Ocular Manifestations of Mpox and Other Poxvirus Infections: Clinical Insights and Emerging Therapeutic and Preventive Strategies
Abstract
:1. Introduction
2. MPXV Variants and Transmission
3. Systemic Manifestations of Mpox
4. Ocular Complication in Poxvirus Infections with Focus on Mpox
4.1. Ocular Complications in Poxvirus Infections
4.2. Clinical Features of Mpox-Related Eye Disease
4.2.1. Overview of Ocular Manifestations
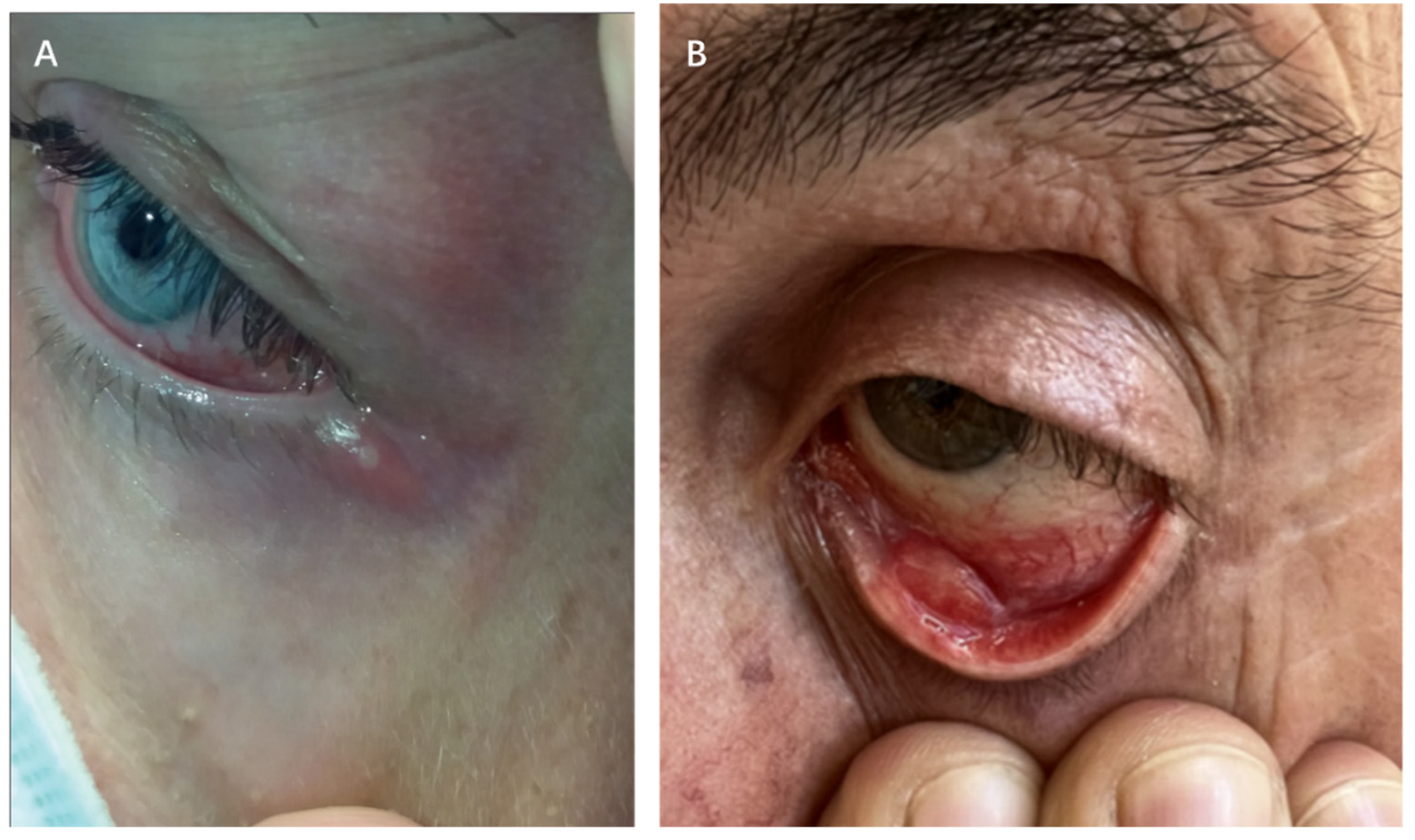
4.2.2. Periocular Manifestations
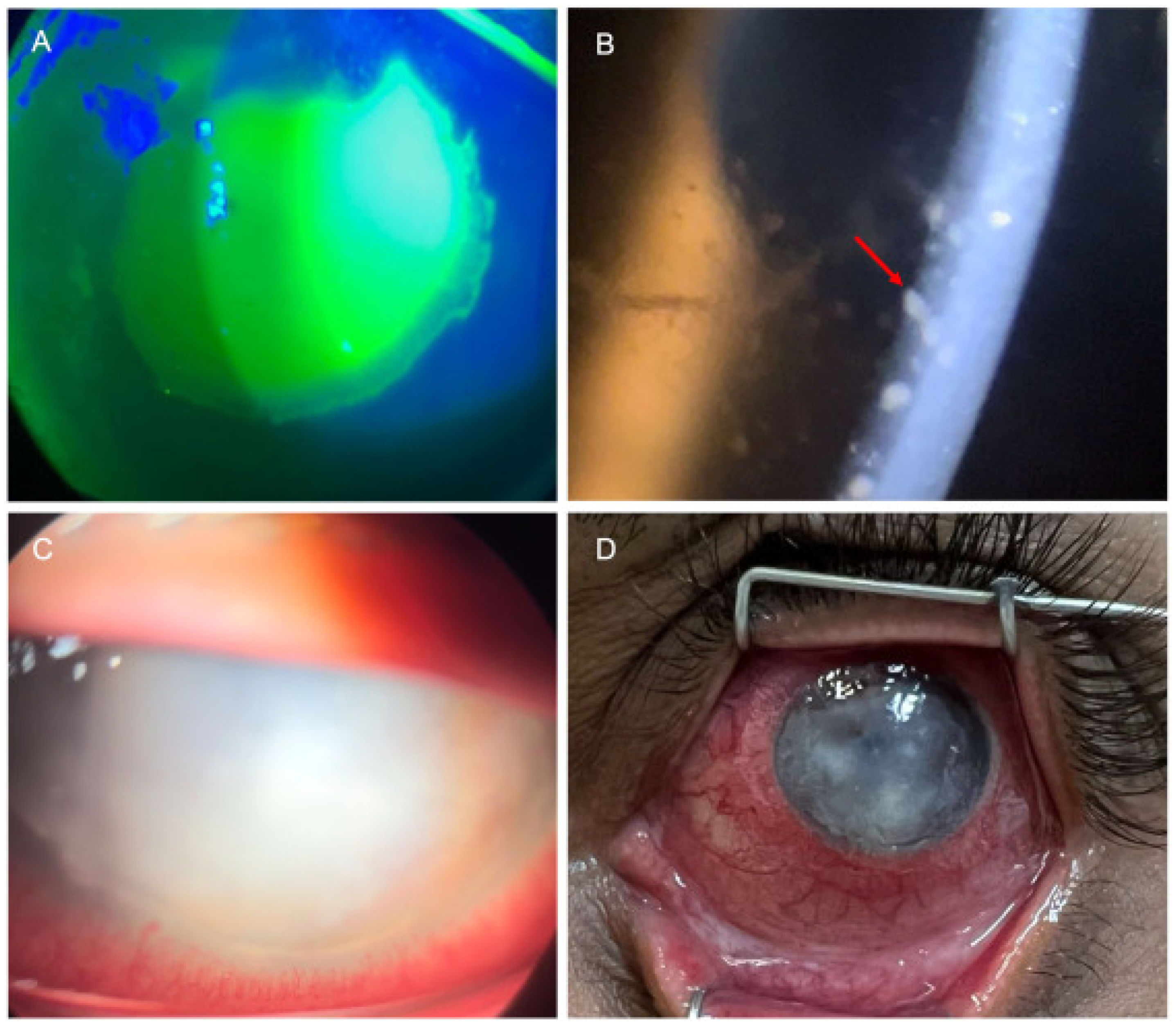
4.2.3. Anterior Segment Manifestations
Conjunctivitis
Keratitis
Scleritis
4.2.4. Intraocular Manifestations
Uveitis
4.2.5. Severe Complications
4.3. Clade-Specific Ocular Manifestations
5. Advances and Challenges in the Diagnosis of Mpox-Related Ocular Complications
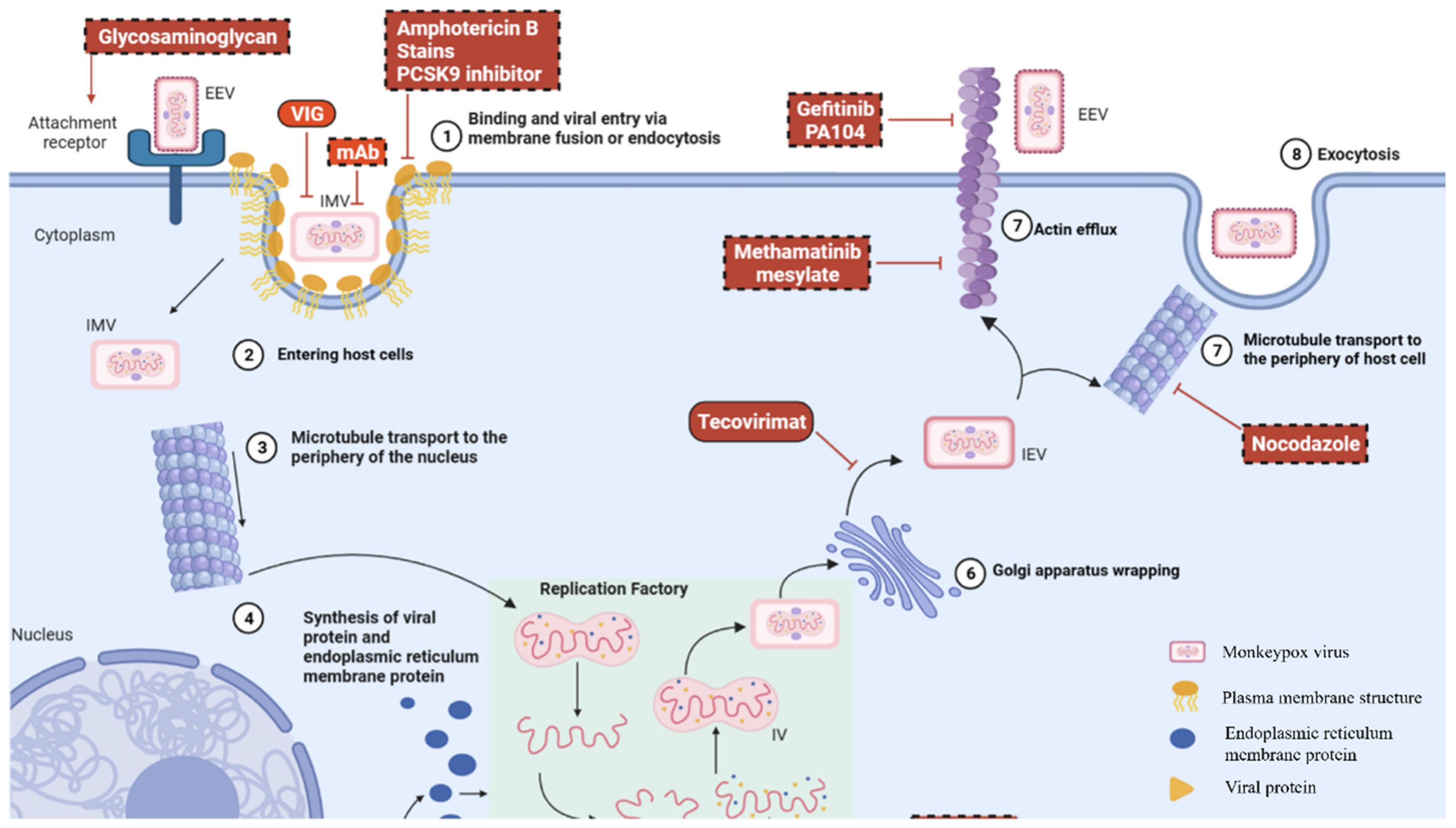
6. Current and Emerging Therapeutic Strategies for Ocular MPXV Infection
6.1. Management of Systemic Mpox
6.2. Ocular Treatment for Mpox
6.3. Vaccine Application for Mpox
7. Future Directions in the Prevention and Management of Mpox Ocular Manifestations
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hata, D.J.; Powell, E.A.; Starolis, M.W.; Realegeno, S.E. What the pox? Review of poxviruses affecting humans. J. Clin. Virol. 2024, 174, 105719. [Google Scholar] [CrossRef] [PubMed]
- Aljabali, A.A.A.; Obeid, M.A.; Nusair, M.B.; Hmedat, A.; Tambuwala, M.M. Monkeypox virus: An emerging epidemic. Microb. Pathog. 2022, 173, 105794. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.L.; Langland, J.O.; Kibler, K.V.; Denzler, K.L.; White, S.D.; Holechek, S.A.; Wong, S.; Huynh, T.; Baskin, C.R. Vaccinia virus vaccines: Past, present and future. Antivir. Res. 2009, 84, 1–13. [Google Scholar] [CrossRef]
- León-Figueroa, D.A.; Bonilla-Aldana, D.K.; Pachar, M.; Romaní, L.; Saldaña-Cumpa, H.M.; Anchay-Zuloeta, C.; Diaz-Torres, M.; Franco-Paredes, C.; Suárez, J.A.; Ramirez, J.D.; et al. The never-ending global emergence of viral zoonoses after COVID-19? The rising concern of monkeypox in Europe, North America and beyond. Travel Med. Infect. Dis. 2022, 49, 102362. [Google Scholar] [CrossRef]
- Hatami, H.; Jamshidi, P.; Arbabi, M.; Safavi-Naini, S.A.A.; Farokh, P.; Izadi-Jorshari, G.; Mohammadzadeh, B.; Nasiri, M.J.; Zandi, M.; Nayebzade, A.; et al. Demographic, Epidemiologic, and Clinical Characteristics of Human Monkeypox Disease Pre- and Post-2022 Outbreaks: A Systematic Review and Meta-Analysis. Biomedicines 2023, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, J.B.; Borio, L.L.; Gostin, L.O. The WHO Declaration of Monkeypox as a Global Public Health Emergency. JAMA 2022, 328, 615–617. [Google Scholar] [CrossRef]
- Zong, Y.; Kamoi, K.; Zhang, J.; Yang, M.; Ohno-Matsui, K. Mpox (Monkeypox) and the Eye: Ocular Manifestation, Diagnosis, Treatment and Vaccination. Viruses 2023, 15, 616. [Google Scholar] [CrossRef]
- Taylor, L. WHO and African CDC declare mpox a public health emergency. BMJ 2024, 386, q1809. [Google Scholar] [CrossRef]
- Azizi, A.; Rose, K.; Kamuyu, G.; Ogbeni, D.; Bernasconi, V. Preparedness and priority research to tackle the mpox outbreak response. Nat. Med. 2025, 31, 14–15. [Google Scholar] [CrossRef]
- Abdelaal, A.; Serhan, H.A.; Mahmoud, M.A.; Rodriguez-Morales, A.J.; Sah, R. Ophthalmic manifestations of monkeypox virus. Eye 2023, 37, 383–385. [Google Scholar] [CrossRef]
