Is Influenza Vaccination Our Best ‘Shot’ at Preventing MACE? Review of Current Evidence, Underlying Mechanisms, and Future Directions
Abstract
1. Introduction
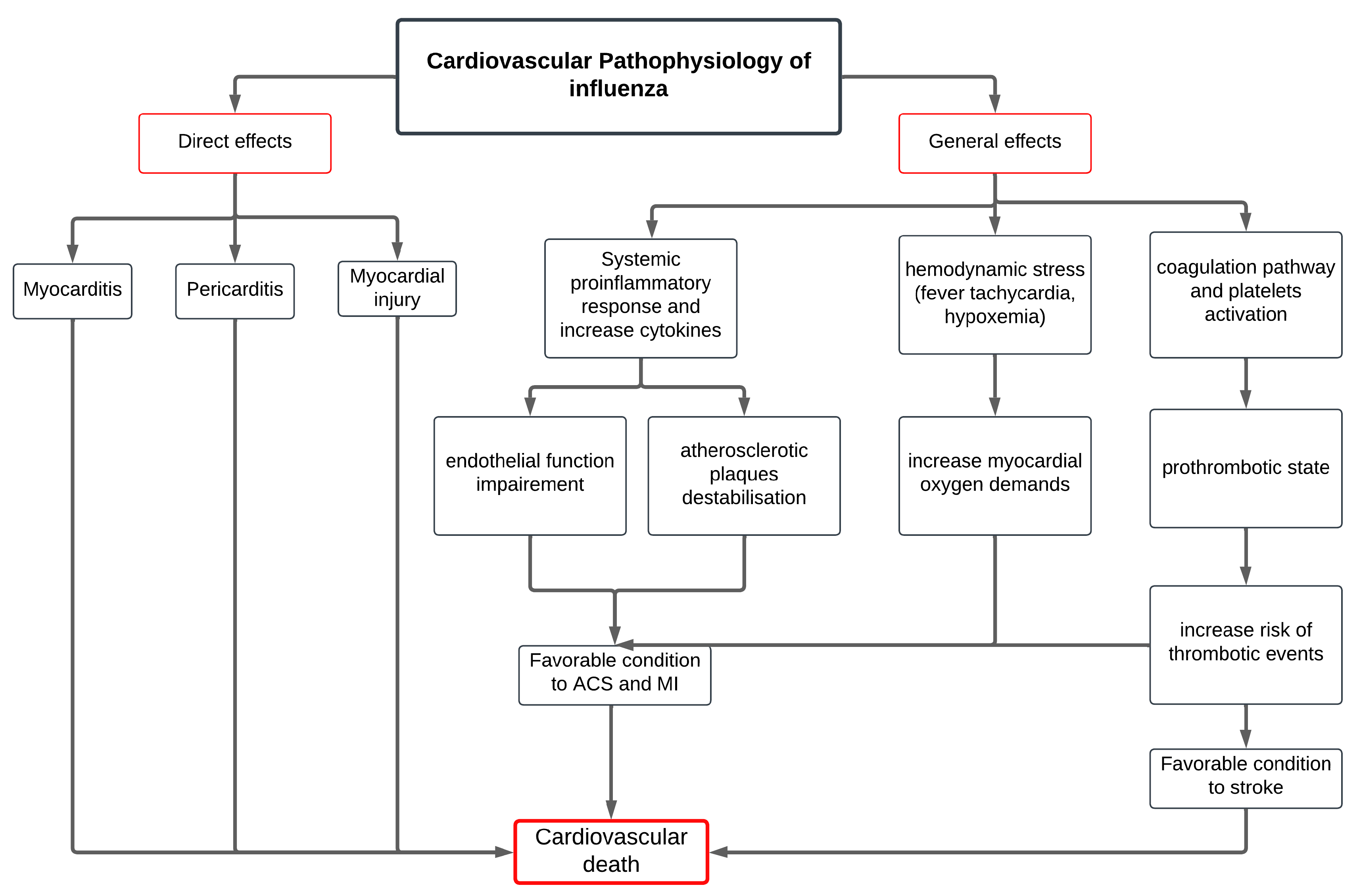
2. Mechanisms Linking Influenza Infection and MACE
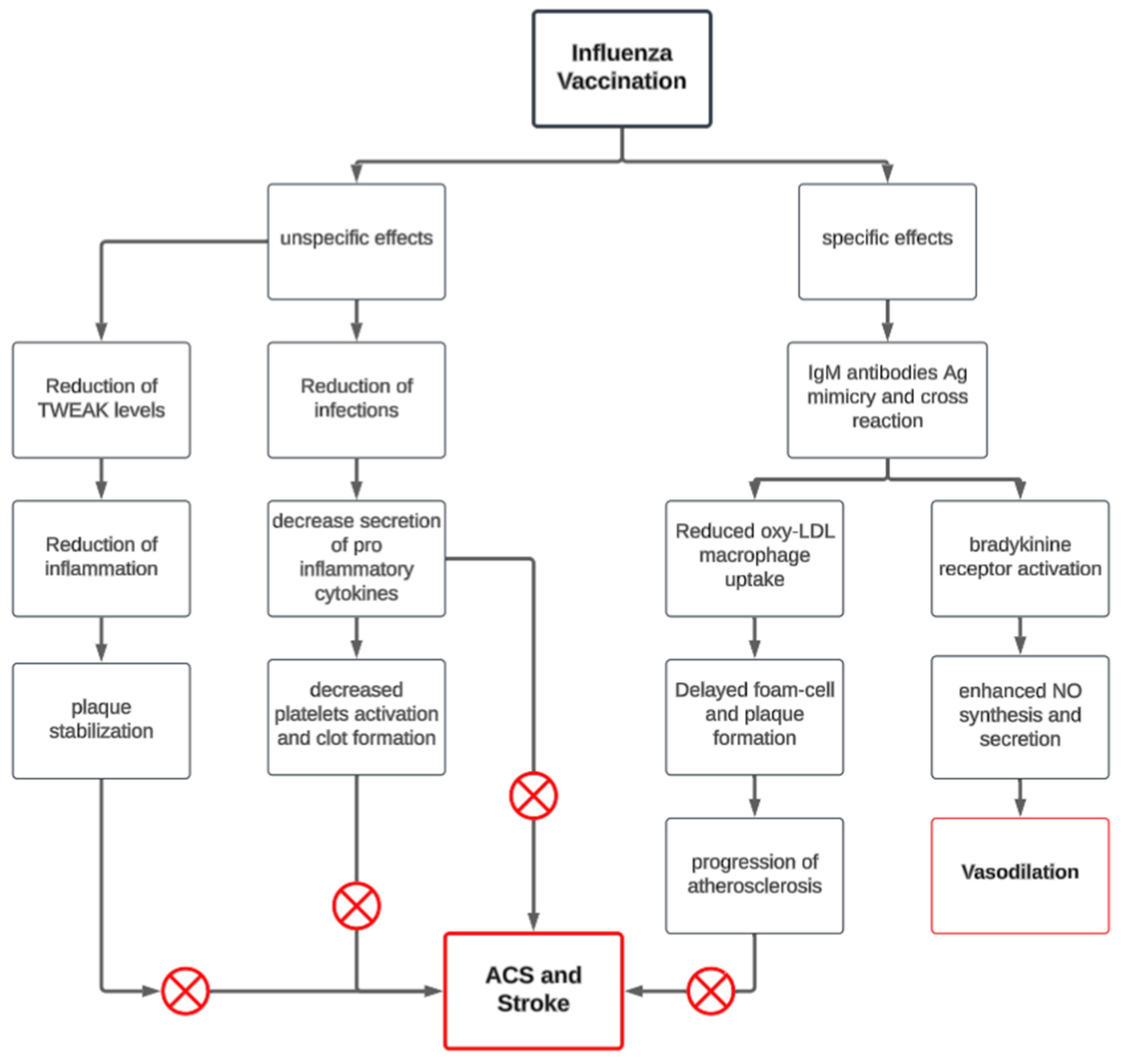
3. Mechanism of Action of Influenza Vaccine on MACE
4. Effects of Influenza Vaccine on MACE
4.1. Effect of Vaccine in Specific Populations
4.1.1. Effects of Influenza Vaccination in High-Risk Patients/Secondary Prevention of MACE
Effect of Influenza Vaccination on Reducing AMI and Cardiovascular Death
Effect of Influenza Vaccination in Reducing Stroke
4.1.2. Effects of Influenza Vaccination in Low-Risk Patients/Primary Prevention/General Population of MACE
4.2. Timing of Influenza Vaccination and Cardiovascular Events
4.2.1. Optimal Seasonal Timing of Vaccination
4.2.2. Post-Cardiovascular Events or Hospitalization Timing
4.2.3. Impact of Annual Vaccination on MACE
4.3. Type of Vaccine and Dosage Considerations
4.3.1. Standard-Dose vs. High-Dose Influenza Vaccination
4.3.2. Adjuvanted Vaccines and Other Considerations
5. Future Directions and Research Gaps
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACS | Acute coronary syndrome |
| AMI | Acute myocardial infarction |
| BKB2R | Bradykinin 2 receptor |
| CI | Confidence interval |
