Discovery of Synergistic Broadly Neutralizing Antibodies Targeting Non-Dominant Epitopes on SARS-CoV-2 RBD and NTD
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Human Samples
2.3. Cell Lines
2.4. Protein Expression and Purification
2.5. Spike-Specific Single B Cell Sorting and PCR
2.6. ELISA
2.7. Pseudovirus Neutralization Assay
2.8. Competition ELISA
2.9. Sequence Analysis
2.10. Calculation of Combination Index
2.11. Construction and Expression of Bispecific Antibodies
3. Results
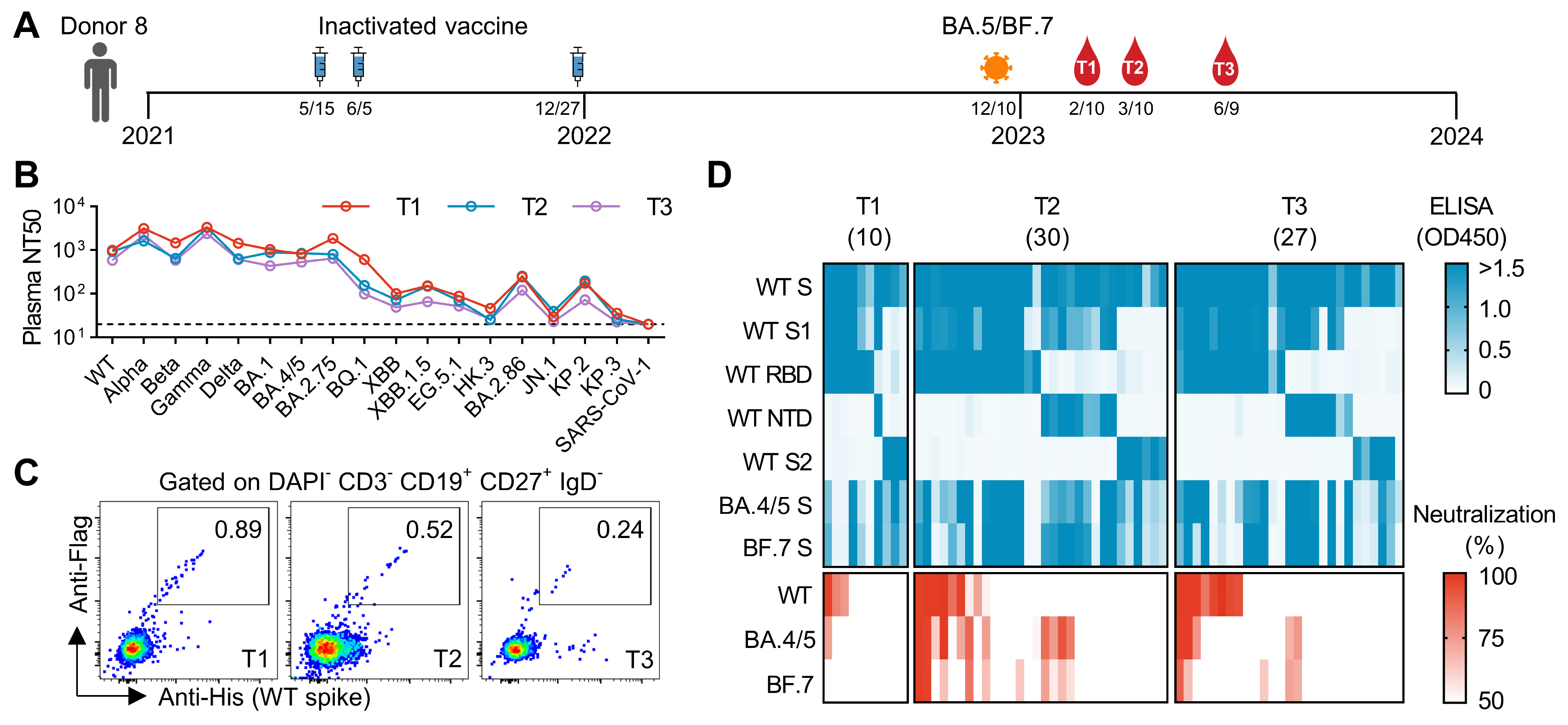
3.1. Initial Screening of mAbs from an Individual Who Experienced Breakthrough Infection After Three Doses of Inactivated Vaccines
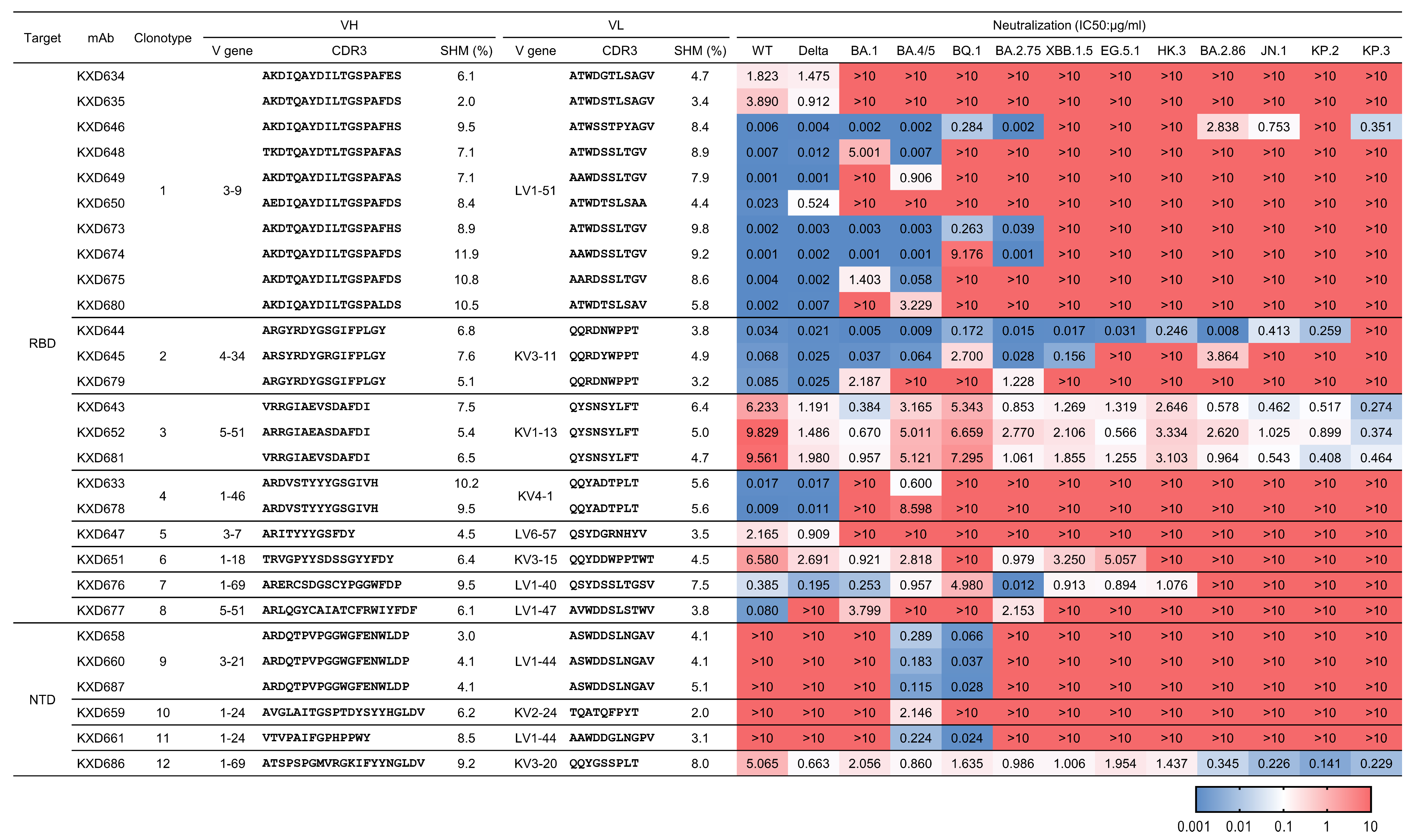
3.2. Identification of Four bnAbs Against SARS-CoV-2
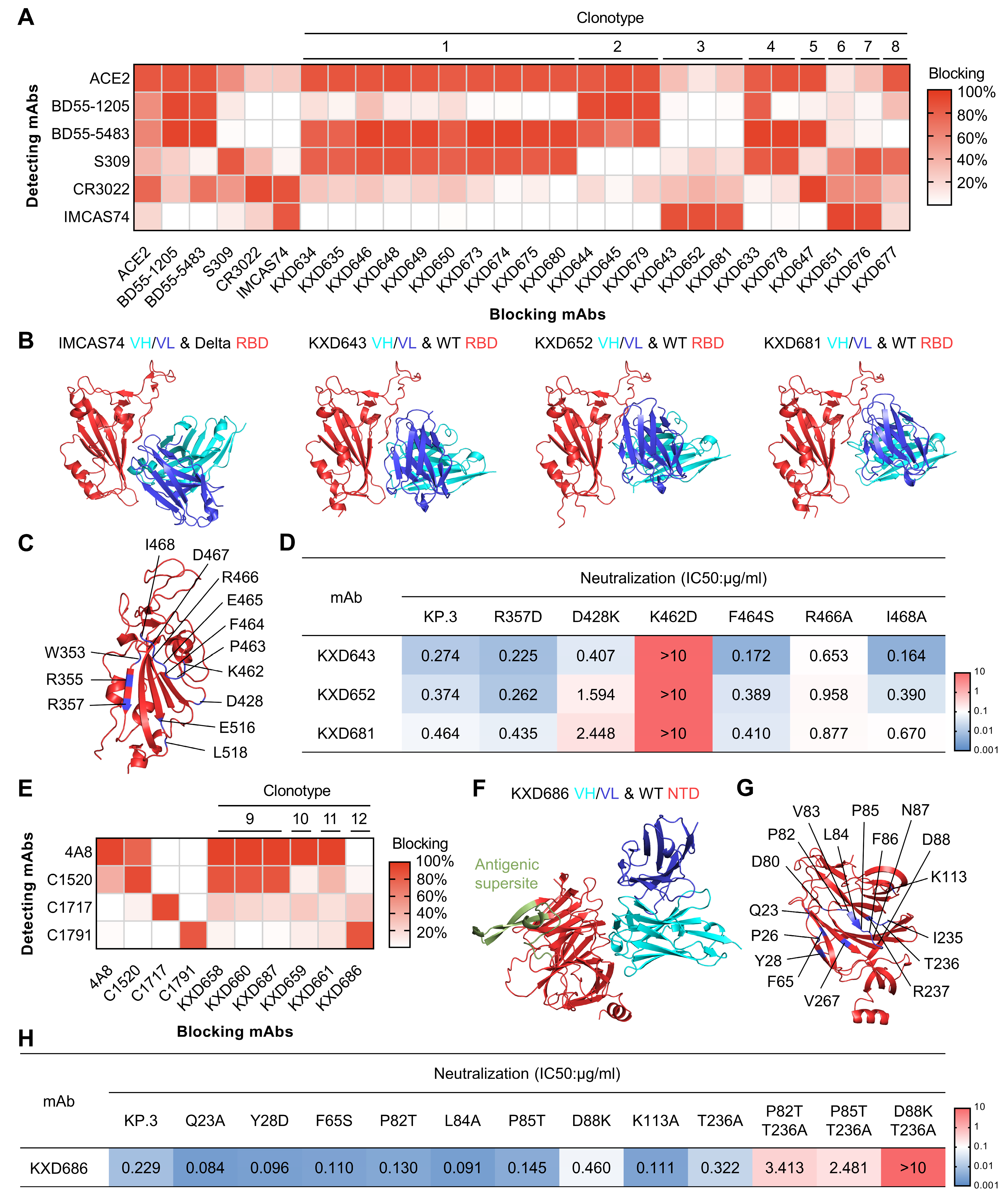
