Circulating Antibodies Against Common Cold Coronaviruses Do Not Interfere with Immune Responses to Primary or Booster SARS-CoV-2 mRNA Vaccines
Abstract
1. Introduction
2. Materials and Methods
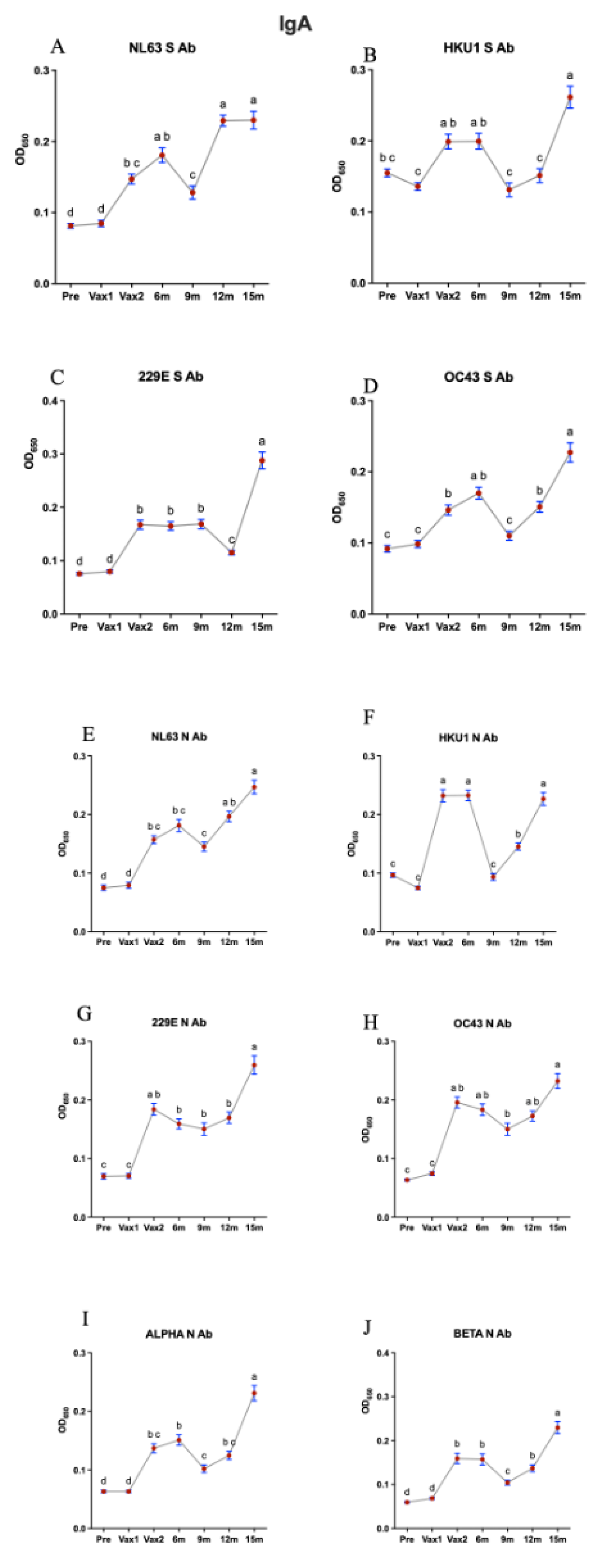
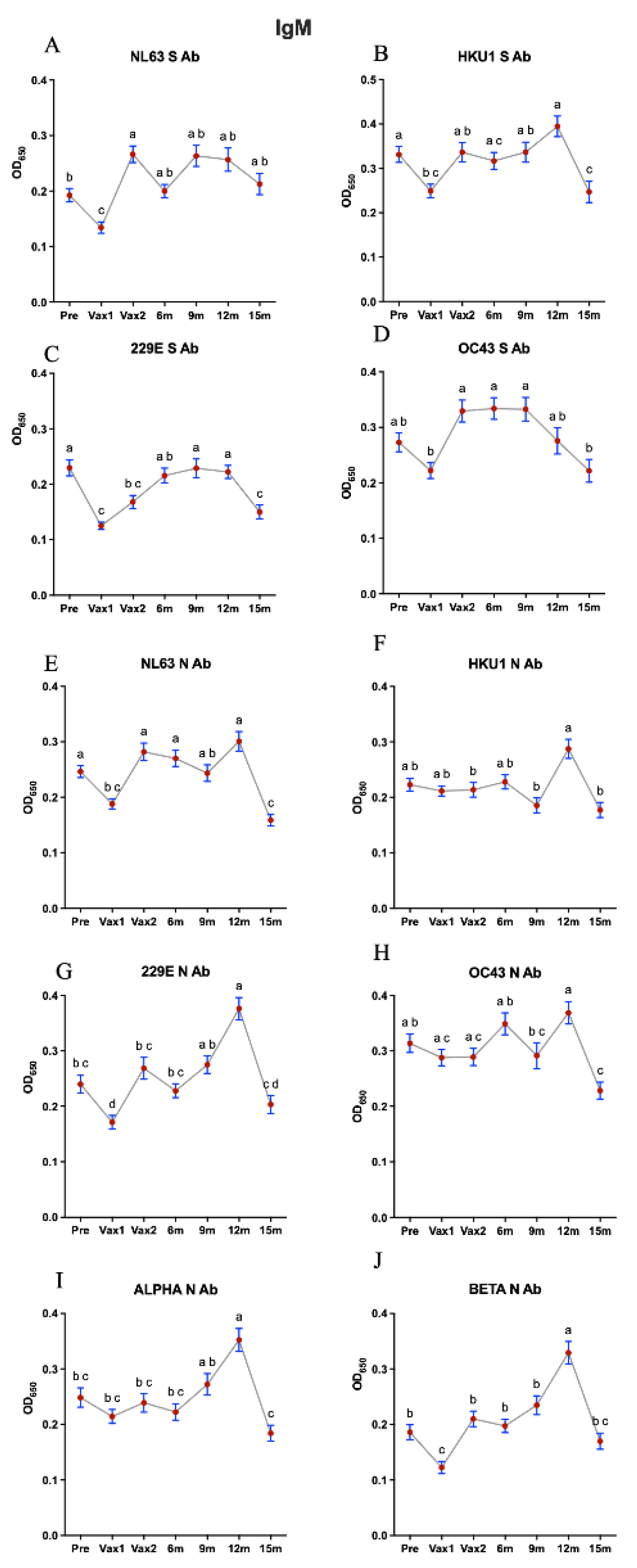
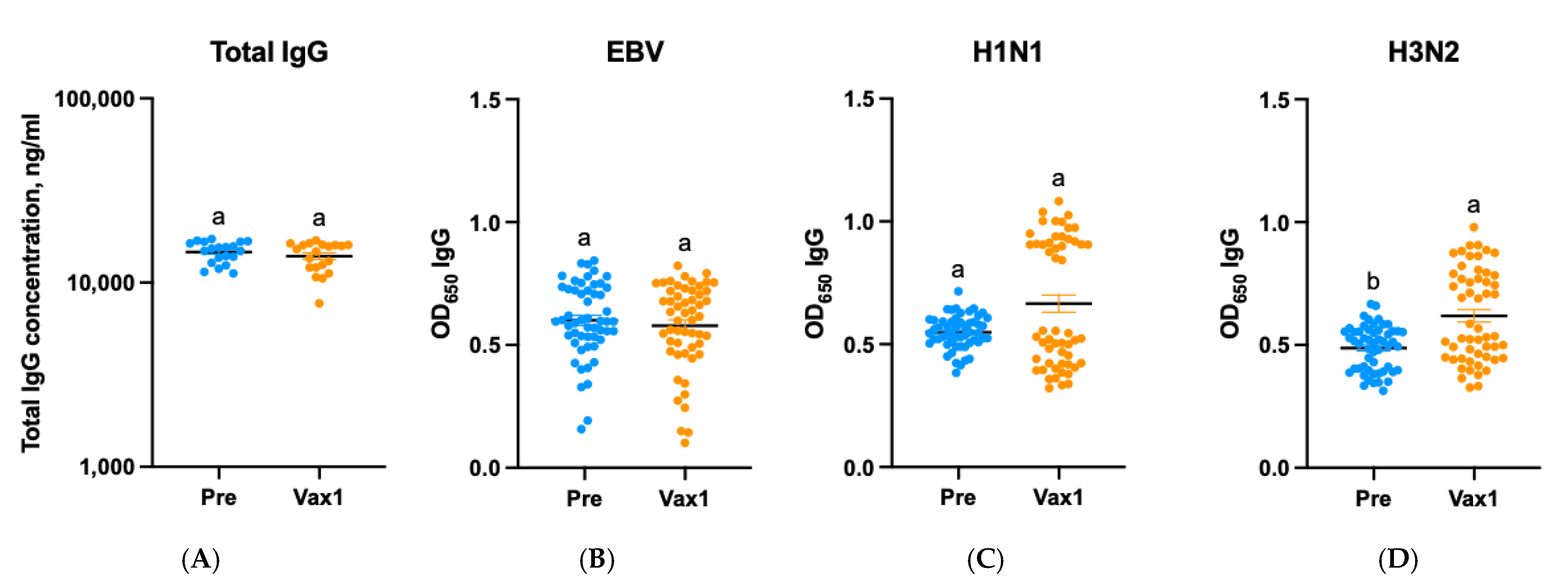
3. Results
4. Discussion
5. Limitations of This Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Narayanan, S.A.; Jamison, D.A.; Guarnieri, J.W.; Zaksas, V.; Topper, M.; Koutnik, A.P.; Park, J.; Clark, K.B.; Enguita, F.J.; Leitão, A.L.; et al. A comprehensive SARS-CoV-2 and COVID-19 review, Part 2: Host extracellular to systemic effects of SARS-CoV-2 infection. Eur. J. Hum. Genet. 2024, 32, 10–20. [Google Scholar] [CrossRef]
- Kiszel, P.; Sík, P.; Miklós, J.; Kajdácsi, E.; Sinkovits, G.; Cervenak, L.; Prohászka, Z. Class switch towards spike protein-specific IgG4 antibodies after SARS-CoV-2 mRNA vaccination depends on prior infection history. Sci. Rep. 2023, 13, 13166. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Kuo, R.L.; Shih, S.R. COVID-19: The first documented coronavirus pandemic in history. Biomed. J. 2020, 43, 328–333. [Google Scholar] [CrossRef]
- Kenney, S.P.; Wang, Q.; Vlasova, A.; Jung, K.; Saif, L. Naturally Occurring Animal Coronaviruses as Models for Studying Highly Pathogenic Human Coronaviral Disease. Vet. Pathol. 2021, 58, 438–452. [Google Scholar] [CrossRef]
- Saif, L. Animal coronaviruses: What can they teach us about the severe acute respiratory syndrome? Rev. Sci. Tech. (Int. Off. Epizoot.) 2004, 23, 643–660. [Google Scholar] [CrossRef] [PubMed]
- Drosten, C.; Gunther, S.; Preiser, W.; van der Werf, S.; Brodt, H.R.; Becker, S.; Rabenau, H.; Panning, M.; Kolesnikova, L.; Fouchier, R.A.; et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, K.; Dees, J.H.; Becker, W.B.; Kapikian, A.Z.; Chanock, R.M. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc. Natl. Acad. Sci. USA 1967, 57, 933–940. [Google Scholar] [CrossRef]
- van Elden, L.J.; van Loon, A.M.; van Alphen, F.; Hendriksen, K.A.; Hoepelman, A.I.; van Kraaij, M.G.; Oosterheert, J.J.; Schipper, P.; Schuurman, R.; Nijhuis, M. Frequent detection of human coronaviruses in clinical specimens from patients with respiratory tract infection by use of a novel real-time reverse-transcriptase polymerase chain reaction. J. Infect. Dis. 2004, 189, 652–657. [Google Scholar] [CrossRef]
- Woo, P.C.; Lau, S.K.; Chu, C.M.; Chan, K.H.; Tsoi, H.W.; Huang, Y.; Wong, B.H.; Poon, R.W.; Cai, J.J.; Luk, W.K.; et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 2005, 79, 884–895. [Google Scholar] [CrossRef]
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Muth, D.; Niemeyer, D.; Drosten, C. Hosts and Sources of Endemic Human Coronaviruses. Adv. Virus Res. 2018, 100, 163–188. [Google Scholar] [CrossRef]
