Persistent Low Anti-HIV Neutralizing Antibody Titers in HIV/HCV Coinfection Despite HCV Cure: A 5-Year Longitudinal Analysis
Abstract
1. Introduction
2. Objective
3. Methods
3.1. Design Study
3.2. Clinical Data and Sample Collection
3.3. Laboratory Assays
3.3.1. Generation of a Panel of Recombinant Viruses
3.3.2. Purification of IgG
3.3.3. HIV Neutralization Assays
4. Statistical Analysis
Statistical Power Analysis
5. Results
5.1. Characteristics of the Study Participants
5.2. Anti-HIV-nAbs Response
5.3. Impact of Variables Related to HIV/HCV Coinfection on Anti-HIV-nAbs Response
6. Discussion
7. Strengths and Limitations of This Study
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 95% CI | 95% confidence interval |
| AMR | arithmetic mean ratio |
| bNAbs | broadly neutralizing antibodies |
| cART | combination antiretroviral therapy |
| DAA | direct-acting antiviral |
| EDTA | ethylenediaminetetraacetic acid |
| Env | envelope glycoprotein |
| FDR | false discovery rate |
| GLM | generalized linear model |
| GLMM | generalized linear mixed model |
| Gt | HCV genotype |
| HCV | hepatitis C virus |
| HIV | human immunodeficiency virus |
| IFN | Interferon |
| IgG | immunoglobulin G |
| II | integrase inhibitors |
| LSM | liver stiffness measurement |
| MDSC | myeloid-derived suppressor cells |
| nAbs | neutralizing antibodies |
| NRTI | nucleoside reverse transcriptase inhibitor |
| OR | odds ratio |
| PEG-IFNα | pegylated interferon alpha |
| PLWH | people living with HIV |
| RNA | ribonucleic acid |
| SVR | sustained virologic response |
| SPSS | statistical package for social sciences |
| Tregs | regulatory T-cells |
| VSV | vesicular stomatitis virus |
Appendix A
Appendix A.1. The GESIDA 3603b Cohort Study Group
Appendix A.2. The ESCORIAL Study Group
References
- Gobran, S.T.; Ancuta, P.; Shoukry, N.H. A Tale of Two Viruses: Immunological Insights Into HCV/HIV Coinfection. Front. Immunol. 2021, 12, 726419. [Google Scholar] [CrossRef] [PubMed]