- Yi-Ting, L.; Chien-Hsien, H.; Hwa-Hsin, F.; Cheng-Kuo, C.; Pai-Huei, P. Monkeypox-related ophthalmic disease. Taiwan J. Ophthalmol. 2024, 14, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Alcamí, A. Pathogenesis of the circulating mpox virus and its adaptation to humans. Proc. Natl. Acad. Sci. USA 2023, 120, e2301662120. [Google Scholar] [CrossRef]
- Català, A.; Clavo-Escribano, P.; Riera-Monroig, J.; Martín-Ezquerra, G.; Fernandez-Gonzalez, P.; Revelles-Peñas, L.; Simon-Gozalbo, A.; Rodríguez-Cuadrado, F.J.; Castells, V.G.; de la Torre Gomar, F.J.; et al. Monkeypox outbreak in Spain: Clinical and epidemiological findings in a prospective cross-sectional study of 185 cases*. Br. J. Dermatol. 2022, 187, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Sarkar, S.; Mishra, R. Mpox and related poxviruses: A literature review of evolution, pathophysiology, and clinical manifestations. Asian Pac. J. Trop. Biomed. 2024, 14, 319–330. [Google Scholar] [CrossRef]
- Lu, J.; Xing, H.; Wang, C.; Tang, M.; Wu, C.; Ye, F.; Yin, L.; Yang, Y.; Tan, W.; Shen, L. Mpox (formerly monkeypox): Pathogenesis, prevention and treatment. Signal Transduct. Target. Ther. 2023, 8, 458. [Google Scholar] [CrossRef]
- Happi, C.; Adetifa, I.; Mbala, P.; Njouom, R.; Nakoune, E.; Happi, A.; Ndodo, N.; Ayansola, O.; Mboowa, G.; Bedford, T.; et al. Urgent need for a non-discriminatory and non-stigmatizing nomenclature for monkeypox virus. PLoS Biol. 2022, 20, e3001769. [Google Scholar] [CrossRef] [PubMed]
- Olawade, D.B.; Wada, O.Z.; Fidelis, S.C.; Oluwole, O.S.; Alisi, C.S.; Orimabuyaku, N.F.; Clement David-Olawade, A. Strengthening Africa’s response to Mpox (monkeypox): Insights from historical outbreaks and the present global spread. Sci. One Health 2024, 3, 100085. [Google Scholar] [CrossRef]
- Van Dijck, C.; Hoff, N.A.; Mbala-Kingebeni, P.; Low, N.; Cevik, M.; Rimoin, A.W.; Kindrachuk, J.; Liesenborghs, L. Emergence of mpox in the post-smallpox era-a narrative review on mpox epidemiology. Clin. Microbiol. Infect. 2023, 29, 1487–1492. [Google Scholar] [CrossRef]
- Cho, C.T.; Wenner, H.A. Monkeypox virus. Bacteriol. Rev. 1973, 37, 1–18. [Google Scholar] [CrossRef]
- Srivastava, S.; Laxmi; Sharma, K.; Sridhar, S.B.; Talath, S.; Shareef, J.; Mehta, R.; Satapathy, P.; Sah, R. Clade Ib: A new emerging threat in the Mpox outbreak. Front. Pharmacol. 2024, 15, 1504154. [Google Scholar] [CrossRef]
- Gigante, C.M.; Korber, B.; Seabolt, M.H.; Wilkins, K.; Davidson, W.; Rao, A.K.; Zhao, H.; Smith, T.G.; Hughes, C.M.; Minhaj, F.; et al. Multiple lineages of monkeypox virus detected in the United States, 2021–2022. Science 2022, 378, 560–565. [Google Scholar] [CrossRef]
- Vakaniaki, E.H.; Kacita, C.; Kinganda-Lusamaki, E.; O’Toole, Á.; Wawina-Bokalanga, T.; Mukadi-Bamuleka, D.; Amuri-Aziza, A.; Malyamungu-Bubala, N.; Mweshi-Kumbana, F.; Mutimbwa-Mambo, L.; et al. Sustained human outbreak of a new MPXV clade I lineage in eastern Democratic Republic of the Congo. Nat. Med. 2024, 30, 2791–2795. [Google Scholar] [CrossRef] [PubMed]
- Masirika, L.M.; Udahemuka, J.C.; Schuele, L.; Ndishimye, P.; Otani, S.; Mbiribindi, J.B.; Marekani, J.M.; Mambo, L.M.; Bubala, N.M.; Boter, M.; et al. Ongoing mpox outbreak in Kamituga, South Kivu province, associated with monkeypox virus of a novel Clade I sub-lineage, Democratic Republic of the Congo, 2024. Eurosurveillance 2024, 29, 2400106. [Google Scholar] [CrossRef] [PubMed]
