Mucosal and Serum Neutralization Immune Responses Elicited by COVID-19 mRNA Vaccination in Vaccinated and Breakthrough-Infection Individuals: A Longitudinal Study from Louisville Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Participant Inclusion and Exclusion Criteria
2.3. Human Samples and Serology
2.4. Cells and Viruses
2.5. Infectious Virus Microneutralization Assay
2.6. Anti-RBD Assay for Wild Type and Variants of Concerns (VOCs)
2.7. Statistics
3. Results
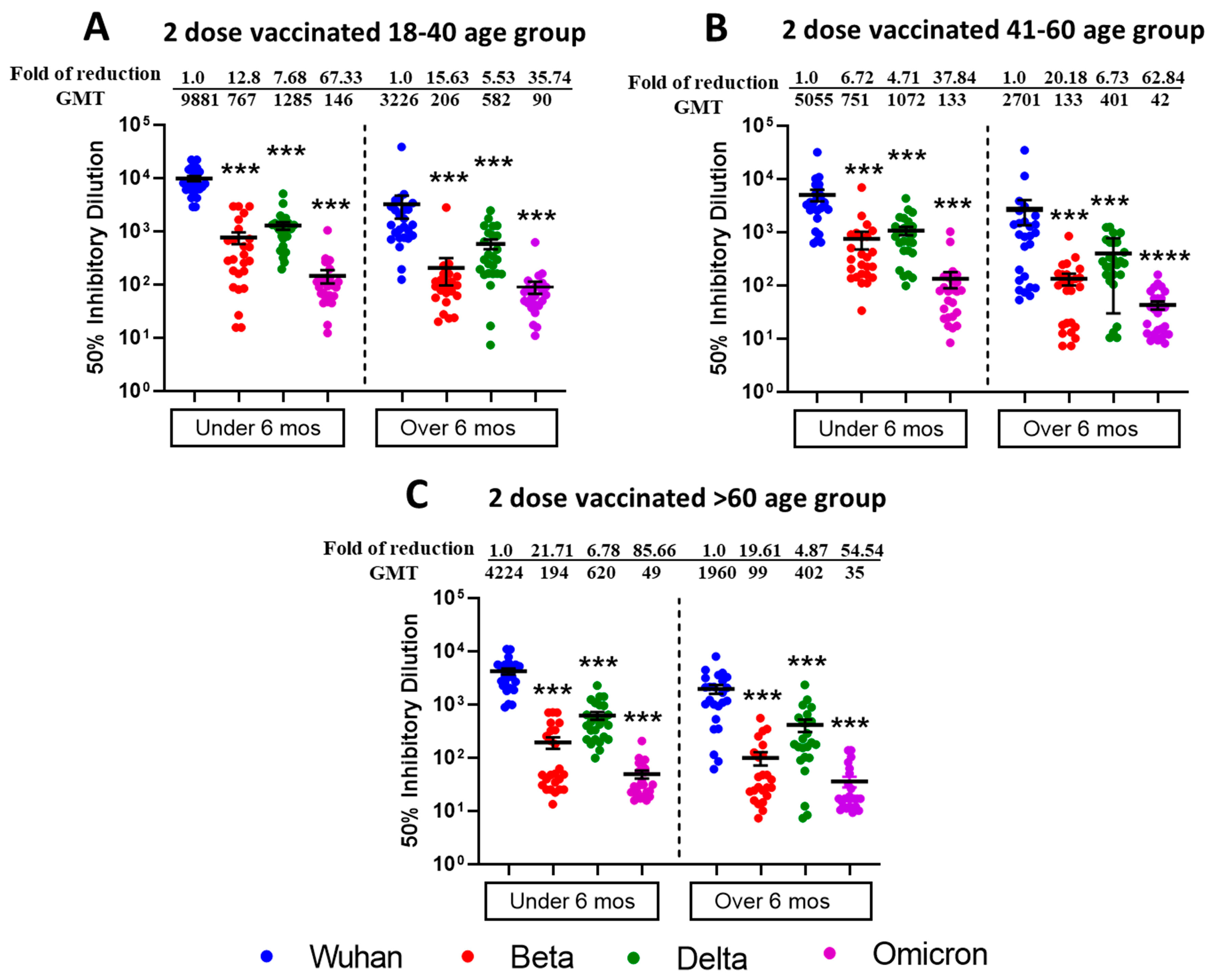
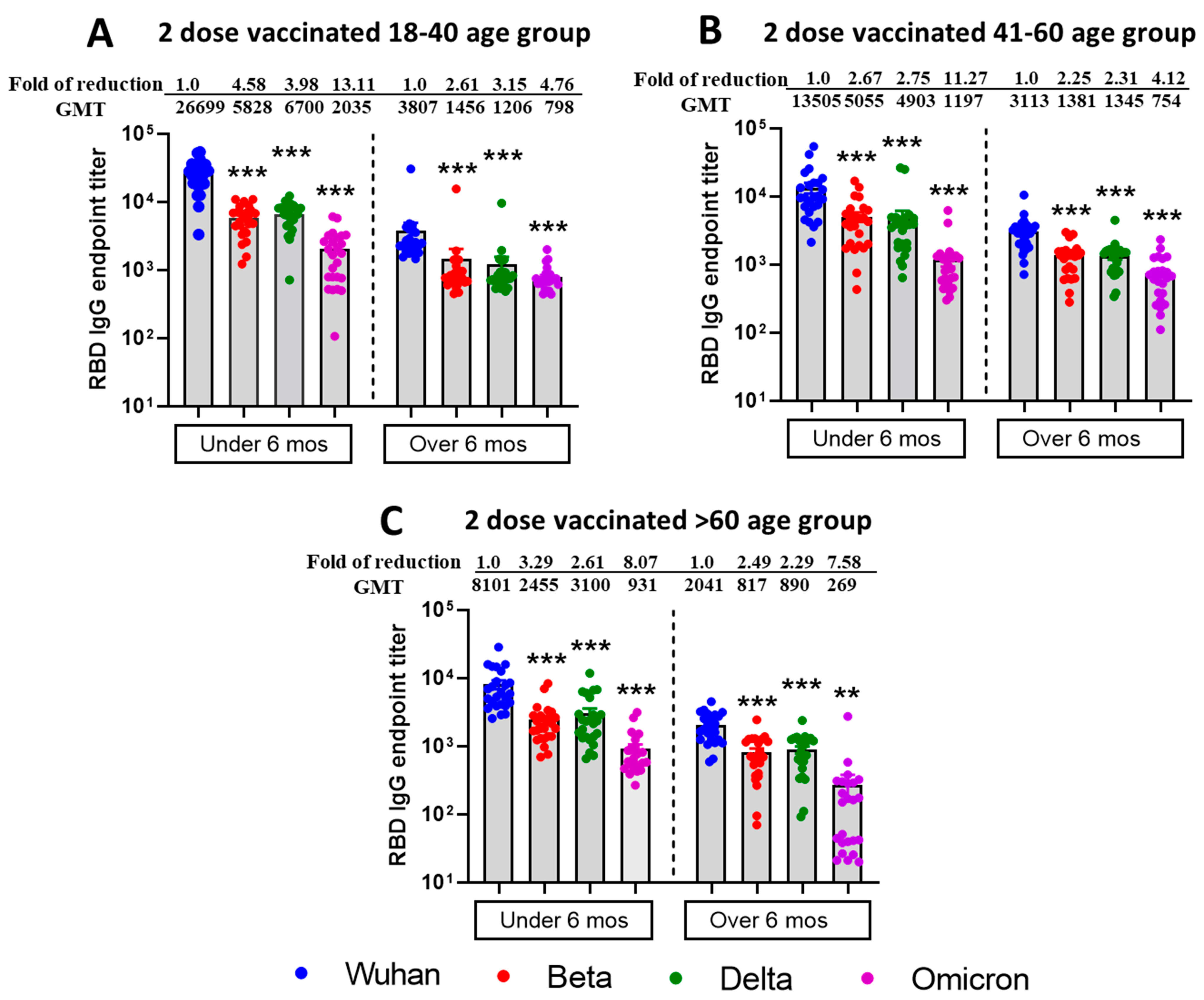
3.1. Reduced Sensitivity of SARS-CoV-2 Variants Beta, Delta, and Omicron BA.1 to Antibody Neutralization from Sera of Pfizer- or Moderna-Vaccinated Individuals
3.2. Increased Efficiency for Virus Microneutralization and IgG Binding to RBD of Different VOCs from Sera Samples Collected from Two-Dose Vaccinated and SARS-CoV-2 Infected (Breakthrough) Individuals
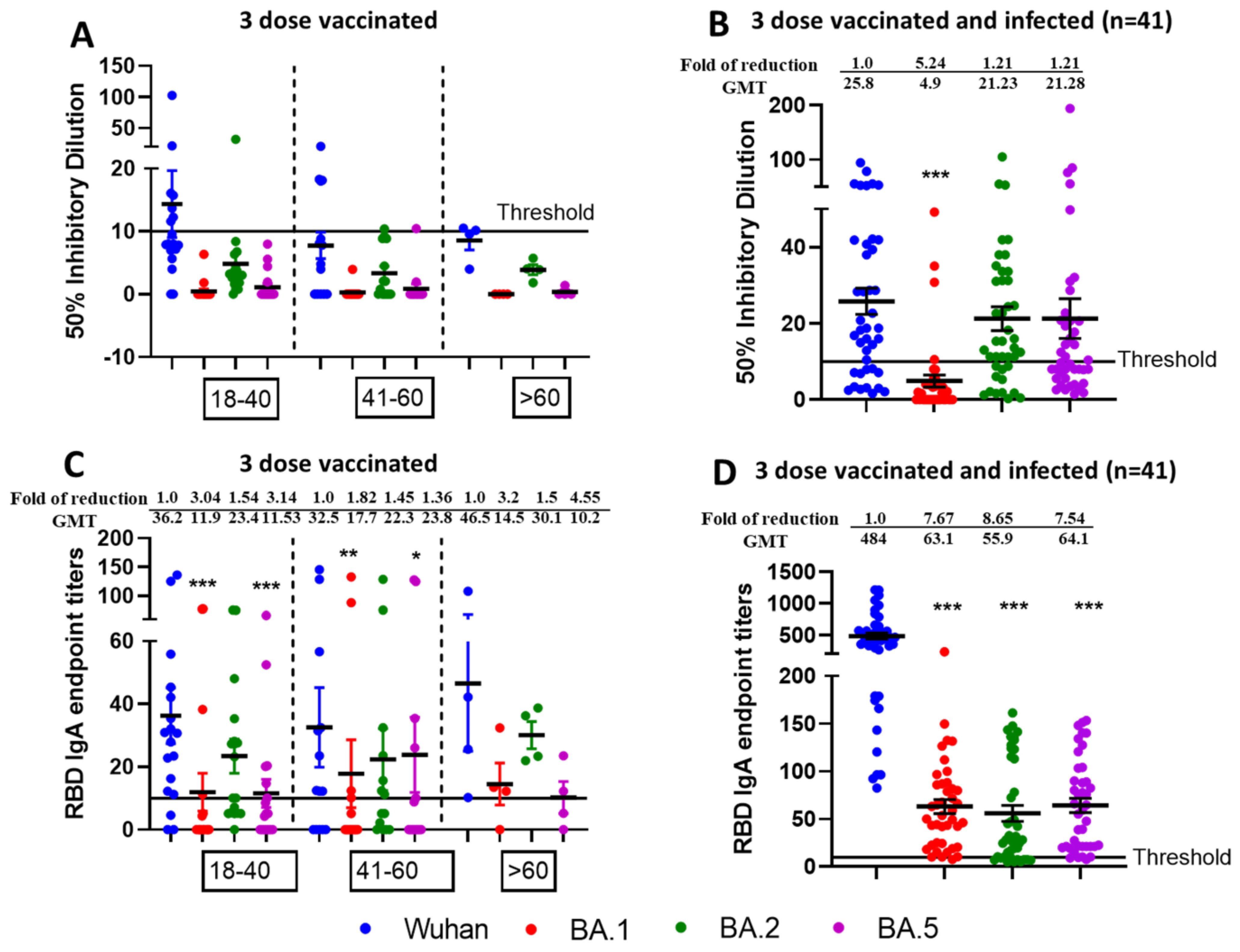
