||
| High income | 22 | 51 |
| Upper-middle income | 16 | 37 |
| Lower-middle income | 5 | 12 |
| The primary location of the first author | ||
| Research Institute | 9 | 21 |
| Research Group | 8 | 19 |
| Hospital or university | 26 | 60 |
| Study Funder | ||
| Pharmaceutical company | 16 | 37 |
| National Fund | 9 | 21 |
| Bill & Melinda Gates Foundation | 4 | 9 |
| Education Fund | 3 | 7 |
| WHO and GAVI | 1 | 2 |
| Did not receive funding | 1 | 2 |
| Did not declare | 9 | 21 |
| Conflict of interest | ||
| Yes | 17 | 40 |
| No | 23 | 53 |
| Not stated | 3 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mongan, S.P.; Byrnes, J.; Kim, H. The Cost-Effectiveness of the Human Papilloma Virus Vaccination in Asia Pacific Countries: What Lessons Can Indonesia Learn?—A Systematic Review. Vaccines 2025, 13, 593. https://doi.org/10.3390/vaccines13060593
Mongan SP, Byrnes J, Kim H. The Cost-Effectiveness of the Human Papilloma Virus Vaccination in Asia Pacific Countries: What Lessons Can Indonesia Learn?—A Systematic Review. Vaccines. 2025; 13(6):593. https://doi.org/10.3390/vaccines13060593
Chicago/Turabian StyleMongan, Suzanna Patricia, Joshua Byrnes, and Hansoo Kim. 2025. "The Cost-Effectiveness of the Human Papilloma Virus Vaccination in Asia Pacific Countries: What Lessons Can Indonesia Learn?—A Systematic Review" Vaccines 13, no. 6: 593. https://doi.org/10.3390/vaccines13060593
APA StyleMongan, S. P., Byrnes, J., & Kim, H. (2025). The Cost-Effectiveness of the Human Papilloma Virus Vaccination in Asia Pacific Countries: What Lessons Can Indonesia Learn?—A Systematic Review. Vaccines, 13(6), 593. https://doi.org/10.3390/vaccines13060593