- Branda, F.; Ceccarelli, G.; Ciccozzi, M.; Scarpa, F. First cases of mpox Clade I outside of Africa: Genetic insights on its evolution. Infect. Dis. 2024, 56, 1003–1005. [Google Scholar] [CrossRef]
- Lee, S.S.; Traore, T.; Zumla, A. The WHO mpox public health emergency of international concern declaration: Need for reprioritisation of global public health responses to combat the MPXV Clade I epidemic. Int. J. Infect. Dis. 2024, 147, 107227. [Google Scholar] [CrossRef]
- Treutiger, C.-J.; Filén, F.; Rehn, M.; Aarum, J.; Jacks, A.; Gisslén, M.; Sturegård, E.; Karlberg, M.L.; Karlsson Lindsjö, O.; Sondén, K. First case of mpox with monkeypox virus clade Ib outside Africa in a r eturning traveller, Sweden, August 2024: Public health measures. Eurosurveillance 2024, 29, 2400740. [Google Scholar] [CrossRef]
- Petersen, E.; Hvid, U.; Tomori, O.; Pedersen, A.G.; Wallinga, J.; Pebody, R.; Cenciarelli, O.; Aavitsland, P.; Van Laeken, D.; Andreasen, V.; et al. Possible scenarios for the spread of mpox outside the endemic focus in Africa. Int. J. Infect. Dis. 2024, 153, 107373. [Google Scholar] [CrossRef]
- Faye, O.; Pratt, C.B.; Faye, M.; Fall, G.; Chitty, J.A.; Diagne, M.M.; Wiley, M.R.; Yinka-Ogunleye, A.F.; Aruna, S.; Etebu, E.N.; et al. Genomic characterisation of human monkeypox virus in Nigeria. Lancet Infect. Dis. 2018, 18, 246. [Google Scholar] [CrossRef]
- Alakunle, E.; Moens, U.; Nchinda, G.; Okeke, M.I. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses 2020, 12, 1257. [Google Scholar] [CrossRef]
- Sejvar, J.J.; Chowdary, Y.; Schomogyi, M.; Stevens, J.; Patel, J.; Karem, K.; Fischer, M.; Kuehnert, M.J.; Zaki, S.R.; Paddock, C.D. Human monkeypox infection: A family cluster in the midwestern United States. J. Infect. Dis. 2004, 190, 1833–1840. [Google Scholar] [CrossRef]
- Vaughan, A.; Aarons, E.; Astbury, J.; Brooks, T.; Chand, M.; Flegg, P.; Hardman, A.; Harper, N.; Jarvis, R.; Mawdsley, S.; et al. Human-to-Human Transmission of Monkeypox Virus, United Kingdom, October 2018. Emerg. Infect. Dis. 2020, 26, 782–785. [Google Scholar] [CrossRef]
- Yinka-Ogunleye, A.; Aruna, O.; Dalhat, M.; Ogoina, D.; McCollum, A.; Disu, Y.; Mamadu, I.; Akinpelu, A.; Ahmad, A.; Burga, J.; et al. Outbreak of human monkeypox in Nigeria in 2017–18: A clinical and epidemiological report. Lancet Infect. Dis. 2019, 19, 872–879. [Google Scholar] [CrossRef]
- Burki, T. What does it mean to declare monkeypox a PHEIC? Lancet Infect. Dis. 2022, 22, 1286–1287. [Google Scholar] [CrossRef] [PubMed]
- Beiras, C.G.; Malembi, E.; Escrig-Sarreta, R.; Ahuka, S.; Mbala, P.; Mavoko, H.M.; Subissi, L.; Abecasis, A.B.; Marks, M.; Mitja, O. Concurrent outbreaks of mpox in Africa-an update. Lancet 2025, 405, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Ogoina, D.; Izibewule, J.H.; Ogunleye, A.; Ederiane, E.; Anebonam, U.; Neni, A.; Oyeyemi, A.; Etebu, E.N.; Ihekweazu, C. The 2017 human monkeypox outbreak in Nigeria-Report of outbreak experience and response in the Niger Delta University Teaching Hospital, Bayelsa State, Nigeria. PLoS ONE 2019, 14, e0214229. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Tegally, H.; Pigott, D.M.; Dasgupta, A.; Sheldon, J.; Wilkinson, E.; Schultheiss, M.; Han, A.; Oglia, M.; Marks, S.; et al. Tracking the 2022 monkeypox outbreak with epidemiological data in real-time. Lancet Infect. Dis. 2022, 22, 941–942. [Google Scholar] [CrossRef]