3.3. Reduced Sensitivity of New SARS-CoV-2 Omicron Variants BA.1 and BA.5, but Not BA.2 to Antibody Neutralization from Sera of Pfizer or Moderna Triple-Vaccinated Individuals
3.4. Higher Neutralization Potential Against New SARS-CoV-2 Omicron Variants BA.1, BA.2, and BA.5, from Sera of Pfizer or Moderna Triple-Vaccinated Individuals and SARS-CoV-2 Infected (Breakthrough) Individuals
3.5. Mucosal IgA Generated Against SARS-CoV-2 Omicron Infection Leading to Enhanced, Effective, and Cross Neutralization Against BA.1, BA.2, and BA.5 from Nasal Swab Samples of Triple Vaccinated and SARS-CoV-2 Infected Individuals
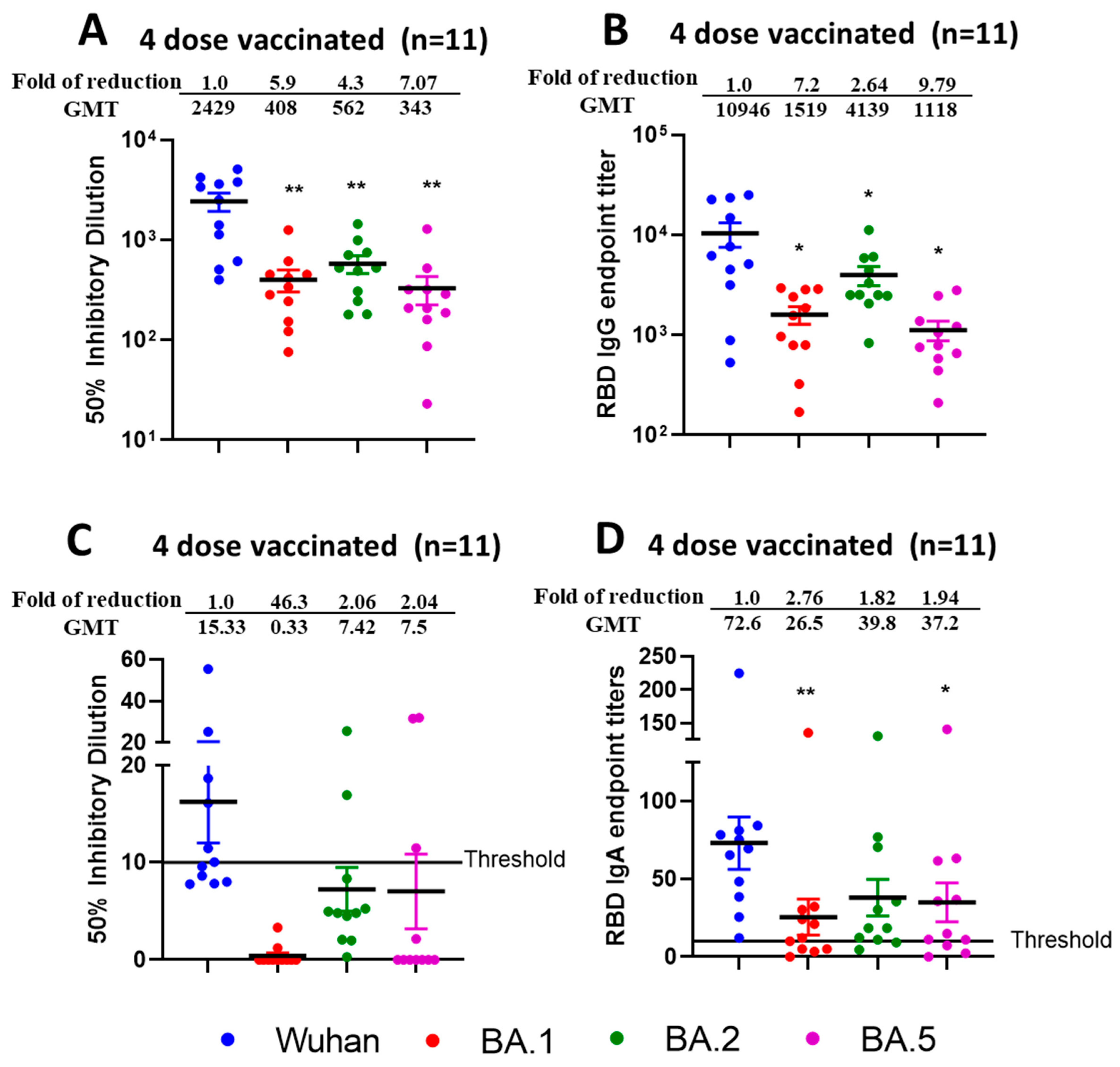
3.6. Reduced Sensitivity of BA.1, BA.2, and BA.5 to Antibody Neutralization from Sera and Nasal Swabs of Double-Boosted (Quadruple-Vaccinated) Individuals
4. Discussion