| COPD | Chronic obstructive pulmonary disease |
| CVD | Cardiovascular disease |
| FLUCAD | Influenza Vaccination in Secondary Prevention from Coronary Ischemic Events study |
| FLUVACS | Flu Vaccination Acute Coronary Syndrome study |
| HF | Heart failure |
| HR | Hazard ratio |
| IAMI | Influenza Vaccination After Myocardial Infarction trial |
| IHD | Ischemic heart disease |
| INVESTED | Influenza Vaccine to Effectively Stop Cardiothoracic Events and Decompensated Heart Failure study |
| IVCAD | Efficacy of Influenza Vaccine in Reducing Cardiovascular Events study |
| LDL | Low-density lipoprotein |
| MACE | Major adverse cardiovascular events |
| MF59-TIV | MF59-adjuvanted trivalent influenza vaccine |
| MI | Myocardial infarction |
| NO | Nitric oxide |
| OR | Odds ratio |
| RCT | Randomized controlled trial |
| RR | Relative risk |
| TWEAK | Tumor necrosis factor-alpha-related weak inducer of apoptosis |
References
- Bosco, E.; Hsueh, L.; McConeghy, K.W.; Gravenstein, S.; Saade, E. Major adverse cardiovascular event definitions used in observational analysis of administrative databases: A systematic review. BMC Med. Res. Methodol. 2021, 21, 241. [Google Scholar] [CrossRef]