- Orviz, E.; Negredo, A.; Ayerdi, O.; Vázquez, A.; Muñoz-Gomez, A.; Monzón, S.; Clavo, P.; Zaballos, A.; Vera, M.; Sánchez, P.; et al. Monkeypox outbreak in Madrid (Spain): Clinical and virological aspects. J. Infect. 2022, 85, 412–417. [Google Scholar] [CrossRef]
- Begley, J.; Kaftan, T.; Song, H.; Fashina, T.; Hartley, C.D.; Nguyen, N.; Crozier, I.; Mwanza, J.C.; Yeh, S. Ocular Complications of Mpox: Evolving Understanding and Future Directions. Int. Ophthalmol. Clin. 2024, 64, 15–22. [Google Scholar] [CrossRef]
- Kaler, J.; Hussain, A.; Flores, G.; Kheiri, S.; Desrosiers, D. Monkeypox: A Comprehensive Review of Transmission, Pathogenesis, and Manifestation. Cureus 2022, 14, e26531. [Google Scholar] [CrossRef]
- Chen, N.; Li, G.; Liszewski, M.K.; Atkinson, J.P.; Jahrling, P.B.; Feng, Z.; Schriewer, J.; Buck, C.; Wang, C.; Lefkowitz, E.J.; et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology 2005, 340, 46–63. [Google Scholar] [CrossRef]
- Titanji, B.K.; Hazra, A.; Zucker, J. Mpox Clinical Presentation, Diagnostic Approaches, and Treatment Strategies: A Review. JAMA 2024, 332, 1652–1662. [Google Scholar] [CrossRef] [PubMed]
- Khamees, A.A.; Awadi, S.; Al-Shami, K.; Alkhoun, H.A.; Al-Eitan, S.F.; Alsheikh, A.M.; Saeed, A.; Al-Zoubi, R.M.; Zoubi, M.S.A. Human monkeypox virus in the shadow of the COVID-19 pandemic. J. Infect. Public Health 2023, 16, 1149–1157. [Google Scholar] [CrossRef]
- McCollum, A.M.; Shelus, V.; Hill, A.; Traore, T.; Onoja, B.; Nakazawa, Y.; Doty, J.B.; Yinka-Ogunleye, A.; Petersen, B.W.; Hutson, C.L.; et al. Epidemiology of Human Mpox—Worldwide, 2018–2021. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 68–72. [Google Scholar] [CrossRef]
- Ogoina, D.; Damon, I.; Nakoune, E. Clinical review of human mpox. Clin. Microbiol. Infect. 2023, 29, 1493–1501. [Google Scholar] [CrossRef]
- Fashina, T.; Huang, Y.; Thomas, J.; Conrady, C.D.; Yeh, S. Ophthalmic Features and Implications of Poxviruses: Lessons from Clinical and Basic Research. Microorganisms 2022, 10, 2487. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.R. Eye Complications of Smallpox. Some Observations During the Recent Epidemic in Cleveland. J. Am. Med. Assoc. 1903, 41, 645–648. [Google Scholar] [CrossRef]
- Semba, R.D. The Ocular Complications of Smallpox and Smallpox Immunization. Arch. Ophthalmol. 2003, 121, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Graef, S.; Kurth, A.; Auw-Haedrich, C.; Plange, N.; Kern, W.V.; Nitsche, A.; Reinhard, T. Clinicopathological Findings in Persistent Corneal Cowpox Infection. JAMA Ophthalmol. 2013, 131, 1089–1091. [Google Scholar] [CrossRef]
- Di Giulio, D.B.; Eckburg, P.B. Human monkeypox: An emerging zoonosis. Lancet Infect. Dis. 2004, 4, 15–25. [Google Scholar] [CrossRef]
- Nguyen, M.; Doan, T.; Seitzman, G.D. Ocular manifestations of mpox. Curr. Opin. Ophthalmol. 2024, 35, 423–429. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Mentreddy, A.; Schallhorn, J.; Chan, M.; Aung, S.; Doernberg, S.B.; Babik, J.; Miles, K.; Yang, K.; Lydon, E.; et al. Isolated Ocular Mpox without Skin Lesions, United States. Emerg. Infect. Dis. 2023, 29, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, N.; Hemani, D.; Paravastu, R.; Ahmad, Z.; Palani, S.N.; Arumugaswami, V.; Kumar, A. Mpox Virus and its ocular surface manifestations. Ocul. Surf. 2024, 34, 108–121. [Google Scholar] [CrossRef]