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.J.; Dong, X.; Liu, G.H.; Gao, Y.D. Risk and Protective Factors for COVID-19 Morbidity, Severity, and Mortality. Clin. Rev. Allergy Immunol. 2023, 64, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Pietzner, M.; Denaxas, S.; Yasmeen, S.; Ulmer, M.A.; Nakanishi, T.; Arnold, M.; Kastenmüller, G.; Hemingway, H.; Langenberg, C. Complex patterns of multimorbidity associated with severe COVID-19 and long COVID. Commun. Med. 2024, 4, 94. [Google Scholar] [CrossRef]
- Alharbi, A.M.; Rabbani, S.I.; Halim Mohamed, A.A.; Almushayti, B.K.; Aldhwayan, N.I.; Almohaimeed, A.T.; Alharbi, A.A.; Alharbi, N.S.; Asdaq, S.M.B.; Alamri, A.S.; et al. Analysis of potential risk factors associated with COVID-19 and hospitalization. Front. Public Health 2022, 10, 921953. [Google Scholar] [CrossRef] [PubMed]
- Mulchandani, R.; Lyngdoh, T.; Kakkar, A.K. Deciphering the COVID-19 cytokine storm: Systematic review and meta-analysis. Eur. J. Clin. Invesig. 2021, 51, e13429. [Google Scholar] [CrossRef]
