COVID-19 Vaccination Enhances the Immunogenicity of Seasonal Influenza Vaccination in the Elderly
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Consent Statement
2.3. Vaccinees and Formulations
2.4. Hemagglutination Inhibition (HAI) Assay
2.5. SARS-CoV-2 Serum Neutralization Assay
2.6. Statistical Analysis
3. Results
3.1. Demographics of Participants
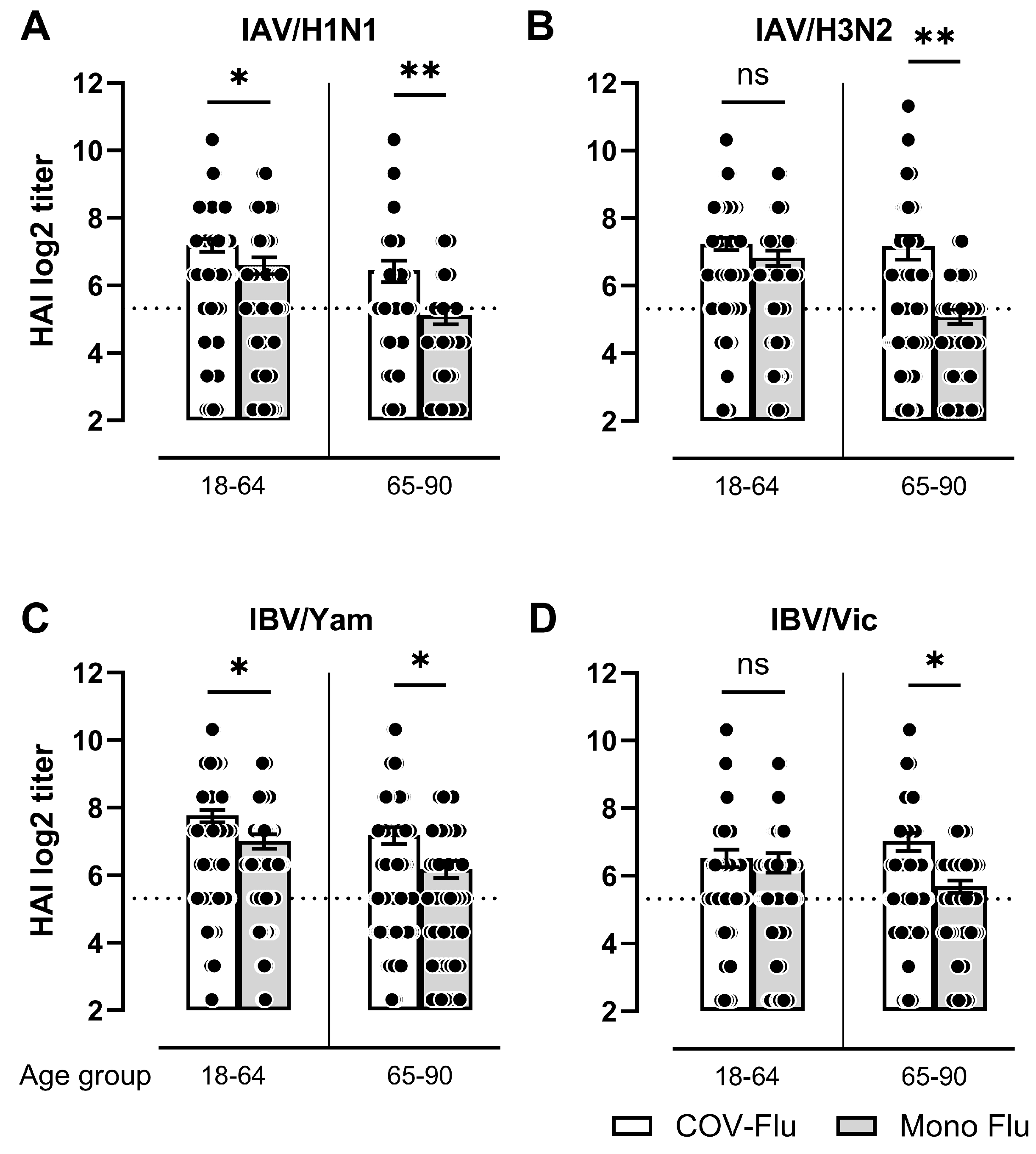
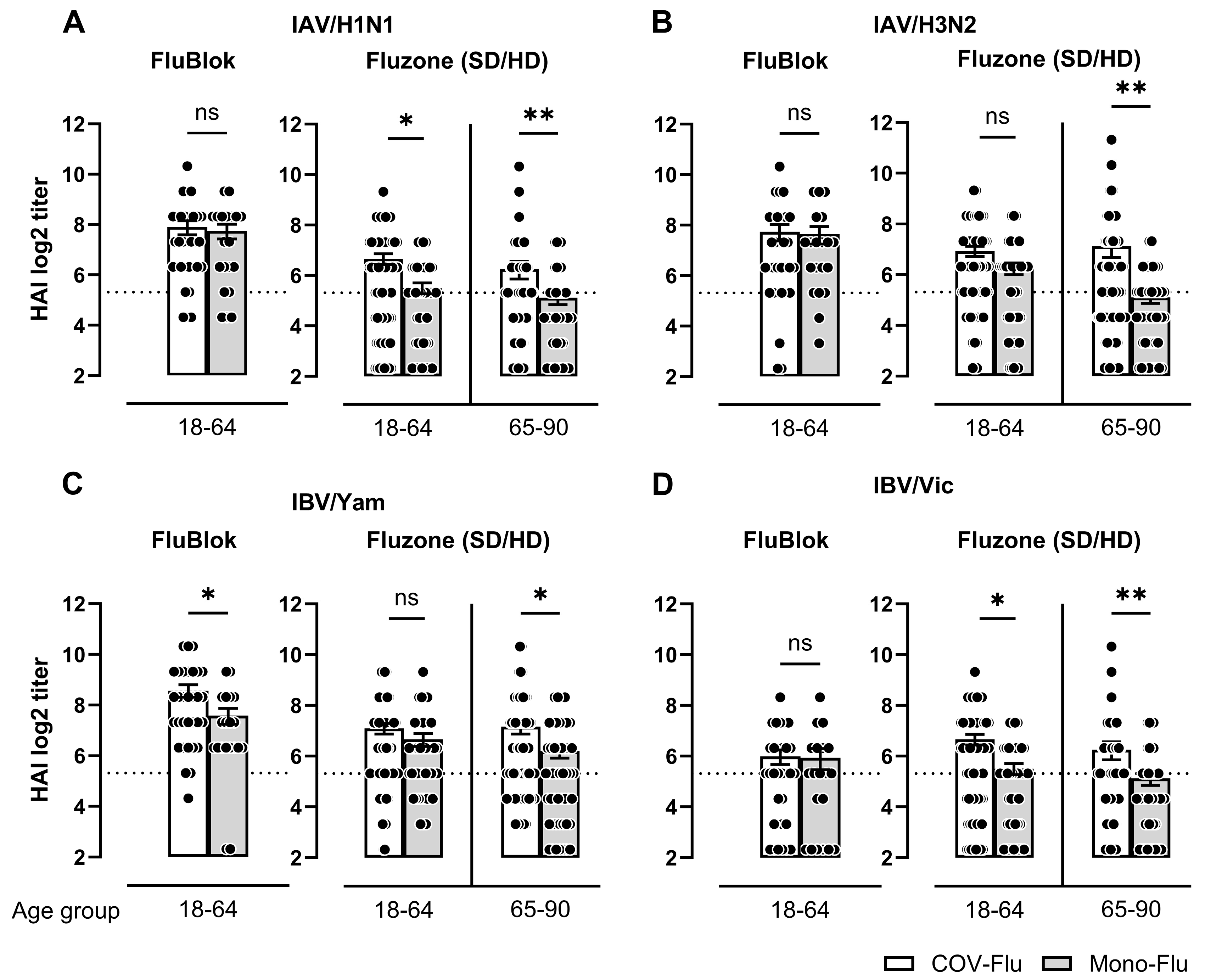
3.2. Comparison of HAI Titers Between COV-Flu and Mono-Flu Participants
3.3. SARS-Cov-2 Virus Neutralization Titer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piroth, L.; Cottenet, J.; Mariet, A.-S.; Bonniaud, P.; Blot, M.; Tubert-Bitter, P.; Quantin, C. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: A nationwide, population-based retrospective cohort study. Lancet Respir. Med. 2021, 9, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, Q.; Chi, J.; Dong, B.; Lv, W.; Shen, L.; Wang, Y. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 99, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Near, A.; Tse, J.; Young-Xu, Y.; Hong, D.K.; Reyes, C.M. Health Resource Burden of Influenza Among the Elderly with Underlying Conditions in the United States. Open Forum. Infect. Dis. 2020, 7, S174–S175. [Google Scholar] [CrossRef]
- Capone, A. Simultaneous circulation of COVID-19 and flu in Italy: Potential combined effects on the risk of death? Int. J. Infect. Dis. 2020, 99, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Alosaimi, B.; Naeem, A.; Hamed, M.E.; Alkadi, H.S.; Alanazi, T.; Al Rehily, S.S.; Almutairi, A.Z.; Zafar, A. Influenza co-infection associated with severity and mortality in COVID-19 patients. Virol. J. 2021, 18, 127. [Google Scholar] [CrossRef]