- Platt, L.; Easterbrook, P.; Gower, E.; McDonald, B.; Sabin, K.; McGowan, C.; Yanny, I.; Razavi, H.; Vickerman, P. Prevalence and burden of HCV co-infection in people living with HIV: A global systematic review and meta-analysis. Lancet Infect. Dis. 2016, 16, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Martinello, M.; Solomon, S.S.; Terrault, N.A.; Dore, G.J. Hepatitis C. Lancet 2023, 402, 1085–1096. [Google Scholar] [CrossRef]
- Lin, W.; Wang, X.; Zhang, J.; Wen, C.; Kang, W.; Mao, L.; Yang, J.; Dou, Y.; Shi, L.; Dang, B.; et al. A simple, feasible, efficient and safe treatment strategy of sofosbuvir/velpatasvir for chronic HCV/HIV-1 coinfected patients regardless of HCV genotypes: A multicenter, open-label study in China. Lancet Reg. Health West. Pac. 2023, 36, 100749. [Google Scholar] [CrossRef]
- Caraballo Cortes, K.; Osuch, S.; Perlejewski, K.; Radkowski, M.; Janiak, M.; Berak, H.; Rauch, A.; Fehr, J.S.; Hoffmann, M.; Gunthard, H.F.; et al. T-Cell Exhaustion in HIV-1/Hepatitis C Virus Coinfection Is Reduced After Successful Treatment of Chronic Hepatitis C. Open Forum Infect. Dis. 2023, 10, ofad514. [Google Scholar] [CrossRef]
- Chiodi, F.; Scarlatti, G. Editorial: HIV-Induced Damage of B Cells and Production of HIV Neutralizing Antibodies. Front. Immunol. 2018, 9, 297. [Google Scholar] [CrossRef]
- Laskus, T.; Kibler, K.V.; Chmielewski, M.; Wilkinson, J.; Adair, D.; Horban, A.; Stanczak, G.; Radkowski, M. Effect of hepatitis C infection on HIV-induced apoptosis. PLoS ONE 2013, 8, e75921. [Google Scholar] [CrossRef]
- Mouquet, H. Humoral immunity in HIV-1 post-treatment controllers. Curr. Opin. HIV AIDS 2025, 20, 80–85. [Google Scholar] [CrossRef]
- Sepulveda-Crespo, D.; Yelamos, M.B.; Diez, C.; Gomez, J.; Hontanon, V.; Torresano-Felipe, F.; Berenguer, J.; Gonzalez-Garcia, J.; Ibanez-Samaniego, L.; Llop, E.; et al. Negative impact of HIV infection on broad-spectrum anti-HCV neutralizing antibody titers in HCV-infected patients with advanced HCV-related cirrhosis. Biomed. Pharmacother. 2022, 150, 113024. [Google Scholar] [CrossRef]
- Garcia-Broncano, P.; Medrano, L.M.; Berenguer, J.; Brochado-Kith, O.; Gonzalez-Garcia, J.; Jimenez-Sousa, M.A.; Quereda, C.; Sanz, J.; Tellez, M.J.; Diaz, L.; et al. Mild profile improvement of immune biomarkers in HIV/HCV-coinfected patients who removed hepatitis C after HCV treatment: A prospective study. J. Infect. 2020, 80, 99–110. [Google Scholar] [CrossRef]