- Blackburn, R.; Zhao, H.; Pebody, R.; Hayward, A.; Warren-Gash, C. Laboratory-Confirmed Respiratory Infections as Predictors of Hospital Admission for Myocardial Infarction and Stroke: Time-Series Analysis of English Data for 2004–2015. Clin. Infect Dis. 2018, 67, 8–17. [Google Scholar] [CrossRef]
- Kwong, J.C.; Schwartz, K.L.; Campitelli, M.A.; Chung, H.; Crowcroft, N.S.; Karnauchow, T.; Katz, K.; Ko, D.T.; McGeer, A.J.; McNally, D.; et al. Acute Myocardial Infarction after Laboratory-Confirmed Influenza Infection. N. Engl. J. Med. 2018, 378, 345–353. [Google Scholar] [CrossRef]
- Ciszewski, A.; Bilinska, Z.T.; Brydak, L.B.; Kepka, C.; Kruk, M.; Romanowska, M.; Ksiezycka, E.; Przyluski, J.; Piotrowski, W.; Maczynska, R.; et al. Influenza vaccination in secondary prevention from coronary ischaemic events in coronary artery disease: FLUCAD study. Eur. Heart J. 2008, 29, 1350–1358. [Google Scholar] [CrossRef]
- Fröbert, O.; Götberg, M.; Erlinge, D.; Akhtar, Z.; Christiansen, E.H.; MacIntyre, C.R.; Oldroyd, K.G.; Motovska, Z.; Erglis, A.; Moer, R.; et al. Clinical impact of influenza vaccination after ST- and non-ST-segment elevation myocardial infarction-insights from the IAMI trial. Am. Heart J. 2023, 255, 82–89. [Google Scholar] [CrossRef]
- CDC. Influenza Vaccination: A Summary for Clinicians. Available online: http://med.iiab.me/modules/en-cdc/www.cdc.gov/flu/professionals/vaccination/vax-summary.htm (accessed on 22 March 2024).