- Miyara, M.; Saichi, M.; Sterlin, D.; Anna, F.; Marot, S.; Mathian, A.; Atif, M.; Quentric, P.; Mohr, A.; Claer, L.; et al. Pre-COVID-19 Immunity to Common Cold Human Coronaviruses Induces a Recall-Type IgG Response to SARS-CoV-2 Antigens Without Cross-Neutralisation. Front. Immunol. 2022, 13, 790334. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.W.; Faulkner, N.; Cornish, G.H.; Rosa, A.; Harvey, R.; Hussain, S.; Ulferts, R.; Earl, C.; Wrobel, A.G.; Benton, D.J.; et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science 2020, 370, 1339–1343. [Google Scholar] [CrossRef]
- Morgenlander, W.R.; Henson, S.N.; Monaco, D.R.; Chen, A.; Littlefield, K.; Bloch, E.M.; Fujimura, E.; Ruczinski, I.; Crowley, A.R.; Natarajan, H.; et al. Antibody responses to endemic coronaviruses modulate COVID-19 convalescent plasma functionality. J. Clin. Investig. 2021, 131, e146927. [Google Scholar] [CrossRef]
- Swadling, L.; Diniz, M.O.; Schmidt, N.M.; Amin, O.E.; Chandran, A.; Shaw, E.; Pade, C.; Gibbons, J.M.; Le Bert, N.; Tan, A.T.; et al. Pre-existing polymerase-specific T cells expand in abortive seronegative SARS-CoV-2. Nature 2022, 601, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Kundu, R.; Narean, J.S.; Wang, L.; Fenn, J.; Pillay, T.; Fernandez, N.D.; Conibear, E.; Koycheva, A.; Davies, M.; Tolosa-Wright, M.; et al. Cross-reactive memory T cells associate with protection against SARS-CoV-2 infection in COVID-19 contacts. Nat. Commun. 2022, 13, 80. [Google Scholar] [CrossRef]
- Loyal, L.; Braun, J.; Henze, L.; Kruse, B.; Dingeldey, M.; Reimer, U.; Kern, F.; Schwarz, T.; Mangold, M.; Unger, C.; et al. Cross-reactive CD4(+) T cells enhance SARS-CoV-2 immune responses upon infection and vaccination. Science 2021, 374, eabh1823. [Google Scholar] [CrossRef]
- Mateus, J.; Dan, J.M.; Zhang, Z.; Rydyznski Moderbacher, C.; Lammers, M.; Goodwin, B.; Sette, A.; Crotty, S.; Weiskopf, D. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science 2021, 374, eabj9853. [Google Scholar] [CrossRef]
- Ogbe, A.; Pace, M.; Bittaye, M.; Tipoe, T.; Adele, S.; Alagaratnam, J.; Aley, P.K.; Ansari, M.A.; Bara, A.; Broadhead, S.; et al. Durability of ChAdOx1 nCoV-19 vaccination in people living with HIV. JCI Insight 2022, 7, e157031. [Google Scholar] [CrossRef]
- Sagar, M.; Reifler, K.; Rossi, M.; Miller, N.S.; Sinha, P.; White, L.F.; Mizgerd, J.P. Recent endemic coronavirus infection is associated with less-severe COVID-19. J. Clin. Investig. 2021, 131, e143380. [Google Scholar] [CrossRef]
- Adhikari, B.; Lee, C.N.; Khadka, V.S.; Deng, Y.; Fukumoto, G.; Thorne, M.; Caires, K.; Odani, J.; Mishra, B. RNA-Sequencing based analysis of bovine endometrium during the maternal recognition of pregnancy. BMC Genom. 2022, 23, 494. [Google Scholar] [CrossRef] [PubMed]
- Tso, F.Y.; Lidenge, S.J.; Peña, P.B.; Clegg, A.A.; Ngowi, J.R.; Mwaiselage, J.; Ngalamika, O.; Julius, P.; West, J.T.; Wood, C. High prevalence of pre-existing serological cross-reactivity against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in sub-Saharan Africa. Int. J. Infect. Dis. 2021, 102, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.M.; Goodwin, E.C.; Verma, A.; Arevalo, C.P.; Bolton, M.J.; Weirick, M.E.; Gouma, S.; McAllister, C.M.; Christensen, S.R.; Weaver, J.; et al. Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection. Cell 2021, 184, 1858–1864 e1810. [Google Scholar] [CrossRef]