3.3. The Four bnAbs Recognize Non-Dominant, While Conserved, Epitopes
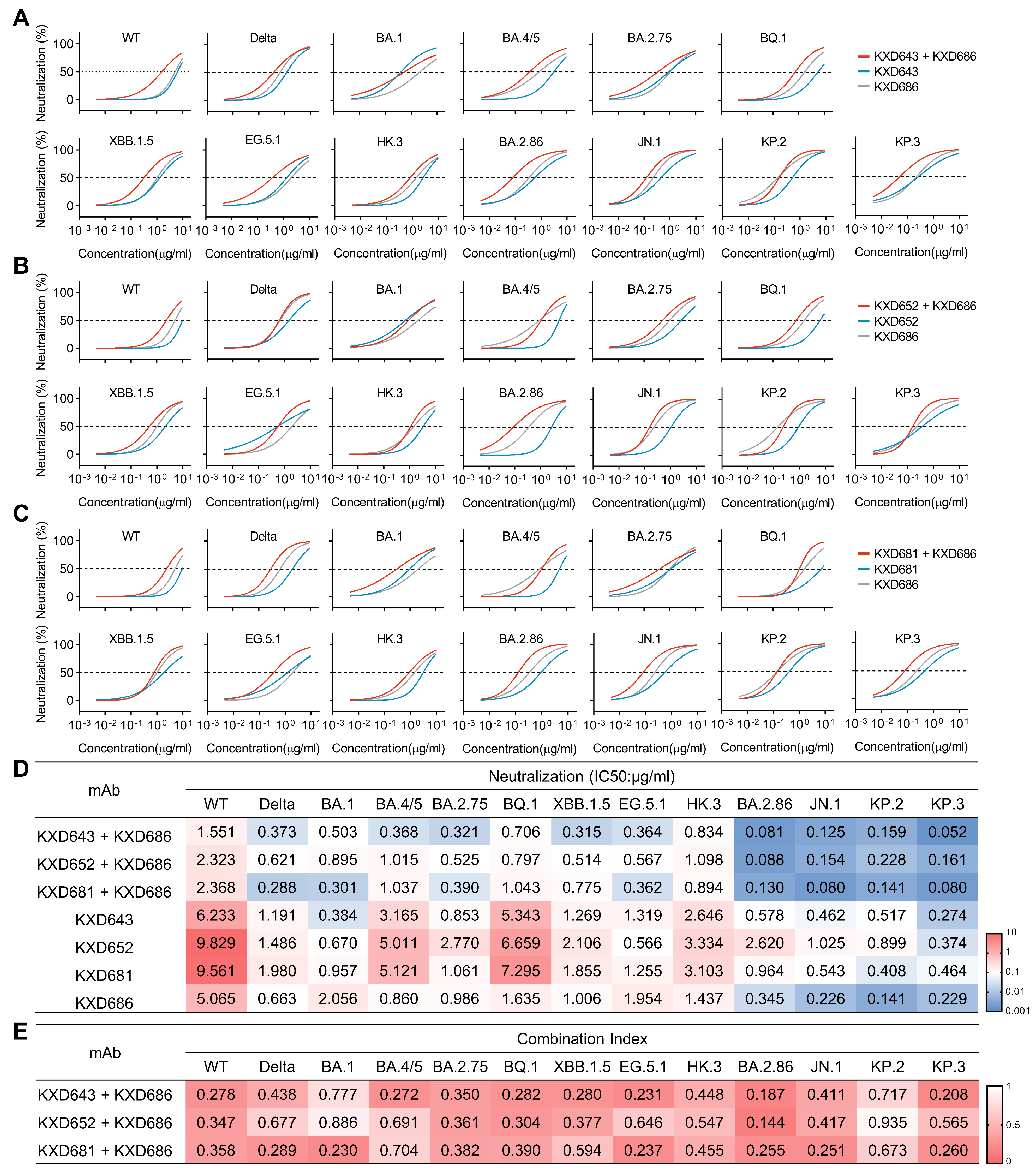
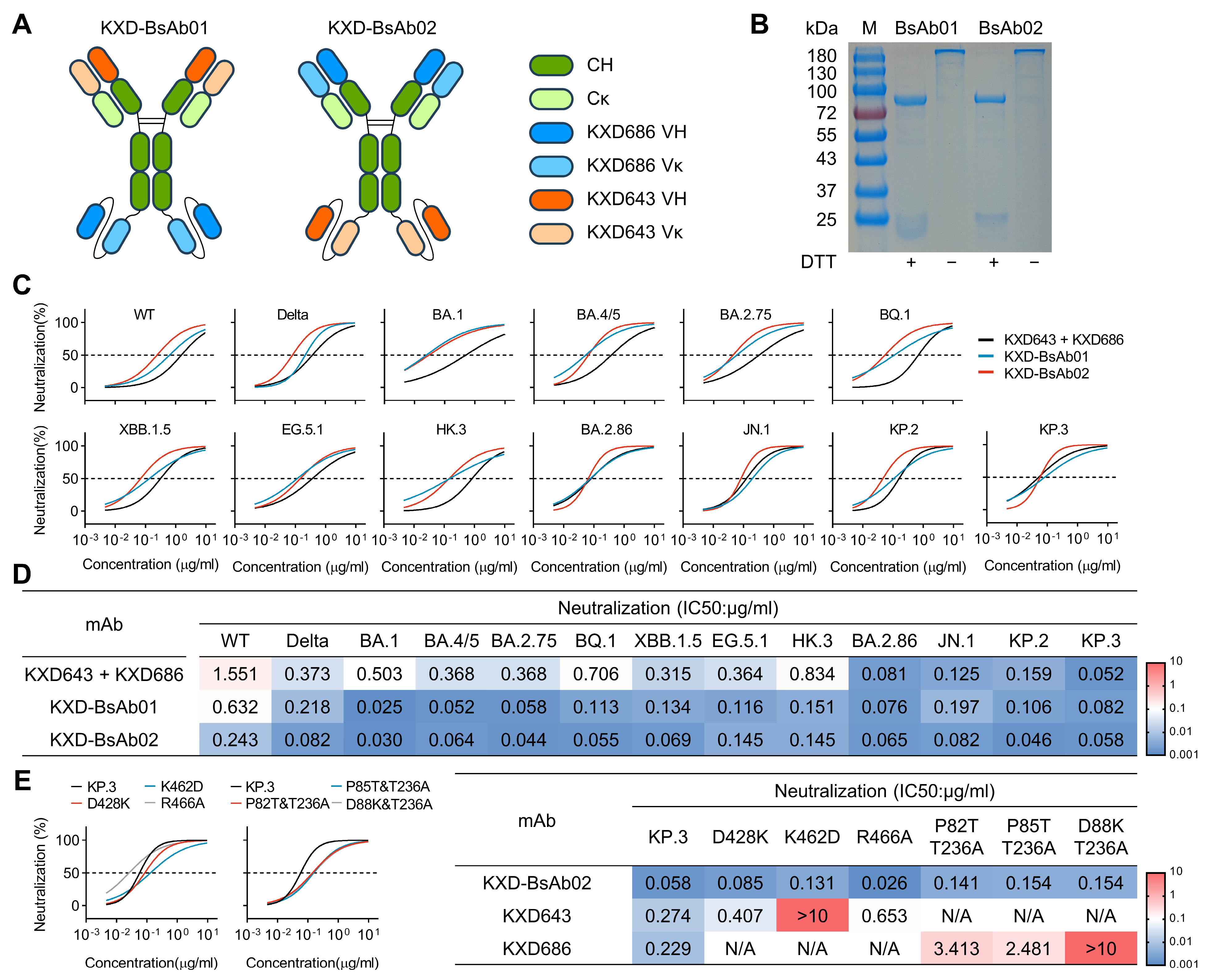
3.4. Antibody Cocktails and Bispecific Antibodies Based on the Four bnAbs Neutralize SARS-CoV-2 More Potently
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gorbalenya, A.E.; Baker, S.C.; Baric, R.S.; de Groot, R.J.; Drosten, C.; Gulyaeva, A.A.; Haagmans, B.L.; Lauber, C.; Leontovich, A.M.; Neuman, B.W.; et al. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; Peacock, S.J.; Barclay, W.S.; de Silva, T.; Towers, G.J.; Robertson, D.L.; et al. SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef] [PubMed]
- COVID Data Tracker. Available online: https://covid.cdc.gov/covid-data-tracker/#variant-proportions (accessed on 10 April 2025).