- Bai, L.; Zhao, Y.; Dong, J.; Liang, S.; Guo, M.; Liu, X.; Wang, X.; Huang, Z.; Sun, X.; Zhang, Z.; et al. Coinfection with influenza A virus enhances SARS-CoV-2 infectivity. Cell Res. 2021, 31, 395–403. [Google Scholar] [CrossRef]
- Chaves, S.S.; Naeger, S.; Lounaci, K.; Zuo, Y.; Loiacono, M.M.; Pilard, Q.; Nealon, J.; Genin, M.; Mahe, C. High-Dose Influenza Vaccine Is Associated with Reduced Mortality Among Older Adults with Breakthrough Influenza Even When There Is Poor Vaccine-Strain Match. Clin. Infect. Dis. 2023, 77, 1032–1042. [Google Scholar] [CrossRef]
- Griffin, J.B.; Haddix, M.; Danza, P.; Fisher, R.; Koo, T.H.; Traub, E.; Gounder, P.; Jarashow, C.; Balter, S. SARS-CoV-2 Infections and Hospitalizations Among Persons Aged ≥ 16 Years, by Vaccination Status—Los Angeles County, California, 1 May–25 July 2021. MMWR Morb. Mortal Wkly. Rep. 2021, 70, 1170–1176. [Google Scholar] [CrossRef]
- World Health Organization (WHO); Melanie Marti, M.F.; Chadwick, C.; Hombach, J.; Wilder-Smith, A.; Desai, S.; O’Brien, K. Coadministration of Seasonal Inactivated Influenza and COVID-19 Vaccines: Interim Guidance. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-coadministration-influenza-vaccines (accessed on 20 March 2025).
- Lazarus, R.; Baos, S.; Cappel-Porter, H.; Carson-Stevens, A.; Clout, M.; Culliford, L.; Emmett, S.R.; Garstang, J.; Gbadamoshi, L.; Hallis, B.; et al. Safety and immunogenicity of concomitant administration of COVID-19 vaccines (ChAdOx1 or BNT162b2) with seasonal influenza vaccines in adults in the UK (ComFluCOV): A multicentre, randomised, controlled, phase 4 trial. Lancet 2021, 398, 2277–2287. [Google Scholar] [CrossRef]