- Toschke, A.M.; Heuschmann, P.U.; Wood, O.; Wolfe, C.D.A. Temporal relationship between influenza infections and subsequent first-ever stroke incidence. Age Ageing 2009, 38, 100–103. [Google Scholar] [CrossRef]
- Warren-Gash, C.; Smeeth, L.; Hayward, A.C. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: A systematic review. Lancet Infect. Dis. 2009, 9, 601–610. [Google Scholar] [CrossRef]
- Fröbert, O.; Götberg, M.; Erlinge, D.; Akhtar, Z.; Christiansen, E.H.; MacIntyre, C.R.; Oldroyd, K.G.; Motovska, Z.; Erglis, A.; Moer, R.; et al. Influenza Vaccination After Myocardial Infarction: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. Circulation 2021, 144, 1476–1484. [Google Scholar] [CrossRef]
- Yedlapati, S.H.; Mendu, A.; Tummala, V.R.; Maganti, S.S.; Nasir, K.; Khan, S.U. Vaccines and cardiovascular outcomes: Lessons learned from influenza epidemics. Eur. Heart J. Suppl. 2023, 25, A17–A24. [Google Scholar] [CrossRef]
- Tanaka, K.; Demchuk, A.M.; Malo, S.; Hill, M.D.; Holodinsky, J.K. Risk of stroke within 3, 7, 14, 21 and 30 days after influenza vaccination in Alberta, Canada: A population-based study-PubMed. Eur. J. Neurol. 2024, 31, e16172. [Google Scholar] [CrossRef]
- Skaarup, K.G.; Modin, D.; Nielsen, L.; Jensen, J.U.S.; Biering-Sorensen, T. Influenza and cardiovascular disease pathophysiology: Strings attached. Eur. Heart J. Suppl. 2023, 25, A5–A11. [Google Scholar] [CrossRef]
- Bermúdez-Fajardo, A.; Oviedo-Orta, E. Influenza vaccination promotes stable atherosclerotic plaques in apoE knockout mice. Atherosclerosis 2011, 217, 97–105. [Google Scholar] [CrossRef]
- Goeijenbier, M.; van Wissen, M.; van de Weg, C.; Jong, E.; Gerdes, V.E.; Meijers, J.C.; Brandjes, D.P.; van Gorp, E.C. Review: Viral infections and mechanisms of thrombosis and bleeding. J. Med. Virol. 2012, 84, 1680–1696. [Google Scholar] [CrossRef]
- Aidoud, A.; Marlet, J.; Angoulvant, D.; Debacq, C.; Gavazzi, G.; Fougère, B. Influenza vaccination as a novel means of pre-venting coronary heart disease: Effectiveness in older adults. Vaccine 2020, 38, 4944–4955. [Google Scholar] [CrossRef]
- Veljkovic, V.; Glisic, S.; Veljkovic, N.; Bojic, T.; Dietrich, U.; Perovic, V.R.; Colombatti, A. Influenza vaccine as prevention for cardio-vascular diseases: Possible molecular mechanism. Vaccine 2014, 32, 6569–6575. [Google Scholar] [CrossRef]
- Mendez-Barbero, N.; Gutierrez-Munoz, C.; Blazquez-Serra, R.; Martin-Ventura, J.L.; Blanco-Colio, L.M. Tumor Necrosis Factor-Like Weak Inducer of Apoptosis (TWEAK)/Fibroblast Growth Factor-Inducible 14 (Fn14) Axis in Cardiovascular Diseases: Progress and Challenges. Cells 2020, 9, 405. [Google Scholar] [CrossRef]
- Pleskov, V.M.; Bannikov, A.I.; Zaĭtsev, I.V. The receptor-mediated endocytosis of influenza viruses and low-density lipoproteins by tissue cells. Vopr. Virusol. 1994, 39, 121–125. [Google Scholar]
- Gurfinkel, E.P.; de la Fuente, R.L.; Mendiz, O.; Mautner, B. Flu vaccination in acute coronary syndromes and planned percutaneous coronary interventions (FLUVACS) Study. Eur. Heart J. 2004, 25, 25–31. [Google Scholar] [CrossRef]