- Lee, C.J.; Woo, W.; Kim, A.Y.; Yon, D.K.; Lee, S.W.; Koyanagi, A.; Kim, M.S.; Tizaoui, K.; Dragioti, E.; Radua, J.; et al. Clinical manifestations of COVID-19 breakthrough infections: A systematic review and meta-analysis. J. Med. Virol. 2022, 94, 4234–4245. [Google Scholar] [CrossRef]
- CDC.gov, Symptoms of COVID-19. 2024. Available online: https://www.cdc.gov/covid/signs-symptoms/index.html (accessed on 15 March 2025).
- Tyagi, S.C.; Singh, M. Multi-organ damage by COVID-19: Congestive (cardio-pulmonary) heart failure, and blood-heart barrier leakage. Mol. Cell Biochem. 2021, 476, 1891–1895. [Google Scholar] [CrossRef]
- Patel, R.; Kaki, M.; Potluri, V.S.; Kahar, P.; Khanna, D. A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson. Hum. Vaccin. Immunother. 2022, 18, 2002083. [Google Scholar] [CrossRef] [PubMed]
- CDC.gov, Staying Up to Date with COVID-19 Vaccines. 2025. Available online: https://www.cdc.gov/covid/vaccines/stay-up-to-date.html (accessed on 15 March 2025).