- Toback, S.; Galiza, E.; Cosgrove, C.; Galloway, J.; Goodman, A.L.; Swift, P.A.; Rajaram, S.; Graves-Jones, A.; Edelman, J.; Burns, F.; et al. Safety, immunogenicity, and efficacy of a COVID-19 vaccine (NVX-CoV2373) co-administered with seasonal influenza vaccines: An exploratory substudy of a randomised, observer-blinded, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2022, 10, 167–179. [Google Scholar] [CrossRef]
- Janssen, C.; Mosnier, A.; Gavazzi, G.; Combadière, B.; Crépey, P.; Gaillat, J.; Launay, O.; Botelho-Nevers, E. Coadministration of seasonal influenza and COVID-19 vaccines: A systematic review of clinical studies. Hum. Vaccin. Immunother. 2022, 18, 2131166. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, L.; Quan, K.; Baber, J.A.; Ho, A.W.Y.; Zhang, Y.; Xu, X.; Lu, C.; Cooper, D.; Koury, K.; Lockhart, S.P.; et al. Safety and Immunogenicity of the BNT162b2 Vaccine Coadministered with Seasonal Inactivated Influenza Vaccine in Adults. Infect. Dis. Ther. 2023, 12, 2241–2258. [Google Scholar] [CrossRef]
- Hall, K.T.; Stone, V.E.; Ojikutu, B. Reactogenicity and Concomitant Administration of the COVID-19 Booster and Influenza Vaccine. JAMA Netw. Open 2022, 5, e2222246. [Google Scholar] [CrossRef]
- King, S.M.; Bryan, S.P.; Hilchey, S.P.; Wang, J.; Zand, M.S. First Impressions Matter: Immune Imprinting and Antibody Cross-Reactivity in Influenza and SARS-CoV-2. Pathogens 2023, 12, 169. [Google Scholar] [CrossRef] [PubMed]
- Simon, V.; Kota, V.; Bloomquist Ryan, F.; Hanley Hannah, B.; Forgacs, D.; Pahwa, S.; Pallikkuth, S.; Miller Loren, G.; Schaenman, J.; Yeaman Michael, R.; et al. PARIS and SPARTA: Finding the Achilles’ Heel of SARS-CoV-2. mSphere 2022, 7, e00179-22. [Google Scholar] [CrossRef] [PubMed]