- Phrommintikul, A.; Kuanprasert, S.; Wongcharoen, W.; Kanjanavanit, R.; Chaiwarith, R.; Sukonthasarn, A. Influenza vaccination reduces cardiovascular events in patients with acute coronary syndrome. Eur. Heart J. 2011, 32, 1730–1735. [Google Scholar] [CrossRef]
- Keshtkar-Jahromi, M.; Vakili, H.; Rahnavardi, M.; Gholamin, S.; Razavi, S.M.; Eskandari, A. The efficacy of influenza vaccination in reducing cardiovascular events in patients with coronary artery diseases: IVCAD study. Clin. Microbiol. Infect. 2009, 15, S395–s396. [Google Scholar]
- Yedlapati, S.H.; Khan, S.U.; Talluri, S.; Lone, A.N.; Khan, M.Z.; Khan, M.S.; Navar, A.M.; Gulati, M.; Johnson, H.; Baum, S.; et al. Effects of Influenza Vaccine on Mortality and Cardiovascular Outcomes in Patients With Cardiovascular Disease: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2021, 10, e019636. [Google Scholar] [CrossRef]
- Clar, C.; Oseni, Z.; Flowers, N.; Keshtkar-Jahromi, M.; Rees, K. Influenza vaccines for preventing cardiovascular disease. Cochrane Database Syst. Rev. 2015, 2015, CD005050. [Google Scholar] [CrossRef]
- Maniar, Y.M.; Al-Abdouh, A.; Michos, E.D. Influenza Vaccination for Cardiovascular Prevention: Further Insights from the IAMI Trial and an Updated Meta-analysis. Curr. Cardiol. Rep. 2022, 24, 1327–1335. [Google Scholar] [CrossRef]
- Zahhar, J.A.; Salamatullah, H.K.; Almutairi, M.B.; Faidah, D.E.; Afif, L.M.; Banjar, T.A.; Alansari, N.; Betar, M.; Alghamdi, S.; Makkawi, S. Influenza vaccine effect on risk of stroke occurrence: A systematic review and meta-analysis. Front. Neurol. 2023, 14, 1324677. [Google Scholar] [CrossRef]
- Holodinsky, J.K.; Zerna, C.; Malo, S.; Svenson, L.W.; Hill, M.D. Association between influenza vaccination and risk of stroke in Alberta, Canada: A population-based study. Lancet Public Health 2022, 7, e914–e922. [Google Scholar] [CrossRef]
- Davidson, J.A.; Banerjee, A.; Douglas, I.; Leyrat, C.; Pebody, R.; McDonald, H.I.; Herrett, E.; Forbes, H.; Smeeth, L.; Warren-Gash, C. Primary prevention of acute cardiovascular events by influenza vaccination: An observational study. Eur. Heart J. 2023, 44, 610–620. [Google Scholar] [CrossRef]
- Behrouzi, B.; Udell, J.A. Universal flu vaccines: A shot at lifelong cardioprotection? Nat. Rev. Cardiol. 2022, 19, 145–146. [Google Scholar] [CrossRef]
- Wu, A.D.; Lindson, N.; Hartmann-Boyce, J.; Wahedi, A.; Hajizadeh, A.; Theodoulou, A.; Thomas, E.T.; Lee, C.; Aveyard, P. Smoking cessation for secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2022, 8, CD014936. [Google Scholar] [CrossRef]
- Kuhn, J.; Olie, V.; Grave, C.; Le Strat, Y.; Bonaldi, C.; Joly, P. Impact of Smoking Reduction Scenarios on the Burden of Myocardial Infarction in the French Population Until 2035. Clin. Epidemiol. 2024, 16, 605–616. [Google Scholar] [CrossRef]
- Kinlay, S.; Young, M.M.; Gagnon, D.R. Smoking and 10-year risk of cardiovascular and non-cardiovascular events after contemporary coronary stenting. Am. J. Prev. Cardiol. 2024, 19, 100718. [Google Scholar] [CrossRef]