- Bacher, P.; Rosati, E.; Esser, D.; Martini, G.R.; Saggau, C.; Schiminsky, E.; Dargvainiene, J.; Schröder, I.; Wieters, I.; Khodamoradi, Y.; et al. Low-Avidity CD4(+) T Cell Responses to SARS-CoV-2 in Unexposed Individuals and Humans with Severe COVID-19. Immunity 2020, 53, 1258–1271.e1255. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, A.L.; Paton, R.S.; Edmans, M.; Youngs, J.; Wellens, J.; Phalora, P.; Fyfe, A.; Belij-Rammerstorfer, S.; Bolton, J.S.; Ball, J.; et al. Fatal COVID-19 outcomes are associated with an antibody response targeting epitopes shared with endemic coronaviruses. JCI Insight 2022, 7, e156372. [Google Scholar] [CrossRef]
- Wratil, P.R.; Schmacke, N.A.; Karakoc, B.; Dulovic, A.; Junker, D.; Becker, M.; Rothbauer, U.; Osterman, A.; Spaeth, P.M.; Ruhle, A.; et al. Evidence for increased SARS-CoV-2 susceptibility and COVID-19 severity related to pre-existing immunity to seasonal coronaviruses. Cell Rep. 2021, 37, 110169. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Cameron, A.; Zhou, Q.; Wiltse, A.; Nayak, J.; Pecora, N.D.; Zand, M.S. IgG Against Human Betacoronavirus Spike Proteins Correlates With SARS-CoV-2 Anti-Spike IgG Responses and COVID-19 Disease Severity. J. Infect. Dis. 2022, 226, 474–484. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Wolf, J.; Brice, D.C.; Sun, Y.; Locke, M.; Cherry, S.; Castellaw, A.H.; Wehenkel, M.; Crawford, J.C.; Zarnitsyna, V.I.; et al. Pre-existing humoral immunity to human common cold coronaviruses negatively impacts the protective SARS-CoV-2 antibody response. Cell Host Microbe 2022, 30, 83–96.e84. [Google Scholar] [CrossRef]
- Monroe, I.; Dale, M.; Schwabe, M.; Schenkel, R.; Schenarts, P.J. The COVID-19 Patient in the Surgical Intensive Care Unit. Surg. Clin. N. Am. 2022, 102, 1–21. [Google Scholar] [CrossRef]
- Waterlow, N.R.; Van Leeuwen, E.; Davies, N.G.; CMMID COVID-19 Working Group; Flasche, S.; Eggo, R.M. How immunity from and interaction with seasonal coronaviruses can shape SARS-CoV-2 epidemiology. Proc. Natl. Acad. Sci. USA 2021, 118, e2108395118. [Google Scholar] [CrossRef]
- Adhikari, B.; Oltz, E.M.; Bednash, J.S.; Horowitz, J.C.; Amimo, J.O.; Raev, S.A.; Fernández, S.; Anghelina, M.; Liu, S.-L.; Rubinstein, M.P.; et al. Increased COVID-19 Mortality and Deficient SARS-CoV-2 Immune Response Are Not Associated with Higher Levels of Endemic Coronavirus Antibodies. Immuno 2023, 3, 330–345. [Google Scholar] [CrossRef]
- Altarawneh, H.N.; Chemaitelly, H.; Ayoub, H.H.; Tang, P.; Hasan, M.R.; Yassine, H.M.; Al-Khatib, H.A.; Al Thani, A.A.; Coyle, P.; Al-Kanaani, Z.; et al. Effects of previous infection, vaccination, and hybrid immunity against symptomatic Alpha, Beta, and Delta SARS-CoV-2 infections: An observational study. EBioMedicine 2023, 95, 104734. [Google Scholar] [CrossRef]