- Harrison, A.G.; Lin, T.; Wang, P.H. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef] [PubMed]
- Wrapp, D.; Wang, N.S.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Bidon, M.; Jaimes, J.A.; Whittaker, G.R.; Daniel, S. Coronavirus membrane fusion mechanism offers a potential target for antiviral development. Antivir. Res. 2020, 178, 104792. [Google Scholar] [CrossRef]
- Liu, H.J.; Wilson, I.A. Protective neutralizing epitopes in SARS-CoV-2. Immunol. Rev. 2022, 310, 76–92. [Google Scholar] [CrossRef]
- Yuan, M.; Wilson, I.A. Structural Immunology of SARS-CoV-2. Immunol. Rev. 2025, 329, e13431. [Google Scholar] [CrossRef]
- Hastie, K.M.; Li, H.Y.; Bedinger, D.; Schendel, S.L.; Dennison, S.M.; Li, K.; Rayaprolu, V.; Yu, X.Y.; Mann, C.; Zandonatti, M.; et al. Defining variant-resistant epitopes targeted by SARS-CoV-2 antibodies: A global consortium study. Science 2021, 374, 472–478. [Google Scholar] [CrossRef]
- Rao, X.; Zhao, R.C.; Tong, Z.; Guo, S.X.; Peng, W.Y.; Liu, K.F.; Li, S.H.; Wu, L.L.; Tong, J.Y.; Chai, Y.; et al. Defining a de novo non- RBM antibody as RBD-8 and its synergistic rescue of immune- evaded antibodies to neutralize Omicron SARS- CoV-2. Proc. Natl. Acad. Sci. USA 2023, 120, e2314193120. [Google Scholar] [CrossRef]
- Chi, X.Y.; Yan, R.H.; Zhang, J.; Zhang, G.Y.; Zhang, Y.Y.; Hao, M.; Zhang, Z.; Fan, P.F.; Dong, Y.Z.; Yang, Y.L.; et al. A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science 2020, 369, 650–655. [Google Scholar] [CrossRef] [PubMed]
- McCallum, M.; De Marco, A.; Lempp, F.; Tortorici, M.A.; Pinto, D.; Walls, A.C.; Beltramello, M.; Chen, A.; Liu, Z.M.; Zatta, F.; et al. N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2. Cell 2021, 184, 2332–2347.e16. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, G.; Guo, Y.C.; Zhou, T.Q.; Gorman, J.; Lee, M.; Rapp, M.; Reddem, E.R.; Yu, J.; Bahna, F.; Bimela, J.; et al. Potent SARS-CoV-2 neutralizing antibodies directed against spike N-terminal domain target a single supersite. Cell Host Microbe 2021, 29, 819–833.e7. [Google Scholar] [CrossRef]
- Wang, Z.J.; Muecksch, F.; Cho, A.; Gaebler, C.; Hoffmann, H.H.; Ramos, V.; Zong, S.; Cipolla, M.; Johnson, B.; Schmidt, F.; et al. Analysis of memory B cells identifies conserved neutralizing epitopes on the N-terminal domain of variant SARS-Cov-2 spike proteins. Immunity 2022, 55, 998–1012.e8. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, Y.; Mellis, I.A.; Wu, M.; Mohri, H.; Gherasim, C.; Valdez, R.; Purpura, L.J.; Yin, M.T.; Gordon, A.; et al. Antibody evasiveness of SARS-CoV-2 subvariants KP.3.1.1 and XEC. Cell Rep. 2025, 44, 115543. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Y.; Jian, F.; Yang, S.; Song, W.; Wang, P.; Yu, L.; Shao, F.; Cao, Y. Enhanced immune evasion of SARS-CoV-2 variants KP.3.1.1 and XEC through N-terminal domain mutations. Lancet Infect. Dis. 2025, 25, e6–e7. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, Y.; Yang, S.; Jian, F.; Song, W.; Yu, L.; Shao, F.; Cao, Y. Virological and antigenic characteristics of SARS-CoV-2 variants LF.7.2.1, NP.1, and LP.8.1. Lancet Infect. Dis. 2025, 25, e128–e130. [Google Scholar] [CrossRef]
- Jian, F.; Wang, J.; Yisimayi, A.; Song, W.; Xu, Y.; Chen, X.; Niu, X.; Yang, S.; Yu, Y.; Wang, P.; et al. Evolving antibody response to SARS-CoV-2 antigenic shift from XBB to JN.1. Nature 2025, 637, 921–929. [Google Scholar] [CrossRef]