- Epstein, K.A.; Viscoli, C.M.; Spence, J.D.; Young, L.H.; Inzucchi, S.E.; Gorman, M.; Gerstenhaber, B.; Guarino, P.D.; Dixit, A.; Furie, K.L.; et al. Smoking cessation and outcome after ischemic stroke or TIA. Neurology 2017, 89, 1723. [Google Scholar] [CrossRef]
- Wu, X.-D.; Ye, X.-Y.; Liu, X.-Y.; Lin, Y.; Lin, X.; Li, Y.-Y.; Ye, B.-H.; Sun, J.-C. Benefits of intensive lipid-lowering therapies in patients with acute coronary syndrome: A systematic review and meta-analysis. Ann. Med. 2024, 56, 2389470. [Google Scholar] [CrossRef]
- Lee, M.; Cheng, C.-Y.; Wu, Y.-L.; Lee, J.-D.; Hsu, C.-Y.; Ovbiagele, B. Association Between Intensity of Low-Density Lipoprotein Cholesterol Reduction With Statin-Based Therapies and Secondary Stroke Prevention: A Meta-analysis of Randomized Clinical Trials. JAMA Neurol. 2022, 79, 349–358. [Google Scholar] [CrossRef]
- Byrne, P.; Cullinan, J.; Smith, A.; Smith, S.M. Statins for the primary prevention of cardiovascular disease: An overview of systematic reviews. BMJ Open 2019, 9, e023085. [Google Scholar] [CrossRef]
- Beta-Blocker Heart Attack Trial Research Group. A Randomized Trial of Propranolol in Patients With Acute Myocardial Infarction: I. Mortality Results. JAMA 1982, 247, 1707–1714. [Google Scholar] [CrossRef]
- Al Hennawi, H.; Khan, M.K.; Rasheed, F.; Rathi, S.; Ali, M.; Ali, A.; Asghar, Z.; Pasha, K.; Ashraf, M.T.; Klugherz, B. Effectiveness of low-dose rivaroxaban in preventing recurrent major adverse cardiovascular events in coronary artery disease: A systematic review and meta-analysis of randomized controlled trials. Coron. Artery Dis. 2024, 35, 614. [Google Scholar] [CrossRef]
- Udell, J.A.; Zawi, R.; Bhatt, D.L.; Keshtkar-Jahromi, M.; Gaughran, F.; Phrommintikul, A.; Ciszewski, A.; Vakili, H.; Hoffman, E.B.; Farkouh, M.E.; et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: A meta-analysis. JAMA 2013, 310, 1711–1720. [Google Scholar] [CrossRef]
- Siriwardena, A.N.; Gwini, S.M.; Coupland, C.A. Influenza vaccination, pneumococcal vaccination and risk of acute myocardial infarction: Matched case-control study. CMAJ 2010, 182, 1617–1623. [Google Scholar] [CrossRef]
- Cox, N. Influenza seasonality: Timing and formulation of vaccines. Bull. World Health Organ. 2014, 92, 311. [Google Scholar] [CrossRef]
- Spencer, J.A.; Smith, M.Z.; Osthus, D.; Alexander, P.C.; Del Valle, S.Y. When to vaccinate for seasonal influenza? Check the peak forecast. Res. Sq. 2024. preprint. [Google Scholar] [CrossRef]
- Anderson, E.J.; Kao, C.; Yildirim, I. Hospitalization Is an Underutilized Opportunity to Vaccinate for Influenza. Mayo Clin. Proc. 2019, 94, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Kaiser Permanente Department of Research & Evaluation. Flu Vaccine Is Safe for Hospitalized Patients. 2019. Available online: https://www.kp-scalresearch.org/flu-vaccine-is-safe-for-hospitalized-patients/ (accessed on 15 April 2025).