- Carlock, M.A.; Allen, J.D.; Hanley, H.B.; Ross, T.M. Longitudinal assessment of human antibody binding to hemagglutinin elicited by split-inactivated influenza vaccination over six consecutive seasons. PLoS ONE 2024, 19, e0301157. [Google Scholar] [CrossRef]
- World Health Organization. Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Shi, H.; Xiaojian, Z.; Pan, G.; Victoria, M.; Pam, F.; Brandi, L.; Stacey, S.-C.; Ross, T.M. Inactivated influenza virus vaccines expressing COBRA hemagglutinin elicited broadly reactive, long-lived protective antibodies. Hum. Vaccines Immunother. 2024, 20, 2356269. [Google Scholar] [CrossRef]
- Committee for Proprietary Medicinal Products (CPMP). Note for Guidance on Harmonisation of Requirements for Influenza Vaccines; The European Agency for the Evaluation of Medicinal Products: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Kalkeri, R.; Cai, Z.; Lin, S.; Farmer, J.; Kuzmichev, Y.V.; Koide, F. SARS-CoV-2 Spike Pseudoviruses: A Useful Tool to Study Virus Entry and Address Emerging Neutralization Escape Phenotypes. Microorganisms 2021, 9, 1744. [Google Scholar] [CrossRef]
- Ferrara, F.; Temperton, N. Pseudotype Neutralization Assays: From Laboratory Bench to Data Analysis. Methods Protoc. 2018, 1, 8. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Estimates of deaths associated with seasonal influenza—United States, 1976–2007. MMWR Morb. Mortal. Wkly. Rep. 2010, 59, 1057. [Google Scholar]
- CDC. Flu and People 65 Years and Older. Available online: https://www.cdc.gov/flu/highrisk/65over.htm (accessed on 23 March 2025).