- Liang, C.Y.; Raju, S.; Liu, Z.; Li, Y.; Asthagiri Arunkumar, G.; Case, J.B.; Scheaffer, S.M.; Zost, S.J.; Acreman, C.M.; Gagne, M.; et al. Imprinting of serum neutralizing antibodies by Wuhan-1 mRNA vaccines. Nature 2024, 630, 950–960. [Google Scholar] [CrossRef]
- Wang, Z.; Li, L.; Du, R.; Chen, X.; Sun, Y.; Qin, R.; Li, Y.; Feng, H.; Hu, L.; Chen, X.; et al. Ancestral SARS-CoV-2 immune imprinting persists on RBD but not NTD after sequential Omicron infections. iScience 2025, 28, 111557. [Google Scholar] [CrossRef]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, X.; Wang, Z.; Li, Y.; Wang, C.; Jiang, L.; Zuo, T. Breakthrough infection elicits hypermutated IGHV3-53/3-66 public antibodies with broad and potent neutralizing activity against SARS-CoV-2 variants including the emerging EG.5 lineages. PLoS Pathog. 2023, 19, e1011856. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Wu, N.C.; Zhu, X.; Lee, C.D.; So, R.T.Y.; Lv, H.; Mok, C.K.P.; Wilson, I.A. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science 2020, 368, 630–633. [Google Scholar] [CrossRef]
- Pinto, D.; Park, Y.J.; Beltramello, M.; Walls, A.C.; Tortorici, M.A.; Bianchi, S.; Jaconi, S.; Culap, K.; Zatta, F.; De Marco, A.; et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 2020, 583, 290–295. [Google Scholar] [CrossRef]
- Cao, Y.; Jian, F.; Zhang, Z.; Yisimayi, A.; Hao, X.; Bao, L.; Yuan, F.; Yu, Y.; Du, S.; Wang, J.; et al. Rational identification of potent and broad sarbecovirus-neutralizing antibody cocktails from SARS convalescents. Cell Rep. 2022, 41, 111845. [Google Scholar] [CrossRef]
- Cao, Y.; Jian, F.; Wang, J.; Yu, Y.; Song, W.; Yisimayi, A.; Wang, J.; An, R.; Chen, X.; Zhang, N.; et al. Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution. Nature 2023, 614, 521–529. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, H.; Zhang, Y.; Yuan, L.; Wang, Y.; Zhang, T.; Wang, S.; Zhang, J.; Yu, H.; Xiong, H.; et al. Three SARS-CoV-2 antibodies provide broad and synergistic neutralization against variants of concern, including Omicron. Cell Rep. 2022, 39, 110862. [Google Scholar] [CrossRef] [PubMed]
- Mast, F.D.; Fridy, P.C.; Ketaren, N.E.; Wang, J.; Jacobs, E.Y.; Olivier, J.P.; Sanyal, T.; Molloy, K.R.; Schmidt, F.; Rutkowska, M.; et al. Highly synergistic combinations of nanobodies that target SARS-CoV-2 and are resistant to escape. Elife 2021, 10, e73027. [Google Scholar] [CrossRef]
- Yao, H.; Sun, Y.; Deng, Y.Q.; Wang, N.; Tan, Y.; Zhang, N.N.; Li, X.F.; Kong, C.; Xu, Y.P.; Chen, Q.; et al. Rational development of a human antibody cocktail that deploys multiple functions to confer Pan-SARS-CoVs protection. Cell Res. 2021, 31, 25–36. [Google Scholar] [CrossRef]
- Starr, T.N.; Czudnochowski, N.; Liu, Z.; Zatta, F.; Park, Y.J.; Addetia, A.; Pinto, D.; Beltramello, M.; Hernandez, P.; Greaney, A.J.; et al. SARS-CoV-2 RBD antibodies that maximize breadth and resistance to escape. Nature 2021, 597, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xue, W.; Zheng, Q.; Song, S.; Yang, C.; Xiong, H.; Zhang, S.; Hong, M.; Zhang, Y.; Yu, H.; et al. Cross-neutralizing antibodies bind a SARS-CoV-2 cryptic site and resist circulating variants. Nat. Commun. 2021, 12, 5652. [Google Scholar] [CrossRef]