- Modin, D.; Jorgensen, M.E.; Gislason, G.; Jensen, J.S.; Kober, L.; Claggett, B.; Hegde, S.M.; Solomon, S.D.; Torp-Pedersen, C.; Biering-Sorensen, T. Influenza Vaccine in Heart Failure. Circulation 2019, 139, 575–586. [Google Scholar] [CrossRef]
- Vardeny, O.; Kim, K.; Udell, J.A.; Joseph, J.; Desai, A.S.; Farkouh, M.E.; Hegde, S.M.; Hernandez, A.F.; McGeer, A.; Talbot, H.K.; et al. Effect of High-Dose Trivalent vs. Standard-Dose Quadrivalent Influenza Vaccine on Mortality or Cardiopulmonary Hospitalization in Patients With High-risk Cardiovascular Disease: A Randomized Clinical Trial. JAMA 2021, 325, 39–49. [Google Scholar] [CrossRef]
- Saade, E.A.; Abul, Y.; McConeghy, K.; Davidson, H.E.; Han, L.; Joyce, N.; Canaday, D.H.; Hsueh, L.; Bosco, E.; Gravenstein, S. High-dose influenza vaccines for the prevention of hospitalization due to cardiovascular events in older adults in the nursing home: Post-hoc analysis of a cluster-randomized trial. Vaccine 2022, 40, 6700–6705. [Google Scholar] [CrossRef]
- Fonseca, H.A.R.; Furtado, R.H.M.; Zimerman, A.; Lemos, P.A.; Franken, M.; Monfardini, F.; Pedrosa, R.P.; Patriota, R.d.L.S.; Passos, L.C.S.; Dall’Orto, F.T.C.; et al. Influenza vaccination strategy in acute coronary syndromes: The VIP-ACS trial. Eur. Heart J. 2022, 43, 4378–4388. [Google Scholar] [CrossRef]
- Lapi, F.; Marconi, E.; Simonetti, M.; Baldo, V.; Rossi, A.; Sessa, A.; Cricelli, C. Adjuvanted versus nonadjuvanted influenza vaccines and risk of hospitalizations for pneumonia and cerebro/cardiovascular events in the elderly. Expert. Rev. Vaccines 2019, 18, 663–670. [Google Scholar] [CrossRef]
| Outcome | Study | Study Type | Results | Statistics |
|---|---|---|---|---|
| Cardiovascular death | FLUVACS [19] | RCT | Significant reduction | HR = 0.34 (95% CI: 0.17–0.71), p = 0.002 |
| Phrommintikul et al. [20] | RCT | No significant reduction | unadjusted HR 0.39 (95% CI: 0.14–1.12), p = 0.088 | |
| IVCAD [21] | RCT | No significant reduction | (29% vs. 26%, p = 0.60) | |
| IAMI [9] | RCT | Significant reduction | HR = 0.59 (95% CI, 0.39–0.90), p = 0.014 | |
| FLUCAD [4] | RCT | No significant reduction | HR = 1.06 (95% CI: 0.15–7.56), p = 0.95 | |
| Yedlapati et al. [22] | Meta-Analysis | Significant reduction | RR = 0.82 (95% CI: 0.80–0.84), p < 0.001 | |
| Clar et al. [23] | Meta-Analysis | Significant reduction | RR = 0.45 (95% CI: 0.26–0.76), p = 0.003 | |
| MACE | Phrommintikul et al. [20] | RCT | Significant reduction in ACS, HF, stroke | HR 0.70 (95% CI: 0.57–0.86), p = 0.088 |