- Lasrado, N.; Barouch, D.H. SARS-CoV-2 Hybrid Immunity: The Best of Both Worlds. J. Infect. Dis. 2023, 228, 1311–1313. [Google Scholar] [CrossRef]
- Guerrini, G.; Gioria, S.; Sauer, A.V.; Lucchesi, S.; Montagnani, F.; Pastore, G.; Ciabattini, A.; Medaglini, D.; Calzolai, L. Monitoring Anti-PEG Antibodies Level upon Repeated Lipid Nanoparticle-Based COVID-19 Vaccine Administration. Int. J. Mol. Sci. 2022, 23, 8838. [Google Scholar] [CrossRef] [PubMed]
- Igyártó, B.Z.; Qin, Z. The mRNA-LNP vaccines—The good, the bad and the ugly? Front. Immunol. 2024, 15, 1336906. [Google Scholar] [CrossRef]
- Rispens, T.; Huijbers, M.G. The unique properties of IgG4 and its roles in health and disease. Nat. Rev. Immunol. 2023, 23, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Motta, R.V.; Culver, E.L. IgG4 autoantibodies and autoantigens in the context of IgG4-autoimmune disease and IgG4-related disease. Front. Immunol. 2024, 15, 1272084. [Google Scholar] [CrossRef]
- Gelderloos, A.T.; Verheul, M.K.; Middelhof, I.; de Zeeuw-Brouwer, M.-L.; van Binnendijk, R.S.; Buisman, A.-M.; van Kasteren, P.B. Repeated COVID-19 mRNA vaccination results in IgG4 class switching and decreased NK cell activation by S1-specific antibodies in older adults. Immun. Ageing 2024, 21, 63. [Google Scholar] [CrossRef]
- Narowski, T.M.; Raphel, K.; Adams, L.E.; Huang, J.; Vielot, N.A.; Jadi, R.; de Silva, A.M.; Baric, R.S.; Lafleur, J.E.; Premkumar, L. SARS-CoV-2 mRNA vaccine induces robust specific and cross-reactive IgG and unequal neutralizing antibodies in naive and previously infected people. Cell Rep. 2022, 38, 110336. [Google Scholar] [CrossRef]
- Karachaliou, M.; Moncunill, G.; Espinosa, A.; Castaño-Vinyals, G.; Rubio, R.; Vidal, M.; Jiménez, A.; Prados, E.; Carreras, A.; Cortés, B.; et al. SARS-CoV-2 infection, vaccination, and antibody response trajectories in adults: A cohort study in Catalonia. BMC Med. 2022, 20, 347. [Google Scholar] [CrossRef] [PubMed]
- Samanovic, M.I.; Cornelius, A.R.; Gray-Gaillard, S.L.; Allen, J.R.; Karmacharya, T.; Wilson, J.P.; Hyman, S.W.; Tuen, M.; Koralov, S.B.; Mulligan, M.J.; et al. Robust immune responses are observed after one dose of BNT162b2 mRNA vaccine dose in SARS-CoV-2–experienced individuals. Sci. Transl. Med. 2022, 14, eabi8961. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.; Carreño, J.M.; Gleason, C.; Monahan, B.; Singh, G.; Abbad, A.; Tcheou, J.; Raskin, A.; Kleiner, G.; van Bakel, H.; et al. SARS-CoV-2-infection- and vaccine-induced antibody responses are long lasting with an initial waning phase followed by a stabilization phase. Immunity 2024, 57, 587–599.e584. [Google Scholar] [CrossRef]
- Đaković Rode, O.; Bodulić, K.; Zember, S.; Cetinić Balent, N.; Novokmet, A.; Čulo, M.; Rašić, Ž.; Mikulić, R.; Markotić, A. Decline of Anti-SARS-CoV-2 IgG Antibody Levels 6 Months after Complete BNT162b2 Vaccination in Healthcare Workers to Levels Observed Following the First Vaccine Dose. Vaccines 2022, 10, 153. [Google Scholar] [CrossRef]