- Xiong, H.; Sun, H.; Wang, S.; Yuan, L.; Liu, L.; Zhu, Y.; Zhang, J.; Huang, Y.; Qi, R.; Jiang, Y.; et al. The neutralizing breadth of antibodies targeting diverse conserved epitopes between SARS-CoV and SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2022, 119, e2204256119. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Zhou, D.; Ginn, H.M.; Duyvesteyn, H.M.E.; Supasa, P.; Case, J.B.; Zhao, Y.; Walter, T.S.; Mentzer, A.J.; Liu, C.; et al. The antigenic anatomy of SARS-CoV-2 receptor binding domain. Cell 2021, 184, 2183–2200.e22. [Google Scholar] [CrossRef]
- Bullen, G.; Galson, J.D.; Hall, G.; Villar, P.; Moreels, L.; Ledsgaard, L.; Mattiuzzo, G.; Bentley, E.M.; Masters, E.W.; Tang, D.; et al. Cross-Reactive SARS-CoV-2 Neutralizing Antibodies From Deep Mining of Early Patient Responses. Front. Immunol. 2021, 12, 678570. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.; Tian, X.; Zhang, X.; Xing, S.; Song, W.; Liu, Q.; Hao, A.; Hu, Y.; Zhang, M.; Ying, T.; et al. Structural Study of SARS-CoV-2 Antibodies Identifies a Broad-Spectrum Antibody That Neutralizes the Omicron Variant by Disassembling the Spike Trimer. J. Virol. 2022, 96, e0048022. [Google Scholar] [CrossRef]
- Dussupt, V.; Sankhala, R.S.; Mendez-Rivera, L.; Townsley, S.M.; Schmidt, F.; Wieczorek, L.; Lal, K.G.; Donofrio, G.C.; Tran, U.; Jackson, N.D.; et al. Low-dose in vivo protection and neutralization across SARS-CoV-2 variants by monoclonal antibody combinations. Nat. Immunol. 2021, 22, 1503–1514. [Google Scholar] [CrossRef]
- Tanaka, S.; Olson, C.A.; Barnes, C.O.; Higashide, W.; Gonzalez, M.; Taft, J.; Richardson, A.; Martin-Fernandez, M.; Bogunovic, D.; Gnanapragasam, P.N.P.; et al. Rapid identification of neutralizing antibodies against SARS-CoV-2 variants by mRNA display. Cell Rep. 2022, 38, 110348. [Google Scholar] [CrossRef]
- Rosen, L.E.; Tortorici, M.A.; De Marco, A.; Pinto, D.; Foreman, W.B.; Taylor, A.L.; Park, Y.J.; Bohan, D.; Rietz, T.; Errico, J.M.; et al. A potent pan-sarbecovirus neutralizing antibody resilient to epitope diversification. Cell 2024, 187, 7196–7213.e26. [Google Scholar] [CrossRef]
- Rappazzo, C.G.; Tse, L.V.; Kaku, C.I.; Wrapp, D.; Sakharkar, M.; Huang, D.; Deveau, L.M.; Yockachonis, T.J.; Herbert, A.S.; Battles, M.B.; et al. Broad and potent activity against SARS-like viruses by an engineered human monoclonal antibody. Science 2021, 371, 823–829. [Google Scholar] [CrossRef]
- Rouet, R.; Mazigi, O.; Walker, G.J.; Langley, D.B.; Sobti, M.; Schofield, P.; Lenthall, H.; Jackson, J.; Ubiparipovic, S.; Henry, J.Y.; et al. Potent SARS-CoV-2 binding and neutralization through maturation of iconic SARS-CoV-1 antibodies. mAbs 2021, 13, 1922134. [Google Scholar] [CrossRef] [PubMed]
- Entzminger, K.C.; Fleming, J.K.; Entzminger, P.D.; Espinosa, L.Y.; Samadi, A.; Hiramoto, Y.; Okumura, S.C.J.; Maruyama, T. Rapid engineering of SARS-CoV-2 therapeutic antibodies to increase breadth of neutralization including BQ.1.1, CA.3.1, CH.1.1, XBB.1.16, and XBB.1.5. Antib. Ther. 2023, 6, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yuan, M.; Keating, C.; Shaabani, N.; Limbo, O.; Joyce, C.; Woehl, J.; Barman, S.; Burns, A.; Tran, Q.; et al. Broadening a SARS-CoV-1-neutralizing antibody for potent SARS-CoV-2 neutralization through directed evolution. Sci. Signal. 2023, 16, eabk3516. [Google Scholar] [CrossRef]