| IAMI [9] | RCT | Significant reduction in AMI | HR = 0.72 (95% CI, 0.52–0.99), p = 0.040 | |
| Clar et al. [23] | Meta-Analysis | No significant reduction in MI | ||
| Yedlapati et al. [22] | Meta-Analysis | Significant reduction | RR = 0.87 (95% CI: 0.80–0.94), p < 0.001 | |
| Maniar et al. [24] | RCT | Significant reduction in MACE risk but No significant reduction for MI | RR 0.75 (95% CI: 0.57–0.97), I2 = 56% (RR, 0.73; 95% CI, 0.52–1.10, I2 = 0%) | |
| FLUVACS [19] | RCT | Significant reduction in MI | HR = 0.59 (95% CI 0.4—0.86), p = 0.004 | |
| FLUCAD [4] | RCT | Significant reduction in coronary ischemic events | HR 0.54 (95% CI: 0.24–1.21), p = 0.13 | |
| Zahhar et al. [25] | Systematic Review/Meta-Analysis | Significant reduction in stroke events | OR = 0.81, 95% CI [0.77–0.86], p = 0.00001 | |
| Holodinsky et al. [26] | Self-controlled case series | Significant reduction in stroke events | HR = 0.775 [95% CI 0.757–0.793] |
| Intervention | Cardiovascular Death | Primary Prevention of MI | Secondary Prevention of MI | Prevention of Stroke |
|---|---|---|---|---|
| Smoking cessation | 14.8–40% [29,30] | 11% [30] | 14.8–36% [29,31] | 30% [32] |
| Statin | 4–8% [33,34] | 21–27% [35] | 13–27% [33,34] | 12–17% [33,34] |
| Beta blockers | 15–31% [3,36] | - | 9% [36] | - |
| Blood-eluting agents | 12% [37] | - | 19% [37] | 39% [37] |
| Influenza vaccine | 18–55% | 6–19% | 13–46% | 19–25% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Khoury, A.; Abou Farah, J.; Saade, E. Is Influenza Vaccination Our Best ‘Shot’ at Preventing MACE? Review of Current Evidence, Underlying Mechanisms, and Future Directions. Vaccines 2025, 13, 522. https://doi.org/10.3390/vaccines13050522
El Khoury A, Abou Farah J, Saade E. Is Influenza Vaccination Our Best ‘Shot’ at Preventing MACE? Review of Current Evidence, Underlying Mechanisms, and Future Directions. Vaccines. 2025; 13(5):522. https://doi.org/10.3390/vaccines13050522
Chicago/Turabian StyleEl Khoury, Alexia, Joy Abou Farah, and Elie Saade. 2025. "Is Influenza Vaccination Our Best ‘Shot’ at Preventing MACE? Review of Current Evidence, Underlying Mechanisms, and Future Directions" Vaccines 13, no. 5: 522. https://doi.org/10.3390/vaccines13050522
APA StyleEl Khoury, A., Abou Farah, J., & Saade, E. (2025). Is Influenza Vaccination Our Best ‘Shot’ at Preventing MACE? Review of Current Evidence, Underlying Mechanisms, and Future Directions. Vaccines, 13(5), 522. https://doi.org/10.3390/vaccines13050522