- Diez, C.; Berenguer, J.; Ibanez-Samaniego, L.; Llop, E.; Perez-Latorre, L.; Catalina, M.V.; Hontanon, V.; Jimenez-Sousa, M.A.; Aldamiz-Echevarria, T.; Martinez, J.; et al. Persistence of Clinically Significant Portal Hypertension After Eradication of Hepatitis C Virus in Patients With Advanced Cirrhosis. Clin. Infect. Dis. 2020, 71, 2726–2729. [Google Scholar] [CrossRef] [PubMed]
- Medina-Ramirez, M.; Sanchez-Merino, V.; Sanchez-Palomino, S.; Merino-Mansilla, A.; Ferreira, C.B.; Perez, I.; Gonzalez, N.; Alvarez, A.; Alcocer-Gonzalez, J.M.; Garcia, F.; et al. Broadly cross-neutralizing antibodies in HIV-1 patients with undetectable viremia. J. Virol. 2011, 85, 5804–5813. [Google Scholar] [CrossRef]
- Ferreira, C.B.; Merino-Mansilla, A.; Llano, A.; Perez, I.; Crespo, I.; Llinas, L.; Garcia, F.; Gatell, J.M.; Yuste, E.; Sanchez-Merino, V. Evolution of broadly cross-reactive HIV-1-neutralizing activity: Therapy-associated decline, positive association with detectable viremia, and partial restoration of B-cell subpopulations. J. Virol. 2013, 87, 12227–12236. [Google Scholar] [CrossRef]
- Sanchez-Merino, V.; Fabra-Garcia, A.; Gonzalez, N.; Nicolas, D.; Merino-Mansilla, A.; Manzardo, C.; Ambrosioni, J.; Schultz, A.; Meyerhans, A.; Mascola, J.R.; et al. Detection of Broadly Neutralizing Activity within the First Months of HIV-1 Infection. J. Virol. 2016, 90, 5231–5245. [Google Scholar] [CrossRef]
- Burrer, R.; Salmon-Ceron, D.; Richert, S.; Pancino, G.; Spiridon, G.; Haessig, S.; Roques, V.; Barre-Sinoussi, F.; Aubertin, A.M.; Moog, C. Immunoglobulin G (IgG) and IgA, but also nonantibody factors, account for in vitro neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by serum and plasma of HIV-infected patients. J. Virol. 2001, 75, 5421–5424. [Google Scholar] [CrossRef]
- Leite, T.F.; Delatorre, E.; Cortes, F.H.; Ferreira, A.C.G.; Cardoso, S.W.; Grinsztejn, B.; de Andrade, M.M.; Veloso, V.G.; Morgado, M.G.; Guimaraes, M.L. Reduction of HIV-1 Reservoir Size and Diversity After 1 Year of cART Among Brazilian Individuals Starting Treatment During Early Stages of Acute Infection. Front. Microbiol. 2019, 10, 145. [Google Scholar] [CrossRef]