- Blomberg, B.B.; Frasca, D. Quantity, not quality, of antibody response decreased in the elderly. JCI 2011, 121, 2981–2983. [Google Scholar] [CrossRef]
- Aydillo, T.; Maria, B.-M.; Amaya, R.-F.; Alba, E.; Celia, S.-R.; Jerónimo, P.; María, D.M.M.-G.; José, S.-C.M.; Javier, S.-C.; Adolfo, G.-S.; et al. Concomitant administration of seasonal influenza and COVID-19 mRNA vaccines. Emerg. Microbes Infect. 2024, 13, 2292068. [Google Scholar] [CrossRef] [PubMed]
- Pattinson, D.; Jester, P.; Gu, C.; Guan, L.; Armbrust, T.; Petrie, J.G.; King, J.P.; Nguyen, H.Q.; Belongia, E.A.; Halfmann, P.; et al. Ipsilateral and contralateral coadministration of influenza and COVID-19 vaccines produce similar antibody responses. eBioMedicine 2024, 103, 105103. [Google Scholar] [CrossRef]
- Nazareth, J.; Martin, C.A.; Pan, D.; Barr, I.G.; Sullivan, S.G.; Peck, H.; Veli, N.; Das, M.; Bryant, L.; George, N.; et al. Immunogenicity of concomitant SARS-CoV-2 and influenza vaccination in UK healthcare workers: A prospective longitudinal observational study. Lancet Reg. Health—Eur. 2024, 44, 101022. [Google Scholar] [CrossRef]
- Richards, K.A.; Moritzky, S.; Shannon, I.; Fitzgerald, T.; Yang, H.; Branche, A.; Topham, D.J.; Treanor, J.J.; Nayak, J.; Sant, A.J. Recombinant HA-based vaccine outperforms split and subunit vaccines in elicitation of influenza-specific CD4 T cells and CD4 T cell-dependent antibody responses in humans. npj Vaccines 2020, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.; Joyner, D.; Mege, N.J.; Cusimano, G.M.; Bell, M.R.; Marcy, J.; Taramangalam, B.; Kim, K.M.; Lin, P.J.C.; Tam, Y.K.; et al. Lipid nanoparticles (LNP) induce activation and maturation of antigen presenting cells in young and aged individuals. Commun. Biol. 2023, 6, 188. [Google Scholar] [CrossRef]
- Xie, C.; Yao, R.; Xia, X. The advances of adjuvants in mRNA vaccines. npj Vaccines 2023, 8, 162. [Google Scholar] [CrossRef]
- Alameh, M.-G.; Tombácz, I.; Bettini, E.; Lederer, K.; Ndeupen, S.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2021, 54, 2877–2892.e2877. [Google Scholar] [CrossRef]
- Swaminathan, G.; Thoryk, E.A.; Cox, K.S.; Meschino, S.; Dubey, S.A.; Vora, K.A.; Celano, R.; Gindy, M.; Casimiro, D.R.; Bett, A.J. A novel lipid nanoparticle adjuvant significantly enhances B cell and T cell responses to sub-unit vaccine antigens. Vaccine 2016, 34, 110–119. [Google Scholar] [CrossRef]
- Gasparini, R.; Pozzi, T.; Montomoli, E.; Fragapane, E.; Senatore, F.; Minutello, M.; Podda, A. Increased immunogenicity of the MF59-adjuvanted influenza vaccine compared to a conventional subunit vaccine in elderly subjects. Eur. J. Epidemiol. 2001, 17, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Shichinohe, S.; Watanabe, T. Advances in Adjuvanted Influenza Vaccines. Vaccines 2023, 11, 1391. [Google Scholar] [CrossRef] [PubMed]
- Lofano, G.; Mancini, F.; Salvatore, G.; Cantisani, R.; Monaci, E.; Carrisi, C.; Tavarini, S.; Sammicheli, C.; Rossi Paccani, S.; Soldaini, E.; et al. Oil-in-Water Emulsion MF59 Increases Germinal Center B Cell Differentiation and Persistence in Response to Vaccination. J. Immunol. 2015, 195, 1617–1627. [Google Scholar] [CrossRef] [PubMed]