- Ogoina, D.; Iroezindu, M.; James, H.I.; Oladokun, R.; Yinka-Ogunleye, A.; Wakama, P.; Otike-Odibi, B.; Usman, L.M.; Obazee, E.; Aruna, O.; et al. Clinical Course and Outcome of Human Monkeypox in Nigeria. Clin. Infect. Dis. 2020, 71, e210–e214. [Google Scholar] [CrossRef] [PubMed]
- Benatti, S.V.; Venturelli, S.; Comi, N.; Borghi, F.; Paolucci, S.; Baldanti, F. Ophthalmic manifestation of monkeypox infection. Lancet Infect. Dis. 2022, 22, 1397. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, D.; Patrocínio, J.; Frade, J.; Brazão, C.; Mancha, D.; Correia, C.; Borges-Costa, J.; Filipe, P. Monkeypox Diagnosis by Cutaneous and Mucosal Findings. Infect. Dis. Rep. 2022, 14, 759–764. [Google Scholar] [CrossRef]
- Kaufman, A.R.; Chodosh, J.; Pineda, R., 2nd. Monkeypox Virus and Ophthalmology-A Primer on the 2022 Monkeypox Outbreak and Monkeypox-Related Ophthalmic Disease. JAMA Ophthalmol. 2023, 141, 78–83. [Google Scholar] [CrossRef]
- Ly-Yang, F.; Miranda-Sánchez, A.; Burgos-Blasco, B.; Fernández-Vigo, J.I.; Gegúndez-Fernández, J.A.; Díaz-Valle, D. Conjunctivitis in an Individual with Monkeypox. JAMA Ophthalmol. 2022, 140, 1022–1024. [Google Scholar] [CrossRef]
- Janseghers, L.; Matamba, M.; Colaert, J.; Vandepitte, J.; Desmyter, J. Fatal monkeypox in a child in Kikwit, Zaire. Ann. Soc. Belg. Med. Trop. 1984, 64, 295–298. [Google Scholar]
- Jarman, E.L.; Alain, M.; Conroy, N.; Omam, L.A. A case report of monkeypox as a result of conflict in the context of a measles campaign. Public Health Pract. 2022, 4, 100312. [Google Scholar] [CrossRef]
- Gandhi, A.P.; Gupta, P.C.; Padhi, B.K.; Sandeep, M.; Suvvari, T.K.; Shamim, M.A.; Satapathy, P.; Sah, R.; León-Figueroa, D.A.; Rodriguez-Morales, A.J.; et al. Ophthalmic Manifestations of the Monkeypox Virus: A Systematic Review and Meta-Analysis. Pathogens 2023, 12, 452. [Google Scholar] [CrossRef]
- Jezek, Z.; Szczeniowski, M.; Paluku, K.M.; Mutombo, M. Human monkeypox: Clinical features of 282 patients. J. Infect. Dis. 1987, 156, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Domínguez García, L.; Gutierrez-Arroyo, A.; Miguel-Buckley, R.; Martin Ucero, A.; Cantizani, J.; Boto-de-los-Bueis, A. Persistent and Severe Mpox Keratitis Despite Systemic and Topical Treatment. Cornea 2024, 43, 777–783. [Google Scholar] [CrossRef]
- Androudi, S.; Kaufman, A.R.; Kouvalakis, A.; Mitsios, A.; Sapounas, S.; Al-Khatib, D.; Schibler, M.; Pineda, R., 2nd; Baglivo, E. Non-Healing Corneal Ulcer and Uveitis Following Monkeypox Disease: Diagnostic and Therapeutic Challenges. Ocul. Immunol. Inflamm. 2024, 32, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Finamor, L.P.S.; Mendes-Correa, M.C.; Rinkevicius, M.; Macedo, G.; Sabino, E.C.; Villas-Boas, L.S.; de Paula, A.V.; de Araujo-Heliodoro, R.H.; da Costa, A.C.; Witkin, S.S.; et al. Ocular manifestations of Monkeypox virus (MPXV) infection with viral persistence in ocular samples: A case series. Int. J. Infect. Dis. 2024, 146, 107071. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.M.; Medeiros, M.; Veloso, V.G.; Biancardi, A.L.; Curi, A.L.L. Monkeypox Infection Causing Conjunctival Vesicles and Anterior Uveitis. Ocul. Immunol. Inflamm. 2024, 32, 266–267. [Google Scholar] [CrossRef]
- Carrubba, S.; Geevarghese, A.; Solli, E.; Guttha, S.; Sims, J.; Sperber, L.; Meehan, S.; Ostrovsky, A. Novel severe oculocutaneous manifestations of human monkeypox virus infection and their historical analogues. Lancet Infect. Dis. 2023, 23, e190–e197. [Google Scholar] [CrossRef]