- Amanatidou, E.; Gkiouliava, A.; Pella, E.; Serafidi, M.; Tsilingiris, D.; Vallianou, N.G.; Karampela, I.; Dalamaga, M. Breakthrough infections after COVID-19 vaccination: Insights, perspectives and challenges. Metabol. Open 2022, 14, 100180. [Google Scholar] [CrossRef]
- Paul, P.; France, A.M.; Aoki, Y.; Batra, D.; Biggerstaff, M.; Dugan, V.; Galloway, S.; Hall, A.J.; Johansson, M.A.; Kondor, R.J.; et al. Genomic Surveillance for SARS-CoV-2 Variants Circulating in the United States, December 2020-May 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 846–850. [Google Scholar] [CrossRef]
- Ma, K.C.; Shirk, P.; Lambrou, A.S.; Hassell, N.; Zheng, X.Y.; Payne, A.B.; Ali, A.R.; Batra, D.; Caravas, J.; Chau, R.; et al. Genomic Surveillance for SARS-CoV-2 Variants: Circulation of Omicron Lineages—United States, January 2022-May 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 651–656. [Google Scholar] [CrossRef]
- Ma, K.C.; Castro, J.; Lambrou, A.S.; Rose, E.B.; Cook, P.W.; Batra, D.; Cubenas, C.; Hughes, L.J.; MacCannell, D.R.; Mandal, P.; et al. Genomic Surveillance for SARS-CoV-2 Variants: Circulation of Omicron XBB and JN.1 Lineages—United States, May 2023-September 2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, D.N.; Arangies, E.; David, G.L.; Armstrong, B.; Scocca, T.Z.; Fedler, J.; Natarajan, R.; Zhou, J.; Jayashankar, L.; Donis, R.; et al. Development of Next-Generation COVID-19 Vaccines: Biomedical Advanced Research and Development Authority (BARDA-)-Supported Phase 2b Study Designs. Clin. Infect. Dis. 2024, 79, 928–935. [Google Scholar] [CrossRef]
- Huang, N.; Pérez, P.; Kato, T.; Mikami, Y.; Okuda, K.; Gilmore, R.C.; Conde, C.D.; Gasmi, B.; Stein, S.; Beach, M.; et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat. Med. 2021, 27, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Fretheim, A.; Elgersma, I.H.; Helleve, A.; Elstrøm, P.; Kacelnik, O.; Hemkens, L.G. Effect of Wearing Glasses on Risk of Infection With SARS-CoV-2 in the Community: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2244495. [Google Scholar] [CrossRef]
- Reinholm, A.; Maljanen, S.; Jalkanen, P.; Altan, E.; Tauriainen, S.; Belik, M.; Skön, M.; Haveri, A.; Österlund, P.; Iakubovskaia, A.; et al. Neutralizing antibodies after the third COVID-19 vaccination in healthcare workers with or without breakthrough infection. Commun. Med. 2024, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Oluka, G.K.; Sembera, J.; Katende, J.S.; Ankunda, V.; Kato, L.; Kurshan, A.; Graham, C.; Seow, J.; Doores, K.J.; Malim, M.H.; et al. Long-Term Immune Consequences of Initial SARS-CoV-2 A.23.1 Exposure: A Longitudinal Study of Antibody Responses and Cross-Neutralization in a Ugandan Cohort. Vaccines 2025, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Puhach, O.; Bellon, M.; Adea, K.; Bekliz, M.; Hosszu-Fellous, K.; Sattonnet, P.; Hulo, N.; Kaiser, L.; Eckerle, I.; Meyer, B. SARS-CoV-2 convalescence and hybrid immunity elicits mucosal immune responses. EBioMedicine 2023, 98, 104893. [Google Scholar] [CrossRef]
- Azzi, L.; Dalla Gasperina, D.; Veronesi, G.; Shallak, M.; Ietto, G.; Iovino, D.; Baj, A.; Gianfagna, F.; Maurino, V.; Focosi, D.; et al. Mucosal immune response in BNT162b2 COVID-19 vaccine recipients. EBioMedicine 2022, 75, 103788. [Google Scholar] [CrossRef]
- Marking, U.; Bladh, O.; Havervall, S.; Svensson, J.; Greilert-Norin, N.; Aguilera, K.; Kihlgren, M.; Salomonsson, A.C.; Månsson, M.; Gallini, R.; et al. 7-month duration of SARS-CoV-2 mucosal immunoglobulin-A responses and protection. Lancet Infect. Dis. 2023, 23, 150–152. [Google Scholar] [CrossRef]
- Kuhlmann, C.; Mayer, C.K.; Claassen, M.; Maponga, T.; Burgers, W.A.; Keeton, R.; Riou, C.; Sutherland, A.D.; Suliman, T.; Shaw, M.L.; et al. Breakthrough infections with SARS-CoV-2 omicron despite mRNA vaccine booster dose. Lancet 2022, 399, 625–626. [Google Scholar] [CrossRef]