- Hong, F.F.; Mellors, J.W. Changes in HIV reservoirs during long-term antiretroviral therapy. Curr. Opin. HIV AIDS 2015, 10, 43–48. [Google Scholar] [CrossRef]
- Bitnun, A.; Ransy, D.G.; Brophy, J.; Kakkar, F.; Hawkes, M.; Samson, L.; Annabi, B.; Pagliuzza, A.; Morand, J.A.; Sauve, L.; et al. Clinical Correlates of Human Immunodeficiency Virus-1 (HIV-1) DNA and Inducible HIV-1 RNA Reservoirs in Peripheral Blood in Children With Perinatally Acquired HIV-1 Infection With Sustained Virologic Suppression for at Least 5 Years. Clin. Infect. Dis. 2020, 70, 859–866. [Google Scholar] [CrossRef]
- Sloan, D.D.; Lam, C.Y.; Irrinki, A.; Liu, L.; Tsai, A.; Pace, C.S.; Kaur, J.; Murry, J.P.; Balakrishnan, M.; Moore, P.A.; et al. Targeting HIV Reservoir in Infected CD4 T Cells by Dual-Affinity Re-targeting Molecules (DARTs) that Bind HIV Envelope and Recruit Cytotoxic T Cells. PLoS Pathog. 2015, 11, e1005233. [Google Scholar] [CrossRef]
- Schommers, P.; Kim, D.S.; Schlotz, M.; Kreer, C.; Eggeling, R.; Hake, A.; Stecher, M.; Park, J.; Radford, C.E.; Dingens, A.S.; et al. Dynamics and durability of HIV-1 neutralization are determined by viral replication. Nat. Med. 2023, 29, 2763–2774. [Google Scholar] [CrossRef]
- Lin, L.Y.; Gantner, P.; Li, S.; Su, B.; Moog, C. Unpredicted Protective Function of Fc-Mediated Inhibitory Antibodies for HIV and SARS-CoV-2 Vaccines. J. Infect. Dis. 2025, 231, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.; Tilahun, Y.; Taha, O.; Alao, H.; Kodys, K.; Catalano, D.; Szabo, G. Increased microRNA-155 expression in the serum and peripheral monocytes in chronic HCV infection. J. Transl. Med. 2012, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Kuniholm, M.H.; O’Brien, T.R.; Prokunina-Olsson, L.; Augenbraun, M.; Plankey, M.; Karim, R.; Sarkar, M.; French, A.L.; Pierce, C.; Strickler, H.D.; et al. Association of Hepatitis C Virus Infection With CD4/CD8 Ratio in HIV-Positive Women. J. Acquir. Immune Defic. Syndr. 2016, 72, 162–170. [Google Scholar] [CrossRef]