- Hughes, C.; McCollum, A.; Pukuta, E.; Karhemere, S.; Nguete, B.; Shongo Lushima, R.; Kabamba, J.; Balilo, M.; Muyembe Tamfum, J.J.; Wemakoy, O.; et al. Ocular complications associated with acute monkeypox virus infection, DRC. Int. J. Infect. Dis. 2014, 21, 276–277. [Google Scholar] [CrossRef]
- Pazos, M.; Riera, J.; Moll-Udina, A.; Catala, A.; Narvaez, S.; Fuertes, I.; Dotti-Boada, M.; Petiti, G.; Izquierdo-Serra, J.; Maldonado, E.; et al. Characteristics and Management of Ocular Involvement in Individuals with Monkeypox Disease. Ophthalmology 2023, 130, 655–658. [Google Scholar] [CrossRef]
- Patel, A.; Bilinska, J.; Tam, J.C.H.; Da Silva Fontoura, D.; Mason, C.Y.; Daunt, A.; Snell, L.B.; Murphy, J.; Potter, J.; Tuudah, C.; et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: Descriptive case series. BMJ 2022, 378, e072410. [Google Scholar] [CrossRef]
- Mailhe, M.; Beaumont, A.-L.; Thy, M.; Le Pluart, D.; Perrineau, S.; Houhou-Fidouh, N.; Deconinck, L.; Bertin, C.; Ferré, V.M.; Cortier, M.; et al. Clinical characteristics of ambulatory and hospitalized patients with monkeypox virus infection: An observational cohort study. Clin. Microbiol. Infect. 2023, 29, 233–239. [Google Scholar] [CrossRef]
- Doan, S.; Houry, R.; Cristea, I.; Boughar, B.; Cochereau, I.; Gabison, E.E.; Guindolet, D. Severe Corneal Involvement Associated With Mpox Infection. JAMA Ophthalmol. 2023, 141, 402–403. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Badillo, P.; Rodriguez-Aldama, J.C.; Gabian-Fortes, L.D.C.; Sifuentes-Renteria, S.; Valdez-Gonzalez, M.T.; Perez-Flores, B.E.; Velasco-Ramos, R.; Fernandez-Vizcaya, O.; Crabtree-Ramirez, B.; Perez-Barragan, E. Mpox-Related Ophthalmic Disease: A Retrospective Observational Study in a Single Center in Mexico. J. Infect. Dis. 2024, 229, S255–S259. [Google Scholar] [CrossRef] [PubMed]
- Ogoina, D.; Dalhat, M.M.; Denue, B.A.; Okowa, M.; Chika-Igwenyi, N.M.; Yusuff, H.A.; Christian, U.C.; Adekanmbi, O.; Ojimba, A.O.; Aremu, J.T.; et al. Clinical characteristics and predictors of human mpox outcome during the 2022 outbreak in Nigeria: A cohort study. Lancet Infect. Dis. 2023, 23, 1418–1428. [Google Scholar] [CrossRef]
- Malembi, E.; Escrig-Sarreta, R.; Ntumba, J.; Beiras, C.G.; Shongo, R.; Bengehya, J.; Nselaka, C.; Pukuta, E.; Mukadi-Bamuleka, D.; Mulopo-Mukanya, N.; et al. Clinical presentation and epidemiological assessment of confirmed human mpox cases in DR Congo: A surveillance-based observational study. Lancet 2025, 405, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Su, S.-B.; Chen, K.-T. A Review of epidemiology, diagnosis, and management of Mpox: The role of One Health. Glob. Health Med. 2025, 7, 01072. [Google Scholar] [CrossRef]
- Kamoi, K.; Watanabe, T.; Uchimaru, K.; Okayama, A.; Kato, S.; Kawamata, T.; Kurozumi-Karube, H.; Horiguchi, N.; Zong, Y.; Yamano, Y.; et al. Updates on HTLV-1 Uveitis. Viruses 2022, 14, 794. [Google Scholar] [CrossRef]
- Cohen-Gihon, I.; Israeli, O.; Shifman, O.; Erez, N.; Melamed, S.; Paran, N.; Beth-Din, A.; Zvi, A. Identification and Whole-Genome Sequencing of a Monkeypox Virus Strain Isolated in Israel. Microbiol. Resour. Announc. 2020, 9. [Google Scholar] [CrossRef]
- Rayati Damavandi, A.; Semnani, F.; Hassanpour, K. A Review of Monkeypox Ocular Manifestations and Complications: Insights for the 2022 Outbreak. Ophthalmol. Ther. 2023, 12, 55–69. [Google Scholar] [CrossRef]