- Cham, J.; Pandey, A.C.; New, J.; Huynh, T.; Hong, L.; Orendain, N.; Topol, E.J.; Nicholson, L.J. 6 month serologic response to the Pfizer-BioNTech COVID-19 vaccine among healthcare workers. PLoS ONE 2022, 17, e0266781. [Google Scholar] [CrossRef]
- Kolaric, B.; Ambriovic-Ristov, A.; Tabain, I.; Vilibic-Cavlek, T. Waning immunity six months after BioNTech/Pfizer COVID-19 vaccination among nursing home residents in Zagreb, Croatia. Croat. Med. J. 2021, 62, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Barbeau, D.J.; Martin, J.M.; Carney, E.; Dougherty, E.; Doyle, J.D.; Dermody, T.S.; Hoberman, A.; Williams, J.V.; Michaels, M.G.; Alcorn, J.F.; et al. Comparative analysis of human immune responses following SARS-CoV-2 vaccination with BNT162b2, mRNA-1273, or Ad26.COV2.S. npj Vaccines 2022, 7, 77. [Google Scholar] [CrossRef]
- Jalkanen, P.; Kolehmainen, P.; Häkkinen, H.K.; Huttunen, M.; Tähtinen, P.A.; Lundberg, R.; Maljanen, S.; Reinholm, A.; Tauriainen, S.; Pakkanen, S.H.; et al. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat. Commun. 2021, 12, 3991. [Google Scholar] [CrossRef] [PubMed]
- Van Elslande, J.; Weemaes, M.; Godderis, L.; Van Pottelbergh, G.; Bossuyt, X.; Vermeersch, P. IgG anti-spike antibody levels in healthcare workers with and without prior COVID-19 up to 3 months after BNT162b2 vaccination. Diagn. Microbiol. Infect. Dis. 2022, 102, 115638. [Google Scholar] [CrossRef]
- McGill, J.R.; Lagassé, H.A.D.; Hernandez, N.; Hopkins, L.; Jankowski, W.; McCormick, Q.; Simhadri, V.; Golding, B.; Sauna, Z.E. A structural homology approach to identify potential cross-reactive antibody responses following SARS-CoV-2 infection. Sci. Rep. 2022, 12, 11388. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Chao, C.H.; Lai, Y.C.; Hsieh, K.H.; Wang, J.R.; Wan, S.W.; Huang, H.J.; Chuang, Y.C.; Chuang, W.J.; Yeh, T.M. Antibodies against the SARS-CoV-2 S1-RBD cross-react with dengue virus and hinder dengue pathogenesis. Front. Immunol. 2022, 13, 941923. [Google Scholar] [CrossRef]
- Jaago, M.; Rahni, A.; Pupina, N.; Pihlak, A.; Sadam, H.; Tuvikene, J.; Avarlaid, A.; Planken, A.; Planken, M.; Haring, L.; et al. Differential patterns of cross-reactive antibody response against SARS-CoV-2 spike protein detected for chronically ill and healthy COVID-19 naive individuals. Sci. Rep. 2022, 12, 16817. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Kharrazian, D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020, 217, 108480. [Google Scholar] [CrossRef] [PubMed]
- Chaisawangwong, W.; Wang, H.; Kouo, T.; Salathe, S.F.; Isser, A.; Bieler, J.G.; Zhang, M.L.; Livingston, N.K.; Li, S.; Horowitz, J.J.; et al. Cross-reactivity of SARS-CoV-2- and influenza A-specific T cells in individuals exposed to SARS-CoV-2. JCI Insight 2022, 7, e158308. [Google Scholar] [CrossRef] [PubMed]