- Shrivastava, S.; Bhatta, M.; Ward, H.; Romani, S.; Lee, R.; Rosenthal, E.; Osinusi, A.; Kohli, A.; Masur, H.; Kottilil, S.; et al. Multitarget Direct-Acting Antiviral Therapy Is Associated With Superior Immunologic Recovery in Patients Coinfected With Human Immunodeficiency Virus and Hepatitis C Virus. Hepatol. Commun. 2018, 2, 1451–1466. [Google Scholar] [CrossRef]
- Soares Correia, R.; Franca, M. An Immunological Non-responder Human Immunodeficiency Virus/Hepatitis C Virus Coinfected Patient: Considerations About a Clinical Case. Cureus 2023, 15, e37063. [Google Scholar] [CrossRef]
- Zhao, J.; Dang, X.; Zhang, P.; Nguyen, L.N.; Cao, D.; Wang, L.; Wu, X.; Morrison, Z.D.; Zhang, Y.; Jia, Z.; et al. Insufficiency of DNA repair enzyme ATM promotes naive CD4 T-cell loss in chronic hepatitis C virus infection. Cell Discov. 2018, 4, 16. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, S.; Sun, J.; Xu, J.; Zhang, X. Irreversible phenotypic perturbation and functional impairment of B cells during HIV-1 infection. Front. Med. 2017, 11, 536–547. [Google Scholar] [CrossRef]
- Moir, S.; Fauci, A.S. B cells in HIV infection and disease. Nat. Rev. Immunol. 2009, 9, 235–245. [Google Scholar] [CrossRef]
- Hamdane, N.; Jühling, F.; Crouchet, E.; El Saghire, H.; Thumann, C.; Oudot, M.A.; Bandiera, S.; Saviano, A.; Ponsolles, C.; Roca Suarez, A.A.; et al. HCV-Induced Epigenetic Changes Associated With Liver Cancer Risk Persist After Sustained Virologic Response. Gastroenterology 2019, 156, 2313–2329.e2317. [Google Scholar] [CrossRef]
- Underwood, A.P.; Gupta, M.; Wu, B.-R.; Eltahla, A.A.; Boo, I.; Wang, J.J.; Agapiou, D.; Abayasingam, A.; Reynaldi, A.; Keoshkerian, E.; et al. B-cell characteristics define HCV reinfection outcome. J. Hepatol. 2024, 81, 415–428. [Google Scholar] [CrossRef]
- Moretti, S.; Mancini, F.; Borsetti, A. Long term immunological perturbations post DAA therapy in chronic HCV/HIV co-infected patients. Biocell 2022, 46, 2695–2699. [Google Scholar] [CrossRef]