- Keith, R.J.; Holm, R.H.; Amraotkar, A.R.; Bezold, M.M.; Brick, J.M.; Bushau-Sprinkle, A.M.; Hamorsky, K.T.; Kitterman, K.T.; Palmer, K.E.; Smith, T.; et al. Stratified Simple Random Sampling Versus Volunteer Community-Wide Sampling for Estimates of COVID-19 Prevalence. Am. J. Public Health 2023, 113, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Lu, L.; Choi, C.Y.; Cai, J.P.; Tsoi, H.W.; Chu, A.W.; Ip, J.D.; Chan, W.M.; Zhang, R.R.; Zhang, X.; et al. Impact of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Variant-Associated Receptor Binding Domain (RBD) Mutations on the Susceptibility to Serum Antibodies Elicited by Coronavirus Disease 2019 (COVID-19) Infection or Vaccination. Clin. Infect. Dis. 2022, 74, 1623–1630. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, H.; Hu, Y.; Zheng, X.; Chang, F.; Liu, Y.; Pan, Z.; Wang, Q.; Tang, F.; Qian, J.; et al. Immune evasion of Omicron variants JN.1, KP.2, and KP.3 to the polyclonal and monoclonal antibodies from COVID-19 convalescents and vaccine recipients. Antiviral Res. 2025, 235, 106092. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Staropoli, I.; Planchais, C.; Yab, E.; Jeyarajah, B.; Rahou, Y.; Prot, M.; Guivel-Benhassine, F.; Lemoine, F.; Enouf, V.; et al. Escape of SARS-CoV-2 Variants KP.1.1, LB.1, and KP3.3 From Approved Monoclonal Antibodies. Pathog. Immun. 2024, 10, 1–11. [Google Scholar] [CrossRef]
- Xu, K.; An, Y.; Liu, X.; Xie, H.; Li, D.; Yang, T.; Duan, M.; Wang, Y.; Zhao, X.; Dai, L.; et al. Neutralization of SARS-CoV-2 KP.1, KP.1.1, KP.2 and KP.3 by human and murine sera. NPJ Vaccines 2024, 9, 215. [Google Scholar] [CrossRef]
- Planas, D.; Saunders, N.; Maes, P.; Guivel-Benhassine, F.; Planchais, C.; Buchrieser, J.; Bolland, W.H.; Porrot, F.; Staropoli, I.; Lemoine, F.; et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022, 602, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ginn, H.M.; Dejnirattisai, W.; Supasa, P.; Wang, B.; Tuekprakhon, A.; Nutalai, R.; Zhou, D.; Mentzer, A.J.; Zhao, Y.; et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell 2021, 184, 4220–4236.e13. [Google Scholar] [CrossRef]
- Wang, P.; Nair, M.S.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; Wang, M.; Yu, J.; Zhang, B.; Kwong, P.D.; et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021, 593, 130–135. [Google Scholar] [CrossRef]
- Zhang, G.F.; Meng, W.; Chen, L.; Ding, L.; Feng, J.; Perez, J.; Ali, A.; Sun, S.; Liu, Z.; Huang, Y.; et al. Neutralizing antibodies to SARS-CoV-2 variants of concern including Delta and Omicron in subjects receiving mRNA-1273, BNT162b2, and Ad26.COV2.S vaccines. J. Med. Virol. 2022, 94, 5678–5690. [Google Scholar] [CrossRef]
- Evans, J.P.; Zeng, C.; Carlin, C.; Lozanski, G.; Saif, L.J.; Oltz, E.M.; Gumina, R.J.; Liu, S.L. Neutralizing antibody responses elicited by SARS-CoV-2 mRNA vaccination wane over time and are boosted by breakthrough infection. Sci. Transl. Med. 2022, 14, eabn8057. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Henry, B.M.; Pighi, L.; Lippi, G. Total anti-SARS-CoV-2 antibodies measured 6 months after Pfizer-BioNTech COVID-19 vaccination in healthcare workers. J. Med. Biochem. 2022, 41, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; St Denis, K.J.; Hoelzemer, A.; Lam, E.C.; Nitido, A.D.; Sheehan, M.L.; Berrios, C.; Ofoman, O.; Chang, C.C.; Hauser, B.M.; et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2022, 185, 457–466.e4. [Google Scholar] [CrossRef]
- Cheng, S.M.S.; Mok, C.K.P.; Leung, Y.W.Y.; Ng, S.S.; Chan, K.C.K.; Ko, F.W.; Chen, C.; Yiu, K.; Lam, B.H.S.; Lau, E.H.Y.; et al. Neutralizing antibodies against the SARS-CoV-2 Omicron variant BA.1 following homologous and heterologous CoronaVac or BNT162b2 vaccination. Nat. Med. 2022, 28, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Abu Jabal, K.; Ben-Amram, H.; Beiruti, K.; Batheesh, Y.; Sussan, C.; Zarka, S.; Edelstein, M. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: Real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021, 26, 2100096. [Google Scholar] [CrossRef] [PubMed]
- Brockman, M.A.; Mwimanzi, F.; Lapointe, H.R.; Sang, Y.; Agafitei, O.; Cheung, P.K.; Ennis, S.; Ng, K.; Basra, S.; Lim, L.Y.; et al. Reduced Magnitude and Durability of Humoral Immune Responses to COVID-19 mRNA Vaccines Among Older Adults. J. Infect. Dis. 2022, 225, 1129–1140. [Google Scholar] [CrossRef]