- Murugavelu, P.; Perween, R.; Shrivastava, T.; Singh, V.; Ahmad Parray, H.; Singh, S.; Chiranjivi, A.K.; Thiruvengadam, R.; Singh, S.; Yadav, N.; et al. Non-neutralizing SARS CoV-2 directed polyclonal antibodies demonstrate cross-reactivity with the HA glycans of influenza virus. Int. Immunopharmacol. 2021, 99, 108020. [Google Scholar] [CrossRef]
- Kanduc, D.; Shoenfeld, Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: Implications for the vaccine. Immunol. Res. 2020, 68, 310–313. [Google Scholar] [CrossRef]
- Bondareva, M.; Budzinski, L.; Durek, P.; Witkowski, M.; Angermair, S.; Ninnemann, J.; Kreye, J.; Letz, P.; Ferreira-Gomes, M.; Semin, I.; et al. Cross-regulation of antibody responses against the SARS-CoV-2 Spike protein and commensal microbiota via molecular mimicry. Cell Host Microbe 2023, 31, 1866–1881 e1810. [Google Scholar] [CrossRef]
- Assis, R.; Jain, A.; Nakajima, R.; Jasinskas, A.; Khan, S.; Palma, A.; Parker, D.M.; Chau, A.; Specimen Collection, G.; Obiero, J.M.; et al. Distinct SARS-CoV-2 antibody reactivity patterns elicited by natural infection and mRNA vaccination. NPJ Vaccines 2021, 6, 132. [Google Scholar] [CrossRef]
- Fraley, E.; LeMaster, C.; Geanes, E.; Banerjee, D.; Khanal, S.; Grundberg, E.; Selvarangan, R.; Bradley, T. Humoral immune responses during SARS-CoV-2 mRNA vaccine administration in seropositive and seronegative individuals. BMC Med. 2021, 19, 169. [Google Scholar] [CrossRef]
- Mysore, V.; Cullere, X.; Settles, M.L.; Ji, X.; Kattan, M.W.; Desjardins, M.; Durbin-Johnson, B.; Gilboa, T.; Baden, L.R.; Walt, D.R.; et al. Protective heterologous T cell immunity in COVID-19 induced by the trivalent MMR and Tdap vaccine antigens. Med 2021, 2, 1050–1071.e1057. [Google Scholar] [CrossRef]
- Irrgang, P.; Gerling, J.; Kocher, K.; Lapuente, D.; Steininger, P.; Habenicht, K.; Wytopil, M.; Beileke, S.; Schäfer, S.; Zhong, J.; et al. Class switch toward noninflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination. Sci. Immunol. 2023, 8, eade2798. [Google Scholar] [CrossRef] [PubMed]
- Lasrado, N.; Collier, A.R.Y.; Miller, J.; Hachmann, N.P.; Liu, J.; Anand, T.; Bondzie, E.A.; Fisher, J.L.; Mazurek, C.R.; Patio, R.C.; et al. Waning immunity and IgG4 responses following bivalent mRNA boosting. Sci. Adv. 2024, 10, eadj9945. [Google Scholar] [CrossRef] [PubMed]
- Kalkeri, R.; Zhu, M.; Cloney-Clark, S.; Plested, J.S.; Parekh, A.; Gorinson, D.; Cai, R.; Mahato, S.; Ramanathan, P.; Aurelia, L.C.; et al. Altered IgG4 antibody response to repeated mRNA versus recombinant protein SARS-CoV-2 vaccines. J. Infect. 2024, 88, 106119. [Google Scholar] [CrossRef] [PubMed]
- Kobbe, R.; Rau, C.; Schulze-Sturm, U.; Stahl, F.; Fonseca-Brito, L.; Diemert, A.; Lütgehetmann, M.; Addo, M.M.; Arck, P.; Weskamm, L.M. Delayed Induction of Noninflammatory SARS-CoV-2 Spike-Specific IgG4 Antibodies Detected 1 Year After BNT162b2 Vaccination in Children. Pediatr. Infect. Dis. J. 2024, 12, 1200–1203. [Google Scholar] [CrossRef]
- Uversky, V.N.; Redwan, E.M.; Makis, W.; Rubio-Casillas, A. IgG4 Antibodies Induced by Repeated Vaccination May Generate Immune Tolerance to the SARS-CoV-2 Spike Protein. Vaccines 2023, 11, 991. [Google Scholar] [CrossRef]