- Meduri, E.; Malclès, A.; Kecik, M. Conjunctivitis with Monkeypox Virus Positive Conjunctival Swabs. Ophthalmology 2022, 129, 1095. [Google Scholar] [CrossRef]
- Ezat, A.A.; Abduljalil, J.M.; Elghareib, A.M.; Samir, A.; Elfiky, A.A. The discovery of novel antivirals for the treatment of mpox: Is drug repurposing the answer? Expert Opin. Drug Discov. 2023, 18, 551–561. [Google Scholar] [CrossRef]
- DeLaurentis, C.E.; Kiser, J.; Zucker, J. New Perspectives on Antimicrobial Agents: Tecovirimat for Treatment of Human Monkeypox Virus. Antimicrob. Agents Chemother. 2022, 66, e0122622. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.T.; Grosenbach, D.W.; Honeychurch, K.M.; Long, P.G.; Hruby, D.E. Overview of the regulatory approval of tecovirimat intravenous formulation for treatment of smallpox: Potential impact on smallpox outbreak response capabilities, and future tecovirimat development potential. Expert Rev. Anti-Infect. Infect. Ther. 2023, 21, 235–242. [Google Scholar] [CrossRef]
- Shabil, M.; Khatib, M.N.; Ballal, S.; Bansal, P.; Tomar, B.S.; Ashraf, A.; Kumar, M.R.; Sinha, A.; Rawat, P.; Gaidhane, A.M.; et al. Effectiveness of Tecovirimat in Mpox Cases: A Systematic Review of Current Evidence. J. Med. Virol. 2024, 96, e70122. [Google Scholar] [CrossRef]
- Milligan, A.L.; Koay, S.Y.; Dunning, J. Monkeypox as an emerging infectious disease: The ophthalmic implications. Br. J. Ophthalmol. 2022, 106, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Rizk, J.G.; Lippi, G.; Henry, B.M.; Forthal, D.N.; Rizk, Y. Prevention and Treatment of Monkeypox. Drugs 2022, 82, 957–963. [Google Scholar] [CrossRef]
- Trawally Flores, A.; Guedes Guedes, I.I.; Espinoza González, J.P.; Jerez Olivera, E.; Siguero Martín, L.; Pérez Álvarez, J. Ocular involvement secondary to Monkeypox virus infection. Arch. Soc. Española Oftalmol. 2024, 99, 33–37. [Google Scholar] [CrossRef]
- Pischel, L.; Martini, B.A.; Yu, N.; Cacesse, D.; Tracy, M.; Kharbanda, K.; Ahmed, N.; Patel, K.M.; Grimshaw, A.A.; Malik, A.A.; et al. Vaccine effectiveness of 3rd generation mpox vaccines against mpox and disease severity: A systematic review and meta-analysis. Vaccine 2024, 42, 126053. [Google Scholar] [CrossRef]
- Rao, A.K.; Petersen, B.W.; Whitehill, F.; Razeq, J.H.; Isaacs, S.N.; Merchlinsky, M.J.; Campos-Outcalt, D.; Morgan, R.L.; Damon, I.; Sanchez, P.J.; et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for Preexposure Vaccination of Persons at Risk for Occupational Exposure to Orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 734–742. [Google Scholar] [CrossRef]
- Wang, X.; Gu, Z.; Sheng, S.; Song, R.; Jin, R. The Current State and Progress of Mpox Vaccine Research. China CDC Wkly. 2024, 6, 118–125. [Google Scholar] [CrossRef]
- Neff, J.M.; Lane, J.M.; Pert, J.H.; Moore, R.; Millar, J.D.; Henderson, D.A. Complications of smallpox vaccination: National survey in the United States, 1963. N. Engl. J. Med. 1967, 276, 125–132. [Google Scholar] [CrossRef]
- Pepose, J.S.; Margolis, T.P.; LaRussa, P.; Pavan-Langston, D. Ocular complications of smallpox vaccination. Am. J. Ophthalmol. 2003, 136, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Cono, J.; Casey, C.G.; Bell, D.M.; Centers for Disease Control and Prevention. Smallpox vaccination and adverse reactions. MMWR Recomm. Rep. 2003, 52, 1–28. [Google Scholar] [PubMed]