- Hall, V.; Foulkes, S.; Insalata, F.; Kirwan, P.; Saei, A.; Atti, A.; Wellington, E.; Khawam, J.; Munro, K.; Cole, M.; et al. Protection against SARS-CoV-2 after Covid-19 Vaccination and Previous Infection. N. Engl. J. Med. 2022, 386, 1207–1220. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Cohen, C.; Hernán, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef]
- Carazo, S.; Skowronski, D.M.; Brisson, M.; Barkati, S.; Sauvageau, C.; Brousseau, N.; Gilca, R.; Fafard, J.; Talbot, D.; Ouakki, M.; et al. Protection against omicron (B.1.1.529) BA.2 reinfection conferred by primary omicron BA.1 or pre-omicron SARS-CoV-2 infection among health-care workers with and without mRNA vaccination: A test-negative case-control study. Lancet Infect. Dis. 2023, 23, 45–55. [Google Scholar] [CrossRef]
- Chatterjee, D.; Tauzin, A.; Marchitto, L.; Gong, S.Y.; Boutin, M.; Bourassa, C.; Beaudoin-Bussières, G.; Bo, Y.; Ding, S.; Laumaea, A.; et al. SARS-CoV-2 Omicron Spike recognition by plasma from individuals receiving BNT162b2 mRNA vaccination with a 16-week interval between doses. Cell Rep. 2022, 38, 110429. [Google Scholar] [CrossRef]
- Tauzin, A.; Gong, S.Y.; Chatterjee, D.; Ding, S.; Painter, M.M.; Goel, R.R.; Beaudoin-Bussières, G.; Marchitto, L.; Boutin, M.; Laumaea, A.; et al. A boost with SARS-CoV-2 BNT162b2 mRNA vaccine elicits strong humoral responses independently of the interval between the first two doses. Cell Rep. 2022, 41, 111554. [Google Scholar] [CrossRef]
- Cao, Y.; Yisimayi, A.; Jian, F.; Song, W.; Xiao, T.; Wang, L.; Du, S.; Wang, J.; Li, Q.; Chen, X.; et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature 2022, 608, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Kurhade, C.; Zou, J.; Xia, H.; Liu, M.; Yang, Q.; Cutler, M.; Cooper, D.; Muik, A.; Sahin, U.; Jansen, K.U.; et al. Neutralization of Omicron sublineages and Deltacron SARS-CoV-2 by three doses of BNT162b2 vaccine or BA.1 infection. Emerg. Microbes Infect. 2022, 11, 1828–1832. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Karim, F.; Ganga, Y.; Bernstein, M.; Jule, Z.; Reedoy, K.; Cele, S.; Lustig, G.; Amoako, D.; Wolter, N.; et al. Omicron BA.4/BA.5 escape neutralizing immunity elicited by BA.1 infection. Nat. Commun. 2022, 13, 4686. [Google Scholar] [CrossRef] [PubMed]
- Quandt, J.; Muik, A.; Salisch, N.; Lui, B.G.; Lutz, S.; Krüger, K.; Wallisch, A.K.; Adams-Quack, P.; Bacher, M.; Finlayson, A.; et al. Omicron BA.1 breakthrough infection drives cross-variant neutralization and memory B cell formation against conserved epitopes. Sci. Immunol. 2022, 7, eabq2427. [Google Scholar] [CrossRef]
- Hansen, C.H.; Friis, N.U.; Bager, P.; Stegger, M.; Fonager, J.; Fomsgaard, A.; Gram, M.A.; Christiansen, L.E.; Ethelberg, S.; Legarth, R.; et al. Risk of reinfection, vaccine protection, and severity of infection with the BA.5 omicron subvariant: A nation-wide population-based study in Denmark. Lancet Infect. Dis. 2023, 23, 167–176. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Nagelkerke, N.; Ayoub, H.H.; Coyle, P.; Tang, P.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Hasan, M.R.; Al-Kanaani, Z.; et al. Duration of immune protection of SARS-CoV-2 natural infection against reinfection. J. Travel. Med. 2022, 29, taac109. [Google Scholar] [CrossRef]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci. Immunol. 2021, 6, eabi6950. [Google Scholar] [CrossRef]
- Loske, J.; Röhmel, J.; Lukassen, S.; Stricker, S.; Magalhães, V.G.; Liebig, J.; Chua, R.L.; Thürmann, L.; Messingschlager, M.; Seegebarth, A.; et al. Pre-activated antiviral innate immunity in the upper airways controls early SARS-CoV-2 infection in children. Nat. Biotechnol. 2022, 40, 319–324. [Google Scholar] [CrossRef]
- Tang, J.; Zeng, C.; Cox, T.M.; Li, C.; Son, Y.M.; Cheon, I.S.; Wu, Y.; Behl, S.; Taylor, J.J.; Chakaraborty, R.; et al. Respiratory mucosal immunity against SARS-CoV-2 after mRNA vaccination. Sci. Immunol. 2022, 7, eadd4853. [Google Scholar] [CrossRef]
- Mitsi, E.; Diniz, M.O.; Reiné, J.; Collins, A.M.; Robinson, R.E.; Hyder-Wright, A.; Farrar, M.; Liatsikos, K.; Hamilton, J.; Onyema, O.; et al. Respiratory mucosal immune memory to SARS-CoV-2 after infection and vaccination. Nat. Commun. 2023, 14, 6815. [Google Scholar] [CrossRef]