- Kadkhoda, K. Post-COVID mRNA-vaccine IgG4 shift: Worrisome? mSphere 2023, 8, e0008523. [Google Scholar] [CrossRef]
- Buhre, J.S.; Pongracz, T.; Künsting, I.; Lixenfeld, A.S.; Wang, W.; Nouta, J.; Lehrian, S.; Schmelter, F.; Lunding, H.B.; Dühring, L. mRNA vaccines against SARS-CoV-2 induce comparably low long-term IgG Fc galactosylation and sialylation levels but increasing long-term IgG4 responses compared to an adenovirus-based vaccine. Front. Immunol. 2023, 13, 1020844. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci. Immunol. 2021, 6, eabi6950. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Immunological memory to SARS-CoV-2 infection and COVID-19 vaccines. Immunol. Rev. 2022, 310, 27–46. [Google Scholar] [CrossRef]
- Rick, A.M.; Laurens, M.B.; Huang, Y.; Yu, C.; Martin, T.C.S.; Rodriguez, C.A.; Rostad, C.A.; Maboa, R.M.; Baden, L.R.; El Sahly, H.M.; et al. Risk of COVID-19 after natural infection or vaccination. EBioMedicine 2023, 96, 104799. [Google Scholar] [CrossRef]
- Jeong, A.Y.; Lee, P.; Lee, M.S.; Kim, D.J. Pre-existing Immunity to Endemic Human Coronaviruses Does Not Affect the Immune Response to SARS-CoV-2 Spike in a Murine Vaccination Model. Immune Netw. 2023, 23, e19. [Google Scholar] [CrossRef] [PubMed]
- Herzberg, J.; Vollmer, T.; Fischer, B.; Becher, H.; Becker, A.K.; Honarpisheh, H.; Guraya, S.Y.; Strate, T.; Knabbe, C. SARS-CoV-2-antibody response in health care workers after vaccination or natural infection in a longitudinal observational study. Vaccine 2022, 40, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Franco-Luiz, A.P.M.; Fernandes, N.; Silva, T.B.S.; Bernardes, W.; Westin, M.R.; Santos, T.G.; Fernandes, G.D.R.; Simoes, T.C.; Silva, E.F.E.; Gava, S.G.; et al. Longitudinal study of humoral immunity against SARS-CoV-2 of health professionals in Brazil: The impact of booster dose and reinfection on antibody dynamics. Front. Immunol. 2023, 14, 1220600. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Shariff, M.A.; Perez Gutierrez, V.; Carreno, J.M.; Yu, B.; Jawed, M.; Gossai, M.; Valdez, E.; Pillai, A.; Venugopal, U.; et al. Longitudinal humoral antibody response to SARS-CoV-2 infection among healthcare workers in a New York City hospital. BMJ Open 2021, 11, e051045. [Google Scholar] [CrossRef]
- Huynen, P.; Gregoire, C.; Gofflot, S.; Seidel, L.; Maes, N.; Vranken, L.; Delcour, S.; Moutschen, M.; Hayette, M.P.; Kolh, P.; et al. Long-term longitudinal evaluation of the prevalence of SARS-CoV-2 antibodies in healthcare and university workers. Sci. Rep. 2022, 12, 5156. [Google Scholar] [CrossRef]
- Adhikari, B.; Bednash, J.S.; Horowitz, J.C.; Rubinstein, M.P.; Vlasova, A.N. Brief research report: Impact of vaccination on antibody responses and mortality from severe COVID-19. Front. Immunol. 2024, 15, 1325243. [Google Scholar] [CrossRef]
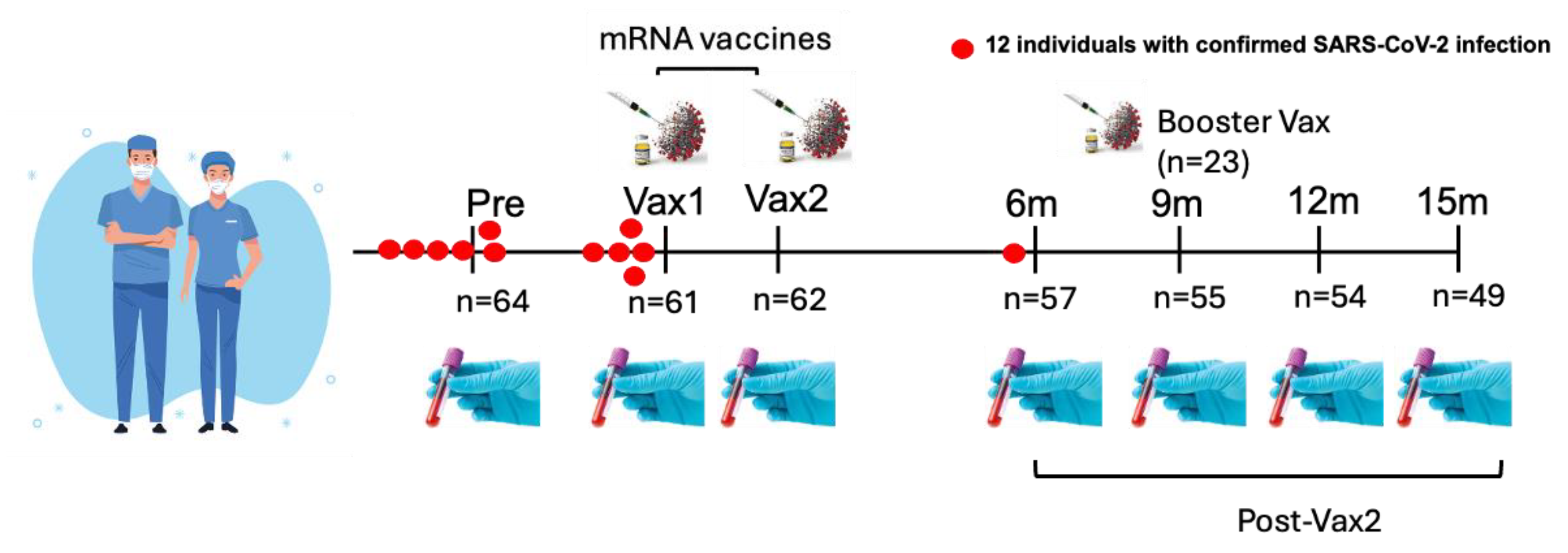


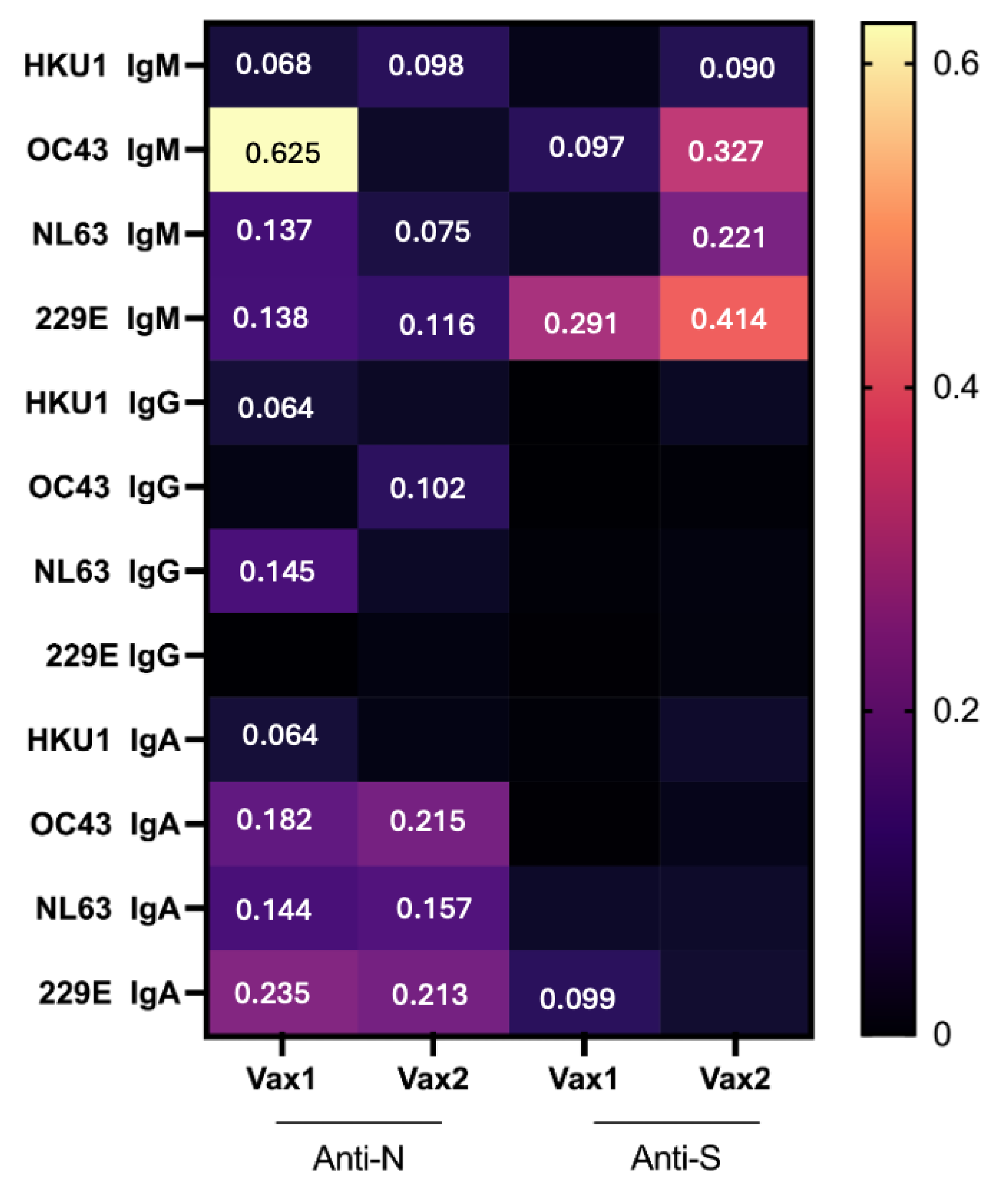
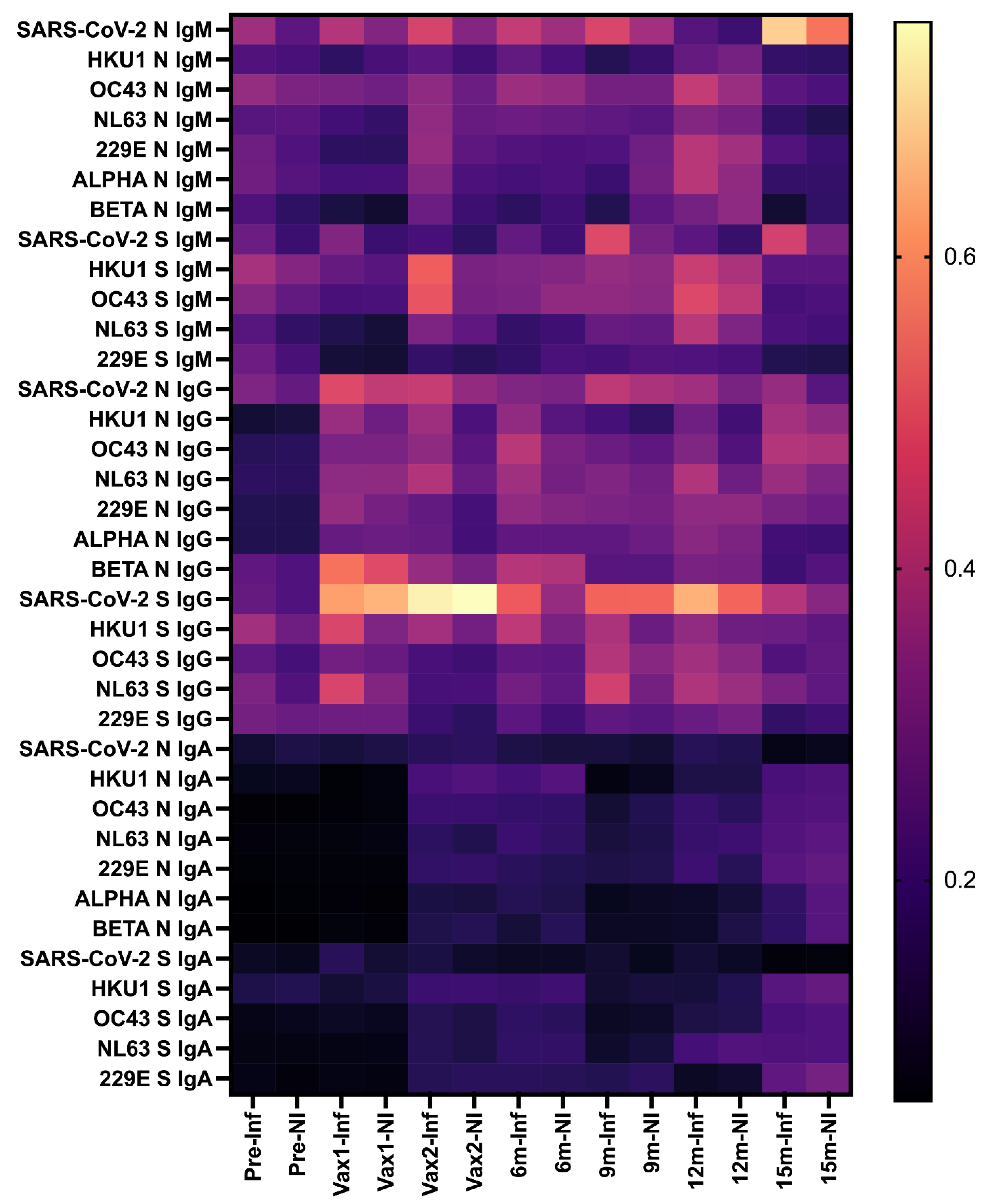