- Malik, S.; Ahmad, T.; Ahsan, O.; Muhammad, K.; Waheed, Y. Recent Developments in Mpox Prevention and Treatment Options. Vaccines 2023, 11, 500. [Google Scholar] [CrossRef]
- Gupta, P.C.; Sharma, N.; Rai, S.; Mishra, P. Use of Smart Silver Nanoparticles in Drug Delivery System. In Metal and Metal-Oxide Based Nanomaterials: Synthesis, Agricultural, Biomedical and Environmental Interventions; Bachheti, R.K., Bachheti, A., Husen, A., Eds.; Springer Nature: Singapore, 2024; pp. 213–241. [Google Scholar]
- Verma, A.; Nazli Khatib, M.; Datt Sharma, G.; Pratap Singh, M.; Bushi, G.; Ballal, S.; Kumar, S.; Bhat, M.; Sharma, S.; Ndabashinze, R. Mpox 2024: New variant, new challenges, and the looming pandemic. Clin. Infect. Pract. 2024, 24, 100394. [Google Scholar] [CrossRef]
| Clade | Region/Study | Year | Ocular Involvement Rate | Population Characteristics |
|---|---|---|---|---|
| Ia | Democratic Republic of the Congo (Hughes et al., 2014) [67] | 2010–2013 | 23.1% conjunctivitis | General population |
| Ia | Democratic Republic of the Congo (Jezek et al., 1987) [61] | 1980–1985 | 4.3% (12/282) keratitis or corneal ulceration | General population |
| IIb | Spain (Català et al., 2022) [13] | 2022 | 1.1% (2/185) Periorbital lesions | Predominantly MSM |
| IIb | Spain (Pazos et al., 2023) [68] | 2023 | 1.0% (9/880) Conjunctivitis, 8.0% (7/880) blepharitis, 0.6% (5/880) eyelid lesions | Predominantly MSM |
| IIb | UK (Patel et al., 2022) [69] | 2022 | 1.0% (2/197) conjunctivitis | Predominantly MSM |
| IIb | France (Mailhe et al., 2023) [70] | 2022 | 0.8% (2/264) ocular involvement: 1 with eyelid lesions; 1 with keratitis, conjunctivitis and blepharitis | Predominantly MSM |
| IIb | France (Doan et al., 2023) [71] | 2023 | 0.3% (2/588) keratitis | Predominantly MSM |
| IIb | Mexico (Rodriguez-Badillo et al., 2024) [72] | 2022 | 6% (6/100) conjunctivitis, 6% (6/100) eyelid lesions, 1% (1/100) episcleritis, 1% (1/100) keratitis | 81.8% of ocular cases were HIV-positive |
| IIb (A lineage) | Nigeria (Ogoina et al., 2023) [73] | 2022–2023 | 6.3% (10/160) keratitis | 5% MSM, M:F ratio 114:46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zong, Y.; Zou, Y.; Yang, M.; Zhang, J.; Ye, Z.; Deng, J.; Ohno-Matsui, K.; Kamoi, K. Ocular Manifestations of Mpox and Other Poxvirus Infections: Clinical Insights and Emerging Therapeutic and Preventive Strategies. Vaccines 2025, 13, 546. https://doi.org/10.3390/vaccines13050546
Zong Y, Zou Y, Yang M, Zhang J, Ye Z, Deng J, Ohno-Matsui K, Kamoi K. Ocular Manifestations of Mpox and Other Poxvirus Infections: Clinical Insights and Emerging Therapeutic and Preventive Strategies. Vaccines. 2025; 13(5):546. https://doi.org/10.3390/vaccines13050546
Chicago/Turabian StyleZong, Yuan, Yaru Zou, Mingming Yang, Jing Zhang, Zizhen Ye, Jiaxin Deng, Kyoko Ohno-Matsui, and Koju Kamoi. 2025. "Ocular Manifestations of Mpox and Other Poxvirus Infections: Clinical Insights and Emerging Therapeutic and Preventive Strategies" Vaccines 13, no. 5: 546. https://doi.org/10.3390/vaccines13050546
APA StyleZong, Y., Zou, Y., Yang, M., Zhang, J., Ye, Z., Deng, J., Ohno-Matsui, K., & Kamoi, K. (2025). Ocular Manifestations of Mpox and Other Poxvirus Infections: Clinical Insights and Emerging Therapeutic and Preventive Strategies. Vaccines, 13(5), 546. https://doi.org/10.3390/vaccines13050546