- Berenguer, J.; Alvarez-Pellicer, J.; Martin, P.M.; Lopez-Aldeguer, J.; Von-Wichmann, M.A.; Quereda, C.; Mallolas, J.; Sanz, J.; Tural, C.; Bellon, J.M.; et al. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology 2009, 50, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Merino, V.; Martin-Serrano, M.; Beltran, M.; Lazaro-Martin, B.; Cervantes, E.; Oltra, M.; Sainz, T.; Garcia, F.; Navarro, M.L.; Yuste, E. The Association of HIV-1 Neutralization in Aviremic Children and Adults with Time to ART Initiation and CD4+/CD8+ Ratios. Vaccines 2023, 12, 8. [Google Scholar] [CrossRef]
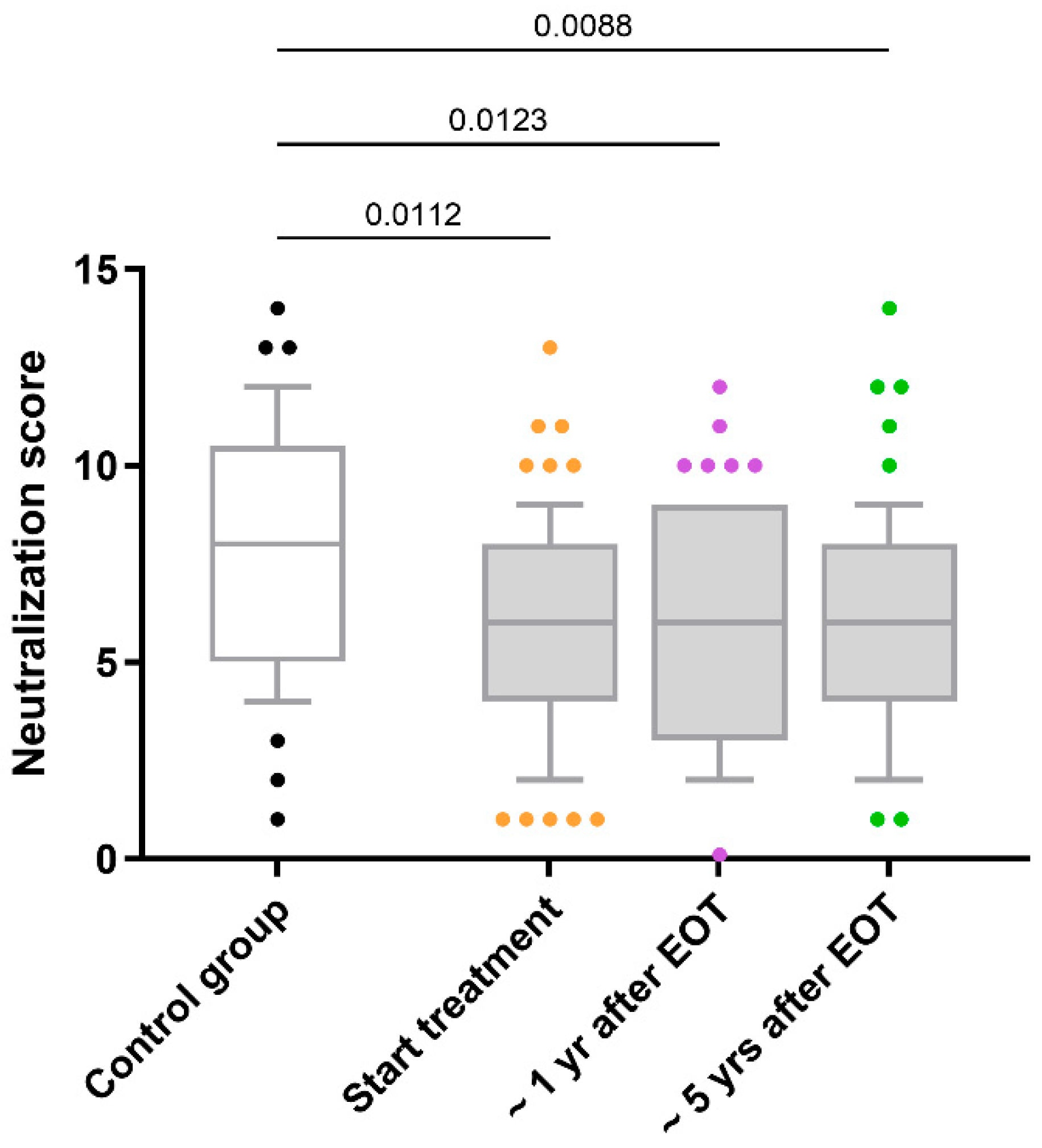
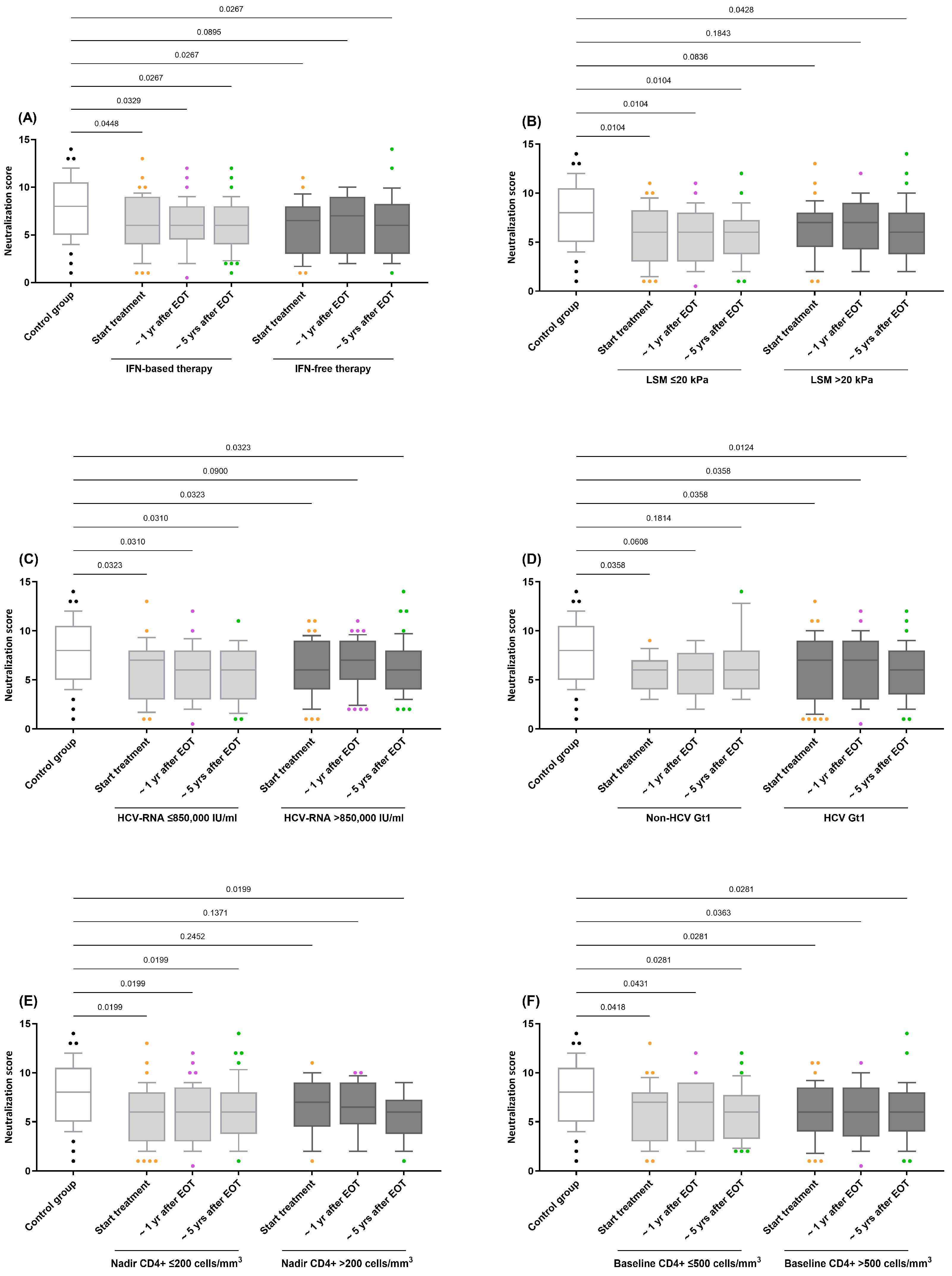
| Characteristics | Data |
|---|---|
| No | 71 |
| Epidemiological data | |
| Age (years), median [IQR] | 51 [47–54] |
| Gender (male), n (%) | 58 (81.7) |
| Body mass index (BMI, kg/m2), median [IQR] | 24.6 [21.5–28.7] |
| Smoking status, n (%) | |
| -Current | 47 (66.2) |
| -Never | 5 (7.0) |
| -Former (>6 months) | 19 (26.8) |
| Alcohol consumption (>50 g/day), n (%) | |
| -Current | 3 (4.2) |
| -Never | 36 (50.7) |
| -Former (>6 months) | 32 (45.1) |
| People who inject drugs, n (%) | |
| -Current | 0 (0) |
| -Never | 16 (22.5) |
| -Former (>6 months) | 55 (77.5) |
| HIV-related markers | |
| Prior acquired immunodeficiency syndrome (AIDS) diagnosis, n (%) | 3 (4.2) |
| Lowest CD4+ count/mm3, median [IQR] | 135.0 [93.0–234.0] |
| CD4+ < 200/mm3 at nadir, n (%) | 21 (30.4) |
| Baseline CD4+ count/mm3, median [IQR] | 518.0 [285.0–731.0] |
| Baseline CD4+ > 500/mm3, n (%) | 37 (52.1) |
| HIV antiretroviral therapy regimen, n (%) | |
| Nucleoside + non-nucleoside reverse transcriptase inhibitors | 19 (26.8) |
| Nucleoside reverse transcriptase + integrase inhibitors | 31 (43.7) |
| Nucleoside reverse transcriptase + protease inhibitors | 16 (22.5) |
| Protease + integrase + non-nucleoside reverse transcriptase inhibitors/maraviroc | 2 (2.8) |
| Other | 3 (4.2) |
| Liver disease indicators | |
| Liver Stiffness Measurement (LSM, kPa), median [IQR] | 21.0 [12.8–35.0] |
| -<12.5 kPa | 17 (23.9) |
| -12.5 kPa to 25 kPa | 22 (31.0) |
| -25 kPa to 40 kPa | 21 (29.6) |
| ->40 kPa | 11 (15.5) |
| Hepatic decompensation, n (%) | 8 (11.3) |
| HCV treatment, n (%) | |
| Previous HCV treatment | 41 (57.7) |
| Initial HCV treatment | |
| -Interferon-based regimen | 45 (63.4) |
| -Interferon-free DAA regimen | 26 (36.6) |
| HCV-related markers | |
| HCV genotype, n (%) | |