| Time Point | Sample Collection Period | Number of Individuals (n) | Details |
|---|---|---|---|
| Pre-vaccination (Pre) | April–May 2020 | 64 | Collected approximately 7–8 months prior to vaccination. |
| Post-vaccination (Vax1) | 13–33 days after 1st dose (Median = 21) | 61 | Collected after the first vaccine dose. Interquartile range (IQR) = 19–23 days. |
| Post-vaccination (Vax2) | 20–31 days after 2nd dose (Median = 26) | 62 | Collected after the second vaccine dose. IQR = 22–28 days. |
| 6-month post-vaccination (6 m) | 6 months post-vaccination | 57 | Collected at the 6-month follow-up. |
| 9-month post-vaccination (9 m) | 9 months post-vaccination | 55 | Collected at the 9-month follow-up. |
| 12-month post-vaccination (12 m) | 12 months post-vaccination | 54 | Collected at the 12-month follow-up. |
| 15-month post-vaccination (15 m) | 15 months post-vaccination | 49 | Collected at the 15-month follow-up. |
| Booster dose (BNT162b2/mRNA-1273) | 1–80 days prior to the 9 m time point | 23 | Total of 23 individuals; BNT162b2 (n = 17), mRNA-1273 (n = 6). Median = 18 days, IQR = 7–29 days before 9 m time point. |
| Second booster (BNT162b2) | Timeframe unknown | 2 | Collected after the second booster dose, timeframe not available. |
| Vaccine type information | - | - | Vax1: 61 individuals (mRNA-1273 n = 28, BNT162b2 n = 33), Vax2: 62 individuals (mRNA-1273 n = 28, BNT162b2 n = 34), boosters: 23 individuals (BNT162b2 n = 17, mRNA-1273 n = 6), 2 received second booster doses (BNT162b2). |
| SARS-CoV-2 infections | - | 12 | Ten confirmed infections 1–9 months prior to 1st dose, one infection within 12 days post-Vax1 (but prior to Vax 1 sampling), one breakthrough infection ~6 months post-Vax2. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adhikari, B.; Oltz, E.M.; Gumina, R.J.; Kick, M.K.; Saif, L.J.; Vlasova, A.N. Circulating Antibodies Against Common Cold Coronaviruses Do Not Interfere with Immune Responses to Primary or Booster SARS-CoV-2 mRNA Vaccines. Vaccines 2025, 13, 547. https://doi.org/10.3390/vaccines13050547
Adhikari B, Oltz EM, Gumina RJ, Kick MK, Saif LJ, Vlasova AN. Circulating Antibodies Against Common Cold Coronaviruses Do Not Interfere with Immune Responses to Primary or Booster SARS-CoV-2 mRNA Vaccines. Vaccines. 2025; 13(5):547. https://doi.org/10.3390/vaccines13050547
Chicago/Turabian StyleAdhikari, Bindu, Eugene M. Oltz, Richard J. Gumina, Maryssa K. Kick, Linda J. Saif, and Anastasia N. Vlasova. 2025. "Circulating Antibodies Against Common Cold Coronaviruses Do Not Interfere with Immune Responses to Primary or Booster SARS-CoV-2 mRNA Vaccines" Vaccines 13, no. 5: 547. https://doi.org/10.3390/vaccines13050547
APA StyleAdhikari, B., Oltz, E. M., Gumina, R. J., Kick, M. K., Saif, L. J., & Vlasova, A. N. (2025). Circulating Antibodies Against Common Cold Coronaviruses Do Not Interfere with Immune Responses to Primary or Booster SARS-CoV-2 mRNA Vaccines. Vaccines, 13(5), 547. https://doi.org/10.3390/vaccines13050547