| UGA6 (2021–2022) a | UGA7 (2022–2023) | UGA8 (2023–2024) | Total UGA6-8 (2021–2024) | SPARTA (2021–2024) b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Demographic Characteristics | COV-Flu c (n = 109) | Mono-Flu d (n = 56) | COV-Flu (n = 61) | Mono-Flu (n = 48) | COV-Flu (n = 31) | Mono-Flu (n = 26) | COV-Flu (n = 201) | Mono-Flu (n = 130) | Mono-COVID-19 e (n = 67) | |
| Sex (%) | ||||||||||
| Male | 37 (34%) | 16 (29%) | 28 (46%) | 21 (44%) | 9 (29%) | 11 (42%) | 74 (37%) | 48 (37%) | 23 (34%) | |
| Female | 72(66%) | 40 (71%) | 33 (54%) | 27 (56%) | 22 (71%) | 15 (58%) | 127 (63%) | 82 (63%) | 44 (66%) | |
| Age group (%) | ||||||||||
| Young adult (18–64) | 66 (61%) | 41 (73%) | 30 (49%) | 23 (48%) | 18 (58%) | 16 (62%) | 114 (57%) | 80 (62%) | 40 (60%) | |
| Elderly (65–90) | 43 (39%) | 15 (27%) | 31 (51%) | 25 (52%) | 13 (42%) | 10 (38%) | 87 (43%) | 50 (38%) | 27 (40%) | |
| Average Age (age range) | ||||||||||
| Young adult (18–64) | 39.3 (18–63) | 39.7 (18–64) | 44.0 (18–63) | 45.2 (19–63) | 48.3 (25–64) | 44.9 (25–64) | 43.9 (18–64) | 43.3 (18–64) | 44.3 (19–64) | |
| Elderly (65–90) | 72.2 (65–86) | 71.3 (65–83) | 71.8 (65–87) | 72.0 (66–84) | 71.7 (65–80) | 71.1 (65–76) | 71.9 (65–87) | 71.5 (65–84) | 73.3 (65–83) | |
| Race/Ethnicity (%) | ||||||||||
| White | 94 (86%) | 45 (80%) | 59 (97%) | 42 (88%) | 28 (90%) | 25 (96%) | 181 (90%) | 112 (86%) | 60 (90%) | |
| Black | 6 (6%) | 2 (4%) | - | 2 (4%) | 2 (7%) | - | 8 (4%) | 4 (3%) | - | |
| Hispanic or Latino | 7 (6%) | 4 (7%) | 2 (3%) | 3 (6%) | 1 (3%) | - | 10 (5%) | 7 (5%) | 2 (3%) | |
| Asian | 2 (2%) | 3 (5%) | - | 1 (2%) | - | 1 (4%) | 2 (1%) | 5 (4%) | 5 (7%) | |
| American Indian or Alaska Native | - | 1 (2%) | - | - | - | - | - | 1 (1%) | - | |
| Mixed (Black, White, Hispanic, or Latino) | - | 1 (2%) | - | - | - | - | - | 1 (1%) | - | |
| Average duration (days) between mRNA COVID-19 vaccine dose and influenza vaccine (day range) | 20.3 (1–88) | - | 27.6 (1–81) | - | 28 (2–87) | - | 24.6 (1–88) | - | - | |
| Average days post-flu vaccination for sera testing (day range) | 29.2 (21–42) | 29.6 (24–42) | 28 (21–40) | 28.6 (24–41) | 28.9 (23–37) | 28.7 (21–35) | 28.8 (21–42) | 29.1 (21–42) | - | |
| Average days post-COVID-19 vaccination for sera testing (day range) | 35.9 (7–87) | - | 48.3 (3–88) | - | 21.5 (4–78) | - | 37.5 (3–88) | - | 46.3 (7–90) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berber, E.; Pantouli, F.; Hanley, H.B.; Ross, T.M. COVID-19 Vaccination Enhances the Immunogenicity of Seasonal Influenza Vaccination in the Elderly. Vaccines 2025, 13, 531. https://doi.org/10.3390/vaccines13050531
Berber E, Pantouli F, Hanley HB, Ross TM. COVID-19 Vaccination Enhances the Immunogenicity of Seasonal Influenza Vaccination in the Elderly. Vaccines. 2025; 13(5):531. https://doi.org/10.3390/vaccines13050531
Chicago/Turabian StyleBerber, Engin, Fani Pantouli, Hannah B. Hanley, and Ted M. Ross. 2025. "COVID-19 Vaccination Enhances the Immunogenicity of Seasonal Influenza Vaccination in the Elderly" Vaccines 13, no. 5: 531. https://doi.org/10.3390/vaccines13050531
APA StyleBerber, E., Pantouli, F., Hanley, H. B., & Ross, T. M. (2025). COVID-19 Vaccination Enhances the Immunogenicity of Seasonal Influenza Vaccination in the Elderly. Vaccines, 13(5), 531. https://doi.org/10.3390/vaccines13050531