- Planas, D.; Staropoli, I.; Porot, F.; Guivel-Benhassine, F.; Handala, L.; Prot, M.; Bolland, W.H.; Puech, J.; Péré, H.; Veyer, D.; et al. Duration of BA.5 neutralization in sera and nasal swabs from SARS-CoV-2 vaccinated individuals, with or without omicron breakthrough infection. Med 2022, 3, 838–847.e3. [Google Scholar] [CrossRef] [PubMed]
- Knisely, J.M.; Buyon, L.E.; Mandt, R.; Farkas, R.; Balasingam, S.; Bok, K.; Buchholz, U.J.; D’Souza, M.P.; Gordon, J.L.; King, D.F.L.; et al. Mucosal vaccines for SARS-CoV-2: Scientific gaps and opportunities-workshop report. NPJ Vaccines 2023, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Pilapitiya, D.; Wheatley, A.K.; Tan, H.X. Mucosal vaccines for SARS-CoV-2: Triumph of hope over experience. eBioMedicine 2023, 92, 104585. [Google Scholar] [CrossRef]
- McMahan, K.; Wegmann, F.; Aid, M.; Sciacca, M.; Liu, J.; Hachmann, N.P.; Miller, J.; Jacob-Dolan, C.; Powers, O.; Hope, D.; et al. Mucosal boosting enhances vaccine protection against SARS-CoV-2 in macaques. Nature 2024, 626, 385–391. [Google Scholar] [CrossRef] [PubMed]


| Vaccinated and Infected (n = 17) | Vaccinated Only (n = 25) | p-Value | |
|---|---|---|---|
| Wuhan | 10,587 (12,245) | 6554 (7292) | 0.212 |
| Beta | 2897 (7872) | 487 (829) | 0.017 |
| Delta | 4688 (7188) | 934 (912) | 0.016 |
| Omicron.BA.1 | 674 (1275) | 118 (166) | 0.097 |
| Infected | Vaccinated | p-Value | |
|---|---|---|---|
| Wuhan | 25,660 (41,375) | 15,253 (14,933) | 0.383 |
| Beta | 2573 (3848) | 3642 (3586) | 0.054 |
| Delta | 6757 (11,896) | 3954 (3561) | 0.522 |
| Omicron.BA.1 | 1444 (2393) | 1417 (1291) | 0.091 |
| Infected | Vaccinated | p-Value | |
|---|---|---|---|
| Wuhan | 1861 (1517) | 1303 (1571) | 0.004 |
| Omicron.BA.1 | 1032 (771) | 248 (296) | <0.001 |
| Omicron.BA.2 | 1522 (1211) | 401 (405) | <0.001 |
| Omicron.BA.5 | 1337 (2092) | 152 (206) | <0.001 |
| Infected | Vaccinated | p-Value | |
|---|---|---|---|
| Wuhan | 4910 (3738) | 5662 (6246) | 0.575 |
| Omicron.BA.1 | 2963 (2922) | 970 (875) | <0.001 |
| Omicron.BA.2 | 5283 (3708) | 1783 (1954) | <0.001 |
| Omicron.BA.5 | 2466 (2011) | 649 (686) | <0.001 |
| Infected | Vaccinated | p-Value | |
|---|---|---|---|
| Wuhan | 26 (22) | 12 (16) | 0.003 |
| Omicron.BA.1 | 5 (10) | 0 (1) | <0.001 |
| Omicron.BA.2 | 21 (20) | 5 (6) | <0.001 |
| Omicron.BA.5 | 21 (33) | 2 (7) | <0.001 |
| Infected | Vaccinated | p-Value | |
|---|---|---|---|
| Wuhan | 484 (314) | 44 (47) | <0.001 |
| Omicron.BA.1 | 63 (47) | 17 (33) | <0.001 |
| Omicron.BA.2 | 56 (54) | 27 (31) | 0.002 |
| Omicron.BA.5 | 64 (47) | 21 (35) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batra, L.; Saxena, D.; Poddar, T.; Zahin, M.; Amraotkar, A.; Bezold, M.M.; Kitterman, K.T.; Deitz, K.A.; Lasnik, A.B.; Keith, R.J.; et al. Mucosal and Serum Neutralization Immune Responses Elicited by COVID-19 mRNA Vaccination in Vaccinated and Breakthrough-Infection Individuals: A Longitudinal Study from Louisville Cohort. Vaccines 2025, 13, 559. https://doi.org/10.3390/vaccines13060559
Batra L, Saxena D, Poddar T, Zahin M, Amraotkar A, Bezold MM, Kitterman KT, Deitz KA, Lasnik AB, Keith RJ, et al. Mucosal and Serum Neutralization Immune Responses Elicited by COVID-19 mRNA Vaccination in Vaccinated and Breakthrough-Infection Individuals: A Longitudinal Study from Louisville Cohort. Vaccines. 2025; 13(6):559. https://doi.org/10.3390/vaccines13060559
Chicago/Turabian StyleBatra, Lalit, Divyasha Saxena, Triparna Poddar, Maryam Zahin, Alok Amraotkar, Megan M. Bezold, Kathleen T. Kitterman, Kailyn A. Deitz, Amanda B. Lasnik, Rachel J. Keith, and et al. 2025. "Mucosal and Serum Neutralization Immune Responses Elicited by COVID-19 mRNA Vaccination in Vaccinated and Breakthrough-Infection Individuals: A Longitudinal Study from Louisville Cohort" Vaccines 13, no. 6: 559. https://doi.org/10.3390/vaccines13060559
APA StyleBatra, L., Saxena, D., Poddar, T., Zahin, M., Amraotkar, A., Bezold, M. M., Kitterman, K. T., Deitz, K. A., Lasnik, A. B., Keith, R. J., Bhatnagar, A., Kong, M., Gabbard, J. D., Severson, W. E., & Palmer, K. E. (2025). Mucosal and Serum Neutralization Immune Responses Elicited by COVID-19 mRNA Vaccination in Vaccinated and Breakthrough-Infection Individuals: A Longitudinal Study from Louisville Cohort. Vaccines, 13(6), 559. https://doi.org/10.3390/vaccines13060559