- Zhu, F.; Rajan, S.; Hayes, C.F.; Kwong, K.Y.; Goncalves, A.R.; Zemla, A.T.; Lau, E.Y.; Zhang, Y.; Cai, Y.; Goforth, J.W.; et al. Preemptive optimization of a clinical antibody for broad neutralization of SARS-CoV-2 variants and robustness against viral escape. Sci. Adv. 2025, 11, eadu0718. [Google Scholar] [CrossRef]
- Mazigi, O.; Langley, D.B.; Henry, J.Y.; Burnett, D.L.; Sobti, M.; Walker, G.J.; Rouet, R.; Balachandran, H.; Lenthall, H.; Jackson, J.; et al. Affinity maturation endows potent activity onto class 6 SARS-CoV-2 broadly neutralizing antibodies. Proc. Natl. Acad. Sci. USA 2025, 122, e2417544121. [Google Scholar] [CrossRef]
- Rubio, A.A.; Baharani, V.A.; Dadonaite, B.; Parada, M.; Abernathy, M.E.; Wang, Z.; Lee, Y.E.; Eso, M.R.; Phung, J.; Ramos, I.; et al. Bispecific antibodies targeting the N-terminal and receptor binding domains potently neutralize SARS-CoV-2 variants of concern. Sci. Transl. Med. 2025, 17, eadq5720. [Google Scholar] [CrossRef]
- Li, Z.; Li, S.; Zhang, G.; Peng, W.; Chang, Z.; Zhang, X.; Fan, Z.; Chai, Y.; Wang, F.; Zhao, X.; et al. An engineered bispecific human monoclonal antibody against SARS-CoV-2. Nat. Immunol. 2022, 23, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hao, A.; Ji, P.; Ma, Y.; Zhang, Z.; Chen, J.; Mao, Q.; Xiong, X.; Rehati, P.; Wang, Y.; et al. A bispecific antibody exhibits broad neutralization against SARS-CoV-2 Omicron variants XBB.1.16, BQ.1.1 and sarbecoviruses. Nat. Commun. 2024, 15, 5127. [Google Scholar] [CrossRef]
- Chang, M.R.; Tomasovic, L.; Kuzmina, N.A.; Ronk, A.J.; Byrne, P.O.; Johnson, R.; Storm, N.; Olmedillas, E.; Hou, Y.J.; Schafer, A.; et al. IgG-like bispecific antibodies with potent and synergistic neutralization against circulating SARS-CoV-2 variants of concern. Nat. Commun. 2022, 13, 5814. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, L.; Gao, X.S.; Zheng, B.Y.; Deng, Y.Q.; Li, J.X.; Feng, R.; Bian, Q.; Guo, X.L.; Wang, N.; et al. A proof of concept for neutralizing antibody-guided vaccine design against SARS-CoV-2. Natl. Sci. Rev. 2021, 8, nwab053. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, H.; Wang, Z.; Li, L.; Li, Y.; Lu, M.; Chen, X.; Hu, L.; Sun, Y.; Du, R.; Qin, R.; et al. Discovery of Synergistic Broadly Neutralizing Antibodies Targeting Non-Dominant Epitopes on SARS-CoV-2 RBD and NTD. Vaccines 2025, 13, 592. https://doi.org/10.3390/vaccines13060592
Feng H, Wang Z, Li L, Li Y, Lu M, Chen X, Hu L, Sun Y, Du R, Qin R, et al. Discovery of Synergistic Broadly Neutralizing Antibodies Targeting Non-Dominant Epitopes on SARS-CoV-2 RBD and NTD. Vaccines. 2025; 13(6):592. https://doi.org/10.3390/vaccines13060592
Chicago/Turabian StyleFeng, Hualong, Zuowei Wang, Ling Li, Yunjian Li, Maosheng Lu, Xixian Chen, Lin Hu, Yi Sun, Ruiping Du, Rongrong Qin, and et al. 2025. "Discovery of Synergistic Broadly Neutralizing Antibodies Targeting Non-Dominant Epitopes on SARS-CoV-2 RBD and NTD" Vaccines 13, no. 6: 592. https://doi.org/10.3390/vaccines13060592
APA StyleFeng, H., Wang, Z., Li, L., Li, Y., Lu, M., Chen, X., Hu, L., Sun, Y., Du, R., Qin, R., Chen, X., Jiang, L., & Zuo, T. (2025). Discovery of Synergistic Broadly Neutralizing Antibodies Targeting Non-Dominant Epitopes on SARS-CoV-2 RBD and NTD. Vaccines, 13(6), 592. https://doi.org/10.3390/vaccines13060592