| -1 | 54 (76.1) |
| -3 | 8 (11.3) |
| -4 | 7 (9.9) |
| -Other/Unknown | 2 (2.8) |
| Log10 HCV-RNA (IU/mL), median [IQR] | 6.1 [5.8–6.6] |
| HCV-RNA ≥ 850,000 IU/mL, n (%) | 44 (62.9) |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HIV vs. HIV/HCV | AMR (95% CI) | p-Value | q-Value | aAMR (95% CI) | p-Value | q-Value |
| Baseline | 0.77 (0.65; 0.91) | 0.003 | 0.004 | 0.81 (0.67; 0.99) | 0.035 | 0.038 |
| ~1 yr after EOT | 0.78 (0.66; 0.93) | 0.005 | 0.005 | 0.82 (0.68; 0.99) | 0.038 | 0.038 |
| ~5 yrs after EOT | 0.76 (0.64; 0.91) | 0.002 | 0.004 | 0.79 (0.64; 0.97) | 0.026 | 0.038 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sepúlveda-Crespo, D.; Sánchez-Merino, V.; Amigot-Sánchez, R.; Rubio-Pérez, A.; Díez, C.; Hontañón, V.; Berenguer, J.; González-García, J.; García, F.; Martínez, I.; et al. Persistent Low Anti-HIV Neutralizing Antibody Titers in HIV/HCV Coinfection Despite HCV Cure: A 5-Year Longitudinal Analysis. Vaccines 2025, 13, 539. https://doi.org/10.3390/vaccines13050539
Sepúlveda-Crespo D, Sánchez-Merino V, Amigot-Sánchez R, Rubio-Pérez A, Díez C, Hontañón V, Berenguer J, González-García J, García F, Martínez I, et al. Persistent Low Anti-HIV Neutralizing Antibody Titers in HIV/HCV Coinfection Despite HCV Cure: A 5-Year Longitudinal Analysis. Vaccines. 2025; 13(5):539. https://doi.org/10.3390/vaccines13050539
Chicago/Turabian StyleSepúlveda-Crespo, Daniel, Víctor Sánchez-Merino, Rafael Amigot-Sánchez, Almudena Rubio-Pérez, Cristina Díez, Víctor Hontañón, Juan Berenguer, Juan González-García, Felipe García, Isidoro Martínez, and et al. 2025. "Persistent Low Anti-HIV Neutralizing Antibody Titers in HIV/HCV Coinfection Despite HCV Cure: A 5-Year Longitudinal Analysis" Vaccines 13, no. 5: 539. https://doi.org/10.3390/vaccines13050539
APA StyleSepúlveda-Crespo, D., Sánchez-Merino, V., Amigot-Sánchez, R., Rubio-Pérez, A., Díez, C., Hontañón, V., Berenguer, J., González-García, J., García, F., Martínez, I., Yuste, E., & Resino, S. (2025). Persistent Low Anti-HIV Neutralizing Antibody Titers in HIV/HCV Coinfection Despite HCV Cure: A 5-Year Longitudinal Analysis. Vaccines, 13(5), 539. https://doi.org/10.3390/vaccines13050539








